Acidithiobacillus thiooxidans DSM 26636: An Alternative for the Bioleaching of Metallic Burrs
Abstract
1. Introduction
2. Results
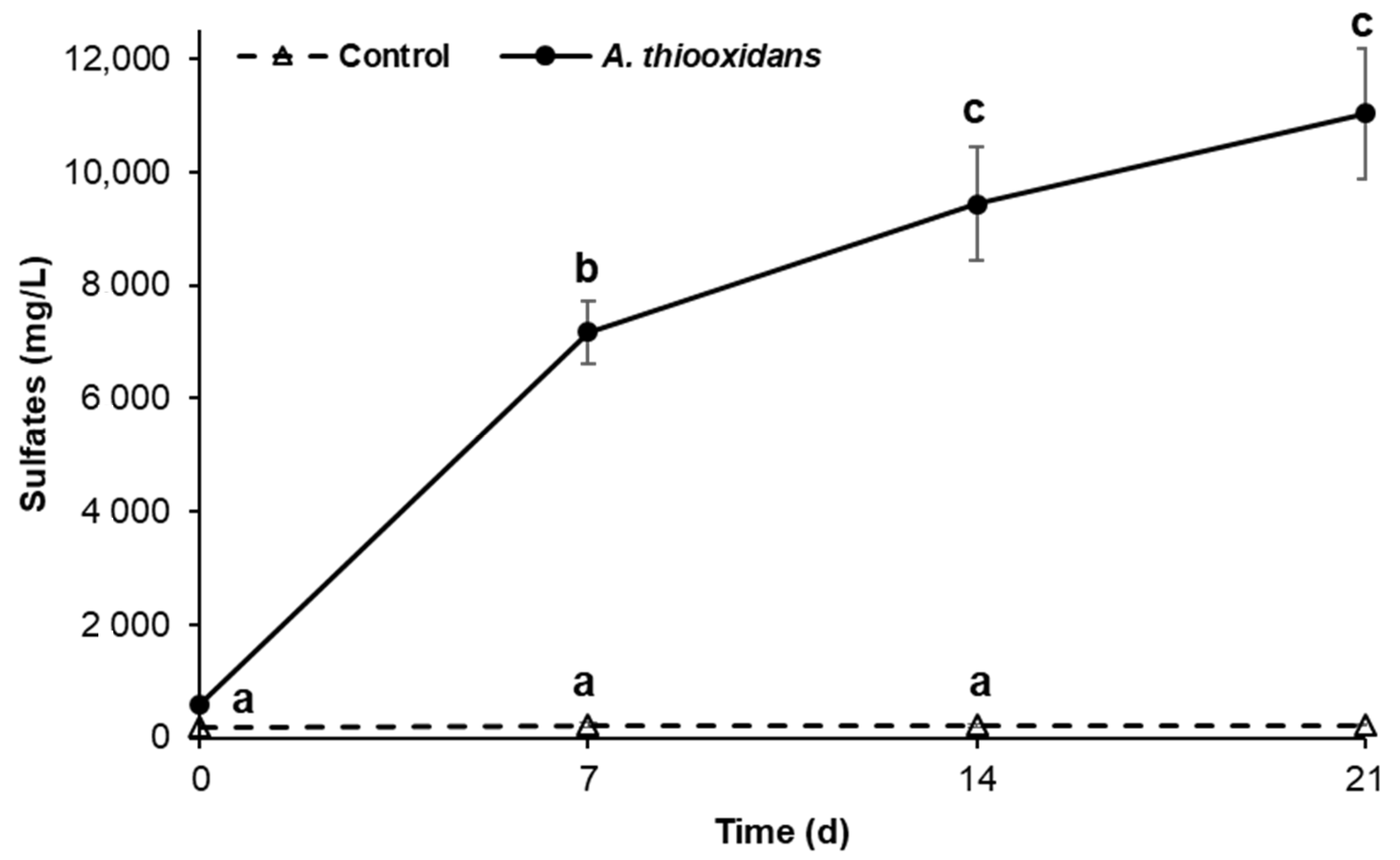
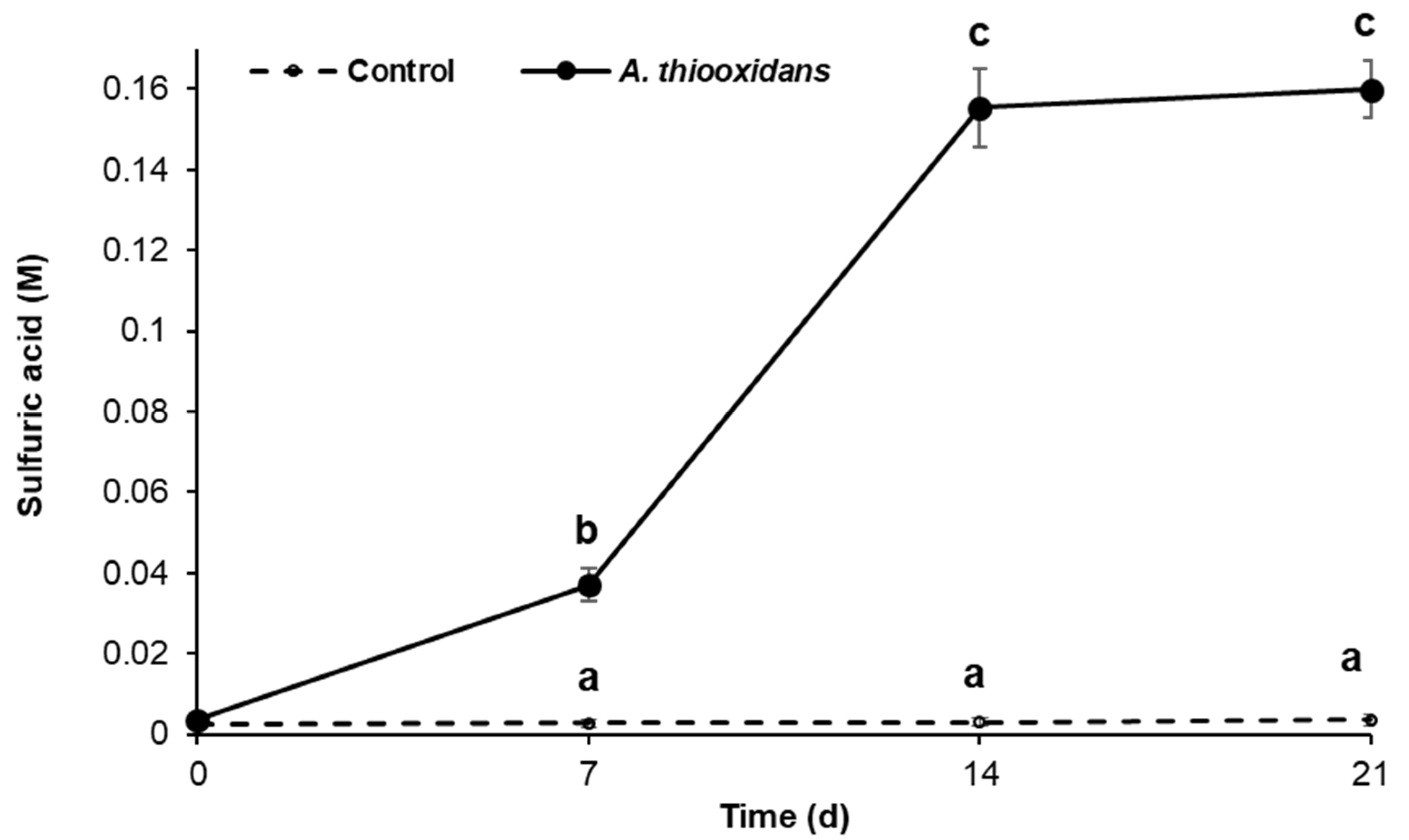
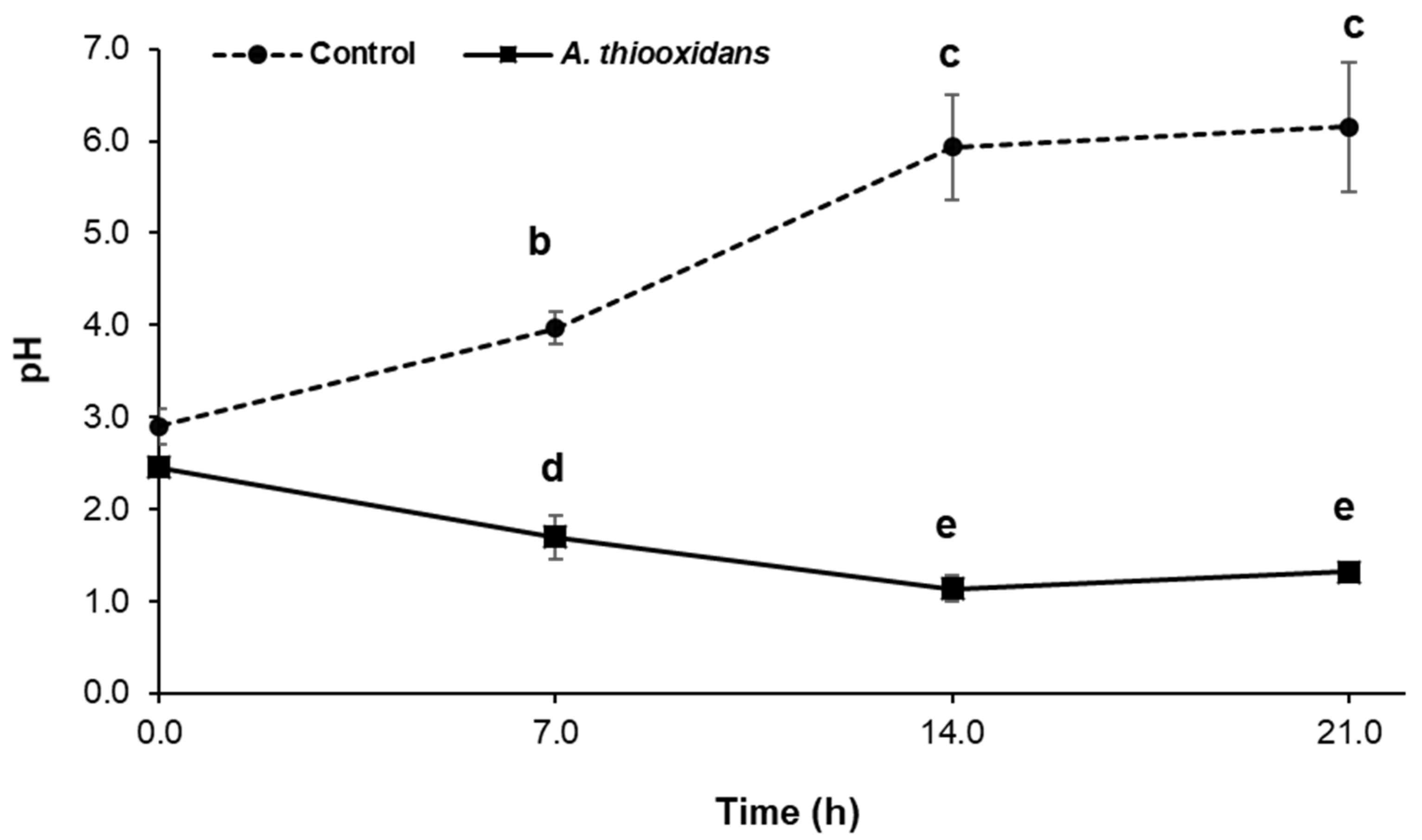
2.1. Sulfur-Oxidizing Activity of Acidithiobacillus Thiooxidans in the Presence of Metal Burrs
2.2. Bioleaching Activity during Metal Burrs Treatment
3. Discussion
4. Materials and Methods
4.1. Bacterium Strains and Growth Condition
4.2. Industrial Waste
4.3. Bioleaching of Metal by Acidithiobacillus thiooxidans DSM 26,636
4.4. Digestion and Analyses of Metals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, C.M.; Choi, Y.H.; Ha, J.H.; Woo, W.S. Eco-friendly technology for recycling of cutting fluids and metal burrs: A review. IJPEM—Green Technol. 2017, 4, 457–468. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Grosse, D.W. Treatment technologies for hazardous wastes: Part IV A review of alternative treatment processes for metal bearing hazardous waste streams. J. Air Pollut. Control. Assoc. 1986, 5, 603–614. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.E. Characteristics and adaptability of iron-and sulfur-oxidizing microrganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 2005, 4, 13. [Google Scholar] [CrossRef]
- Mishra, D.; Rhee, Y.H. Microbial leaching of metals from solid industrial wastes. J. Microbiol. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Rozas, E.E.; Mendes, M.A.; Nascimento, C.A.O.; Espinosa, D.C.R.; Oliveira, R.; Olivera, G.; Custodio, M.R. Bioleaching of electronic waste using bacteria isolated from the marine sponge Hymeniacidon heliophila (Porifera). J. Hazard. Mater. 