Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity
Abstract
:1. Introduction
2. Protecting-Group Chemistry and Selective Modification of Saccharide Hydroxyls
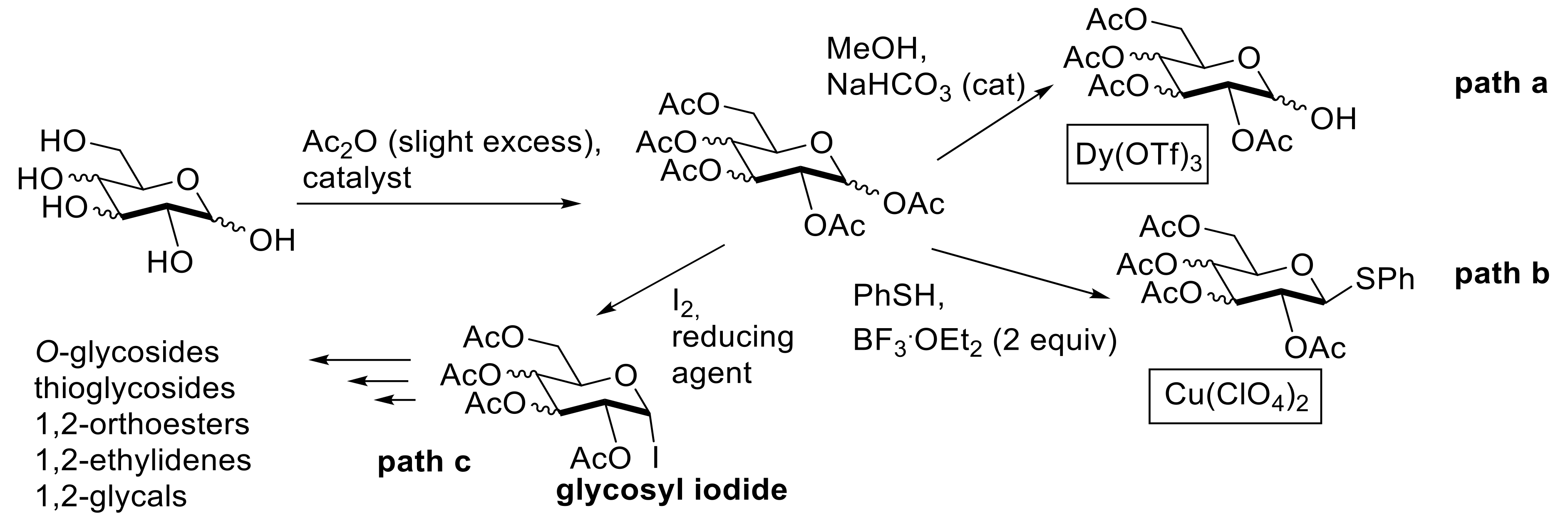
2.1. Acylation Reactions
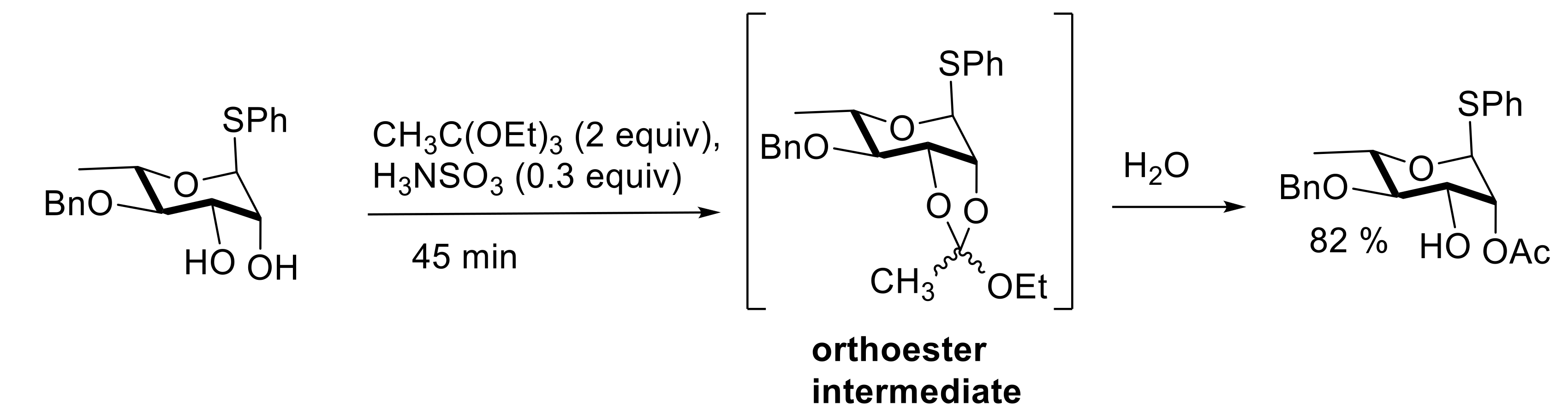
2.2. Acetalation Reactions
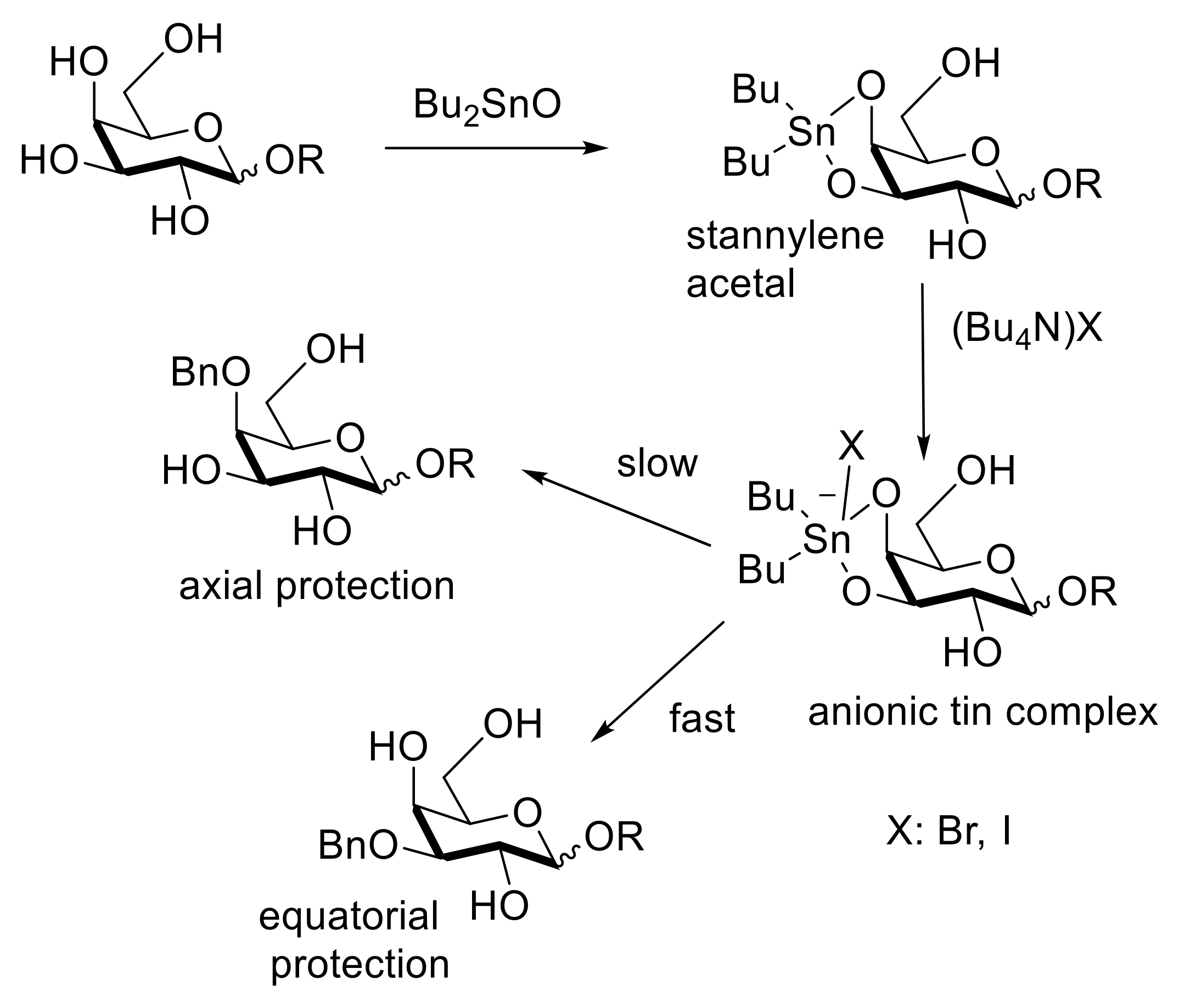
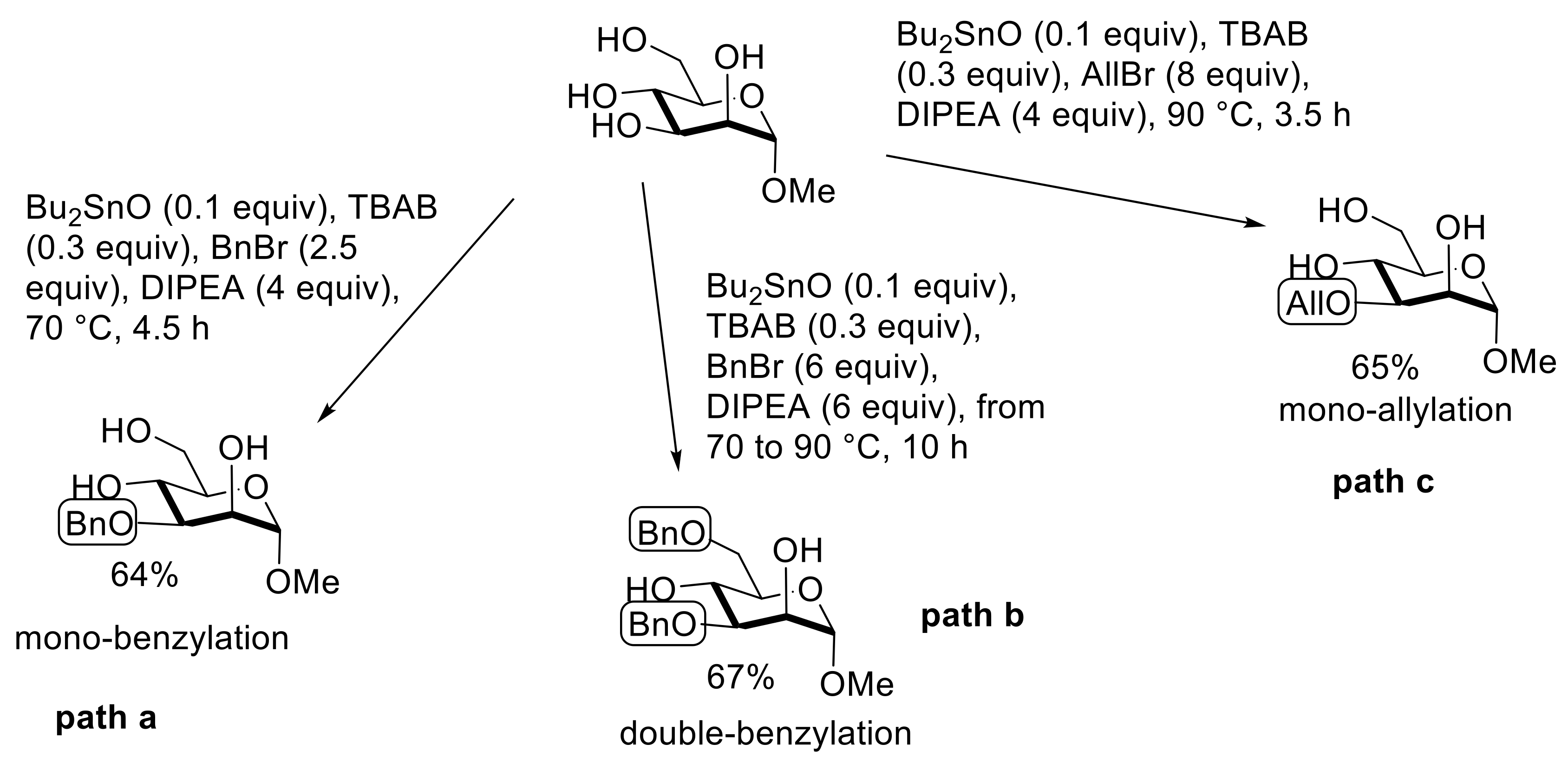
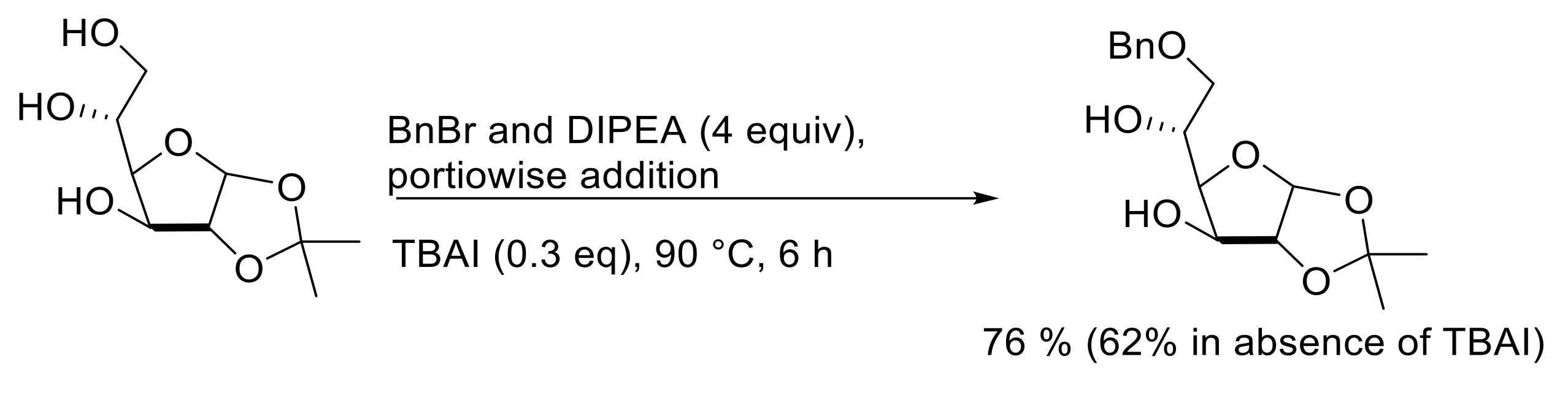
2.3. Selective Modifications Based on Formation of Ether Linkages (Benzylation, Allylation, Silylation, Tritylation)
3. Solvent-Free Halogenation of Saccharide Substrates
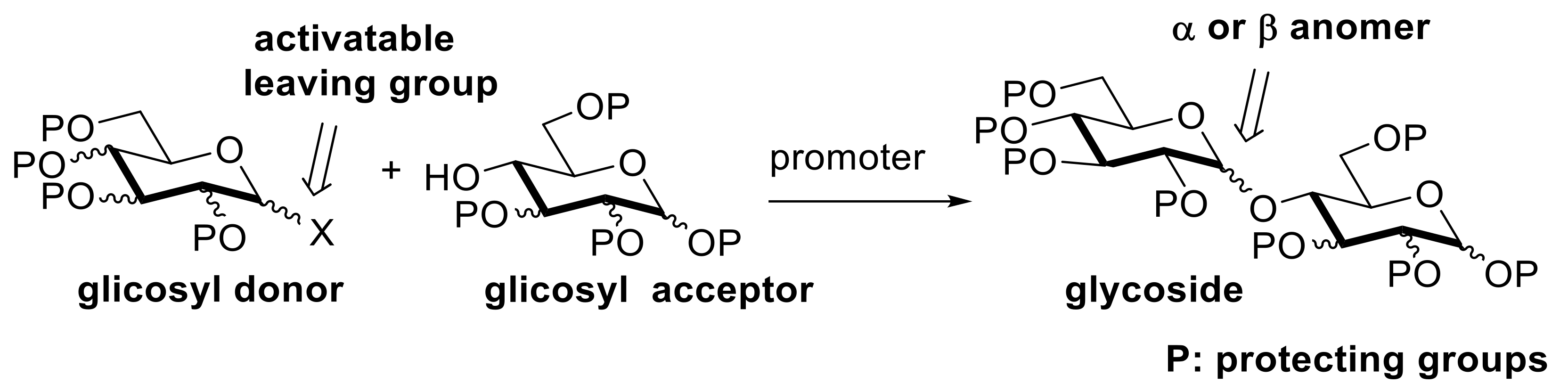
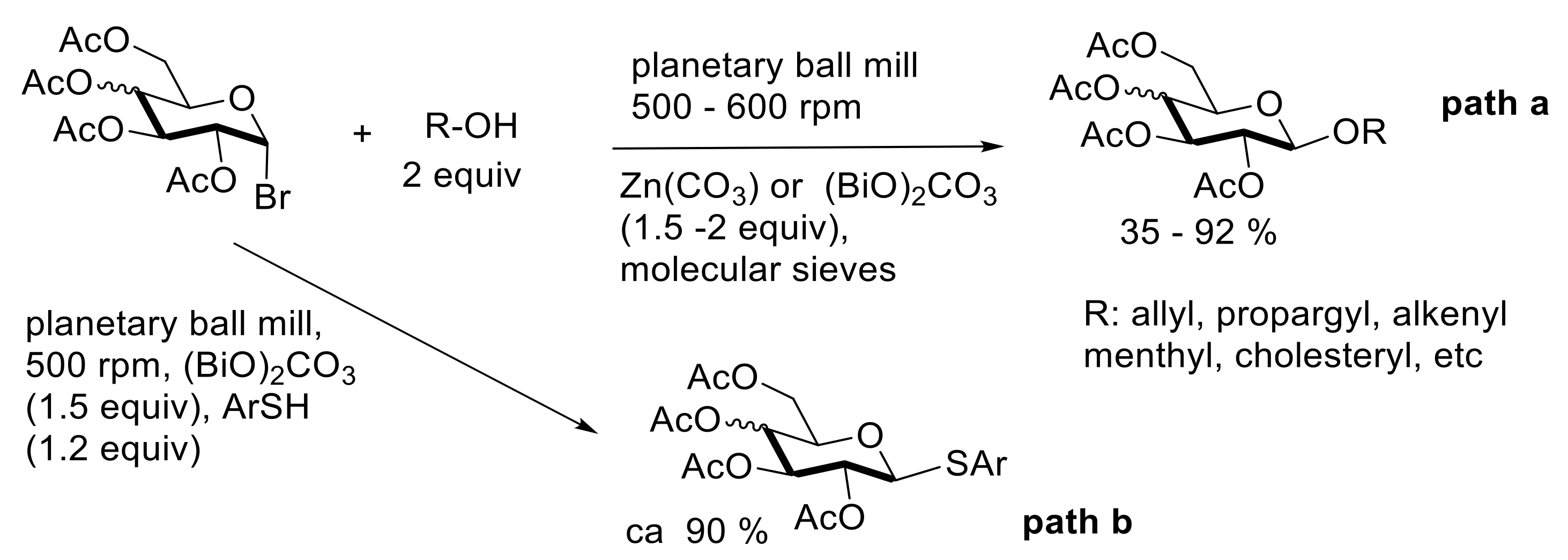
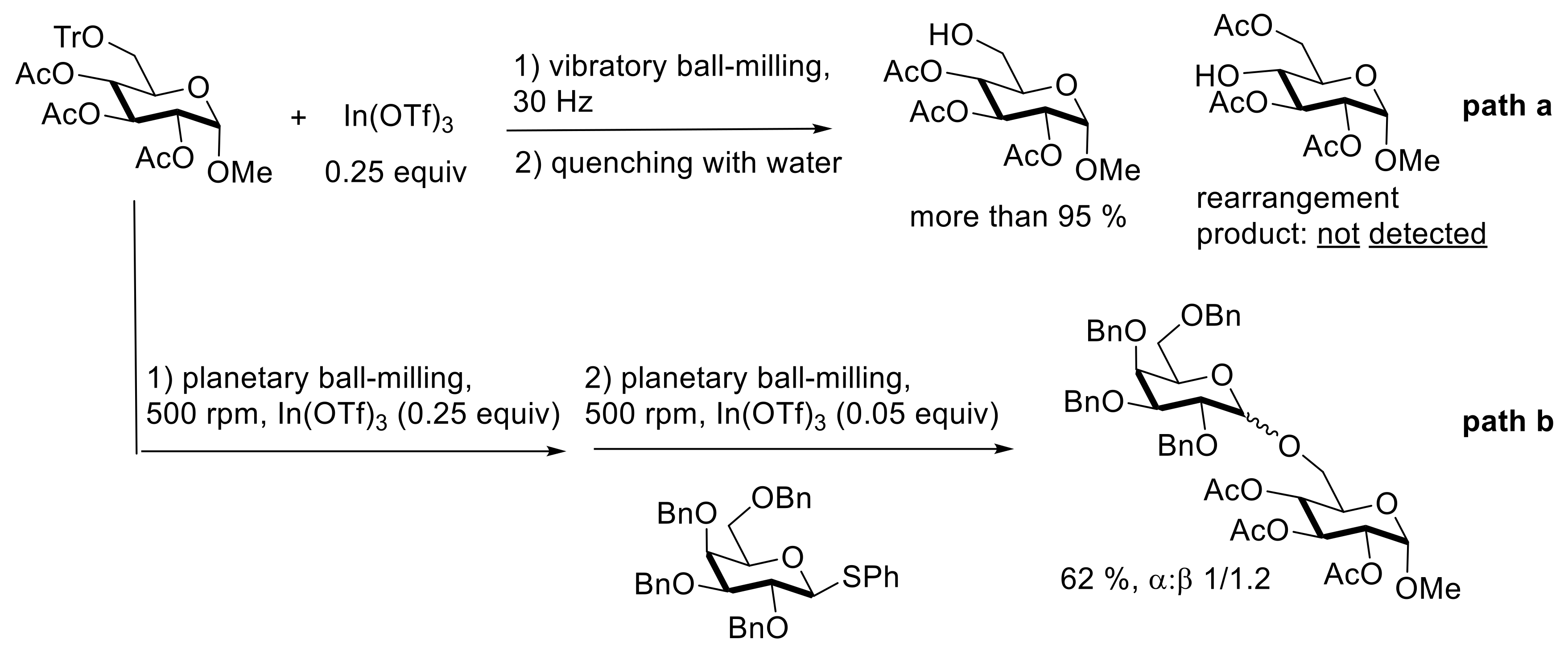
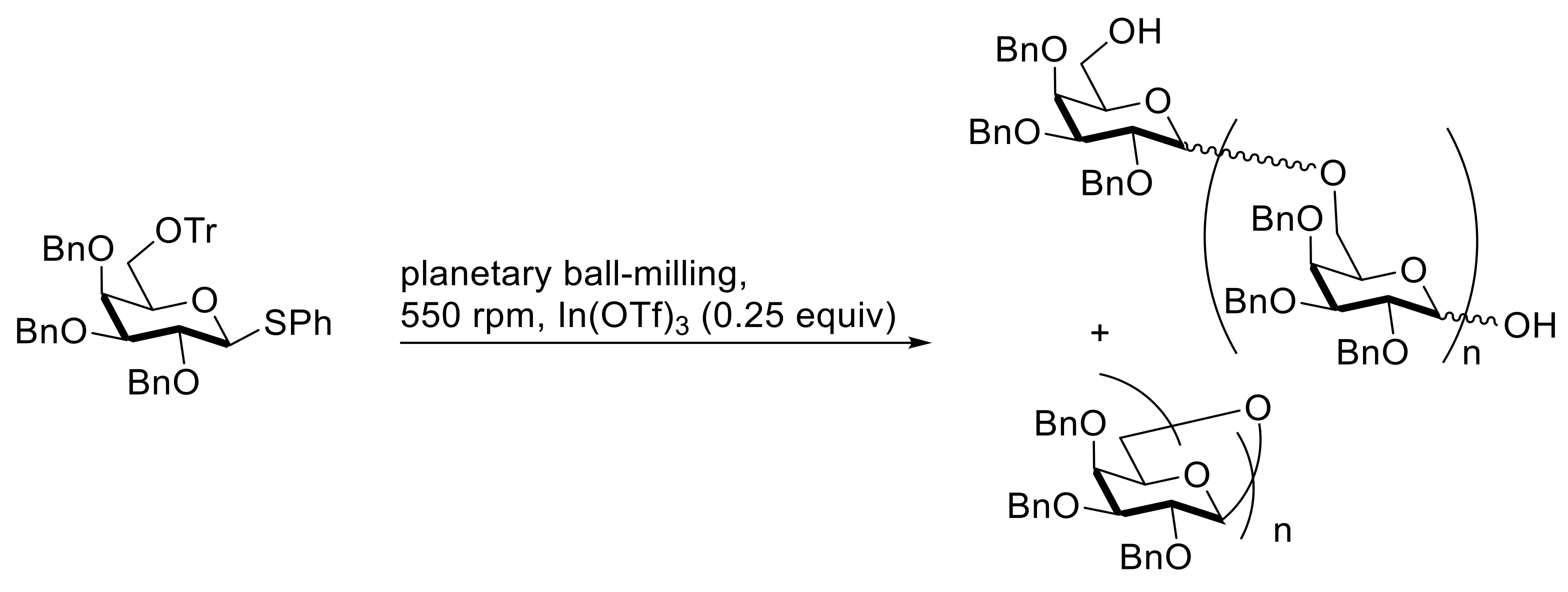
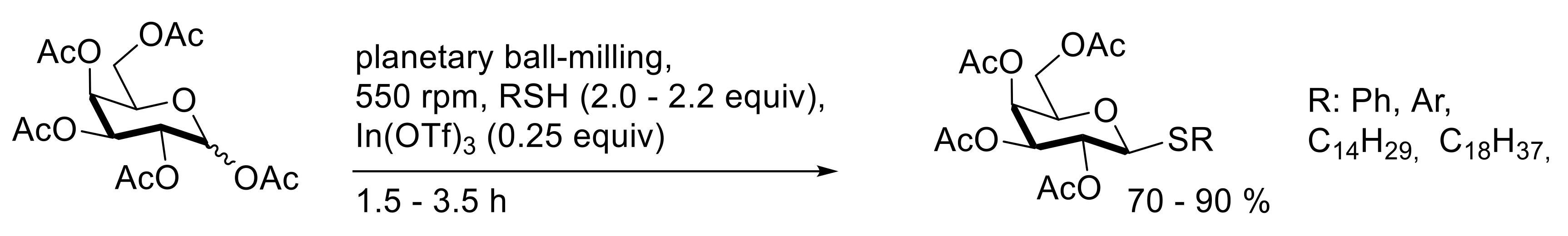
4. Solvent-Free Glycosidations
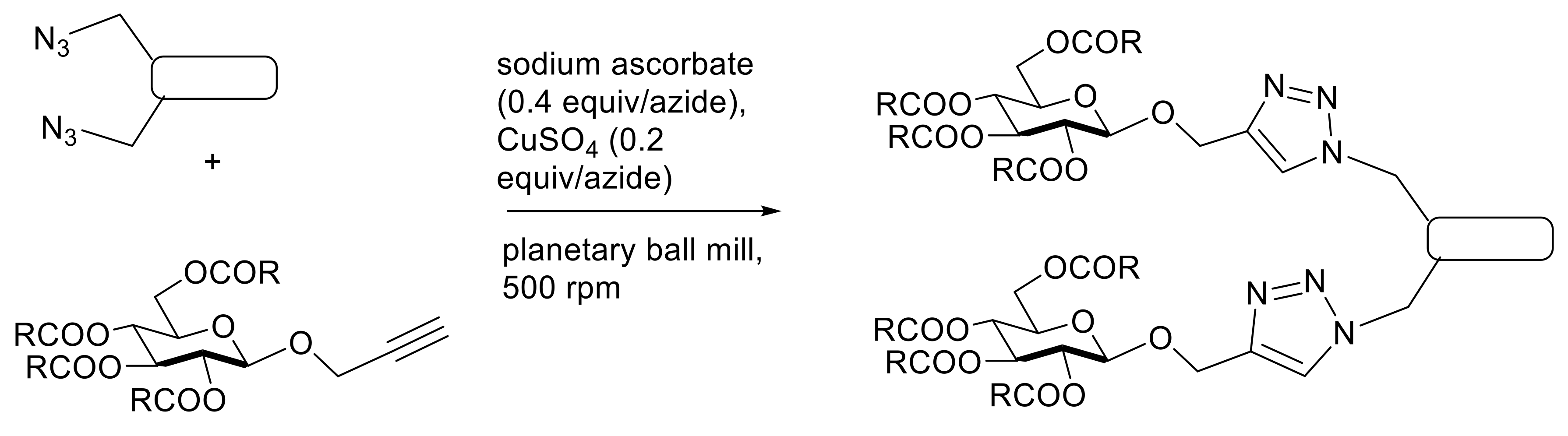
5. Application of Solvent-Free Approaches to Glycoconjugation Reactions
6. Other Solvent-Free Condensation Reactions on Free Carbohydrates
7. Solvent-Free Approaches for Recovery and Elaboration of Biomass-Derived Carbohydrates
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Varma, R.S. Greener and sustainable trends in synthesis of organics and nanomaterials ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar]
- Si, A.; Misra, A.K. Recent Trends in Carbohydrate Chemistry, Volume 1: Synthesis, Structure and Function of Carbohydrates; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–71. [Google Scholar]
