Plasma-Catalysis for Volatile Organic Compounds Decomposition: Complexity of the Reaction Pathways during Acetaldehyde Removal
Abstract
1. Introduction
2. Results
2.1. Acetaldehyde Decomposition Using the Plasma-Driven Catalysis Process
2.2. Acetaldehyde Adsorption on Ag/TiO2/SiO2
2.3. Surface Species Formed during the PDC Process
3. Discussion
3.1. Acetaldehyde Adsorption Mechanism
3.2. Surface Species Formed during the PDC Process: Formation Mechanism
3.3. Proposed Simplified Mechanism for Acetaldehyde Decomposition Using the Plasma/Catalysis Process
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Plasma/Catalysis Process
4.3. DRIFTS Measurements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, K.; Ji, J.; Huang, H.; He, M. Efficient activation of Pd/CeO2 catalyst by non-thermal plasma for complete oxidation of indoor formaldehyde at room temperature. Chemosphere 2020, 246, 125762. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, M.Y.; Ryu, S.; Park, Y.-K. Acetaldehyde oxidation under high humidity using a catalytic non-thermal plasma system over Mn-loaded Y zeolites. Mater. Lett. 2020, 262, 127051. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Nguyen Dinh, M.T.; Nuns, N.; Giraudon, J.M.; De Geyter, N.; Leys, C.; Lamonier, J.F.; Morent, R. Combination of non-thermal plasma and Pd/LaMnO3 for dilute trichloroethylene abatement. Chem. Eng. J. 2016, 283, 668–675. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Eloy, P.; Gaigneaux, E.M.; Parvulescu, V.I. Plasma-assisted catalysis for volatile organic compounds abatement. Appl. Catal. B Environ. 2005, 61, 12–20. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, T.L.; Wang, M.Y.; Li, X.M. Removal of low-concentration BTX in air using a combined plasma catalysis system. Chemosphere 2009, 75, 1301–1306. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; He, C.; Shen, Z.; Wang, T. Synergistic effects and mechanism of a non-thermal plasma catalysis system in volatile organic compound removal: A review. Catal. Sci. Technol. 2018, 8, 936–954. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Xu, W.; Wang, N.; Xiao, H.; Sun, Y.; Huang, H.; Yu, L.; Fu, M.; Wu, J.; et al. The Mechanism of Non-thermal Plasma Catalysis on Volatile Organic Compounds Removal. Catal. Surv. Asia 2018, 22, 73–94. [Google Scholar] [CrossRef]
- Schiavon, M.; Torretta, V.; Casazza, A.; Ragazzi, M. Non-thermal Plasma as an Innovative Option for the Abatement of Volatile Organic Compounds: A Review. Water Air Soil Pollut. 2017, 228, 388. [Google Scholar] [CrossRef]
- Perillo, R.; Ferracin, E.; Giardina, A.; Marotta, E.; Paradisi, C. Efficiency, products and mechanisms of ethyl acetate oxidative degradation in air non-thermal plasma. J. Phys. D Appl. Phys. 2019, 52, 295206. [Google Scholar] [CrossRef]
- Bal, K.M.; Huygh, S.; Bogaerts, A.; Neyts, E.C. Effect of plasma-induced surface charging on catalytic processes: Application to CO2 activation. Plasma Sources Sci. Technol. 2018, 27, 024001. [Google Scholar] [CrossRef]