2017, 329, 120–130. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Gómez-Ramírez, M.; Plata-González, A.; Fierros-Romero, G.; Rojas-Avelizapa, N.G. Novel filamentous fungi for metal removal from spent catalyst. Adv. Mater. Res. 2015, 1130, 673–676. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Li, X.; He, Z.; Liang, Y.; Guo, X.; Hu, Q.; Xiao, Y.; Cong, J.; Ma, L.; et al. Whole-genome sequencing reveals novel insights into sulfur oxidation in the extremophile Acidithiobacillus thiooxidans. BMC Microbiol. 2014, 14, 179. [Google Scholar] [CrossRef]
- Gómez-Ramírez, M.; Rivas-Castillo, A.; Rodríguez-Pozos, I.; Avalos-Zuñiga, R.A.; Rojas-Avelizapa, N.G. Feasibility study of mine tailing’s tretament by Acidithiobacillus thiooxidans DSM26636. World Acad. Sci. Eng. Technol. 2018, 12, 468–471. [Google Scholar] [CrossRef]
- Rivas-Castillo, A.M.; Gómez-Ramirez, M.; Rodríguez-Pozos, I.; Rojas-Avelizapa, N.G. Bioleaching of metals contained in spent catalysts by Acidithiobacillus thiooxidans DSM 26636. World Acad. Sci. Eng. Technol. 2018, 12, 430–434. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Hasan, M.; Mishra, Y.K.; Pandey, A.K.; Tiwary, B.N.; Kuhad, R.C.; Gupta, V.K.; Thakur, U.K. Environmentally sound system for E-waste: Biotechnological perspectives. Curr. Res. Biotechnol. 2019, 1, 58–64. [Google Scholar] [CrossRef]
- Li, Q.; Yang, B.; Zhu, J.; Jiang, H.; Li, J.; Zhang, R.; Sand, W. Comparative analysis of attachment to chalcopyrite of three mesophilic iron and/or sulfur-oxidizing acidophiles. Minerals 2018, 8, 406. [Google Scholar] [CrossRef]