- Boons, G.J.; Hale, K.J. Organic Synthesis with Carbohydrates; Sheffield Academic Press: Sheffield, UK, 2000. [Google Scholar]
- Nicolaou, K.; Hale, C.R.H.; Nilewskia, C.; Ioannidou, H.A. Constructing molecular complexity and diversity: Total synthesis of natural products of biological and medicinal importance. Chem. Soc. Rev. 2012, 41, 5185–5238. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tejada, A.; Canada, F.J.; Jimenez-Barbero, J. Recent developments in synthetic carbohydrate-based diagnostics, vaccines, and therapeutics. Chem. Eur. J. 2015, 21, 10616–10628. [Google Scholar] [CrossRef]
- Prathap, A.; Sureshan, K.M. Sugar-based organogelators for various applications. Langmuir 2019, 35, 6005–6014. [Google Scholar] [CrossRef]
- Hsu, C.H.; Hung, S.C.; Wu, C.Y.; Wong, C.H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. 2011, 50, 11872–11923. [Google Scholar] [CrossRef]
- Das, R.; Mukhopadhyay, B. Chemical O-glycosylations: An overview. ChemistryOpen 2016, 5, 401–433. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.S.; Wang, C.C.; Sabbavarapu, N.M.; Podilapu, A.R.; Liao, P.H.; Hung, S.C. “One-pot” protection, glycosylation, and protection–glycosylation strategies of carbohydrates. Chem. Rev. 2018, 118, 8025–8104. [Google Scholar] [CrossRef]
- Adinolfi, M.; Iadonisi, A.; Schiattarella, M. An easy approach for the acetylation of saccharidic alcohols. Applicability for regioselective protections. Tetrahedron Lett. 2003, 44, 4661–4663. [Google Scholar]
