Direct Alcoholysis of Carbohydrate Precursors and Real Cellulosic Biomasses to Alkyl Levulinates: A Critical Review
Abstract
1. Introduction
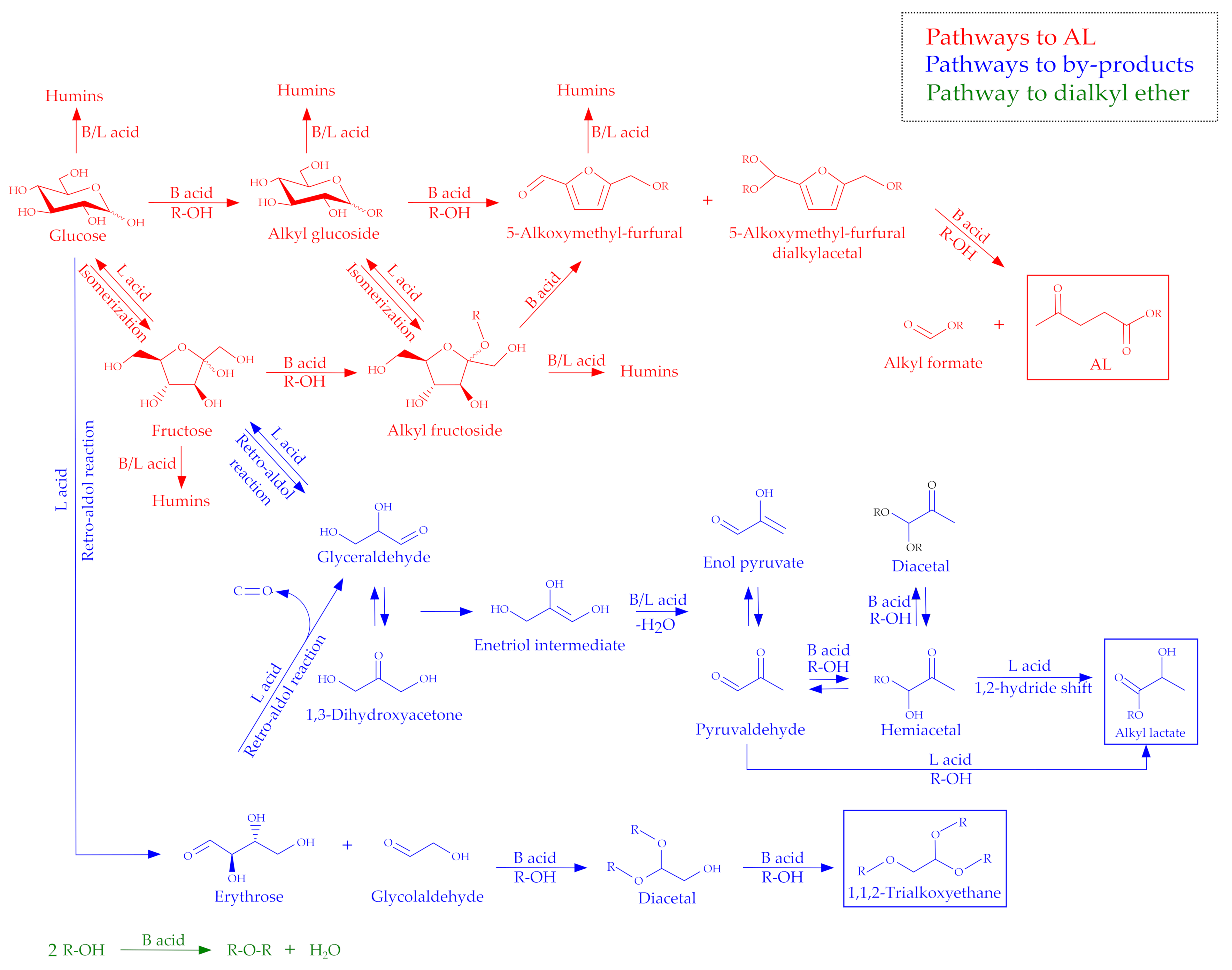
1.1. Reaction Mechanisms
1.2. Possible AL Applications and Aim of the Review
2. AL Synthesis from Model Compounds
2.1. ML Synthesis from Model Carbohydrates
2.2. EL Synthesis from Model Carbohydrates
2.3. PL Synthesis from Model Carbohydrates
2.4. BL and Longer-Chain AL (PeL and HL) Synthesis from Model Carbohydrates
3. AL Synthesis from Real Biomass
3.1. ML Synthesis from Real Biomass
3.2. EL Synthesis from Real Biomass
3.3. PL, BL and Longer-Chain AL (PeL and HL) Synthesisfrom Real Biomass
4. Considerations on the Catalysis Issues, Main Process Bottlenecks and Improvable Aspects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pileidis, F.D.; Titirici, M.-M. Levulinic acid biorefineries: New challenges for efficient utilization of biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Raspolli Galletti, A.M. New frontiers in the catalytic synthesis of levulinic acid: From sugars to raw and waste biomass as starting feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic production of levulinic acid (LA) from actual biomass. Molecules 2019, 24, 2760. [Google Scholar] [CrossRef] [PubMed]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Girisuta, B.; Heeres, H.J. Levulinic acid from biomass: Synthesis and applications. In Production of Platform Chemicals from Sustainable Resources; Fang, Z., Smith, R.L., Qi, X., Eds.; Springer Nature: Singapore, 2017; pp. 143–169. [Google Scholar] [CrossRef]
- Hayes, G.C.; Becer, C.R. Levulinic acid: A sustainable platform chemical for novel polymer architectures. Polym. Chem. 2020, 11, 4068–4077. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. A review on catalytic synthesis of energy rich fuel additive levulinate compounds from biomass derived levulinic acid. Fuel Process. Technol. 2020, 197, 106213–106231. [Google Scholar] [CrossRef]
- Freitas, F.A.; Licursi, D.; Lachter, E.R.; Raspolli Galletti, A.M.; Antonetti, C.; Brito, T.C.; Nascimento, R.S.V. Heterogeneous catalysis for the ketalisation of ethyl levulinate with 1,2-dodecanediol: Opening the way to a new class of bio-degradable surfactants. Catal. Commun. 2016, 73, 84–87. [Google Scholar] [CrossRef]
- Garcia-Ortiz, A.; Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Transforming methyl levulinate into biosurfactants and biolubricants by chemoselective reductive etherification with fatty alcohols. ChemSusChem 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of levulinic acid and alkyl levulinates into biofuels and high-value chemicals. Green Chem. 2017, 19, 5527–5547. [Google Scholar] [CrossRef]
- Biancalana, L.; Fulignati, S.; Antonetti, S.; Zacchini, S.; Provinciali, G.; Pampaloni, G.; Raspolli Galletti, A.M.; Marchetti, F. Ruthenium p-cymene complexes with α-diimine ligands as catalytic precursors for the transfer hydrogenation of ethyl levulinate to γ-valerolactone. New J. Chem. 2018, 42, 17574–17586. [Google Scholar] [CrossRef]
- Van der Waal, J.C.; De Jong, E. Avantium Chemicals: The high potential for the levulinic product tree. In Industrial Biorenewables: A Practital Viewpoint; de María, P.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 97–120. [Google Scholar] [CrossRef]
- Shrikhande, S.; Babu, G.U.B.; Ahmad, Z.; Patle, D.S. Intensification and analysis of ethyl levulinate production process having a reactive distillation through vapor recompression and bottom flash techniques. Chem. Eng. Process. 2020, 156, 108081. [Google Scholar] [CrossRef]
- Ahmad, E.; Alam, I.; Pant, K.K.; Haider, M.A. Catalytic and mechanistic insights into the production of ethyl levulinate from biorenewable feedstocks. Green Chem. 2016, 18, 4804–4823. [Google Scholar] [CrossRef]
- Ethyl Levulinate Market Size, Share & Trends Analysis Report by Application (Flavors, Fragrance), by Region (North America, Europe, Asia Pacific, Middle East & Africa, Central & South America) and Segment Forecast, 2015–2022. Available online: https://www.grandviewresearch.com/industry-analysis/ethyl-levulinate-market (accessed on 1 September 2020).
- Silva, J.F.L.; Grekin, R.; Mariano, A.P.; MacielFilho, R. Making levulinic acid and ethyl levulinate economically viable: A worldwide technoeconomic and environmental assessment of possible routes. Energy Technol. 2018, 6, 613–639. [Google Scholar] [CrossRef]
- Ding, D.; Xi, J.; Wang, J.; Liu, X.; Lu, G.; Wang, Y. Production of methyl levulinate from cellulose: Selectivity and mechanism study. Green Chem. 2015, 17, 4037–4044. [Google Scholar] [CrossRef]
- Antonetti, C.; Bonari, E.; Licursi, D.; Nassi, N.; Raspolli Galletti, A.M. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 2015, 20, 21232–21253. [Google Scholar] [CrossRef]
- Rivas, S.; Raspolli Galletti, A.M.; Antonetti, C.; Santos, V.; Parajó, J.C. Sustainable conversion of Pinus pinaster wood into biofuel precursors: A biorefinery approach. Fuel 2016, 164, 51–58. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A promising technology for conversion of lignocellulose and platform chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. High-yield conversion of plant biomass into the key value-added feedstocks 5-(hydroxymethyl)furfural, levulinic acid, and levulinic esters via 5-(chloromethyl)furfural. Green Chem. 2010, 12, 370–373. [Google Scholar] [CrossRef]
- Mascal, M. 5-(Chloromethyl)furfural is the new HMF: Functionally equivalent but more practical in terms of its production from biomass. ChemSusChem 2015, 8, 3391–3395. [Google Scholar] [CrossRef]
- Mascal, M. 5-(Chloromethyl)furfural (CMF): A platform for transforming cellulose into commercial products. ACS Sustain. Chem. Eng. 2019, 7, 5588–5601. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Y.; Zhao, G.; Chen, X.; Han, L.; Xiao, W. Impact of biomass feedstock variability on acid-catalyzed alcoholysis performance. Fuel Process. Technol. 2018, 180, 14–22. [Google Scholar] [CrossRef]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Brønsted acids on catalytic hydrothermal decomposition of hexose to levulinic acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Tominaga, K.; Mori, A.; Fukushima, Y.; Shimada, S.; Sato, K. Mixed-acid systems for the catalytic synthesis of methyl levulinate from cellulose. Green Chem. 2011, 13, 810–812. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Riisager, A. Solid acid catalysed formation of ethyl levulinate and ethyl glucopyranoside from mono- and disaccharides. Catal. Commun. 2012, 17, 71–75. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, H.; Nan, J.; Wu, L.; Yang, X.; Su, Y.; Lu, T.; Xu, J. Conversion of carbohydrate biomass to methyl levulinate with Al2(SO4)3 as a simple, cheap and efficient catalyst. Catal. Commun. 2014, 50, 13–16. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Riisager, A.; Taarning, E.; Meier, S. Combined function of Brønsted and Lewis acidity in the zeolite-catalyzed isomerization of glucose to fructose in alcohols. ChemCatChem 2016, 8, 3107–3111. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balua, A.M.; Van der Waal, J.C.; Luque, R. Catalytic insights into the production of biomass-derived side products methyl levulinate, furfural and humins. Catal. Today 2018, 302, 2–15. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Yang, T.; Lin, Y.-T.; Zhu, Y.-Z.; Li, L.-C.; Pan, H. Facile and high-yield synthesis of methyl levulinate from cellulose. Green Chem. 2018, 20, 1323–1334. [Google Scholar] [CrossRef]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Raspolli Galletti, A.M. Turning point toward the sustainable production of 5-hydroxymethyl-2-furaldehyde in water: Metal salts for its synthesis from fructose and inulin. ACS Sustain. Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Ju, R.; He, X.; Zhang, Y.; Tang, S.; Ma, L. Condensation of α-carbonyl aldehydes leads to the formation of solid humins during the hydrothermal degradation of carbohydrates. ACS Omega 2019, 4, 7330–7343. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, C.-Z. Levulinic esters from the acid-catalysed reactions of sugars and alcohols as part of a bio-refinery. Green Chem. 2011, 13, 1676–1679. [Google Scholar] [CrossRef]
- Hu, X.; Lievens, C.; Larcher, A.; Li, C.Z. Reaction pathways of glucose during esterification: Effects of reaction parameters on the formation of humin type polymers. Bioresour. Technol. 2011, 102, 10104–10113. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Liu, Q.; Cen, H.; Ju, R.; He, X.; Ma, L. Formation of humins during degradation of carbohydrates and furfural derivatives in various solvents. Biomass Convers. Biorefin. 2020, 10, 277–287. [Google Scholar] [CrossRef]
- Cheng, Z.; Everhart, J.L.; Tsilomelekis, G.; Nikolakis, V.; Saha, B.; Vlachos, D.G. Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem. 2018, 20, 997–1006. [Google Scholar] [CrossRef]
- Zhu, W.; Chang, C.; Ma, C.; Du, F. Kinetics of glucose ethanolysis catalyzed by extremely low sulfuric acid in ethanol medium. Chin. J. Chem. Eng. 2014, 22, 238–242. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Liu, X.; Xia, Q.; Wang, Y. Comprehensive understanding the role of Brønsted and Lewis acid sites in glucose conversion to 5-hydromethylfurfural. ChemCatChem 2017, 9, 2739–2746. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and applications of alkyl levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Comment on processes for the direct conversion of cellulose or cellulosic biomass into levulinate esters. ChemSusChem 2010, 3, 1349–1351. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Li, H. Extremely low sulfuric acid catalyst system for synthesis of methyl levulinate from glucose. Ind. Crops Prod. 2012, 40, 136–144. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Li, H.; Yang, Q. Conversion of carbohydrates biomass into levulinate esters using heterogeneous catalysts. Appl. Energy 2011, 88, 4590–4596. [Google Scholar] [CrossRef]