- Zhai, Q.; Yuan, C. Separating manufacturing metal burrs for recycling through a combined hydrodynamic and electromagnetic approach. In Leveraging Technology for a Sustainable Word, Proceedings of the 19th CIRP Conference on Life Cycle Engineering, Berleley, CA, USA, 23–25 May 2012; Springer: Berlin/Heidelberg, Germany, 2012; pp. 167–172. [Google Scholar]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A Review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Das, S.; Natarajan, G.; Ting, Y.-P. Bio-Extraction of Precious Metals from Urban Solid Waste. AIP Conf. Proc. 2017, 1805, 020004. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Zhang, C.-J.; Yang, C.-L.; Gao, X.-Y.; Lin, C.-M.; Li, Y.-Q.; Li, Y.; et al. Sulfur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9, 3290. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramirez, M.; Zarco-Tovar, K.; Aburto, J.; De León, R.G.; Rojas-Avelizapa, N.G. Microbial treatment of sulfur-contaminated industrial wastes. J. Environ. Sci. Health Part A 2014, 49, 228–232. [Google Scholar] [CrossRef]
- Rojas-Avelizapa, N.G.; Hipólito-Juárez, I.V.; Gómez-Ramírez, M. Biological treatment of coal combustion wastes by Acidithiobacillus thiooxidans DSM 26636. Mex. J. Biotechnol. 2018, 3, 54–67. [Google Scholar] [CrossRef]
- Gómez-Ramírez, M.; Rojas-Avelizapa, N.G.; Hernández-Gama, R.; Tenorio-Sánchez, S.A.; López-Villegas, E.O. Potential use of Bacillus Genera for metals removal from spent catalysts. J. Environ. Sci. Health Part A 2019, 54, 701–710. [Google Scholar] [CrossRef]