- Song, H.; Hu, F.; Peng, Y.; Li, K.; Bai, S.; Li, J. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar] [CrossRef]
- Barakat, C.; Gravejat, P.; Guaitella, O.; Thevenet, F.; Rousseau, A. Oxidation of isopropanol and acetone adsorbed on TiO2 under plasma generated ozone flow: Gas phase and adsorbed species monitoring. Appl. Catal. B Environ. 2014, 147, 302–313. [Google Scholar] [CrossRef]
- Xu, W.; Xu, X.; Wu, J.; Fu, M.; Chen, L.; Wang, N.; Xiao, H.; Chen, X.; Ye, D. Removal of toluene in adsorption-discharge plasma systems over a nickel modified SBA-15 catalyst. RSC Adv. 2016, 6, 104104–104111. [Google Scholar] [CrossRef]
- Sultana, S.; Vandenbroucke, A.M.; Mora, M.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J.; Leys, C.; De Geyter, N.; Morent, R. Post plasma-catalysis for trichloroethylene decomposition over CeO2 catalyst: Synergistic effect and stability test. Appl. Catal. B Environ. 2019, 253, 49–59. [Google Scholar] [CrossRef]
- Rivallan, M.; Fourre, E.; Aiello, S.; Tatibouet, J.M.; Thibault-Starzyk, F. Insights into the Mechanisms of Isopropanol Conversion on γ-Al2O3 by Dielectric Barrier Discharge. Plasma Process. Polym. 2012, 9, 850–854. [Google Scholar] [CrossRef]
- Stere, C.E.; Adress, W.; Burch, R.; Chansai, S.; Goguet, A.; Graham, W.G.; Hardacre, C. Probing a Non-Thermal Plasma Activated Heterogeneously Catalyzed Reaction Using in Situ DRIFTS-MS. ACS Catal. 2015, 5, 956–964. [Google Scholar] [CrossRef]
- Manabe, R.; Okada, S.; Inagaki, R.; Oshima, K.; Ogo, S.; Sekine, Y. Surface Protonics Promotes Catalysis. Sci. Rep. 2016, 6, 38007. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Chen, P.; Wang, B.; Wu, J.; Fu, M.; Chen, L.; Ye, D. Reverse water-gas shift in a packed bed DBD reactor: Investigation of metal-support interface towards a better understanding of plasma catalysis. Appl. Catal. A Gen. 2020, 591, 117407. [Google Scholar] [CrossRef]
- Xu, S.; Chansai, S.; Stere, C.; Inceesungvorn, B.; Goguet, A.; Wangkawong, K.; Taylor, S.F.R.; Al-Janabi, N.; Hardacre, C.; Martin, P.A.; et al. Sustaining metal–organic frameworks for water–gas shift catalysis by non-thermal plasma. Nat. Catal. 2019, 2, 142–148. [Google Scholar] [CrossRef]
- Xu, S.; Chansai, S.; Shao, Y.; Xu, S.; Wang, Y.-c.; Haigh, S.; Mu, Y.; Jiao, Y.; Stere, C.E.; Chen, H.; et al. Mechanistic study of non-thermal plasma assisted CO2 hydrogenation over Ru supported on MgAl layered double hydroxide. Appl. Catal. B Environ. 2020, 268, 118752. [Google Scholar] [CrossRef]
- Kim, H.-H.; Ogata, A.; Schiorlin, M.; Marotta, E.; Paradisi, C. Oxygen Isotope (18O2) Evidence on the Role of Oxygen in the Plasma-Driven Catalysis of VOC Oxidation. Catal. Lett. 2010, 141, 277–282. [Google Scholar] [CrossRef]