- Huq, N.A.; Huo, X.; Hafenstine, G.R.; Tifft, S.M.; Stunkel, J.; Christensen, E.D.; Fioroni, G.M.; Fouts, L.; McCormick, R.L.; Cherry, P.A.; et al. Performance-advantaged ether diesel bioblendstock production by a priori design. Proc. Natl. Acad. Sci. USA 2019, 116, 26421–26430. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, G.; Khan, T.S.; Agarwal, M.; Haider, M.A. Reformulation of gasoline to replace aromatics by biomass-derived alkyl levulinates. ACS Sustain. Chem. Eng. 2017, 5, 7118–7127. [Google Scholar] [CrossRef]
- Christensen, E.; Williams, A.; Paul, S.; Burton, S.; McCormick, R.L. Properties and performance of levulinate esters as diesel blend components. Energy Fuels 2011, 25, 5422–5428. [Google Scholar] [CrossRef]
- Tabrizi, M.M.; Chermahini, A.N.; Mohammadbagheri, Z. Synthesis of hexyl levulinate as a potential fuel additive from levulinic acid over a solid acid catalyst. J. Environ. Chem. Eng. 2019, 7, 103420–103426. [Google Scholar] [CrossRef]
- Jia, S.; Ma, J.; Wang, D.; Wang, K.; Zheng, Q.; Song, C.; Guo, X. Fast and efficient upgrading of levulinic acid into long-chain alkyl levulinate fuel additives with a tungsten salt catalyst at low temperature. Sustain. Energy Fuels 2020, 4, 2018–2025. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Green synthesis of amyl levulinate using lipase in the solvent free system: Optimization, mechanism and thermodynamics studies. Catal. Today 2020. In press. [Google Scholar] [CrossRef]
- Mukesh, C.; Nikjoo, D.; Mikkola, J.-P. Production of C-14 levulinate ester from glucose fermentation liquors catalyzed by acidic ionic liquids in a solvent-free self-biphasic system. ACS Omega 2020, 5, 4828–4835. [Google Scholar] [CrossRef]
- Antonetti, C.; Gori, S.; Licursi, D.; Pasini, G.; Frigo, S.; López, M.; Parajó, J.C.; Raspolli Galetti, A.M. One-pot alcoholysis of the lignocellulosic Eucalyptus nitens biomass to n-butyl levulinate, a valuable additive for diesel motor fuel. Catalysts 2020, 10, 509. [Google Scholar] [CrossRef]
- Kremer, F.; Pischinger, S. Butyl ethers and levulinates. In Biofuels from Lignocellulosic Biomass: Innovations beyond Bioethanol; Boot, M., Ed.; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 87–104. [Google Scholar] [CrossRef]
- Ma, Y.; Tan, W.; Wang, K.; Wang, J.; Jiang, J.; Xu, J. An insight into the selective conversion of bamboo biomass to ethyl glycosides. ACS Sustain. Chem. Eng. 2017, 5, 5880–5886. [Google Scholar] [CrossRef]
- Chang, C.; Deng, L.; Xu, G. Efficient conversion of wheat straw into methyl levulinate catalyzed by cheap metal sulfate in a biorefinery concept. Ind. Crops Prod. 2018, 117, 197–204. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Tan, W.; Jiang, J.; Xu, J.; Wang, K. Directional liquefaction of lignocellulosic biomass for value added monosaccharides and aromatic compounds. Ind. Crops Prod. 2019, 135, 251–259. [Google Scholar] [CrossRef]
- Renault, L.; Pessel, F.; Benvegnu, T. Surfactants based on green/blue sugars: Towards new functionalities in formulations. In Carbohydrate Chemistry: Chemical and Biological Approaches; Rauter, A.P., Lindhorst, T., Queneau, Y., Eds.; RSC: Croydon, UK, 2018; Volume 43, pp. 196–244. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Liu, K.; Hong, M.; You, R.; Qu, P.; Chen, M. Subcritical methanolysis of starch and transglycosidation to produce dodecyl polyglucosides. ACS Omega 2019, 4, 16372–16377. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, Y.-Z.; Chen, Y.; Yang, C.-Y.; Yang, Y.-Y. One-step alcoholysis of lignin into small-molecular aromatics: Influence of temperature, solvent, and catalyst. Biotechnol. Rep. 2019, 24, e00363. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Raspolli Galletti, A.M. New intensification strategies for the direct conversion of real biomass into platform and fine chemicals: What are the main improvable key aspects? Catalysts 2020, 10, 961. [Google Scholar] [CrossRef]
- Peng, L.; Li, H.; Xi, L.; Chen, K.; Chen, H. Facile and efficient conversion of furfuryl alcohol into n-butyl levulinate catalyzed by extremely low acid concentration. BioResources 2014, 9, 3825–3834. [Google Scholar] [CrossRef]
- Xiros, C.; Janssen, M.; Byström, R.; Børresen, B.T.; Cannella, D.; Jørgensen, H.; Koppram, R.; Larsson, C.; Olsson, L.; Tillman, A.M.; et al. Toward a sustainable biorefinery using high-gravity technology. Biofuels Bioprod. Biorefin. 2017, 11, 15–27. [Google Scholar] [CrossRef]
- Chappaz, A.; Lai, J.; De Oliveira Vigier, K.; Morvan, D.; Wischert, R.; Corbet, M.; Doumert, B.; Trivelli, X.; Liebens, A.; Jerome, F. Selective conversion of concentrated feeds of furfuryl alcohol to alkyl levulinates catalyzed by metal triflates. ACS Sustain. Chem. Eng. 2018, 6, 4405–4411. [Google Scholar] [CrossRef]
- Covinich, L.G.; Clauser, N.M.; Felissia, F.E.; Vallejos, M.E.; Area, M.C. The challenge of converting biomass polysaccharides into levulinic acid through heterogeneous catalytic processes. Biofuels Bioprod. Biorefin. 2020, 14, 417–445. [Google Scholar] [CrossRef]
- Peng, L.; Li, H.; Lin, L.; Chen, K. Effect of metal salts existence during the acid-catalyzed conversion of glucose in methanol medium. Catal. Commun. 2015, 59, 10–13. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Hou, T.; Han, L.; Xiao, W. Catalysis performance comparison of a Brønsted acid H2SO4 and a Lewis acid Al2(SO4)3 in methyl levulinate production from biomass carbohydrates. J. Energy Chem. 2018, 27, 552–558. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, J.; Hse, C.-Y.; Yang, Z.; Wang, K.; Ye, J.; Xu, J. Selective catalytic conversion of waste lignocellulosic biomass for renewable value-added chemicals via directional microwave-assisted liquefaction. Sustain. Energy Fuels 2018, 2, 1035–1047. [Google Scholar] [CrossRef]
- Garves, K. Synthesis of Alkoxymethylfurfurals and Alkyl Levulinates from Cellulose or Lignocelluloses or Starch and. Alcohols. Patent DE3621517A1, 7 January 1988. [Google Scholar]
- Li, H.; Peng, L.; Lin, L.; Chen, K.; Zhang, H. Synthesis, isolation and characterization of methyl levulinate from cellulose catalyzed by extremely low concentration acid. J. Energy Chem. 2013, 22, 895–901. [Google Scholar] [CrossRef]
- Wu, X.; Fu, J.; Lu, X. One-pot preparation of methyl levulinate from catalytic alcoholysis of cellulose in near-critical methanol. Carbohydr. Res. 2012, 358, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, J.R.; Oliveira, M.C.; Fernandes, A.C. HReO4 as highly efficient and selective catalyst for the conversion of carbohydrates into value added chemicals. Mol. Catal. 2019, 465, 87–94. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, L.; Shao, Y.; Li, Q.; Fan, H.; Gao, G.; Zhang, S.; Liu, Q.; Wang, Y.; Hua, X. Conversion of monosaccharides into levulinic acid/esters: Impacts of metal sulfate addition and the reaction medium. J. Chem. Technol. Biotechnol. 2019, 94, 3676–3686. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Mei, J.; Zhao, G.; Lyu, Q.; Lyu, X.; Lyu, H.; Han, L.; Xiao, W. Ball milling for cellulose depolymerization and alcoholysis to produce methyl levulinate at mild temperature. Fuel Process. Technol. 2019, 188, 129–136. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, D.; Yang, J.; Yang, X.; Su, Y.; Lu, T. Conversion of recalcitrant cellulose to alkyl levulinates and levulinic acid via oxidation pretreatment combined with alcoholysis over Al2(SO4)3. Cellulose 2020, 27, 1451–1463. [Google Scholar] [CrossRef]
- Deng, W.P.; Liu, M.; Zhang, Q.H.; Tan, X.S.; Wang, Y. Acid-catalysed direct transformation of cellulose into methyl glucosides in methanol at moderate temperatures. Chem. Commun. 2010, 46, 2668–2670. [Google Scholar] [CrossRef]
- Pöpken, T.; Götze, L.; Gmehling, J. Reaction kinetics and chemical equilibrium of homogeneously and heterogeneously catalyzed acetic acid esterification with methanol and methyl acetate hydrolysis. Ind. Eng. Chem. Res. 2000, 39, 2601–2611. [Google Scholar] [CrossRef]
- Nemoto, K.; Tominaga, K.; Sato, K. Straightforward synthesis of levulinic acid ester from lignocellulosic biomass resources. Chem. Lett. 2014, 43, 1327–1329. [Google Scholar] [CrossRef]
- Tominaga, K.; Nemoto, K.; Kamimura, Y.; Yamada, A.; Yamamotoc, Y.; Sato, K. A practical and efficient synthesis of methyl levulinate from cellulosic biomass catalyzed by an aluminum-based mixed acid catalyst system. RSC Adv. 2016, 6, 65119–65124. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Huang, X.; Chen, L.; Ma, L.; Li, X. Conversion of fructose into 5-hydroxymetylfurfural and alkyl levulinates catalyzed by sulfonic acid-functionalized carbon materials. Green Chem. 2013, 15, 2895–2903. [Google Scholar] [CrossRef]
- Pan, H.; Liu, X.; Zhang, H.; Yang, K.; Huang, S.; Yang, S. Multi-SO3H functionalized mesoporous polymeric acid catalyst for biodiesel production and fructose-to-biodiesel additive conversion. Renew. Energy 2017, 107, 245–252. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, H.; Cui, L.; Bai, Y.; Bian, J.; Lu, T.; Su, Y.; Yang, X. Promotion effect of mesopore on the conversion of carbohydrates to methyl levulinate over H-USY zeolite. Catal. Commun. 2015, 71, 74–78. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, L.; Chao, J.; Zhao, H.; Lu, T.; Su, Y.; Yang, X.; Xu, J. Direct catalytic conversion of carbohydrates to methyl levulinate: Synergy of solid Brønsted acid and Lewis acid. Appl. Catal. B Environ. 2018, 220, 589–596. [Google Scholar] [CrossRef]
- Yu, F.; Zhong, R.; Chong, H.; Smet, M.; Dehaen, W.; Sels, B.F. Fast catalytic conversion of recalcitrant cellulose into alkyl levulinates and levulinic acid in the presence of soluble and recoverable sulfonated hyperbranched poly(aryleneoxindole)s. Green Chem. 2017, 19, 153–163. [Google Scholar] [CrossRef]