- Gómez, R.M.; Tenorio, S.S.A. Parameters invoolved in biotreatment of solid waste containing metals. In Biotechnology for Treatment of Wastes Containing Metals; Rojas, A.N.G., Ed.; River Publishers: Gistrup, Denmark, 2019; pp. 43–64. [Google Scholar]
- Asghari, I.; Mousavi, S.M. Effects of key parameters in recycling of metals from petroleum refinery waste catalysts in bioleaching process. Rev. Environ. Sci. Biotechnol. 2014, 13, 139–161. [Google Scholar] [CrossRef]
- Andrzejewska-Górecka, D.A.; Poniatowska, A.; Macherzynski, B.; Wojewódka, D.; Sierakowski, M. Relese of critical metals from furnace waste using the process of bioleaching in various variants. Arch. Enviorn. Prot. 2019, 45, 72–78. [Google Scholar] [CrossRef]
- Yamanea, L.H.; Moraesa, V.T.; Tenórioa, J.A.S.; Espinosaa, D.C.R. Influence of bacterial adaptation on copper bioleaching from printed circuit boards. Adv. Biotech. Micro 2018, 9, 49–54. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio) chemistry of bacterial leaching-direct vs. Indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Laing, G.D.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Asghari, I.; Mousavi, S.M.; Amiri, F.; Tavassoli, S. Bioleaching of spent refinery catalysts: A review. J. Ind. Eng. Chem. 2013, 19, 1069–1081. [Google Scholar] [CrossRef]
- Hocheng, H.; Jadhav, U. Process of Biological Machining. In Handbook of Manufacturing Engineering and Technology; Nee, A.Y.C., Ed.; Springer: London, UK, 2015; p. 1691. [Google Scholar]