- Christensen, P.A.; Mashhadani, Z.T.A.W.; Md Ali, A.H.B.; Manning, D.A.C.; Carroll, M.A.; Martin, P.A. An in situ FTIR study of the plasma- and thermally-driven reaction of isopropyl alcohol at CeO2: Evidence for a loose transition state involving Ce3+? PCCP 2019, 21, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Tatibouët, J.-M.; Fourré, E. Operando DRIFT Spectroscopy Characterization of Intermediate Species on Catalysts Surface in VOC Removal from Air by Non-thermal Plasma Assisted Catalysis. Plasma Chem. Plasma Process. 2016, 36, 901–915. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, X.; Thevenet, F.; Rousseau, A. Dynamic probing of plasma-catalytic surface processes: Oxidation of toluene on CeO2. Plasma Process. Polym. 2017, 14, 1600114. [Google Scholar] [CrossRef]
- Jia, Z.; Rousseau, A. Sorbent track: Quantitative monitoring of adsorbed VOCs under in-situ plasma exposure. Sci. Rep. 2016, 6, 31888. [Google Scholar] [CrossRef]
- Jia, Z.; Vega-González, A.; Amar, M.B.; Hassouni, K.; Tieng, S.; Touchard, S.; Kanaev, A.; Duten, X. Acetaldehyde removal using a diphasic process coupling a silver-based nano-structured catalyst and a plasma at atmospheric pressure. Catal. Today 2013, 208, 82–89. [Google Scholar] [CrossRef]
- Sauce, S.; Vega-González, A.; Jia, Z.; Touchard, S.; Hassouni, K.; Kanaev, A.; Duten, X. New insights in understanding plasma-catalysis reaction pathways: Study of the catalytic ozonation of an acetaldehyde saturated Ag/TiO2/SiO2 catalyst. Eur. Phys. J. Appl. Phys. 2015, 71, 20805. [Google Scholar] [CrossRef]
- Klett, C.; Duten, X.; Tieng, S.; Touchard, S.; Jestin, P.; Hassouni, K.; Vega-González, A. Acetaldehyde removal using an atmospheric non-thermal plasma combined with a packed bed: Role of the adsorption process. J. Hazard. Mater. 2014, 279C, 356–364. [Google Scholar] [CrossRef]
- Mann, A.K.P.; Wu, Z.; Calaza, F.C.; Overbury, S.H. Adsorption and Reaction of Acetaldehyde on Shape-Controlled CeO2 Nanocrystals: Elucidation of Structure–Function Relationships. ACS Catal. 2014, 4, 2437–2448. [Google Scholar] [CrossRef]
- Singh, M.; Zhou, N.; Paul, D.K.; Klabunde, K.J. IR spectral evidence of aldol condensation: Acetaldehyde adsorption over TiO2 surface. J. Catal. 2008, 260, 371–379. [Google Scholar] [CrossRef]
- Idriss, H.; Diagne, C.; Hindermann, J.P.; Kiennemann, A.; Barteau, M.A. Reactions of Acetaldehyde on CeO2 and CeO2-supported catalysts. J. Catal. 1995, 155, 219–237. [Google Scholar] [CrossRef]
- Rekoske, J.E.; Barteau, M.A. Competition between Acetaldehyde and Crotonaldehyde during Adsorption and Reaction on Anatase and Rutile Titanium Dioxide. Langmuir ACS J. Surf. Colloids 1999, 15, 11. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Sushkevich, V.L.; Ivanova, I.I. Study of acetaldehyde condensation chemistry over magnesia and zirconia supported on silica. J. Mol. Catal. A Chem. 2010, 333, 85–93. [Google Scholar] [CrossRef]
- Natal Santiago, M.A.; Hill, J.S.; Dumesic, J.A. Studies of the adsorption of acetaldehyde, methyl acetate, ethyl acetate, and methyl trifluoroacetate on silica. J. Mol. Catal. A Chem. 1999, 140, 16. [Google Scholar] [CrossRef]