- Dora, S.; Bhaskar, T.; Singh, R.; Naik, D.V.; Adhikari, D.K. Effective catalytic conversion of cellulose into high yields of methyl glucosides over sulfonated carbon based catalyst. Bioresour. Technol. 2012, 120, 318–321. [Google Scholar] [CrossRef]
- Palermo, V.; Sathicq, A.G.; Romanelli, G.P. Suitable transformation of compounds present in biomass using heteropoly compounds as catalysts. Curr. Opin. Green Sustain. 2020, 25, 100362–100368. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Thomas, H.J.; Climent, M.J.; Romanelli, G.P.; Iborra, S. Heteropoly compounds as catalysts for biomass product transformations. Catal. Rev. 2016, 58, 497–586. [Google Scholar] [CrossRef]
- Misono, M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 13, 1141–1152. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.-L.; Wu, H.-Z.; Dong, W.-S. An efficient catalyst for the conversion of fructose into methyl levulinate. Catal. Lett. 2013, 143, 1346–1353. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wang, S.; Li, X.; Wang, X.; Shi, J. Hydrolysis and alcoholysis of polysaccharides with high efficiency catalyzed by a (C16TA)xH6-xP2W18O62 nanoassembly. RSC Adv. 2015, 5, 94155–94163. [Google Scholar] [CrossRef]
- Deng, W.; Liu, M.; Zhang, Q.; Wang, Y. Direct transformation of cellulose into methyl and ethyl glucosides in methanol and ethanol media catalyzed by heteropolyacids. Catal. Today 2011, 164, 461–466. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xue, L.; Wang, S.; Wang, X.; Jiang, Z. Catalyzing cascade production of methyl levulinate from polysaccharides using heteropolyacids HnPW11MO39 with Brønsted/Lewis acidic sites. ACS Sustain. Chem. Eng. 2018, 6, 165–176. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Chaves, D.M.; da Silva, M.J. One-pot synthesis of alkyl levulinates from biomass derivative carbohydrates in tin(II) exchanged silicotungstates-catalyzed reactions. Cellulose 2019, 26, 7953–7969. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Lyu, X.; Zhao, G.; Zhao, T.; Han, L.; Xiao, W. Aluminum phosphotungstate as a promising bifunctional catalyst for biomass carbohydrate transformation to methyl levulinate under mild conditions. J. Clean. Prod. 2019, 215, 712–720. [Google Scholar] [CrossRef]
- Rataboul, F.; Essayem, N. Cellulose reactivity in supercritical methanol in the presence of solid acid catalysts: Direct synthesis of methyl-levulinate. Ind. Eng. Chem. Res. 2011, 50, 799–805. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Chen, L. Efficient production of 5-hydroxymethylfurfural and alkyl levulinate from biomass carbohydrate using ionic liquid-based polyoxometalate salts. RCS Adv. 2014, 4, 4194–4202. [Google Scholar] [CrossRef]
- Song, C.; Liu, S.; Peng, X.; Long, J.; Lou, W.; Li, X. Catalytic conversion of carbohydrates to levulinate ester over heteropolyanion-based ionic liquids. ChemSusChem 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Li, Y.; Zhao, W.; Wu, W.; Li, H.; He, C.; Yang, S. Phosphotungstic acid heterogenized by assembly with pyridines for efficient catalytic conversion of fructose to methyl levulinate. RSC Adv. 2018, 8, 16585–16592. [Google Scholar] [CrossRef]
- Fang, C.; Li, Y.; Yu, Z.; Li, H.; Yang, S. Efficient catalytic upgrade of fructose to alkyl levulinates with phenylpyridine-phosphotungstate solid hybrids. Curr. Green Chem. 2019, 6, 44–52. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Li, Y.; Bawa, M.; Wang, S.; Wang, X.; Jiang, Z. First triple-functional polyoxometalate Cs10.6[H2.4GeNb13O41] for highly selective production of methyl levulinate directly from cellulose. Cellulose 2018, 25, 6405–6419. [Google Scholar] [CrossRef]
- Serrano, D.P.; Melero, J.A.; Morales, G.; Iglesias, J.; Pizarro, P. Progress in the design of zeolite catalysts for biomass conversion into biofuels and bio-based chemicals. Catal. Rev. 2018, 60, 1–70. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Riisager, A. Zeolite catalyzed transformation of carbohydrates to alkyl levulinates. ChemCatChem 2013, 5, 1754–1757. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Luo, J.; Yang, S. Direct conversion of biomass components to the biofuel methyl levulinate catalyzed by acid–base bifunctional zirconia-zeolites. Appl. Catal. B Environ. 2017, 200, 182–191. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Gao, B.; Lu, T.; Zhou, L. Conversion of glucose to methyl levulinate over Sn-Al-β zeolite: Role of Sn and mesoporosity. Catal. Commun. 2019, 130, 105783–105786. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, X.; Zhang, Y.; Jiang, L.; Zhou, L.; Lu, T.; Su, Y. Efficient conversion of cellulose to methyl levulinate over heteropoly acid promoted by Sn-Beta zeolite. Cellulose 2019, 26, 9135–9147. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.B.; Wang, X.-Q.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Glucose conversion to methyl levulinate catalyzed by metal ion-exchanged montmorillonites. Appl. Clay Sci. 2017, 141, 118–124. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.-Q.; Yang, B.-B.; Liu, C.-L.; Xu, C.-L.; Dong, W.-S. Highly efficient conversion of glucose into methyl levulinate catalyzed by tin-exchanged montmorillonite. Renew. Energy 2018, 120, 231–240. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Zou, W.; Yue, H.; Tian, G.; Feng, S. Conversion of carbohydrates to methyl levulinate catalyzed by sulfated montmorillonite. Catal. Commun. 2015, 62, 67–70. [Google Scholar] [CrossRef]
- Lai, F.; Luo, J.; Jiang, D.; Su, T.; Zhang, F. Iron(III)-modified tungstophosphoric acid supported on silica-pillared montmorillonite as catalysts for fructose conversion to methyl levulinate. J. Chem. Technol. Biotechnol. 2018, 93, 557–568. [Google Scholar] [CrossRef]
- Shao, Y.; Du, W.; Gao, Z.; Sun, K.; Zhang, Z.; Li, Q.; Zhang, L.; Zhang, S.; Liu, Q.; Hu, X. Sulfated TiO2nanosheets catalyzing conversion of biomass derivatives: Influences of the sulfation on distribution of Brønsted and Lewis acidic sites. J. Chem. Technol. Biotechnol. 2020, 95, 1337–1347. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, F.-S. Nanoporous catalysts for biomass conversion. Green Chem. 2015, 17, 24–39. [Google Scholar] [CrossRef]
- Kuo, C.H.; Poyraz, A.S.; Jin, L.; Meng, Y.; Pahalagedara, L.; Chen, S.-Y.; Kriz, D.A.; Guild, C.; Gudz, A.; Suib, S.L. Heterogeneous acidic TiO2 nanoparticles for efficient conversion of biomass derived carbohydrates. Green Chem. 2014, 16, 785–791. [Google Scholar] [CrossRef]
- Morales, G.; Osatiashtiani, A.; Hernández, B.; Iglesias, J.; Melero, J.A.; Panigua, M.; Brown, D.R.; Granollers, M.; Lee, A.F.; Wilson, K. Conformal sulfated zirconia monolayer catalysts for the one-pot synthesis of ethyl levulinate from glucose. Chem. Commun. 2014, 50, 11742–11745. [Google Scholar] [CrossRef]
- Njagi, E.C.; Genuino, H.C.; Kuo, C.-H.; Dharmarathna, S.; Gudz, A.; Suib, S.L. High-yield selective conversion of carbohydrates to methyl levulinate using mesoporous sulfated titania-based catalysts. Microporous. Mesoporous. Mater. 2015, 202, 68–72. [Google Scholar] [CrossRef]
- Quereshi, S.; Ahmad, E.; Pant, K.K.K.; Duta, S. Insights into microwave-assisted synthesis of 5-ethoxymethylfurfural and ethyl levulinate using tungsten disulfide as a catalyst. ACS Sustain. Chem. Eng. 2020, 8, 1721–1729. [Google Scholar] [CrossRef]
- Xu, G.; Chen, B.; Zheng, Z.; Li, K.; Tao, H. One-pot ethanolysis of carbohydrates to promising biofuels: 5-ethoxymethylfurfural and ethyl levulinate. Asia-Pac. J. Chem. Eng. 2017, 12, 527–535. [Google Scholar] [CrossRef]
- Xu, G.Z.; Chang, C.; Zhu, W.N.; Li, B.; Ma, X.J.; Du, F.G. A comparative study on direct production of ethyl levulinate from glucose in ethanol media catalyzed by different acid catalysts. Chem. Pap. 2013, 67, 1355–1363. [Google Scholar] [CrossRef]
- Bosilji, M.; Schmidt, J.; Fischer, A.; White, R.J. One pot conversion of glucose to ethyl levulinate over a porous hydrothermal acid catalyst in green solvents. RSC Adv. 2019, 9, 20341–20344. [Google Scholar] [CrossRef]
- Xu, G.; Chang, C.; Fang, S.; Ma, X. Cellulose reactivity in ethanol at elevate temperature and the kinetics of one-pot preparation of ethyl levulinate from cellulose. Renew. Energy 2015, 78, 583–589. [Google Scholar] [CrossRef]
- Ming, C.; Sun, K.; Shao, Y.; Zhang, Z.; Zhang, S.; Liu, Q.; Wang, Y.; Hu, S.; Xiang, J.; Hu, X. Conversion of Cellulose to Levulinic Acid/Ester over an Acid Catalyst: Impacts of Dispersion of Hydrogen Ions on Polymerization Reactions. Energy Fuels 2019, 33, 11187–11199. [Google Scholar] [CrossRef]
- Bodachivskyi, I.; Kuzhimapambil, U.; Williams, D.B.G. Metal triflates are tunable acidic catalysts for high yielding conversion of cellulosic biomass into ethyl levulinate. Fuel Process. Technol. 2019, 195, 106159–106164. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Tian, L.; He, Y.; Wang, H.; Deng, F. One-pot alcoholysis of carbohydrates to biofuel 5-ethoyxymethylfurfural and 5-methoxymethylfurfural via a sulfonic porous polymer. Fuel Process. Technol. 2019, 193, 39–47. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, J.; Li, D.; Yuan, H.; Chen, Y. Hyper-cross-linked polymer based carbonaceous materials as efficient catalysts for ethyl levulinate production from carbohydrates. J. Chem. Technol. Biotechnol. 2019, 94, 3073–3083. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J. Modified solid acids derived from biomass based cellulose for one-step conversion of carbohydrates into ethyl levulinate. J. Energy Chem. 2016, 25, 747–753. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Maneechakr, P.; Samart, C.; Kongparakul, S.; Guan, G.; Bayu, A. Direct conversion of sugar into ethyl levulinate catalyzed by selective heterogeneous acid under co-solvent system. Catal. Commun. 2020, 143, 106058–106061. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, G.; Chang, J.; Chang, C.; Bai, J.; Fang, S.; Liu, Z. Direct production of ethyl levulinate from carbohydrates catalyzed by H-ZSM-5 supported phosphotungstic acid. BioResources 2015, 10, 2223–2234. [Google Scholar] [CrossRef]