- Yan, Y.L.; Guo, J.R.; Liang, C.F. Sequential Dy(OTf)3-catalyzed solvent-free per-O-acetylation and regioselective anomeric de-O-acetylation of carbohydrates. Chem. Asian J. 2017, 12, 2471–2479. [Google Scholar] [CrossRef]
- Chatterjee, D.; Paul, A.; Rajkamala; Yadav, S. Cu(ClO4)2.6H2O catalyzed solvent free per-O-acetylation and sequential one-pot conversions of sugars to thioglycosides. RSC Adv. 2015, 5, 29669–29674. [Google Scholar] [CrossRef]
- Kartha, K.P.R.; Field, R.A. Iodine: A versatile reagent in carbohydrate chemistry. IV. Per-O-acetylation, regioselective acylation and acetolysis. Tetrahedron 1997, 53, 11753–11766. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhou, J.; Li, J.; Shi, C.; Huang, T.; Wang, Z.; Tang, J. H2SO4-SiO2: Highly efficient and reusable catalyst for per-O-acetylation of aarbohydrates under solvent-free conditions. J. Carbohydr. Chem. 2011, 30, 165–177. [Google Scholar] [CrossRef]
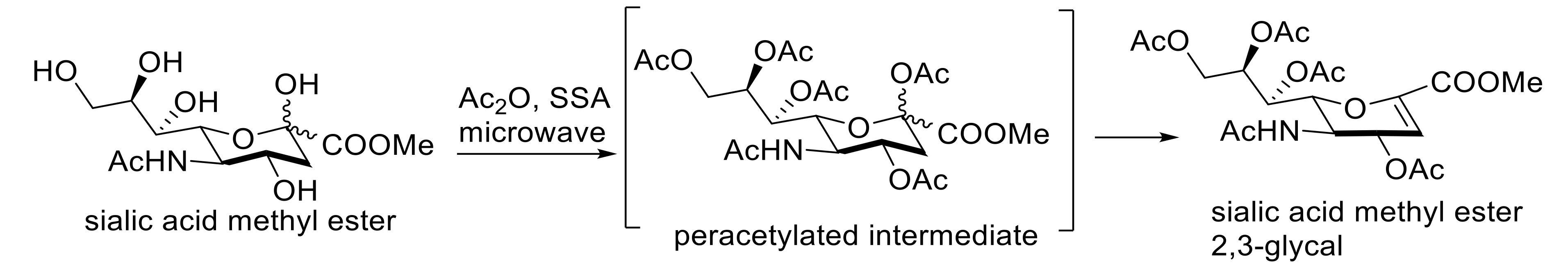
- Paragas, E.M.; Monreal, I.A.; Vasil, C.M.; Saludes, J.P. One-pot SSA-catalyzed beta-elimination: An efficient and inexpensive protocol for easy access to the glycal of sialic acid. Carbohydr. Res. 2015, 402, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ch, R.; Tyagi, M.; Patil, P.R.; Kartha, K.P.R. DABCO: An efficient promoter for the acetylation of carbohydrates and other substances under solvent-free conditions. Tetrahedron Lett. 2011, 52, 5841–5846. [Google Scholar] [CrossRef]