- Maluckov, B.S. The catalytic role of Acidithiobacillus ferrooxidans for metals extraction from mining—Metallurgical resource. Biodivers. Int. J. 2017, 1, 109–119. [Google Scholar] [CrossRef]
- Nareshkumar, R.; Nagendran, R.; Parvathi, K. Bioleaching of heavy metals from contaminated soil using Acidithiobacillus thiooxidans: Effect of sulfur/soil ratio. World J. Microbiol. Biotechnol. 2008, 24, 1539–1546. [Google Scholar] [CrossRef]
- Ferreira, F.P.; Sérvulo, E.F.C.; da Costa, A.C.A.; Ferreira, D.M.; Godoy, M.L.D.P.; Oliveira, F.J.S. Bioleaching of metals from a spent diesel hydrodesulfurization catalyst employing Acidithiobacillus Thiooxidans FG-01. Braz. J. Chem. Eng. 2017, 34, 119–129. [Google Scholar] [CrossRef]
- Hocheng, H.; Hong, T.; Jadhav, U. Microbial leaching of waste solder for recovery of metal. Appl. Biochem. Biotechnol. 2014, 173, 193–204. [Google Scholar] [CrossRef]
- Rojas-Avelizapa, N.G.; Gómez-Ramírez, M.; Hernández-Gama, R.; Aburto, J.; García de León, R. Isolation and selection of sulfur-oxidizing bacteria for the treatment of sulfur-containing hazardous wastes. Chem. Biochem. Eng. Q. 2013, 27, 109–117. [Google Scholar]
- Takakuwa, S.; Nishiwaki, T.; Hosoda, K.; Tominaga, N.; Iwasaki, H. Promoting effect of molybdate on the growth of a sulfur-oxidizing bacterium, Thiobagillus thiooxidans. J. Gen. Appl. Microbiol. 1977, 23, 163–173. [Google Scholar] [CrossRef]
- Secretaria de Comercio y Foment Industrial. Norma Mexicana NMX-AA-074-1981. Analysis of Water—Determination of Sulfate Ion. Available online: https://agua.org.mx/biblioteca/nmx-aa-074-1981-analisis-de-agua-determinacion-del-ion-sulfato (accessed on 30 January 2020).