- Gupta, D.; Mukesh, C.; Pant, K.K. Topotactic transformation of homogeneous phosphotungastomolybdic acid materials to heterogeneous solid acid catalyst for carbohydrate conversion to alkyl methylfurfural and alkyl levulinate. RSC Adv. 2020, 10, 705–718. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, G.; Chang, C.; Fang, S.; Liu, Z.; Du, F. Direct conversion of carbohydrates into ethyl levulinate with potassium phosphotunstate as an efficient catalyst. Catalysts 2015, 5, 1897–1910. [Google Scholar] [CrossRef]
- Srinivasa, R.B.; Krishna, K.P.; Dhana, L.D.; Lingaiah, N. One pot selective transformation of biomass derived chemicals towards alkyl levulinates over titanium exchanged heteropoly tungstate catalysts. Catal. Today 2018, 309, 269–275. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Yang, S. Direct conversion of sugars and ethyl levulinate into γ-valerolactone with superparamegnetic acid-base bifunctional ZrFeOxnanocatalysts. ACS Sustain. Chem. Eng. 2016, 4, 236–246. [Google Scholar] [CrossRef]
- Song, D.; Zhang, Q.; Sun, Y.; Zhang, P.; Guo, Y.H.; Hu, J.L. Design of ordered mesoporous sulfonic acid functionalized ZrO2/organosilica bifunctional catalysts for direct catalytic conversion of glucose to ethyl levulinate. ChemCatChem 2018, 10, 4953–4965. [Google Scholar] [CrossRef]
- Chang, C.; Xu, G.; Zhu, W.; Bai, J.; Fang, S. One-pot production of a liquid biofuel candidate—Ethyl levulinate from glucose and furfural residue using a combination of extremely low sulfuric acid and zeolite USY. Fuel 2015, 140, 365–379. [Google Scholar] [CrossRef]
- Mulik, N.; Niphadkar, P.; Bokade, V. Synergetic combination of H2ZrPW12O40 and Sn-Beta as potential solid acid catalyst for direct one-step transformation of glucose to ethyl levulinate, a biofuel additive. Environ. Prog. Sustain. Energy 2019, 38, 13173–13178. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Zhang, J.; Shi, J.; Liu, S. Solid acid catalyzed glucose conversion to ethyl levulinate. Appl. Catal. A Gen. 2011, 397, 259–265. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, H. An alumina-coated UiO-66 nanocrystalline solid superacid with high acid density as a catalyst for ethyl levulinate synthesis. J. Chem. Technol. Biotechnol. 2020. In press. [Google Scholar] [CrossRef]
- Babaei, Z.; Chermahini, A.N.; Dinari, M. Alumina-coated mesoporous silica SBA-15 as a solid catalyst for catalytic conversion of fructose into liquid biofuel candidate ethyl levulinate. Chem. Eng. J. 2018, 352, 45–52. [Google Scholar] [CrossRef]
- Jorge, E.Y.C.; Lima, C.G.S.; Lima, T.M.; Marchini, L.; Gawande, M.B.; Tomanec, O.; Varma, R.S.; Paixão, M.W. Sulfonated dendritic mesoporous silica nanospheres: A metal-free Lewis acid catalyst for the upgrading of carbohydrates. Green Chem. 2020, 22, 1754–1762. [Google Scholar] [CrossRef]
- Heda, J.; Niphadkar, P.; Bokade, V. Efficient synergetic combination of H-USY and SnO2 for direct conversion of glucose into ethyl levulinate (biofuel additive). Energy Fuels 2019, 33, 2319–2327. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Van Buu, O.N.; Riisager, A. Conversion of mono- and disaccharides to ethyl levulinate and ethyl pyranoside with sulfonic acid-functionalized ionic liquids. ChemSusChem 2011, 4, 723–726. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B. Acidic ionic liquid catalyzed one-pot conversion of cellulose to ethyl levulinate and levulinic acid in ethanol-water solvent system. BioEnergy Res. 2014, 7, 1237–1243. [Google Scholar] [CrossRef]
- Garves, K. Acid catalyzed degradation of cellulose in alcohols. J. Wood Chem. Technol. 1988, 8, 121–134. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Yamaguchi, M.; Kubo, S.; Yamada, T. Direct preparation of butyl levulinate by a single solvolysis process of cellulose. J. Wood Sci. 2013, 59, 179–182. [Google Scholar] [CrossRef]
- Démolis, A.; Eternot, M.; Essayem, N.; Rataboul, F. Influence of butanol isomers on the reactivity of cellulose towards the synthesis of butyl levulinates catalyzed by liquid and solid acid catalysts. New J. Chem. 2016, 40, 3747–3754. [Google Scholar] [CrossRef]
- An, R.; Xu, G.; Chang, C.; Bai, J.; Fang, S. Efficient one-pot synthesis of n-butyl levulinate from carbohydrates catalyzed by Fe2(SO4)3. J. Energy Chem. 2017, 26, 556–563. [Google Scholar] [CrossRef]
- Deng, L.; Chang, C.; An, R.; Qi, X.; Xu, G. Metal sulfates-catalyzed butanolysis of cellulose: Butyl levulinate production and optimization. Cellulose 2017, 24, 5403–5415. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Liu, D.; Zhuang, J.; Pang, C. Conversion of biomass sugars to butyl levulinate over combined catalyst of solid acid and other acid. Adv. Mater. Res. 2014, 955–959, 779–784. [Google Scholar] [CrossRef]
- Ma, H.; Long, J.-X.; Wang, F.-R.; Wang, L.-F.; Li, X.-H. Conversion of cellulose to butyl levulinate in bio-butanol medium catalyzed by acidic ionic liquids. Acta Phys. Chim. Sin. 2015, 31, 973–979. [Google Scholar] [CrossRef]
- Yamada, T.; Yamaguchi, M.; Kubo, S.; Hishikawa, Y. Direct production of alkyl levulinates from cellulosic biomass by a single step acidic solvolysis system at ambient atmospheric pressure. BioResources 2015, 10, 4961–4969. [Google Scholar] [CrossRef]
- Mohammadbagheri, Z.; Chermahini, N. Direct production of hexyl levulinate as a potential fuel additive from glucose catalyzed by modified dendritic fibrous nanosilica. Renew. Energy 2020, 147, 2229–2237. [Google Scholar] [CrossRef]
- Démolis, A.; Eternot, M.; Essayem, N.; Rataboul, F. New insights into the reactivity of biomass with butenes for the synthesis of butyl levulinates. ChemSusChem 2017, 10, 2612–2617. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Zhang, Y.; Qu, T.; Han, L. Product analysis for microwave-assisted methanolysis of lignocellulose. Energy Fuels 2016, 30, 8246–8251. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Li, H.; Chen, K. Acid-catalyzed direct synthesis of methyl levulinate from paper sludge in methanol medium. BioResources 2013, 8, 5896–5907. [Google Scholar] [CrossRef]
- Kim, T.-H.; Oh, Y.-K.; Lee, Y.W.; Chang, Y.K. Levulinate production from algal cell hydrolysis using in situ transesterification. Algal Res. 2017, 26, 431–435. [Google Scholar] [CrossRef]
- Li, P.; Du, Z.; Chang, C.; Zhao, S.; Xu, G.; Xu, C.C. Efficient catalytic conversion of waste peanut shells into liquid biofuel: An artificial intelligence approach. Energy Fuels 2020, 34, 1791–1801. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, X.; Xu, L.; Wang, Y.; Long, J. Catalytic conversion of duckweed to methyl levulinate in the presence of acidic ionic liquids. Bioresour. Technol. 2018, 268, 488–495. [Google Scholar] [CrossRef]
- Le Van Mao, R.; Zhao, Q.; Dima, G.; Petraccone, D. New process for the acid-catalyzed conversion of cellulosic biomass (AC3B) into alkyl levulinates and other esters using a unique one-pot system of reaction and product extraction. Catal. Lett. 2011, 141, 271–276. [Google Scholar] [CrossRef]
- Chang, C.; Xu, G.; Jiang, X. Production of ethyl levulinate by direct conversion of wheat straw in ethanol media. Bioresour. Technol. 2012, 121, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.S.; Kjelden, M.R.; Schlag, A.J.; Sharma, R.K. Levulinate esters from biomass wastes. In Chemicals and Materials from Renewable Resources; Bozell, J., Ed.; American Chemical Society: Washington, DC, USA, 2001; Volume 784, pp. 51–63. [Google Scholar]
- Yang, J.; Park, J.; Son, J.; Kim, B.; Lee, J.W. Enhanced ethyl levulinate production from citrus peels through an in-situ hydrothermal reaction. Bioresour. Technol. Rep. 2018, 2, 84–87. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Song, C.; Liu, S.; Li, X.; Long, J. Intensified levulinic acid/ester production from cassava by one-pot cascade prehydrolysis and delignification. Appl. Energy 2017, 204, 1094–1100. [Google Scholar] [CrossRef]
- Gong, C.; Wei, J.; Tang, X.; Zeng, X.; Sun, Y.; Lin, L. Production of levulinic and ethyl levulinate from cellulosic pulp derived from the cooking of lignocellulosic biomass with active oxygen and solid alkali. Korean J. Chem. Eng. 2019, 36, 740–752. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Hou, T.; Liu, H.; Han, L.; Xiao, W. Efficient microwave-assisted production of biofuel ethyl levulinate from corn stover in ethanol medium. J. Energy Chem. 2018, 27, 890–897. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.Z.; Hou, T.; Chen, X.; Gao, C.; Han, L.; Xiao, W. Mechanical deconstruction of corn stover as an entry process to facilitate the microwave-assisted production of ethyl levulinate. Fuel Process. Technol. 2018, 174, 53–60. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Q.; Chen, L.; Wang, T.; Ma, L.; Chen, G. Efficient production of ethyl levulinate from cassava over Al2(SO4)3 catalyst in ethanol-water system. J. Energy Chem. 2017, 26, 115–120. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Antonopoulou, G.; Braguglia, C.; Campanale, C.; Gallipoli, A.; Lyberatos, G.; Ntaikou, I.; Pastore, C. Lewis-Brønsted acid catalyzed ethanolysis of the organic fraction of municipal solid waste for efficient production of biofuels. Biores. Technol. 2018, 266, 297–305. [Google Scholar] [CrossRef]
- Bianchi, D.; Romano, A.M. Process for the Production of Esters of Levulinic Acid from. Biomasses. Patent WO2009156842A1, 26 June 2008. [Google Scholar]