- Giri, S.K.; Gour, R.; Kartha, K.P.R. Diazepinium perchlorate: A neutral catalyst for mild, solvent-free acetylation of carbohydrates and other substances. RSC Adv. 2017, 7, 13653–13667. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.K.; Kartha, K.P.R. Acyl transfer reactions of carbohydrates, alcohols, phenols, thiols and thiophenols under green reaction conditions. RSC Adv. 2015, 5, 11687–11696. [Google Scholar] [CrossRef]
- Giri, S.K.; Verma, M.; Kartha, K.P.R. Indium(III) triflate: A highly efficient catalyst for reactions of sugars. J. Carbohydr. Chem. 2008, 27, 464–478. [Google Scholar] [CrossRef]
- Roy, B.; Dasgupta, S.; Rajput, V.K.; Mukhopadhyay, B. Samarium trifluoromethanesulfonate: An efficient moisture tolerant acylation catalyst under solvent-free condition. J. Carbohydr. Chem. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Lee, J.C.; Taic, C.A.; Hung, S.C. Sc(OTf)3-catalyzed acetolysis of 1,6-anhydro-β-hexopyranoses and solvent-free per-acetylation of hexoses. Tetrahedron Lett. 2002, 43, 851–855. [Google Scholar] [CrossRef]
- Santra, A.; Guchhait, G.; Misra, A.K. Efficient acylation and sulfation of carbohydrates using sulfamic acid, a mild, eco-friendly catalyst under organic solvent-free conditions. Green Chem. 2011, 13, 1345–1351. [Google Scholar] [CrossRef]