- Cerruti, C.; Curutchet, G.; Donati, E. Bio-dissolution of spent nickel–cadmium batteries using Thiobacillus ferrooxidans. J. Biotechnol. 1986, 2, 209–219. [Google Scholar] [CrossRef]

| Element | Content in mg/kg |
|---|---|
| Fe | 410,159.5 ± 21,163.2 |
| Cr | 39,242.3 ± 2428.2 |
| Si | 13,819.0 ± 5460.8 |
| Al | 11,126.9 ± 13,030.5 |
| Mo | 9664.9 ± 573.4 |
| V | 7453.1 ± 440.6 |
| Mn | 4737.9 ± 248.2 |
| Mg | 1894.0 ± 1287.6 |
| Ni | 1611.4 ± 229.1 |
| Cu | 829.9 ± 122.7 |
| Sb | 450.0 ± 47.4 |
| Li | 298.3 ± 7.0 |
| Zn | 176.2 ± 76.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marlenne, G.-R.; Fernanda, M.-V.; Norma G, R.-A. Acidithiobacillus thiooxidans DSM 26636: An Alternative for the Bioleaching of Metallic Burrs. Catalysts 2020, 10, 1230. https://doi.org/10.3390/catal10111230
Marlenne G-R, Fernanda M-V, Norma G R-A. Acidithiobacillus thiooxidans DSM 26636: An Alternative for the Bioleaching of Metallic Burrs. Catalysts. 2020; 10(11):1230. https://doi.org/10.3390/catal10111230
Chicago/Turabian StyleMarlenne, Gómez-Ramírez, Moreno-Villanueva Fernanda, and Rojas-Avelizapa Norma G. 2020. "Acidithiobacillus thiooxidans DSM 26636: An Alternative for the Bioleaching of Metallic Burrs" Catalysts 10, no. 11: 1230. https://doi.org/10.3390/catal10111230
APA StyleMarlenne, G.-R., Fernanda, M.-V., & Norma G, R.-A. (2020). Acidithiobacillus thiooxidans DSM 26636: An Alternative for the Bioleaching of Metallic Burrs. Catalysts, 10(11), 1230. https://doi.org/10.3390/catal10111230