- Finkelstein-Shapiro, D.; Buchbinder, A.M.; Vijayan, B.; Bhattacharyya, K.; Weitz, E.; Geiger, F.M.; Gray, K.A. Identification of Binding Sites for Acetaldehyde Adsorption on Titania Nanorod Surfaces Using CIMS. Langmuir ACS J. Surf. Colloids 2011, 27, 14842–14848. [Google Scholar] [CrossRef] [PubMed]
- Raskó, J.; Kiss, J. Adsorption and surface reactions of acetaldehyde on alumina-supported noble metal catalysts. Catal. Lett. 2005, 101, 71–77. [Google Scholar] [CrossRef]
- Chang, C.-A.; Ray, B.; Paul, D.K.; Demydov, D.; Klabunde, K.J. Photocatalytic reaction of acetaldehyde over SrTiO3 nanoparticles. J. Mol. Catal. A Chem. 2008, 281, 99–106. [Google Scholar] [CrossRef]
- Young, Z.D.; Hanspal, S.; Davis, R.J. Aldol Condensation of Acetaldehyde over Titania, Hydroxyapatite, and Magnesia. ACS Catal. 2016, 6, 3193–3202. [Google Scholar] [CrossRef]
- Hauchecorne, B.; Terrens, D.; Verbruggen, S.; Martens, J.A.; Van Langenhove, H.; Demeestere, K.; Lenaerts, S. Elucidating the photocatalytic degradation pathway of acetaldehyde: An FTIR in situ study under atmospheric conditions. Appl. Catal. B Environ. 2011, 106, 630–638. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Pasupulety, L. Surface Reactions of Acetone on Al2O3, TiO2, ZrO2, and CeO2: IR Spectroscopic Assessment of Impacts of the Surface Acid−Base Properties. Langmuir ACS J. Surf. Colloids 2001, 17, 768–774. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Kasimayan, U.; Nadarajan, A.; Singaravelu, C.M.; Pan, G.T.; Kandasamy, J.; Yang, T.C.; Lin, J.H. In-situ DRIFT investigation of photocatalytic reduction and oxidation properties of SiO2@alpha-Fe2O3 core-shell decorated RGO nanocomposite. Sci. Rep. 2020, 10, 2128. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chuang, S. In situ IR study of adsorbed species and photogenerated electrons during photocatalytic oxidation of ethanol on TiO2. J. Catal. 2007, 246, 118–126. [Google Scholar] [CrossRef]
- Lukaski, A.C.; Muggli, D.S. Photocatalytic oxidation of methyl formate on TiO2: A transient DRIFTS study. J. Catal. 2004, 223, 250–261. [Google Scholar] [CrossRef]
- Backes, M.J.; Lukaski, A.C.; Muggli, D.S. Active sites and effects of H2O and temperature on the photocatalytic oxidation of 13C-acetic acid on TiO2. Appl. Catal. B Environ. 2005, 61, 21–35. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Wu, W.-C.; Huang, M.-C.; Huang, I.-C.; Lin, J.-L. FTIR Study of Adsorption and Reactions of Methyl Formate on Powdered TiO2. J. Catal. 1999, 185, 12. [Google Scholar] [CrossRef]
- Coronado, J.M.; Kataoka, S.; Tejedor-Tejedor, I.; Anderson, M.A. Dynamic phenomena during the photocatalytic oxidation of ethanol and acetone over nanocrystalline TiO2: Simultaneous FTIR analysis of gas and surface species. J. Catal. 2003, 219, 219–230. [Google Scholar] [CrossRef]
- Gazsi, A.; Koós, A.; Bánsági, T.; Solymosi, F. Adsorption and decomposition of ethanol on supported Au catalysts. Catal. Today 2011, 160, 70–78. [Google Scholar] [CrossRef]