- Wu, L.; Yin, Z. Sulfonic acid functionalized nano γ-Al2O3 catalyzed per-O-acetylated of carbohydrates. Carbohydr. Res. 2013, 365, 14–19. [Google Scholar] [CrossRef]
- Meloncelli, P.J.; Martin, A.D.; Lowary, T.L. Glycosyl iodides. History and recent advances. Carbohydr. Res. 2009, 344, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, M.; Iadonisi, A.; Pastore, A.; Valerio, S. The I2/Et3SiH system: A versatile combination with multiple applications in carbohydrate chemistry. Pure Appl. Chem. 2012, 84, 1–10. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Kartha, K.P.R.; Russell, D.A.; Field, R.A. Streamlined synthesis of per-O-acetylated sugars, glycosyl iodides, or thioglycosides from unprotected reducing sugars. J. Org. Chem. 2004, 69, 7758–7760. [Google Scholar] [CrossRef] [PubMed]
- Valerio, S.; Iadonisi, A.; Adinolfi, M.; Ravidà, A. Novel approaches for the synthesis and activation of thio- and selenoglycoside donors. J. Org. Chem. 2007, 72, 6097–6106. [Google Scholar] [CrossRef]
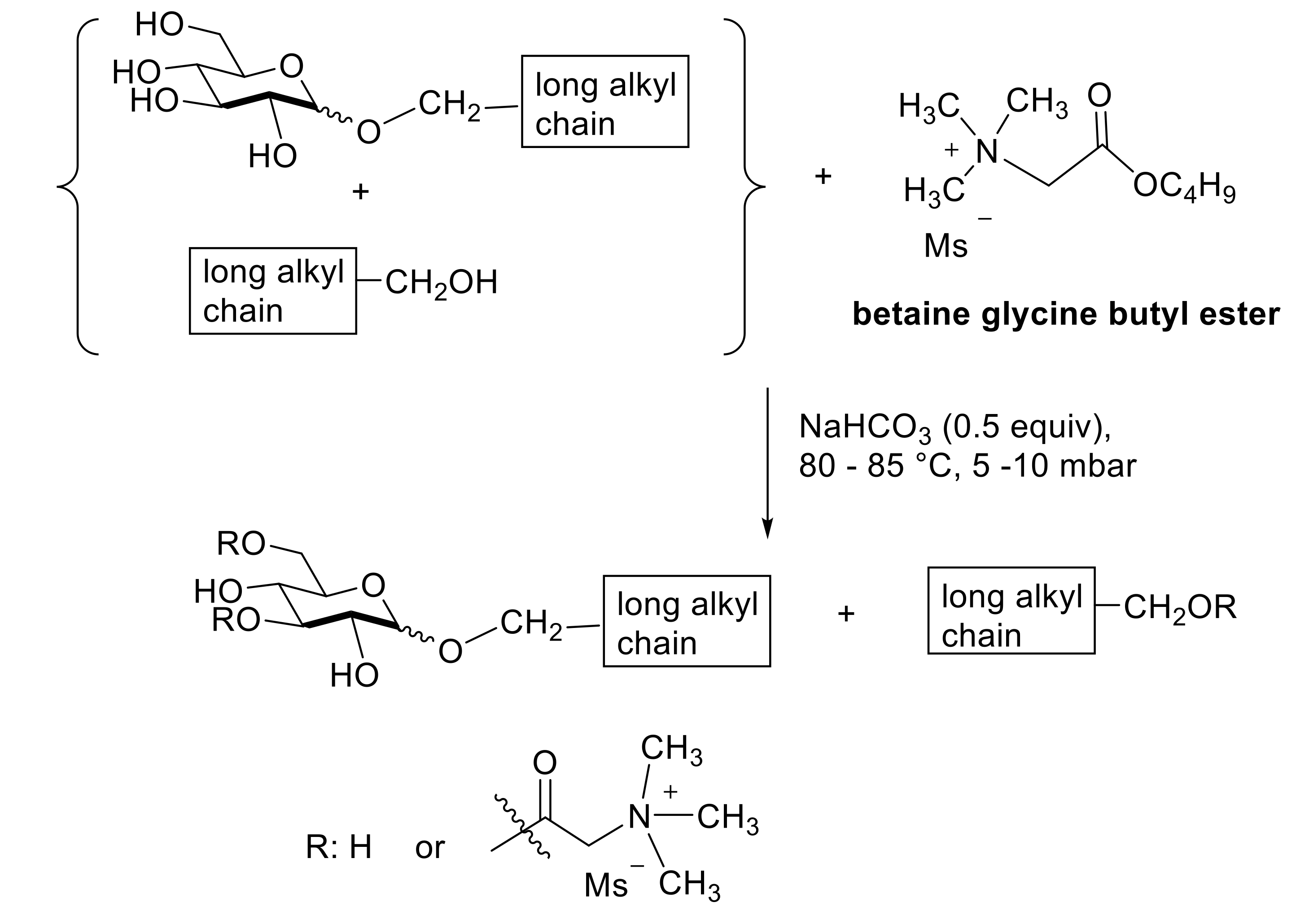
- Perusse, D.; Guegan, J.P.; Rolland, H.; Guilbotc, J.; Benvegnu, T. Efficient solvent-free cationization of alkylpolyglycoside based surfactant compositions using natural glycine betaine. Green Chem. 2016, 18, 1664–1673. [Google Scholar] [CrossRef]
- Ogawa, S.; Endo, A.; Kitahara, N.; Yamagishi, T.; Aoyagi, S.; Hara, S. Factors determining the reaction temperature of the solvent-free enzymatic synthesis of trehalose esters. Carbohydr. Res. 2019, 482, 107739. [Google Scholar] [CrossRef]