- Guan, Q.; Lei, T.; Wang, Z.; Xu, H.; Lin, L.; Chen, G.; Li, X.; Li, Z. Preparation of ethyl levulinate from wheat straw catalyzed by sulfonate ionic liquid. Ind. Crops Prod. 2018, 113, 150–156. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Conversion of oil palm biomass to ethyl levulinate via ionic liquids. Chem. Eng. Trans. 2017, 56, 1021–1026. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. One-pot conversion of oil palm empty fruit bunch and mesocarp fiber biomass to levulinic acid and upgrading to ethyl levulinate via indium trichloride-ionic liquids. J. Clean. Prod. 2017, 168, 1251–1261. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Optimisation studies on the conversion of oil palm biomass to levulinic acid and ethyl levulinate via indium thrichloride-ionic liquids: A response surface methodology approach. Ind. Crops Prod. 2019, 128, 221–234. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Zhang, Y.; Lu, M.; Lyu, H.; Han, L.; Xiao, W. Alcoholysis of ball-milled corn stover: The enhanced conversion of carbohydrates into biobased chemicals over combination catalysts of [Bmim-SO3H][HSO4] and Al2(SO4)3. Energy Fuels 2020, 34, 7085–7093. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Gori, S.; Caposciutti, G.; Pasini, G.; Antonelli, M.; Frigo, S. Biomass Alcoholysis to Butyl Levulinate and Valorization as Additive in ci Internal Combustion Engine. In Proceedings of the European Biomass Conference and Exhibition (EUBCE), Lisbon, Portugal, 27–30 May 2019; pp. 1399–1403. [Google Scholar] [CrossRef]
- Tian, M.; McCormick, R.L.; Luecke, J.; de Jong, E.; van der Waal, J.C.; van Klink, G.P.M.; Boot, M.D. Anti-knock quality of sugar derived levulinic esters and cyclic ethers. Fuel 2017, 202, 414–425. [Google Scholar] [CrossRef]
- Unlu, D.; Boz, N.; Ilgen, O.; Hilmioglu, N. Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate. Open Chem. 2018, 16, 647–652. [Google Scholar] [CrossRef]
- No, S.-Y. Other drop-in liquid biofuels. In Application of Liquid Biofuels to Internal Combustion Engines; No, S.-Y., Ed.; Springer Nature: Singapore, 2019; pp. 405–450. [Google Scholar] [CrossRef]
- Herskowitz, M.; Landau, M.; Reizner, Y. Diesel Fuel from Vegetable and Animal Oils Blended with Alkyl. Levulinates. Patent WO 2010/106536A1, 23 September 2010. [Google Scholar]
- Sivalakshmi, S.; Balusamy, T. Effect of biodiesel and its blends with diethyl ether on the combustion, performance and emissions from a diesel engine. Fuels 2013, 106, 106–110. [Google Scholar] [CrossRef]


| Entry | Feedstock | Catalyst | Cat.(g)/MeOH (g) 1 | T (°C) | t (h) | Heat 2 | YML (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ML_1 | Fructose | H2SO4 (100 wt%) | 0.2/15.8 | 200 | 2 | Conv. | 85 | [66] |
| ML_2 | Fructose | H2SO4 (96 wt%) | 0.2/5.0 | 160 | 0.5 | MW | 90 | [67] |
| ML_3 | Fructose | H2SO4 (96 wt%) | 0.3/8.0 | 160 | 0.67 | MW | 93 | [68] |
| ML_4 | Glucose | H2SO4 (96 wt%) | 0.2/5.0 | 160 | 1 | MW | 70 | [67] |
| ML_5 | Glucose | H2SO4 (96 wt%) | 0.3/8.0 | 160 | 0.67 | MW | 72 | [68] |
| ML_6 | Cellulose | H2SO4 (100 wt%) | 0.2/6.9 | 179 | 0.25 | Conv. | 31 | [69] |
| ML_7 | Cellulose | H2SO4 (100 wt%) | 0.2/3.4 3 | 194 | 0.083 | Conv. | 45 | [69] |
| ML_8 | Cellulose | H2SO4 (100 wt%) | 0.1/39.6 | 210 | 2 | Conv. | 50 | [70] |
| ML_9 | Cellulose | H2SO4 (100 wt%) | 0.1/39.5 | 190 | 4.2 | Conv. | 55 | [71] |
| ML_10 | Cellulose | H2SO4 (100 wt%) | 0.2/39.5 | 180 | 4 | Conv. | 42 | [71] |
| ML_11 | Cellulose | H2SO4 (96 wt%) | 0.3/8.0 | 180 | 0.67 | MW | 46 | [68] |
| ML_12 | Cellulose | H2SO4 (96 wt%) | 0.2/5.0 | 160 | 1 | MW | 70 | [67] |
| ML_13 | Fructose | HReO4 | 0.1/22.2 | 160 | 16 | Conv. | 76 | [72] |
| ML_14 | Fructose | Fe2(SO4)3 | 2.2/9.0 | 170 | 2 | Conv. | 25 | [73] |
| ML_15 | Glucose | Fe2(SO4)3 | 2.2/9.0 | 170 | 2 | Conv. | 26 | [73] |
| ML_16 | Glucose | La2(SO4)3 | 3.2/9.0 | 170 | 2 | Conv. | 30 | [73] |
| ML_17 | Glucose | Fe2(SO4)3 | 0.8/15.8 | 200 | 2 | Conv. | 43 | [66] |
| ML_18 | Glucose | Al2(SO4)3 | 0.7/15.8 | 200 | 2 | Conv. | 54 | [66] |
| ML_19 | Fructose | Al2(SO4)3 | 0.6/29.8 | 160 | 1 | MW | 70 | [67] |
| ML_20 | Glucose | Al2(SO4)3 | 0.6/29.8 | 160 | 1 | MW | 70 | [67] |
| ML_21 | Cellulose | Al2(SO4)3 | 0.6/29.8 | 160 | 1 | MW | 20 | [67] |
| ML_22 | Cellulose | Al2(SO4)3 | 0.6/29.8 | 160 | 4 | MW | 49 | [67] |
| ML_23 | Cellulose | Al2(SO4)3 | 0.6/29.8 | 180 | 1 | MW | 61 | [67] |
| ML_24 | Glucose | Al2(SO4)3 | 0.4/35.0 | 160 | 2.5 | Conv. | 64 | [29] |
| ML_25 | Sucrose | Al2(SO4)3 | 0.4/35.0 | 160 | 2.5 | Conv. | 55 | [29] |
| ML_26 | Cellulose | Al2(SO4)3 | 0.4/35.0 | 180 | 5 | Conv. | 44 | [29] |
| ML_27 | Sucrose | Al2(SO4)3 | 0.4/22.4 4 | 180 | 0.25 | MW | 83 | [32] |
| ML_28 | Cellulose | Al2(SO4)3 | 0.4/22.4 4 | 170 | 1 | MW | 44 | [32] |
| ML_29 | Cellulose | Al2(SO4)3 | 0.4/22.4 4 | 180 | 0.67 | MW | 71 | [32] |
| ML_30 | BM-cellulose 5 | Al2(SO4)3 | 0.6/29.8 | 160 | 1 | MW | 58 | [74] |
| ML_31 | BM-cellulose 5 | Al2(SO4)3 | 0.6/29.8 | 170 | 0.75 | MW | 65 | [74] |
| ML_32 | Cellulose 6 | Al2(SO4)3·18H2O | 0.6/35.0 | 180 | 3 | Conv. | 52 | [75] |
| Entry | Feedstock | Catalyst | Cat.(g)/MeOH(g) 1 | T (°C) | t (h) | YML (mol%) | Ref. |
|---|---|---|---|---|---|---|---|
| ML_33 | Cellulose | PTSA 2 | 0.05/39.5 | 180 | 5 | 20 | [27] |
| ML_34 | Cellulose | PTSA 2 | 0.12/6.9 | 210 | 0.5 | 34 | [69] |
| ML_35 | Cellulose | PTSA 2 | 0.35/39.5 | 180 | 4 | 46 | [71] |
| ML_36 | Cellulose | In(OTf)3 | 0.03/39.5 | 180 | 5 | 52 | [27] |
| ML_37 | Cellulose | PTSA 2 + In(OTf)3 | 0.05 + 0.03/39.5 | 180 | 5 | 70 | [27] |
| ML_38 | Cellulose | 2-NSA 3 + In(OTf)3 | 0.05 + 0.03/39.5 | 180 | 5 | 75 | [27] |
| ML_39 | Glucose | BSA 4 + In(OTf)3 | n.a.5 | 180 | 5 | 70 | [78] |
| ML_40 | Mannose | BSA 4 + In(OTf)3 | n.a.5 | 180 | 5 | 76 | [78] |
| ML_41 | Galactose | BSA 4 + In(OTf)3 | n.a.5 | 180 | 5 | 70 | [78] |
| ML_42 | Cellulose | PTSA 2 + Al(OEt)3 | 0.08 + 0.01/39.5 | 180 | 5 | 69 | [79] |
| ML_43 | Cellulose | PTSA 2 + Al(acac)3 | 0.08 + 0.02/39.5 | 180 | 5 | 72 | [79] |
| ML_44 | Cellulose | 2-NSA 3 + Al(OH)3 | 0.10 + 0.01/39.5 | 180 | 5 | 74 | [79] |
| ML_45 | Fructose | Amberlyst-15 | 0.40/64.0 | 100 | 24 | 54 | [80] |
| ML_46 | Fructose | Amberlyst-15 | 0.28/48.3 | 170 | 15 | 68 | [81] |
| ML_47 | Glucose | Amberlyst-15 | 0.27/32.4 | 160 | 5 | 12 | [82] |
| ML_48 | Glucose | Amberlyst-15 | 0.54/32.4 | 160 | 5 | 75 | [83] |
| ML_49 | Fructose | Nafion NR50 | 0.28/48.3 | 170 | 15 | 73 | [81] |
| ML_50 | Fructose | PD-En-SO3H 6 | 0.28/48.3 | 170 | 15 | 78 | [81] |
| ML_51 | Fructose | PSSA-g-CNT 7 | 0.40/64.0 | 100 | 24 | 69 | [80] |
| ML_52 | Fructose | PSSA-g-CNF 8 | 0.40/64.0 | 100 | 24 | 53 | [80] |
| ML_53 | Fructose | BSA-g-CMK-5 9 | 0.40/64.0 | 100 | 24 | 49 | [80] |
| ML_54 | Fructose | BSA-g-CNT 10 | 0.40/64.0 | 100 | 24 | 12 | [80] |
| ML_55 | Fructose | 5-Cl-SHPAO 11 | 0.25/20.0 | 160 | 1 | 79 | [84] |
| ML_56 | Glucose | 5-Cl-SHPAO 11 | 0.25/20.0 | 160 | 12 | 60 | [84] |
| ML_57 | Inulin | 5-Cl-SHPAO 11 | 0.25/20.0 | 160 | 8 | 71 | [84] |
| ML_58 | Cellulose | Sulfonated char | 0.50/31.6 | 200 | 1.25 | 30 | [85] |
| ML_59 | Cellulose | Sulfonated char | 0.50/31.6 | 225 | 0.75 | 30 | [85] |
| Entry | Feedstock | Catalyst | Cat.(g)/MeOH(g) 1 | T (°C) | t (h) | Heat 2 | YML (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ML_60 | Fructose | H3PW12O40 | 0.6/32.0 | 130 | 2 | Conv. | 60 | [89] |
| ML_61 | Cellulose | H3PW12O40 | 2.0/63.0 | 160 | 5 | Conv. | 42 | [90] |
| ML_62 | Cellulose | H6P2W18O62 | 3.1/63.0 | 160 | 5 | Conv. | 52 | [90] |
| ML_63 | Cellulose | H4SiW12O40 | 0.1/31.6 | 195 | 1 | Conv. | 12 | [91] |
| ML_64 | Sucrose | H5PW11TiO40 | 1.5/42.0 | 100 | 5 | Conv. | 58 | [92] |
| ML_65 | Cellobiose | H5PW11TiO40 | 1.5/42.0 | 120 | 5 | Conv. | 51 | [92] |
| ML_66 | Starch | H5PW11TiO40 | 1.5/42.0 | 130 | 5 | Conv. | 47 | [92] |
| ML_67 | Cellulose | H5PW11TiO40 | 1.5/42.0 | 160 | 7 | Conv. | 51 | [92] |
| ML_68 | Cellulose | H5PW11TiO40 | 1.5/42.0 | 160 | 2 | MW | 63 | [92] |
| ML_69 | Fructose | FePW12O40 | 0.6/32.0 | 130 | 2 | Conv. | 74 | [89] |
| ML_70 | Glucose | FePW12O40 | 0.6/32.0 | 130 | 2 | Conv. | 14 | [89] |
| ML_71 | Sucrose | FePW12O40 | 0.6/32.0 | 130 | 2 | Conv. | 44 | [89] |
| ML_72 | Inulin | FePW12O40 | 0.6/32.0 | 130 | 2 | Conv. | 92 | [89] |
| ML_73 | Cellulose | FePW12O40 | 0.6/32.0 | 220 | 2 | Conv. | 14 | [89] |
| ML_74 | Fructose | Sn2SiW12O40 | 1.7/65.8 | 150 | 3 | Conv. | 57 | [93] |
| ML_75 | Fructose | AlPW12O40 | 0.6/7.5 | 160 | 0.5 | MW | 70 | [94] |
| ML_76 | Glucose | AlPW12O40 | 0.6/7.5 | 160 | 0.5 | MW | 64 | [94] |
| ML_77 | Sucrose | AlPW12O40 | 0.6/7.5 | 160 | 0.5 | MW | 65 | [94] |
| ML_78 | Cellulose | AlPW12O40 | 0.6/7.5 | 160 | 0.5 | MW | 45 | [94] |
| ML_79 | Cellulose | Cs2.5H0.5PW12O40 | 0.4/71.4 | 300 | 0.02 | Conv. | 20 | [95] |
| ML_80 | Fructose | [TMEDAPS]3 [PW12O40]2 3 | 1.0/64.0 | 120 | 12 | Conv. | 80 | [96] |
| ML_81 | Sucrose | [PyPS]3PW12O40 4 | 5.0/46.5 | 150 | 4.5 | Conv. | 76 | [97] |
| ML_82 | Glucose | [PyPS]3PW12O40 4 | 9.4/87.8 | 150 | 4 | Conv. | 59 | [97] |
| ML_83 | Starch | [PyPS]3PW12O40 4 | 10.6/98.8 | 150 | 5 | Conv. | 51 | [97] |
| ML_84 | Cellulose | [PyPS]3PW12O40 4 | 10.6/98.8 | 150 | 5 | Conv. | 71 | [97] |
| ML_85 | Cellulose | [C16TA]H5P2W18O62 5 | 3.3/63.0 | 160 | 7 | Conv. | 58 | [90] |
| ML_86 | Fructose | 3-FPyPW 6 | 0.5/22.2 | 120 | 10 | Conv. | 82 | [98] |
| ML_87 | Fructose | 3-PhPyPW 7 | 0.5/22.2 | 140 | 8 | Conv. | 71 | [99] |
| ML_88 | Fructose | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 130 | 3 | Conv. | 53 | [100] |
| ML_89 | Glucose | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 130 | 3 | Conv. | 85 | [100] |
| ML_90 | Starch | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 150 | 4 | Conv. | 60 | [100] |
| ML_91 | Cellulose | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 165 | 10 | Conv. | 46 | [100] |
| ML_92 | Fructose | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 110 | 1 | MW | 55 | [100] |