- Liao, L.-F.; Lien, C.-F.; Lin, J.-L. FTIR study of adsorption and photoreactions of acetic acid on TiO2. Phys. Chem. Chem. Phys. 2001, 3, 7. [Google Scholar] [CrossRef]
- Bratož, S.; Hadži, D.; Sheppard, N. The infra-red absorption bands associated with the COOH and COOD groups in dimeric carboxylic acid—II: The region from 3700 to 1500 cm−1. Spectrochim. Acta 1956, 8, 249–261. [Google Scholar] [CrossRef]
- Raskó, J. Adsorption and reaction of formaldehyde on TiO2-supported Rh catalysts studied by FTIR and mass spectrometry. J. Catal. 2004, 226, 183–191. [Google Scholar] [CrossRef]
- Idriss, H.; Kim, K.S.; Barteau, M.A. Surface-dependent pathways for formaldehyde oxidation and reduction on TiO2(001). Surf. Sci. 1992, 262, 113–127. [Google Scholar] [CrossRef]
- Tóth, M.; Varga, E.; Oszkó, A.; Baán, K.; Kiss, J.; Erdőhelyi, A. Partial oxidation of ethanol on supported Rh catalysts: Effect of the oxide support. J. Mol. Catal. A Chem. 2016, 411, 377–387. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Tejedor-Tejedor, I.; Coronado, J.M.; Anderson, M.A.; Soria, J. Operando FTIR study of the photocatalytic oxidation of acetone in air over TiO2–ZrO2 thin films. Catal. Today 2009, 143, 364–373. [Google Scholar] [CrossRef]
- Silva, A.; Barandas, A.; Costa, L.; Borges, L.; Mattos, L.; Noronha, F. Partial oxidation of ethanol on Ru/Y2O3 and Pd/Y2O3 catalysts for hydrogen production. Catal. Today 2007, 129, 297–304. [Google Scholar] [CrossRef]
- Gazsi, A.; Schubert, G.; Pusztai, P.; Solymosi, F. Photocatalytic decomposition of formic acid and methyl formate on TiO2 doped with N and promoted with Au. Production of H2. Int. J. Hydrogen Energy 2013, 38, 7756–7766. [Google Scholar] [CrossRef]
- Araña, J.; Garriga i Cabo, C.; Doña-Rodríguez, J.M.; González-Díaz, O.; Herrera-Melián, J.A.; Pérez-Peña, J. FTIR study of formic acid interaction with TiO2 and TiO2 doped with Pd and Cu in photocatalytic processes. Appl. Surf. Sci. 2004, 239, 60–71. [Google Scholar] [CrossRef]
- Miller, K.L.; Lee, C.W.; Falconer, J.L.; Medlin, J.W. Effect of water on formic acid photocatalytic decomposition on TiO2 and Pt/TiO2. J. Catal. 2010, 275, 294–299. [Google Scholar] [CrossRef]
- Liao, L.-F.; Wu, W.-C.; Chen, C.-Y.; Lin, J.-L. Photooxidation of Formic Acid vs. Formate and Ethanol vs. Ethoxy on TiO2 and Effect of Adsorbed Water on the Rates of Formate and Formic Acid Photooxidation. J. Phys. Chem. B 2001, 105, 7678–7685. [Google Scholar] [CrossRef]
- Li, C.; Domen, K.; Maruya, K.; Onishi, T. Spectroscopic Identification of Adsorbed Species Derived from Adsorption and Decomposition of Formic Acid, Methanol, and Formaldehyde on Cerium Oxide. J. Catal. 1990, 125, 11. [Google Scholar] [CrossRef]