- Ye, R.; Pyo, S.H.; Hayes, D.G. Lipase-catalyzed synthesis of saccharide–fatty acid esters using suspensions of saccharide crystals in solvent-free media. J. Am. Oil Chem. Soc. 2010, 87, 281–293. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G. Optimization of the solvent-free lipase-catalyzed synthesis of fructose-oleic acid ester through programming of water removal. J. Am. Oil Chem. Soc. 2011, 88, 1351–1359. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G. Lipase-catalyzed synthesis of saccharide-fatty acid esters utilizing solvent-free suspensions: Effect of acyl donors and acceptors, and enzyme activity retention. J. Am. Oil Chem. Soc. 2012, 89, 455–463. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G. Solvent-free lipase-catalysed synthesis of saccharide-fatty acid esters: Closed-loop bioreactor system with in situ formation of metastable suspensions. Biocatal. Biotransform. 2012, 30, 209–216. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G.; Burton, R.; Liu, A.J.; Harte, F.M.; Wang, Y.M. Solvent-free lipase-catalyzed synthesis of technical-grade sugar esters and evaluation of their physicochemical and bioactive properties. Catalysts 2016, 6, 78. [Google Scholar] [CrossRef]
- Strukil, V. Mechanochemical organic synthesis: The art of making chemistry green. Synlett 2018, 29, 1281–1288. [Google Scholar] [CrossRef]