| ML_93 | Glucose | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 110 | 1.5 | MW | 89 | [100] |
| ML_94 | Starch | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 120 | 3 | MW | 60 | [100] |
| ML_95 | Cellulose | Cs10.6[H2.4GeNb13O41] | 1.3/48.0 | 165 | 3 | MW | 50 | [100] |
| Entry | Feedstock | Catalyst | Cat.(g)/MeOH(g) 1 | T(°C) | t (h) | Heat 2 | YML(mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ML_96 | Glucose | H-β | 0.3/32.4 | 160 | 5 | Conv. | 17 | [82] |
| ML_97 | Cellulose | HZSM-5 | 0.4/71.4 | 300 | 0.02 | Conv. | 12 | [95] |
| ML_98 | Cellulose | HY | 0.4/71.4 | 300 | 0.02 | Conv. | 17 | [95] |
| ML_99 | Fructose | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 51 | [102] |
| ML_100 | Glucose | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 49 | [102] |
| ML_101 | Cellobiose | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 53 | [102] |
| ML_102 | Maltose | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 51 | [102] |
| ML_103 | Inulin | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 50 | [102] |
| ML_104 | Starch | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 31 | [102] |
| ML_105 | Cellulose | H-USY (6) | 0.6/31.6 | 160 | 20 | Conv. | 13 | [102] |
| ML_106 | Fructose | H-USY-0.2 | 0.3/32.4 | 160 | 5 | Conv. | 40 | [82] |
| ML_107 | Glucose | H-USY-0.2 | 0.3/32.4 | 160 | 5 | Conv. | 32 | [82] |
| ML_108 | Mannose | H-USY-0.2 | 0.3/32.4 | 160 | 5 | Conv. | 21 | [82] |
| ML_109 | Sucrose | H-USY-0.2 | 0.3/32.4 | 160 | 5 | Conv. | 38 | [82] |
| ML_110 | Cellobiose | H-USY-0.2 | 0.3/32.4 | 160 | 5 | Conv. | 20 | [82] |
| ML_111 | Glucose | ZrY6 (0.5) | 0.3/40.0 | 180 | 3 | MW | 68 | [103] |
| ML_112 | Mannose | ZrY6 (0.5) | 0.3/40.0 | 180 | 3 | MW | 70 | [103] |
| ML_113 | Galactose | ZrY6 (0.5) | 0.3/40.0 | 180 | 3 | MW | 73 | [103] |
| ML_114 | Sucrose | ZrY6 (0.5) | 0.3/40.0 | 180 | 3 | MW | 78 | [103] |
| ML_115 | Cellobiose | ZrY6 (0.5) | 0.3/40.0 | 180 | 3 | MW | 46 | [103] |
| ML_116 | Starch | ZrY6 (0.5) | 0.3/40.0 | 180 | 6 | MW | 53 | [103] |
| ML_117 | Cellulose | ZrY6 (0.5) | 0.3/40.0 | 180 | 6 | MW | 27 | [103] |
| ML_118 | Glucose | Sn-Al-Beta | 0.6/33.3 | 160 | 5 | Conv. | 41 | [104] |
| ML_119 | Mannose | Sn-Beta + H4SiW12O40 | 0.2 + 0.3/29.0 | 150 | 3 | Conv. | 65 | [105] |
| ML_120 | Starch | Sn-Beta + H4SiW12O40 | 0.2 + 0.3/29.0 | 150 | 5 | Conv. | 58 | [105] |
| ML_121 | Cellulose | Sn-Beta + H4SiW12O40 | 0.2 + 0.3/29.0 | 160 | 10 | Conv. | 62 | [105] |
| Entry | Feedstock | Catalyst | Cat.(g)/MeOH(g) 1 | T(°C) | t (h) | YML(mol%) | Ref. |
|---|---|---|---|---|---|---|---|
| ML_122 | Glucose | Al-clay | 0.5/80.0 | 220 | 6 | 61 | [107] |
| ML_123 | Glucose | Cu-clay | 0.5/80.0 | 220 | 6 | 59 | [107] |
| ML_124 | Glucose | In-clay | 0.5/80.0 | 220 | 6 | 52 | [107] |
| ML_125 | Fructose | Sn-clay | 0.5/80.0 | 220 | 6 | 66 | [108] |
| ML_126 | Glucose | Sn-clay | 0.5/80.0 | 220 | 6 | 60 | [108] |
| ML_127 | Sucrose | Sn-clay | 0.5/80.0 | 220 | 6 | 62 | [108] |
| ML_128 | Inulin | Sn-clay | 0.5/80.0 | 220 | 6 | 55 | [108] |
| ML_129 | Starch | Sn-clay | 0.5/80.0 | 220 | 6 | 46 | [108] |
| ML_130 | Cellulose | Sn-clay | 0.5/80.0 | 220 | 6 | 19 | [108] |
| ML_131 | Fructose | 20-SO42−/clay | 0.8/87.8 | 200 | 4 | 65 | [109] |
| ML_132 | Glucose | 20-SO42−/clay | 0.8/87.8 | 200 | 4 | 48 | [109] |
| ML_133 | Sucrose | 20-SO42−/clay | 0.8/87.8 | 200 | 4 | 60 | [109] |
| ML_134 | Starch | 20-SO42−/clay | 0.8/87.8 | 200 | 4 | 41 | [109] |
| ML_135 | Cellulose | 20-SO42−/clay | 0.8/87.8 | 200 | 4 | 24 | [109] |
| ML_136 | Fructose | 4-HPWFe-MMTSi 2 | 0.6/24.8 | 180 | 1 | 74 | [110] |
| Entry | Feedstock | Catalyst | Cat.(g)/MeOH(g) 1 | T(°C) | t (h) | Heat 2 | YML(mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ML_137 | Fructose | TiO2nanop. | 0.6/87.8 | 175 | 1 | Conv. | 80 | [113] |
| ML_138 | Glucose | TiO2nanop. | 0.6/87.8 | 175 | 9 | Conv. | 61 | [113] |
| ML_139 | Starch | TiO2nanop. | 1.1/175.6 | 175 | 20 | Conv. | 40 | [113] |
| ML_140 | Cellulose | TiO2nanop. | 1.1/175.6 | 175 | 20 | Conv. | 42 | [113] |
| ML_141 | Fructose | SO42−/TiO2 | 0.5/15.8 | 200 | 2 | Conv. | 59 | [44] |
| ML_142 | Glucose | SO42−/TiO2 | 0.5/15.8 | 200 | 2 | Conv. | 33 | [44] |
| ML_143 | Sucrose | SO42−/TiO2 | 0.5/15.8 | 200 | 2 | Conv. | 43 | [44] |
| ML_144 | Starch | SO42−/TiO2 | 0.5/15.8 | 200 | 2 | Conv. | 28 | [44] |
| ML_145 | Cellulose | SO42−/TiO2 | 0.5/15.8 | 200 | 2 | Conv. | 10 | [44] |
| ML_146 | Glucose | GraftedSO42−/ZrO2/SBA-15 | 0.5/18.0 | 140 | 24 | Conv. | 24 | [114] |
| ML_147 | Fructose | SO42−/TiO2-ZrO2 | 0.6/87.8 | 200 | 1 | Conv. | 71 | [115] |
| ML_148 | Glucose | SO42−/TiO2-ZrO2 | 0.6/87.8 | 200 | 1 | Conv. | 23 | [115] |
| ML_149 | Sucrose | SO42−/TiO2-ZrO2 | 0.6/87.8 | 200 | 1 | Conv. | 54 | [115] |
| ML_150 | Fructose | SO42−/ZrO2 + Sn-Beta | 0.4 + 0.1/32.4 | 160 | 5 | Conv. | 54 | [83] |
| ML_151 | Glucose | SO42−/ZrO2+ Sn-Beta | 0.4 + 0.1/32.4 | 160 | 5 | Conv. | 59 | [83] |
| ML_152 | Mannose | SO42−/ZrO2 + Sn-Beta | 0.4 + 0.1/32.4 | 160 | 5 | Conv. | 55 | [83] |
| ML_153 | Sucrose | SO42−/ZrO2 + Sn-Beta | 0.4 + 0.1/32.4 | 160 | 5 | Conv. | 31 | [83] |
| ML_154 | Cellobiose | SO42−/ZrO2+ Sn-Beta | 0.4 + 0.1/32.4 | 160 | 5 | Conv. | 37 | [83] |
| ML_155 | Cellulose | NbPO4 | 0.2/19.0 3 | 180 | 24 | Conv. | 56 | [18] |
| ML_156 | Fructose | WS2 | 0.4/22.2 | 160 | 0.25 | MW | 37 | [116] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | Heat 2 | YEL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| EL_1 | Fructose | H2SO4 (100 wt%) | 0.04/39.2 | 200 | 1.5 | Conv. | 72 | [117] |
| EL_2 | Glucose | H2SO4 (100 wt%) | 0.01/39.4 | 180 | 0.5 | Conv. | 45 | [118] |
| EL_3 | Glucose | H2SO4 (100 wt%) | 0.02/26.5 3 | 200 | 6 | Conv. | 34 | [119] |
| EL_4 | Glucose | H2SO4 (100 wt%) | 0.04/39.2 | 200 | 1.5 | Conv. | 37 | [117] |
| EL_5 | Sucrose | H2SO4 (100 wt%) | 0.04/39.2 | 200 | 1.5 | Conv. | 40 | [117] |
| EL_6 | Inulin | H2SO4 (100 wt%) | 0.04/39.2 | 200 | 1.5 | Conv. | 51 | [117] |
| EL_7 | Cellulose | H2SO4 (100 wt%) | 0.04/39.2 | 200 | 1.5 | Conv. | 25 | [117] |
| EL_8 | Cellulose | H2SO4 (100 wt%) | 0.09/6.9 | 190 | 0.25 | Conv. | 12 | [69] |
| EL_9 | Cellulose | H2SO4 (100 wt%) | 0.13/11.8 | 190 | 0.5 | Conv. | 43 | [120] |
| EL_10 | Cellulose | H2SO4 (96 wt%) | 0.42/10.0 | 170 | 2 | Conv. | 51 | [121] |
| EL_11 | Fructose | HReO4 | 0.14/21.7 | 160 | 16 | Conv. | 80 | [72] |
| EL_12 | Glucose | HReO4 | 0.14/21.7 | 160 | 16 | Conv. | 27 | [72] |
| EL_13 | Sucrose | HReO4 | 0.14/21.7 | 160 | 16 | Conv. | 52 | [72] |
| EL_14 | Inulin | HReO4 | 0.14/21.7 | 160 | 16 | Conv. | 65 | [72] |
| EL_15 | Fructose | Fe2(SO4)3 | 2.24/9.0 | 170 | 2 | Conv. | 29 | [73] |
| EL_16 | Glucose | Fe2(SO4)3 | 2.24/9.0 | 170 | 2 | Conv. | 39 | [73] |
| EL_17 | Glucose | La2(SO4)3 | 2.24/9.0 | 170 | 2 | Conv. | 29 | [73] |
| EL_18 | Glucose | Ce(SO4)2 | 2.24/9.0 | 170 | 2 | Conv. | 29 | [73] |
| EL_19 | Cellulose | Al2(SO4)3 | 1.00/34.7 | 180 | 5 | Conv. | 45 | [75] |
| EL_20 | Cellulose | Al2(SO4)3 | 0.40/22.0 4 | 180 | 0.6 | MW | 54 | [32] |
| EL_21 | Cellulose | Al2(SO4)3 | 0.40/22.0 4 | 180 | 0.9 | MW | 70 | [32] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | Heat 2 | YEL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| EL_22 | Cellulose | Al(OTf)3 | 0.47/64.0 | 160 | 4 | Conv. | 32 | [122] |
| EL_23 | Cellulose | In(OTf)3 | 0.56/64.0 | 160 | 4 | Conv. | 20 | [122] |
| EL_24 | Cellulose | Sn(OTf)2 | 0.42/64.0 | 160 | 4 | Conv. | 23 | [122] |
| EL_25 | Cellulose | Hf(OTf)4 | 0.77/64.0 | 160 | 4 | Conv. | 24 | [122] |
| EL_26 | Cellulose | Y(OTf)3 + H3PO4 (100 wt%) | 0.60 + 0.10/64.0 | 180 | 2 | Conv. | 75 | [122] |
| EL_27 | Fructose | PSDVB-SO3H 3 | 0.90/80.0 | 120 | 2 | Conv. | 26 | [123] |
| EL_28 | Fructose | Amberlyst-15 | 0.40/64.0 | 120 | 24 | Conv. | 73 | [80] |
| EL_29 | Fructose | Amberlyst-15 | 0.80/78.0 | 150 | 3.5 | Conv. | 75 | [124] |
| EL_30 | Cellulose | Acid resin D008 | 2.40/10.0 | 170 | 2 | Conv. | 20 | [121] |
| EL_31 | Fructose | PSSA-g-CNT 4 | 0.40/64.0 | 120 | 24 | Conv. | 84 | [80] |
| EL_32 | Fructose | PSSA-g-CNF 5 | 0.40/64.0 | 120 | 24 | Conv. | 69 | [80] |
| EL_33 | Fructose | BSA-g-CMK-5 6 | 0.40/64.0 | 120 | 24 | Conv. | 60 | [80] |
| EL_34 | Fructose | BSA-g-CNT 7 | 0.40/64.0 | 120 | 24 | Conv. | 45 | [80] |
| EL_35 | Fructose | HDS-3.6 8 | 0.80/78.0 | 150 | 3.5 | Conv. | 70 | [124] |
| EL_36 | Glucose | HDS-3.6 8 | 0.40/390.0 | 170 | 8 | Conv. | 25 | [124] |
| EL_37 | Inulin | HDS-3.6 8 | 0.40/390.0 | 170 | 6 | Conv. | 51 | [124] |
| EL_38 | Starch | HDS-3.6 8 | 0.40/390.0 | 170 | 8 | Conv. | 18 | [124] |
| EL_39 | Cellulose | HDS-3.6 8 | 0.40/390.0 | 170 | 10 | Conv. | 12 | [124] |
| EL_40 | Fructose | 5-Cl-SHPAO 9 | 0.25/20.0 | 160 | 1 | Conv. | 68 | [84] |
| EL_41 | Glucose | 5-Cl-SHPAO 9 | 0.25/20.0 | 160 | 8 | Conv. | 61 | [84] |
| EL_42 | Sucrose | 5-Cl-SHPAO 9 | 0.25/20.0 | 160 | 6 | Conv. | 62 | [84] |
| EL_43 | Cellobiose | 5-Cl-SHPAO 9 | 0.25/20.0 | 160 | 8 | Conv. | 60 | [84] |
| EL_44 | Cellulose | 5-Cl-SHPAO 9 | 0.25/20.0 | 160 | 8 | Conv. | 58 | [84] |
| EL_45 | Fructose | AC-Fe-SO3H 10 | 0.50/52.7 | 195 | 3 | Conv. | 58 | [125] |
| EL_46 | Fructose | AC-Fe-SO3H 10 | 0.50/52.7 | 200 | 3 | Conv. | 47 | [125] |
| EL_47 | Glucose | AC-Fe-SO3H 10 | 0.50/52.7 | 200 | 3 | Conv. | 19 | [125] |
| EL_48 | Sucrose | AC-Fe-SO3H 10 | 0.50/52.7 | 200 | 3 | Conv. | 28 | [125] |
| EL_49 | Inulin | AC-Fe-SO3H 10 | 0.50/52.7 | 200 | 3 | Conv. | 35 | [125] |
| EL_50 | Starch | AC-Fe-SO3H 10 | 0.50/52.7 | 200 | 3 | Conv. | 12 | [125] |
| EL_51 | Sucrose | 20 wt% Zn-SC 11 | 0.50/47.3 12 | 100 | 12 | Conv. | 64 | [126] |
| EL_52 | Sucrose | 20 wt% Zn-SC 11 | 0.50/47.3 12 | 100 | 1 | US | 72 | [126] |
| EL_53 | Glucose | Sulfonated char | 0.20/26.5 13 | 200 | 6 | Conv. | 37 | [119] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | Heat 2 | YEL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| EL_54 | Fructose | H3PW12O40 | 0.20/39.4 | 160 | 2 | Conv. | 50 | [127] |