- Xie, B.; Wong, R.J.; Tan, T.H.; Higham, M.; Gibson, E.K.; Decarolis, D.; Callison, J.; Aguey-Zinsou, K.F.; Bowker, M.; Catlow, C.R.A.; et al. Synergistic ultraviolet and visible light photo-activation enables intensified low-temperature methanol synthesis over copper/zinc oxide/alumina. Nat. Commun. 2020, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, V.A.; Eremeev, N.F.; Sadovskaya, E.M.; Chesalov, Y.A.; Pavlova, S.N.; Rogov, V.A.; Simonov, M.N.; Bobin, A.S.; Glazneva, T.S.; Smal, E.A.; et al. Detailed Mechanism of Ethanol Transformation into Syngas on Catalysts Based on Mesoporous MgAl2O4 Support Loaded with Ru + Ni/(PrCeZrO or MnCr2O4) Active Components. Top. Catal. 2020, 63, 166–177. [Google Scholar] [CrossRef]
- Topalian, Z.; Stefanov, B.I.; Granqvist, C.G.; Österlund, L. Adsorption and photo-oxidation of acetaldehyde on TiO2 and sulfate-modified TiO2: Studies by in situ FTIR spectroscopy and micro-kinetic modeling. J. Catal. 2013, 307, 265–274. [Google Scholar] [CrossRef]
- Raskó, J.; Kecskés, T.; Kiss, J. FT-IR and mass spectrometric studies on the interaction of acetaldehyde with TiO2-supported noble metal catalysts. Appl. Catal. A Gen. 2005, 287, 244–251. [Google Scholar] [CrossRef]
- Idriss, H.; Kim, K.S.; Barteau, M.A. Carbon-Carbon Bond Formation via Aldolization of Acetaldehyde on Single Crystal and Polycrystalline TiO2 Surfaces. J. Catal. 1993, 139, 15. [Google Scholar] [CrossRef]
- Kim, K.S.; Barteau, M.A. Structure and Composition Requirements for Deoxygenation, Dehydration, and Ketonization Reactions of Carboxylic Acids on TiO2(001) Single-Crystal Surfaces. J. Catal. 1990, 125, 23. [Google Scholar] [CrossRef]
- Sheng, P.Y.; Bowmaker, G.A.; Idriss, H. The Reactions of Ethanol over Au/CeO2. Appl. Catal. A Gen. 2004, 261, 171–181. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Toste, F.D.; Bell, A.T. Mechanism and Kinetics of Isobutene Formation from Ethanol and Acetone over ZnxZryOz. ACS Catal. 2019, 9, 10588–10604. [Google Scholar] [CrossRef]
- Velasquez Ochoa, J.; Farci, E.; Cavani, F.; Sinisi, F.; Artiglia, L.; Agnoli, S.; Granozzi, G.; Paganini, M.C.; Malfatti, L. CeOx/TiO2 (Rutile) Nanocomposites for the Low-Temperature Dehydrogenation of Ethanol to Acetaldehyde: A Diffuse Reflectance Infrared Fourier Transform Spectroscopy–Mass Spectrometry Study. ACS Appl. Nano Mater. 2019, 2, 3434–3443. [Google Scholar] [CrossRef]
- Bokhimi, X.; Zanella, R.; Maturano, V.; Morales, A. Nanocrystalline Ag, and Au–Ag alloys supported on titania for CO oxidation reaction. Mater. Chem. Phys. 2013, 138, 490–499. [Google Scholar] [CrossRef]
- Zhao, D.Z.; Shi, C.; Li, X.S.; Zhu, A.M.; Jang, B.W. Enhanced effect of water vapor on complete oxidation of formaldehyde in air with ozone over MnOx catalysts at room temperature. J. Hazard. Mater. 2012, 239–240, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F.F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Li, H.; Liu, T.; Sang, S.; Chen, S.; Duan, L.; Zeng, L.; Xiang, W.; Gong, J. Chemical looping oxidative steam reforming of methanol: A new pathway for auto-thermal conversion. Appl. Catal. B Environ. 2020, 269, 118758. [Google Scholar] [CrossRef]