- Perez-Venegas, M.; Juaristi, E. Mechanochemical and mechanoenzymatic synthesis of pharmacologically active compounds: A green perspective. ACS Sustain. Chem. Eng. 2020, 8, 8881–8893. [Google Scholar] [CrossRef]
- Chen, J.; ·Li, Y.; Chen, X.; Mai, Y.; Gao, M.; Zhang, J.; Wang, X. Efficient solvent-free synthesis of sucrose esters via sand-milling pretreatment on solid-liquid mixtures. J. Surfact. Deterg. 2019, 22, 1515–1520. [Google Scholar] [CrossRef]
- Wutts, P.G.M. Greene’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Traboni, S.; Bedini, E.; Giordano, M.; Iadonisi, A. Three solvent-free catalytic approaches to the acetal functionalization of carbohydrates and their applicability to one-pot generation of orthogonally protected building blocks. Adv. Synth. Catal. 2015, 357, 3562–3572. [Google Scholar] [CrossRef]
- Vessella, G.; Casillo, A.; Fabozzi, A.; Traboni, S.; Iadonisi, A.; Corsaro, M.M.; Bedini, E. Synthesis of the tetrasaccharide repeating unit of the cryoprotectant capsular polysaccharide from Colwellia psychrerythraea 34H. Org. Biomol. Chem. 2019, 17, 3129–3140. [Google Scholar] [CrossRef]
- Zhu, Y.; Tyrikos-Ergas, T.; Schiefelbein, K.; Grafmueller, A.; Seeberger, P.H.; Delbianco, M. Automated access to well-defined ionic oligosaccharides. Org. Biomol. Chem. 2020, 18, 1349–1353. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Boyd, R.J.; Grindley, T.B. Role of fluoride in accelerating the reactions of dialkylstannylene. J. Org. Chem. 2015, 80, 2989–3002. [Google Scholar] [CrossRef]