| EL_55 | Fructose | HPW4Mo10Ox | n.a. 3 | 170 | 0.3 | MW | 74 | [128] |
| EL_56 | Glucose | HPW4Mo10Ox | n.a. 3 | 180 | 0.5 | MW | 62 | [128] |
| EL_57 | Cellulose | H4SiW12O40 | 0.10/31.6 | 180 | 1 | Conv. | 19 | [91] |
| EL_58 | Fructose | KH2PW12O40 | 0.75/35.5 4 | 150 | 2 | Conv. | 69 | [129] |
| EL_59 | Glucose | KH2PW12O40 | 0.75/35.5 4 | 150 | 2 | Conv. | 15 | [129] |
| EL_60 | Sucrose | KH2PW12O40 | 0.75/35.5 4 | 150 | 2 | Conv. | 35 | [129] |
| EL_61 | Inulin | KH2PW12O40 | 0.75/35.5 4 | 150 | 2 | Conv. | 52 | [129] |
| EL_62 | Cellulose | KH2PW12O40 | 0.75/35.5 4 | 220 | 2 | Conv. | 15 | [129] |
| EL_63 | Fructose | Ti0.75PW12O40 | 0.43/17.5 | 120 | 6 | Conv. | 63 | [130] |
| EL_64 | Glucose | Ti0.75PW12O40 | 0.43/17.5 | 120 | 6 | Conv. | 21 | [130] |
| EL_65 | Fructose | Sn2SiW12O40 | 1.67/65.8 | 150 | 2 | Conv. | 71 | [93] |
| EL_66 | Sucrose | Sn2SiW12O40 | 0.91/35.9 | 150 | 2 | Conv. | 78 | [93] |
| EL_67 | Inulin | Sn2SiW12O40 | 2.00/79.0 | 150 | 2 | Conv. | 61 | [93] |
| EL_68 | Fructose | 3-PhPyPW 5 | 0.50/21.7 | 140 | 8 | Conv. | 30 | [99] |
| EL_69 | Fructose | [TMEDAPS]3 [PW12O40]2 6 | 1.00/64.0 | 120 | 12 | Conv. | 80 | [96] |
| EL_70 | Glucose | [TMEDAPS]3 [PW12O40]2 6 | 1.00/64.0 | 150 | 24 | Conv. | 20 | [96] |
| EL_71 | Sucrose | [TMEDAPS]3 [PW12O40]2 6 | 1.00/64.0 | 120 | 12 | Conv. | 45 | [96] |
| EL_72 | Cellobiose | [TMEDAPS]3 [PW12O40]2 6 | 1.00/64.0 | 150 | 24 | Conv. | 18 | [96] |
| EL_73 | Inulin | [TMEDAPS]3 [PW12O40]2 6 | 1.00/64.0 | 120 | 12 | Conv. | 67 | [96] |
| EL_74 | Cellulose | [TMEDAPS]3 [PW12O40]2 6 | 1.00/64.0 | 150 | 24 | Conv. | 14 | [96] |
| EL_75 | Cellulose | [PyPS]3PW12O40 7 | 10.49/97.5 | 150 | 5 | Conv. | 57 | [97] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | YEL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|
| EL_76 | Fructose | HY | 0.31/18.2 | 230 | 3 | 53 | [131] |
| EL_77 | Glucose | HY | 1.00/64.0 | 170 | 12 | 39 | [132] |
| EL_78 | Glucose | H-β (19) | 0.60/31.6 | 160 | 20 | 28 | [102] |
| EL_79 | Fructose | H-USY (6) | 0.60/31.6 | 160 | 20 | 40 | [102] |
| EL_80 | Glucose | H-USY (6) | 0.60/31.6 | 160 | 20 | 41 | [102] |
| EL_81 | Mannose | H-USY (6) | 0.60/31.6 | 160 | 20 | 44 | [102] |
| EL_82 | Sucrose | H-USY (6) | 0.63/32.9 | 160 | 20 | 35 | [102] |
| EL_83 | Maltose | H-USY (6) | 0.60/31.6 | 160 | 20 | 47 | [102] |
| EL_84 | Cellobiose | H-USY (6) | 0.63/32.9 | 160 | 20 | 44 | [102] |
| EL_85 | Inulin | H-USY (6) | 0.68/35.9 | 160 | 20 | 39 | [102] |
| EL_86 | Glucose | USY | 0.03/39.4 | 180 | 2 | 47 | [118] |
| EL_87 | Glucose | USY + H2SO4 (100 wt%) | 0.02 + 0.001/39.4 | 180 | 2 | 51 | [133] |
| EL_88 | Glucose | Sn-β + ZrH2PW12O40 | 0.40 + 0.10/26.4 | 180 | 3 | 54 | [134] |
| EL_89 | Fructose | H3PW12O40/H-ZSM-5 2 | 1.50/39.2 | 160 | 2 | 43 | [127] |
| EL_90 | Glucose | H3PW12O40/H-ZSM-5 2 | 1.50/39.2 | 160 | 2 | 19 | [127] |
| EL_91 | Sucrose | H3PW12O40/H-ZSM-5 2 | 1.50/39.2 | 160 | 2 | 27 | [127] |
| EL_92 | Inulin | H3PW12O40/H-ZSM-5 2 | 1.50/39.2 | 160 | 2 | 37 | [127] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | Heat 2 | YEL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| EL_93 | Fructose | TiO2 nanoparticles | 0.56/87.8 | 150 | 3 | Conv. | 71 | [113] |
| EL_94 | Fructose | MCM-41 | 0.80/78.0 | 150 | 3.5 | Conv. | 25 | [124] |
| EL_95 | Fructose | SO42−/ZrO2 | 0.80/78.0 | 150 | 3.5 | Conv. | 44 | [124] |
| EL_96 | Glucose | SO42−/ZrO2 | 0.02/15.8 | 200 | 3 | Conv. | 29 | [135] |
| EL_97 | Glucose | SO42−/ZrO2@Al2O3 | 0.50/61.1 | 200 | 5 | Conv. | 37 | [136] |
| EL_98 | Glucose | Grafted SO42−/ZrO2/SBA-15 | 0.50/25.6 | 140 | 24 | Conv. | 31 | [114] |
| EL_99 | Glucose | SO42−/ZrO2-PMO-SO3H 2 | 1.00/64.0 | 170 | 12 | Conv. | 42 | [132] |
| EL_100 | Fructose | Al2O3/SBA-15 | 1.00/78.0 | 190 | 4 | Conv. | 58 | [137] |
| EL_101 | Fructose | DMSi-SA 3 | 0.81/13.3 | 170 | 24 | Conv. | 83 | [138] |
| EL_102 | Glucose | DMSi-SA 3 | 0.81/13.3 | 170 | 24 | Conv. | 62 | [138] |
| EL_103 | Sucrose | DMSi-SA 3 | 0.81/13.3 | 170 | 24 | Conv. | 90 | [138] |
| EL_104 | Glucose | SnO2 + H-USY | 0.03 + 0.48/15.8 | 180 | 3 | Conv. | 81 | [139] |
| EL_105 | Fructose | WS2 | 0.40/21.7 | 160 | 0.5 | MW | 23 | [116] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | YEL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|
| EL_106 | Fructose | [BMIm-SO3H][HSO4] 2 | 0.11/14.8 | 140 | 24 | 68 | [140] |
| EL_107 | Sucrose | [BMIm-SO3H][HSO4] 2 | 0.11/14.8 | 140 | 24 | 41 | [140] |
| EL_108 | Fructose | [BPyr-SO3H][HSO4] 3 | 0.11/14.8 | 140 | 24 | 70 | [140] |
| EL_109 | Sucrose | [BPyr-SO3H][HSO4] 3 | 0.11/14.8 | 140 | 24 | 43 | [140] |
| EL_110 | Fructose | [NEt3B-SO3H][HSO4] 4 | 0.15/14.8 | 140 | 24 | 74 | [140] |
| EL_111 | Sucrose | [NEt3B-SO3H][HSO4] 4 | 0.15/14.8 | 140 | 24 | 41 | [140] |
| EL_112 | Fructose | [BMIm-SO3H][NTf2] 5 | 0.19/14.8 | 140 | 24 | 77 | [140] |
| EL_113 | Sucrose | [BMIm-SO3H][NTf2] 5 | 0.19/14.8 | 140 | 24 | 43 | [140] |
| EL_114 | Fructose | [BMIm-SO3H][OMs] 6 | 0.11/14.8 | 140 | 24 | 67 | [140] |
| EL_115 | Sucrose | [BMIm-SO3H][OMs] 6 | 0.11/14.8 | 140 | 24 | 40 | [140] |
| EL_116 | Fructose | [BMIm-SO3H][OTf] 7 | 0.15/14.8 | 140 | 24 | 69 | [140] |
| EL_117 | Sucrose | [BMIm-SO3H][OTf] 7 | 0.15/14.8 | 140 | 24 | 42 | [140] |
| EL_118 | Cellulose | [PSMIm][Cl] 8 | 0.33/2.1 9 | 170 | 12 | 19 | [141] |
| Entry | Feedstock | Catalyst | Cat.(g)/PrOH(g) 1 | T (°C) | t (h) | Heat 2 | YPL (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PL_1 | Cellulose | H2SO4 (100 wt%) | 0.18/7.0 (n) 3 | 190 | 0.25 | Conv. | 35 | [69] |
| PL_2 | Cellulose | H2SO4 (96 wt%) | 0.42/10.0 (i) 4 | 170 | 2 | Conv. | 41 | [121] |
| PL_3 | Fructose | HReO4 | 0.14/21.7 (i) 4 | 160 | 16 | Conv. | 22 | [72] |
| PL_4 | Fructose | Fe2(SO4)3 | 2.24/9.0 (i) 4 | 170 | 2 | Conv. | 61 | [73] |
| PL_5 | Glucose | Fe2(SO4)3 | 2.24/9.0 (i) 4 | 170 | 2 | Conv. | 55 | [73] |
| PL_6 | Cellulose | Al2(SO4)3 | 0.42/22.0 (i) 4 | 180 | 1.3 | MW | 54 | [32] |
| PL_7 | Fructose | Amberlyst-15 | 0.40/64.0 (n) 3 | 120 | 24 | Conv. | 80 | [80] |
| PL_8 | Cellulose | Acid resin D008 | 2.40/10.0 (i) 4 | 170 | 2 | Conv. | 16 | [121] |
| PL_9 | Fructose | PSSA-g-CNT 5 | 0.40/64.0 (n) 3 | 120 | 24 | Conv. | 86 | [80] |
| PL_10 | Fructose | PSSA-g-CNF 6 | 0.40/64.0 (n) 3 | 120 | 24 | Conv. | 75 | [80] |
| PL_11 | Fructose | BSA-g-CMK-5 7 | 0.40/64.0 (n) 3 | 120 | 24 | Conv. | 68 | [80] |
| PL_12 | Fructose | BSA-g-CNT 8 | 0.40/64.0 (n) 3 | 120 | 24 | Conv. | 54 | [80] |
| PL_13 | Cellulose | 5-Cl-SHPAO 9 | 0.25/20.0 (n) 3 | 160 | 8 | Conv. | 60 | [84] |
| PL_14 | Glucose | H-USY (6) | 0.60/32.0 (n) 3 | 160 | 20 | Conv. | 17 | [102] |
| PL_15 | Fructose | Sn2SiW12O40 | 1.67/66.7 (n) 3 | 150 | 2 | Conv. | 74 | [93] |
| PL_16 | Fructose | 3-PhPyPW 10 | 0.50/22.2 (n) 3 | 140 | 8 | Conv. | 22 | [99] |
| PL_17 | Fructose | [TMEDAPS]3 [PW12O40]2 11 | 1.00/64.0 (n) 3 | 120 | 12 | Conv. | 83 | [96] |
| PL_18 | Cellulose | [PyPS]3PW12O40 12 | 10.49/98.7 (n) 3 | 150 | 5 | Conv. | 37 | [97] |
| PL_19 | Cellulose | [PyPS]3PW12O40 12 | 10.49/98.7 (i) 4 | 150 | 5 | Conv. | 22 | [97] |
| PL_20 | Fructose | TiO2 nanoparticles | 0.56/88.9 (n) 3 | 150 | 3 | Conv. | 78 | [113] |
| PL_21 | Fructose | TiO2 nanoparticles | 0.56/88.9 (i) 4 | 150 | 3 | Conv. | 13 | [113] |
| PL_22 | Glucose | Grafted SO42−/ZrO2/SBA-15 | 0.50/25.6 (i) 4 | 140 | 24 | Conv. | 10 | [114] |
| Entry | Feedstock | Catalyst | Cat.(g)/Alcohol (g) 1 | T (°C) | t (h) | Heat 2 | Y (mol%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BL_1 | Cellulose | H2SO4 (100 wt%) | 0.17/7.5 (n) 3 | 210 | 0.17 | Conv. | 40 | [142] |
| BL_2 | Cellulose | H2SO4 (100 wt%) | 0.18/7.0 (i) 4 | 210 | 0.25 | Conv. | 40 | [69] |
| BL_3 | Cellulose | H2SO4 (96 wt%) | 25.00/100.0 (n) 3 | 130 | 20 | Conv. | 60 | [143] |
| BL_4 | Cellulose | H2SO4 (96 wt%) | 42.86/100.0 (n) 3 | 130 | 5 | Conv. | 60 | [143] |
| BL_5 | Cellulose | H2SO4 (100 wt%) | 0.25/40.0 (n) 3 | 200 | 0.5 | Conv. | 50 | [144] |
| BL_6 | Cellulose | H2SO4 (100 wt%) | 0.25/40.0 (i) 4 | 200 | 0.5 | Conv. | 45 | [144] |
| BL_7 | Cellulose | H2SO4 (100 wt%) | 0.25/40.0 (s) 5 | 200 | 0.5 | Conv. | 13 | [144] |
| BL_8 | Fructose | Fe2(SO4)3 | 0.20/32.4 (n) 3 | 190 | 3 | Conv. | 63 | [145] |
| BL_9 | Glucose | Fe2(SO4)3 | 0.20/32.4 (n) 3 | 190 | 3 | Conv. | 40 | [145] |
| BL_10 | Sucrose | Fe2(SO4)3 | 0.20/32.4 (n) 3 | 190 | 3 | Conv. | 50 | [145] |
| BL_11 | Inulin | Fe2(SO4)3 | 0.20/32.4 (n) 3 | 190 | 3 | Conv. | 57 | [145] |
| BL_12 | Cellulose | Fe2(SO4)3 | 0.20/32.4 (n) 3 | 220 | 3 | Conv. | 30 | [145] |
| BL_13 | Cellulose | Fe2(SO4)3 +Al2(SO4)3 | 0.48 + 0.025/40.8 (n) 3 | 194 | 3 | Conv. | 40 | [146] |
| BL_14 | Cellulose | Cs2HPW12O40 | 0.40/40.0 (n) 3 | 200 | 1 | Conv. | 12 | [144] |
| BL_15 | Fructose | 3-PhPyPW 6 | 0.50/22.2 (n) 3 | 140 | 8 | Conv. | 18 | [99] |
| BL_16 | Glucose | Zeolite H-USY (6) | 0.60/32.4 (n) 3 | 160 | 20 | Conv. | 12 | [102] |
| BL_17 | Cellulose | SO42−/ZrO2 | 0.40/40.0 (n) 3 | 200 | 1 | Conv. | 13 | [144] |
| BL_18 | Glucose | SO42−/SnO2-ZrO2 | 0.44/16.2 (n) 3 | 200 | 2 | Conv. | 33 | [147] |
| BL_19 | Cellulose | SO42−/SnO2-ZrO2 | 0.44/16.2 (n) 3 | 200 | 2 | Conv. | 10 | [147] |
| BL_20 | Glucose | SO42−/SnO2-ZrO2 + (COOH)2 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 36 | [147] |
| BL_21 | Glucose | SO42−/SnO2-ZrO2 + H2SO4 (100 wt%) | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 40 | [147] |
| BL_22 | Cellulose | SO42−/SnO2-ZrO2 + H2SO4 (100 wt%) | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 28 | [147] |
| BL_23 | Glucose | SO42−/SnO2-ZrO2 + Fe2(SO4)3 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 35 | [147] |
| BL_24 | Cellulose | SO42−/SnO2-ZrO2 + Fe2(SO4)3 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 18 | [147] |