- Manzoli, M.; Chiorino, A.; Boccuzzi, F. Decomposition and combined reforming of methanol to hydrogen: A FTIR and QMS study on Cu and Au catalysts supported on ZnO and TiO2. Appl. Catal. B Environ. 2005, 57, 201–209. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.-J.; Ge, Q. Effect of Pt Clusters on Methanol Adsorption and Dissociation over Perfect and Defective Anatase TiO2(101) Surface. J. Phys. Chem. C 2009, 113, 20674–20682. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous Gold Catalysts for Selective Gas-Phase Oxidative Coupling of Methanol at Low Temperature. Science 2010, 327, 319. [Google Scholar] [CrossRef]
- Klett, C.; Touchard, S.; Vega-González, A.; Redolfi, M.; Bonnin, X.; Hassouni, K.; Duten, X. Experimental and modeling study of the oxidation of acetaldehyde in an atmospheric-pressure pulsed corona discharge. Plasma Sources Sci. Technol. 2012, 21, 045001. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Yang, X.; Wu, Y. Catalytic oxidation of methanol to methyl formate over silver? A new purpose of a traditional catalysis system. Catal. Lett. 2005, 100, 205–211. [Google Scholar] [CrossRef]
- Rebsdat, S.; Mayer, D.; Ethylene Oxide. Ethylene Oxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2001; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a10_117 (accessed on 28 July 2020).
- Li, Y.; Yue, B.; Yan, S.; Yang, W.; Xie, Z.; Chen, Q.; He, H. Preparation of Ethylene Glycol via Catalytic Hydration with Highly Efficient Supported Niobia Catalyst. Catal. Lett. 2004, 95, 163–166. [Google Scholar] [CrossRef]
- Kandasamy, S.; Samudrala, S.P.; Bhattacharya, S. The route towards sustainable production of ethylene glycol from a renewable resource, biodiesel waste: A review. Catal. Sci. Technol. 2019, 9, 567–577. [Google Scholar] [CrossRef]
- Yu, W.; Mellinger, Z.J.; Barteau, M.A.; Chen, J.G. Comparison of Reaction Pathways of Ethylene Glycol, Acetaldehyde, and Acetic Acid on Tungsten Carbide and Ni-Modified Tungsten Carbide Surfaces. J. Phys. Chem. C 2012, 116, 5720–5729. [Google Scholar] [CrossRef]
- McManus, J.R.; Martono, E.; Vohs, J.M. Selective Deoxygenation of Aldehydes: The Reaction of Acetaldehyde and Glycolaldehyde on Zn/Pt(111) Bimetallic Surfaces. ACS Catal. 2013, 3, 1739–1750. [Google Scholar] [CrossRef]
- Neitzel, A.; Lykhach, Y.; Johánek, V.; Tsud, N.; Skála, T.; Prince, K.C.; Matolín, V.; Libuda, J. Role of Oxygen in Acetic Acid Decomposition on Pt(111). J. Phys. Chem. C 2014, 118, 14316–14325. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ko, A.-N. Vapor phase reactions of acetaldehyde over type X zeolites. Appl. Catal. A Gen. 2000, 190, 149–155. [Google Scholar] [CrossRef]
- Boamah, M.D.; Sullivan, K.K.; Shulenberger, K.E.; Soe, C.M.; Jacob, L.M.; Yhee, F.C.; Atkinson, K.E.; Boyer, M.C.; Haines, D.R.; Arumainayagam, C.R. Low-energy electron-induced chemistry of condensed methanol: Implications for the interstellar synthesis of prebiotic molecules. Faraday Discuss. 2014, 168, 249. [Google Scholar] [CrossRef] [PubMed]
- Busca, G. Infrared studies of the reactive adsorption of organic molecules over metal oxides and of the mechanisms of their heterogeneously-catalyzed oxidation. Catal. Today 1996, 27, 40. [Google Scholar] [CrossRef]
- Kim, M.; Park, E.; Jurng, J. Oxidation of gaseous formaldehyde with ozone over MnOx/TiO2 catalysts at room temperature (25 °C). Powder Technol. 2018, 325, 368–372. [Google Scholar] [CrossRef]
- Wittstock, A.; Biener, J.; Bäumer, M. Nanoporous Gold: A Novel Catalyst with Tunable Properties. ECS Trans. 2010, 28, 1–13. [Google Scholar] [CrossRef]
- Jia, Z.; Ben Amar, M.; Brinza, O.; Astafiev, A.; Nadtochenko, V.; Evlyukhin, A.B.; Chichkov, B.N.; Duten, X.; Kanaev, A. Growth of Silver Nanoclusters on Monolayer Nanoparticulate Titanium-oxo-alkoxy Coatings. J. Phys. Chem. C 2012, 116, 17239–17247. [Google Scholar] [CrossRef]
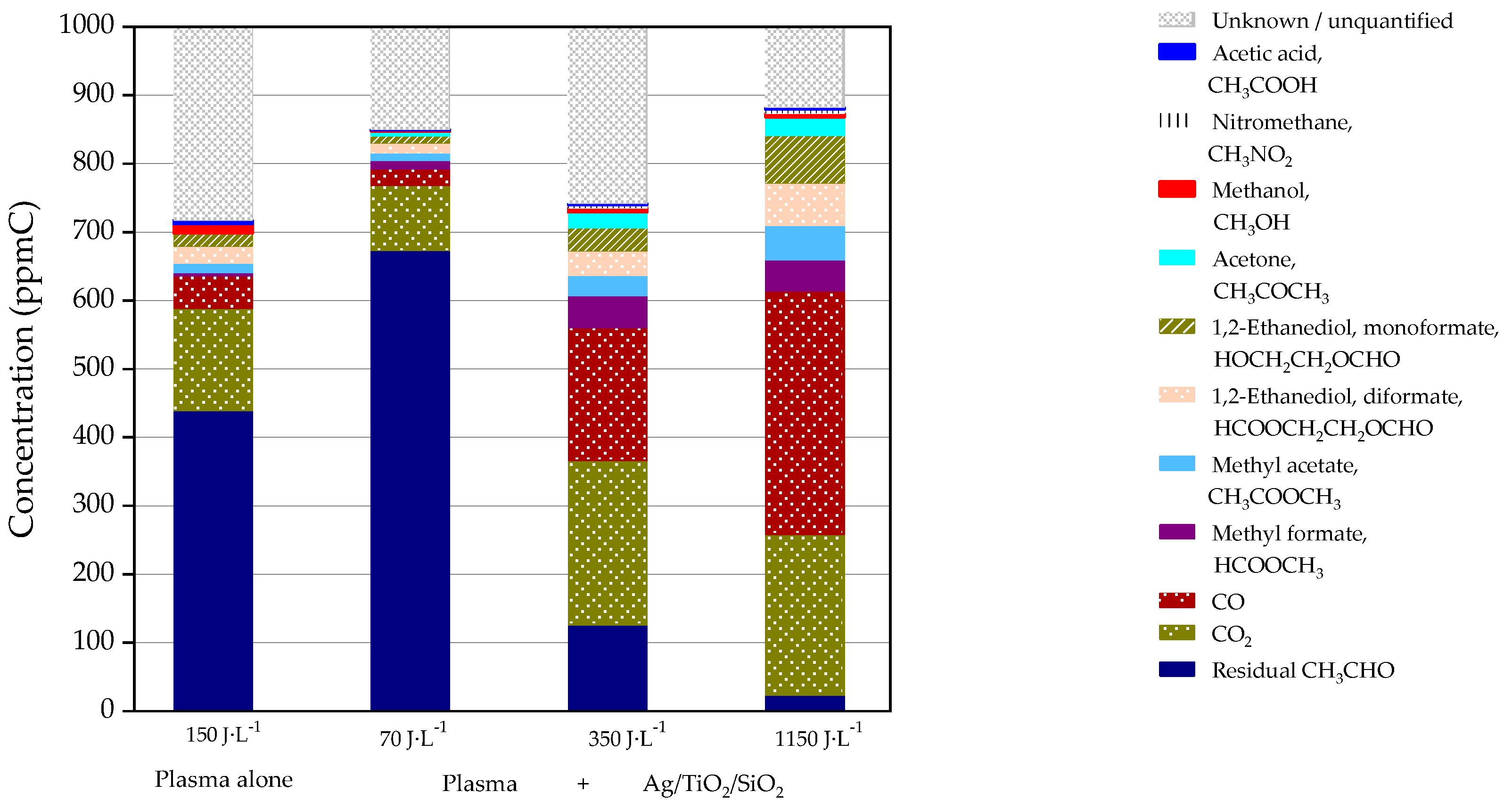
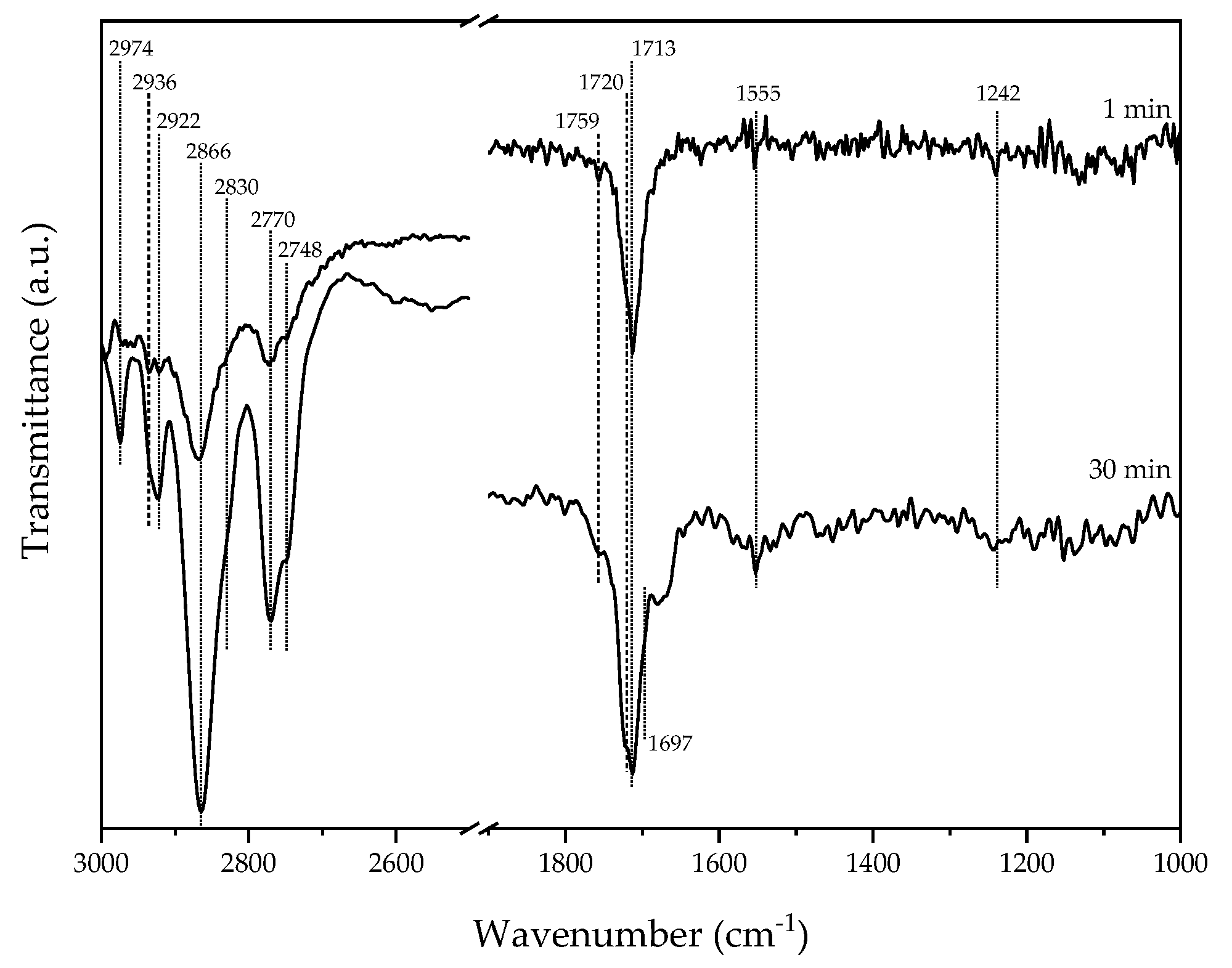
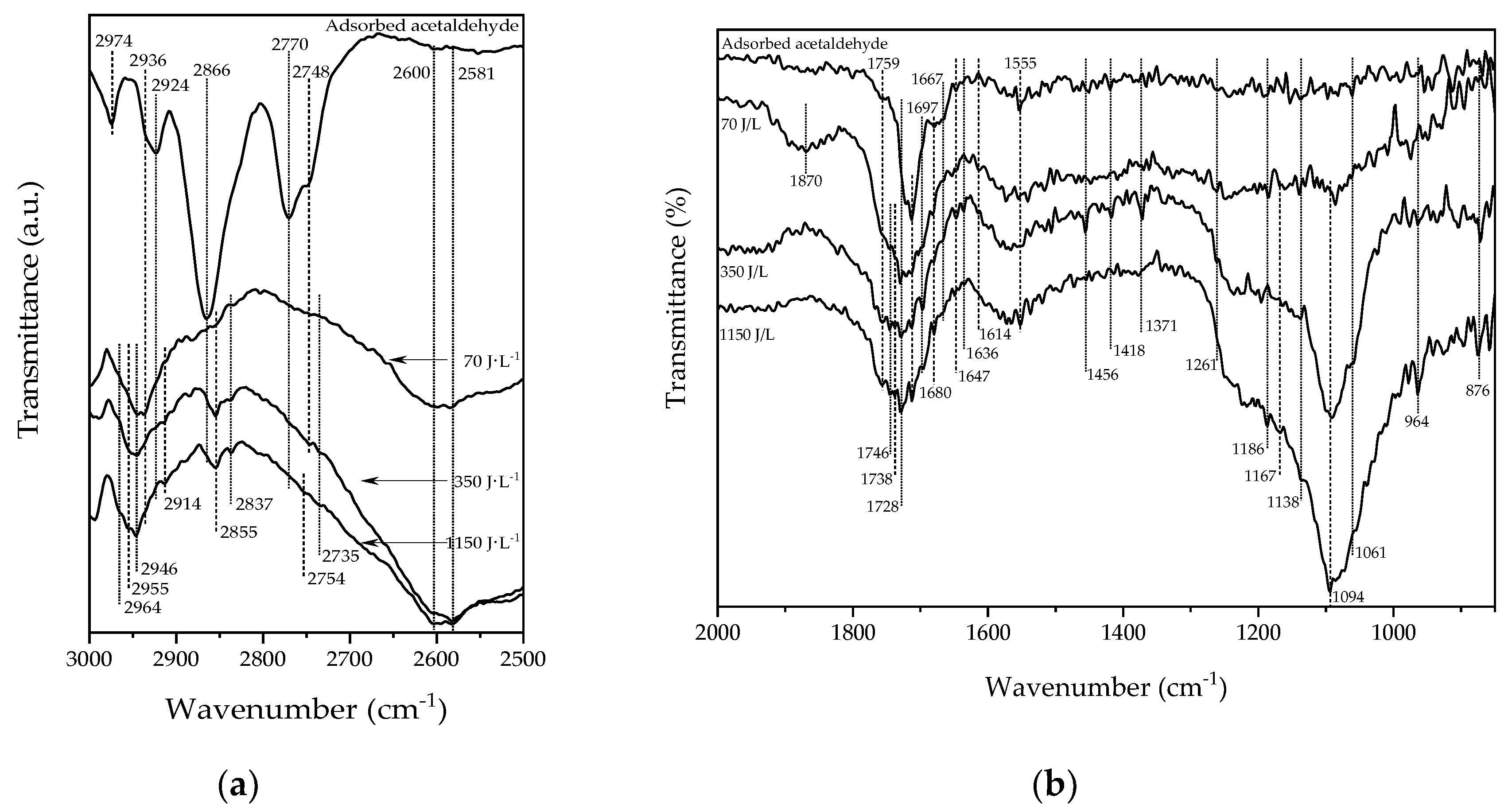

| Specific Input Energy (SIE) (J·L−1) | Ag/TiO2/SiO2 | Plasma Alone | Plasma + Ag/TiO2/SiO2 | ||
|---|---|---|---|---|---|
| Alone | 150 | 70 | 350 | 1150 | |
| Acetaldehyde removal (%) | 0 | 55 | 33 | 87 | 98 |
| Vibrational Mode | Vibrational Assignments (cm−1) | Reference | |
|---|---|---|---|
| Gas Phase CH3CHO | CH3CHO Adsorbed on Ag/TiO2/SiO2 | ||
| νas(CH3) | 2967 | 2974 | [30] |
| νas(CH2) | 2936 | [30,31,32] | |
| νs(CH3) | 2923 | 2922 | [30,33] |
| 2ν6A’ Fermi | 2840 | 2866 | [34] |
| νs(CH2) | 2830 | [30] | |
| ν(CH)η1-acetaldehyde | 2736, 2704 | 2770, 2748 | [31,33,35] |
| ν(C=O) | 1735 | 1713 | [31] |
| νas(COO) | 1555 | [36] | |
| Surface Species | IR Band (cm−1) | Reference |
|---|---|---|
| Acetate | 1560–1540/1450–1418 | [33,39,46,54,55,56] |
| Formate | 1870–1828/1590–1550/1380–1350 | [42,44,45,47,54,55,57,58,59,60] |
| Methoxy | 964/1100–1030 | [61,62] |
| Ethoxy | 1456/1380–1390/1190–1090/1065–1050 | [37,49,56,63] |
| Carbonate | 1680/1614/1520/1430/1310 | [42] |
| Acetic acid | 1736/1675/1535/1453/1415/1341/1296/1025–1050 | [44,46,56] |
| Formaldehyde | 1767–1746/1727–1713/1418/1260 | [42,43,52,55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-González, A.; Duten, X.; Sauce, S. Plasma-Catalysis for Volatile Organic Compounds Decomposition: Complexity of the Reaction Pathways during Acetaldehyde Removal. Catalysts 2020, 10, 1146. https://doi.org/10.3390/catal10101146
Vega-González A, Duten X, Sauce S. Plasma-Catalysis for Volatile Organic Compounds Decomposition: Complexity of the Reaction Pathways during Acetaldehyde Removal. Catalysts. 2020; 10(10):1146. https://doi.org/10.3390/catal10101146
Chicago/Turabian StyleVega-González, Arlette, Xavier Duten, and Sonia Sauce. 2020. "Plasma-Catalysis for Volatile Organic Compounds Decomposition: Complexity of the Reaction Pathways during Acetaldehyde Removal" Catalysts 10, no. 10: 1146. https://doi.org/10.3390/catal10101146
APA StyleVega-González, A., Duten, X., & Sauce, S. (2020). Plasma-Catalysis for Volatile Organic Compounds Decomposition: Complexity of the Reaction Pathways during Acetaldehyde Removal. Catalysts, 10(10), 1146. https://doi.org/10.3390/catal10101146