| BL_25 | Glucose | SO42−/SnO2-ZrO2 + CuSO4 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 39 | [147] |
| BL_26 | Cellulose | SO42−/SnO2-ZrO2 + CuSO4 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 19 | [147] |
| BL_27 | Glucose | SO42−/SnO2-ZrO2 + PTSA 7 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 33 | [147] |
| BL_28 | Cellulose | SO42−/SnO2-ZrO2 + PTSA 7 | 0.44 + 0.01/16.2 (n) 3 | 200 | 2 | Conv. | 23 | [147] |
| BL_29 | Cellulose | [C4H8SO3Hmim]HSO4 8 | 0.10/10.0 (n) 3 | 180 | 0.75 | Conv. | 31 | [148] |
| PeL_1 | Cellulose | H2SO4 (100 wt%) | 25.4/101.8 | 138 | 3 | Conv. | 69 | [149] |
| PeL_2 | Fructose | WS2 | 0.39/21.7 | 160 | 0.25 | MW | 5 | [116] |
| HL_1 | Cellulose | H2SO4 (100 wt%) | 25.4/101.8 | 157 | 1 | Conv. | 60 | [149] |
| HL_2 | Glucose | Zn/DFNS 9 | 0.71/58.6 | 200 | 5 | Conv. | 55 | [150] |
| Entry | Feedstock | Catalyst | Cat.(g)/MeOH(g) 1 | T (°C) | t (h) | Heat 2 | YML (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ML_157 | Bamboo | HCl (37 wt%) | 0.20/8.0 | 180 | 0.5 | MW | 2 | [68] |
| ML_158 | Bamboo | H2SO4 (96 wt%) | 0.20/5.0 | 180 | 0.5 | MW | 25 | [68] |
| ML_159 | Bamboo | H2SO4 (96 wt%) | 0.25/8.0 | 180 | 0.67 | MW | 29 | [68] |
| ML_160 | Straw | H2SO4 (96 wt%) | 0.25/8.0 | 180 | 0.67 | MW | 22 | [68] |
| ML_161 | Eucalyptus | H2SO4 (96 wt%) | 0.25/8.0 | 180 | 0.67 | MW | 24 | [68] |
| ML_162 | Poplar | H2SO4 (96 wt%) | 0.25/8.0 | 180 | 0.67 | MW | 25 | [68] |
| ML_163 | Pine | H2SO4 (96 wt%) | 0.25/8.0 | 180 | 0.67 | MW | 26 | [68] |
| ML_164 | Bagasse | H2SO4 (96 wt%) | 0.25/8.0 | 180 | 0.67 | MW | 28 | [68] |
| ML_165 | Corn stover | H2SO4 (96 wt%) | 0.17/5.0 | 160 | 1 | MW | 9 | [152] |
| ML_166 | Corn stover | H2SO4 (100 wt%) | 0.003/29.8 | 160 | 1 | MW | 9 | [74] |
| ML_167 | BM-corn stover 3 | H2SO4 (100 wt%) | 0.003/29.8 | 160 | 1 | MW | 11 | [74] |
| ML_168 | Paper sludge | H2SO4 (100 wt%) | 0.06/15.8 | 222 | 3.58 | Conv. | 27 | [153] |
| ML_169 | Chlorella sp. KR-1 | H2SO4 (100 wt%) | 2.75/6.5 | 130 | 2 | Conv. | 7 | [154] |
| ML_170 | Nannochloropsisgaditana | H2SO4 (100 wt%) | 2.75/6.5 | 130 | 2 | Conv. | 2 | [154] |
| ML_171 | Corn stover | Al2(SO4)3 | 0.33/29.8 | 160 | 1 | MW | 8 | [74] |
| ML_172 | BM-corn stover 3 | Al2(SO4)3 | 0.33/29.8 | 160 | 1 | MW | 12 | [74] |
| ML_173 | Wheat straw | CuSO4 | 0.16/7.1 | 182 | 3.3 | Conv. | 16 | [55] |
| ML_174 | Pretreated wheat straw 4 | CuSO4 | 0.15/7.1 | 183 | 3.9 | Conv. | 20 | [55] |
| ML_175 | Peanut shells | Al2(SO4)3 + H2SO4 (100 wt%) | 0.51 + 0.02/13.2 | 160 | 3.6 | Conv. | 18 | [155] |
| ML_176 | Bamboo | PTSA 5 | 0.20/8.0 | 180 | 0.5 | MW | 19 | [68] |
| ML_177 | Cedar | Al(acac)3 + PTSA 5 | 0.12 + 0.06/31.6 | 180 | 5 | Conv. | 28 | [79] |
| ML_178 | Eucalyptus | Al(acac)3 + PTSA 5 | 0.12 + 0.06/31.6 | 180 | 5 | Conv. | 25 | [79] |
| ML_179 | Cedar | In(OTf)3 + BSA 6 | 0.04 + 0.06/31.6 | 200 | 5 | Conv. | 31 | [78] |
| ML_180 | Pine | In(OTf)3 + BSA 6 | 0.04 + 0.06/31.6 | 200 | 5 | Conv. | 26 | [78] |
| ML_181 | Eucalyptus | In(OTf)3 + BSA 6 | 0.04 + 0.06/31.6 | 200 | 5 | Conv. | 24 | [78] |
| ML_182 | Bagasse | In(OTf)3 + BSA 6 | 0.04 + 0.06/31.6 | 200 | 5 | Conv. | 25 | [78] |
| ML_183 | Bamboo | H3PW12O40 | 0.20/8.0 | 180 | 0.5 | MW | 15 | [68] |
| ML_184 | Corn straw | [PyPS]3PW12O40 7 | 3.40/31.6 | 170 | 4.5 | Conv. | 18 | [97] |
| ML_185 | Bagasse | [PyPS]3PW12O40 7 | 3.40/31.6 | 170 | 4 | Conv. | 14 | [97] |
| ML_186 | Bamboo | [HSO3BMIM]HSO4 8 | 0.20/8.0 | 180 | 0.5 | MW | 19 | [68] |
| ML_187 | Duckweed | [C3H6SO3HPy]HSO4 | 0.80/31.6 | 170 | 5 | Conv. | 24 | [156] |
| Entry | Feedstock | Catalyst | Cat.(g)/EtOH(g) 1 | T (°C) | t (h) | Heat 2 | YEL (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| EL_119 | Grey pine wood | H2SO4 (96 wt%) | 0.04/5.0 | 190 | 1.6 | Conv. | 16 | [157] |
| EL_120 | Paper pulp | H2SO4 (96 wt%) | 0.04/5.0 | 190 | 1.6 | Conv. | 26 | [157] |
| EL_121 | Switchgrass | H2SO4 (96 wt%) | 0.04/5.0 | 190 | 1.6 | Conv. | 14 | [157] |
| EL_122 | Wheat straw | H2SO4 (100 wt%) | 0.51/19.8 | 183 | 0.6 | Conv. | 18 | [158] |
| EL_123 | Chipped laminated particleboard | H2SO4 (96 wt%) | 0.19/7.4 | 200 | 0.5 | Conv. | 24 | [159] |
| EL_124 | Chlorella sp. KR-1 | H2SO4 (100 wt%) | 2.75/6.5 | 130 | 2 | Conv. | 11 | [154] |
| EL_125 | Nannochloropsisgaditana | H2SO4 (100 wt%) | 2.75/6.5 | 130 | 2 | Conv. | 3 | [154] |
| EL_126 | Mandarin peels | H2SO4 (100 wt%) | 0.90/5.3 3 | 150 | 2 | Conv. | 28 | [160] |
| EL_127 | Cassava | H2SO4 (96 wt%) | 0.25/3.9 4 | 160 | 3 | Conv. | 14 | [161] |
| EL_128 | Cassava 5 | H2SO4 (96 wt%) | 0.25/3.9 4 | 160 | 3 | Conv. | 21 | [161] |
| EL_129 | Cassava 5 | H2SO4 (96 wt%) | 0.17/5.3 6 | 160 | 5 | Conv. | 27 | [161] |
| EL_130 | Bamboo | H2SO4 (96 wt%) | 0.04/30.1 | 210 | 2.1 | Conv. | 51 | [162] |
| EL_131 | Bamboo 7 | H2SO4 (96 wt%) | 0.04/30.1 | 210 | 2.1 | Conv. | 63 | [162] |
| EL_132 | Corn stover | H2SO4 (96 wt%) | 0.42/15.0 | 190 | 0.5 | Conv. | 7 | [163] |
| EL_133 | Corn stover | H2SO4 (96 wt%) | 0.42/15.0 | 190 | 0.5 | MW | 17 | [163] |
| EL_134 | Corn stover | H2SO4 (96 wt%) | 0.20/20.0 | 180 | 0.5 | MW | 13 | [164] |
| EL_135 | Corn stover 8 | H2SO4 (96 wt%) | 0.20/20.0 | 180 | 0.5 | MW | 14 | [164] |
| EL_136 | Corn stover | H2SO4 (96 wt%) | 0.15/5.0 | 180 | 0.5 | MW | 12 | [25] |
| EL_137 | Wheat straw | H2SO4 (96 wt%) | 0.15/5.0 | 180 | 0.5 | MW | 11 | [25] |
| EL_138 | Rice straw | H2SO4 (96 wt%) | 0.15/5.0 | 180 | 0.5 | MW | 11 | [25] |
| EL_139 | Rape straw | H2SO4 (96 wt%) | 0.15/5.0 | 180 | 0.5 | MW | 6 | [25] |
| EL_140 | Poplar wood | H2SO4 (96 wt%) | 0.15/5.0 | 180 | 0.5 | MW | 9 | [25] |
| EL_141 | Cassava | H2SO4 (96 wt%) | 0.10/19.0 | 200 | 6 | Conv. | 31 | [165] |
| EL_142 | Cassava | NaHSO4 | 0.10/19.0 | 200 | 6 | Conv. | 15 | [165] |
| EL_143 | Cassava | Al2(SO4)3 | 0.10/19.0 | 200 | 6 | Conv. | 36 | [165] |
| EL_144 | Cassava | Fe2(SO4)3 | 0.10/19.0 | 200 | 6 | Conv. | 9 | [165] |
| EL_145 | DHFW 9 | H2SO4 (96 wt%) +AlCl3·6H2O | 0.16 + 0.16/15.8 | 180 | 4 | Conv. | 15 | [166] |
| EL_146 | KW 10 | H2SO4 (96 wt%) +AlCl3·6H2O | 0.16 + 0.16/15.8 | 180 | 4 | Conv. | 32 | [166] |
| EL_147 | FVS 11 | H2SO4 (96 wt%) +AlCl3·6H2O | 0.16 + 0.16/15.8 | 180 | 4 | Conv. | 11 | [166] |
| EL_148 | OFMSW 12 | H2SO4 (96 wt%) +AlCl3·6H2O | 0.16 + 0.16/15.8 | 180 | 4 | Conv. | 14 | [166] |
| EL_149 | Coniferous wood | 1,5-NSA 13 | 0.20/7.9 | 200 | 4 | Conv. | 46 | [167] |
| EL_150 | Coniferous wood | 2-NSA 14 | 0.20/7.9 | 200 | 4 | Conv. | 49 | [167] |
| EL_151 | Furfural residue | USY + H2SO4 (96 wt%) | 0.03 + 0.04/39.9 | 219 | 1.8 | Conv. | 19 | [133] |
| EL_152 | Wheat straw | [HSO3-BMIM] [HSO4] | 0.26/15.0 | 200 | 1 | Conv. | 16 | [168] |
| EL_153 | OPEFB 15,16 | [HMIM][HSO4] | n.a. 17 | 90 | 12 | Conv. | 12 | [169] |
| EL_154 | OPMF 18,16 | [HMIM][HSO4] | n.a. 17 | 90 | 12 | Conv. | 14 | [169] |
| EL_155 | OPEFB 15,19 | [HMIM][HSO4] + InCl3 | n.a. 17 | 90 | 10 | Conv. | 13 | [170] |
| EL_156 | OPMF 18,19 | [HMIM][HSO4] + InCl3 | n.a. 17 | 90 | 10 | Conv. | 15 | [170] |
| EL_157 | OPEFB 15,20 | [HMIM][HSO4] + InCl3 | n.a. 17 | 105 | 12.2 | Conv. | 19 | [171] |
| EL_158 | OPMF 18,20 | [HMIM][HSO4] + InCl3 | n.a. 17 | 105 | 12.2 | Conv. | 21 | [171] |
| EL_159 | Corn stover 21 | [HSO3-BMIM][HSO4] + Al2(SO4)3 | 0.51 + 0.14/20.0 | 170 | 2 | MW | 11 | [172] |
| Entry | Feedstock | Catalyst | Cat.(g)/Alcohol (g) 1 | T (°C) | t (h) | Heat 2 | Y (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PL_23 | Coniferous wood | 2-NSA 3 | 0.20/8.0 (i) 4 | 200 | 4 | Conv. | 46 | [167] |
| PL_24 | Duckweed | [C3H6SO3HPy]HSO4 5 | 0.80/32.0 (n) 6 | 170 | 5 | Conv. | 20 | [156] |
| BL_30 | Softwood Kraft pulp | H2SO4(100 wt%) | 42.86/100.0 (n) 7 | 117 | 3 | Conv. | 53 | [149] |
| BL_31 | Hardwood Kraft pulp | H2SO4(100 wt%) | 42.86/100.0 (n) 7 | 117 | 3 | Conv. | 43 | [149] |
| BL_32 | Papermaking sludge “A” | H2SO4(100 wt%) | 42.86/100.0 (n) 7 | 117 | 3 | Conv. | 11 | [149] |
| BL_33 | ADW Eucalyptus Nitens 8 | H2SO4(100 wt%) | 0.10/3.9 (n) 7 | 183 | 2.4 | MW | 38 | [52] |
| BL_34 | Arundo donax | H2SO4(100 wt%) | 0.10/6.0 (n) 7 | 190 | 0.25 | MW | 37 | [173] |
| BL_35 | Coniferous wood | 2-NSA 3 | 0.20/8.1 (n) 7 | 200 | 4 | Conv. | 43 | [167] |
| PeL_3 | Softwood Kraft pulp | H2SO4(100 wt%) | 25.4/101.8 | 138 | 3 | Conv. | 62 | [149] |
| PeL_4 | Hardwood Kraft pulp | H2SO4(100 wt%) | 25.4/101.8 | 138 | 3 | Conv. | 56 | [149] |
| PeL_5 | Papermaking sludge “G” | H2SO4(100 wt%) | 25.4/101.8 | 138 | 3 | Conv. | 45 | [149] |
| HL_3 | Softwood Kraft pulp | H2SO4(100 wt%) | 25.4/101.8 | 157 | 1 | Conv. | 59 | [149] |
| HL_4 | Hardwood Kraft pulp | H2SO4(100 wt%) | 25.4/101.8 | 157 | 1 | Conv. | 54 | [149] |
| HL_5 | Papermaking sludge “G” | H2SO4(100 wt%) | 25.4/101.8 | 157 | 1 | Conv. | 42 | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raspolli Galletti, A.M.; Antonetti, C.; Fulignati, S.; Licursi, D. Direct Alcoholysis of Carbohydrate Precursors and Real Cellulosic Biomasses to Alkyl Levulinates: A Critical Review. Catalysts 2020, 10, 1221. https://doi.org/10.3390/catal10101221
Raspolli Galletti AM, Antonetti C, Fulignati S, Licursi D. Direct Alcoholysis of Carbohydrate Precursors and Real Cellulosic Biomasses to Alkyl Levulinates: A Critical Review. Catalysts. 2020; 10(10):1221. https://doi.org/10.3390/catal10101221
Chicago/Turabian StyleRaspolli Galletti, Anna Maria, Claudia Antonetti, Sara Fulignati, and Domenico Licursi. 2020. "Direct Alcoholysis of Carbohydrate Precursors and Real Cellulosic Biomasses to Alkyl Levulinates: A Critical Review" Catalysts 10, no. 10: 1221. https://doi.org/10.3390/catal10101221
APA StyleRaspolli Galletti, A. M., Antonetti, C., Fulignati, S., & Licursi, D. (2020). Direct Alcoholysis of Carbohydrate Precursors and Real Cellulosic Biomasses to Alkyl Levulinates: A Critical Review. Catalysts, 10(10), 1221. https://doi.org/10.3390/catal10101221