- David, S.; Hanessian, S. Regioselective manipulation of hydroxyl groups via organotin derivatives. Tetrahedron 1985, 41, 643–663. [Google Scholar] [CrossRef]
- Grindley, T.B. Applications of tin-containing intermediates to carbohydrate chemistry. Adv. Carbohydr. Chem. Biochem. 1998, 53, 17–142. [Google Scholar]
- Giordano, M.; Iadonisi, A. Tin-mediated regioselective benzylation and allylation of polyols: Applicability of a catalytic approach under solvent-free conditions. J. Org. Chem. 2014, 79, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Iadonisi, A. A practical approach to regioselective O-benzylation of primary positions of polyols. Tetrahedron Lett. 2013, 54, 1550–1552. [Google Scholar] [CrossRef]
- Gathirwa, J.W.; Maki, T. Benzylation of hydroxy groups with tertiary amine as a base. Tetrahedron 2012, 68, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lu, Y.; Zhou, Y.; Ren, B.; Pei, Y.; Dong, H.; Pei, Z. Regioselective benzylation of diols and polyols by catalytic amounts of an organotin reagent. Adv. Synth. Catal. 2014, 356, 1735–1740. [Google Scholar] [CrossRef]
- Saikam, V.; Dara, S.; Yadav, M.; Singh, P.P.; Vishwakarma, R.A. Dimethyltin dichloride catalyzed regioselective alkylation of cis-1,2-diols at room temperature. J. Org. Chem. 2015, 80, 11916–11925. [Google Scholar] [CrossRef]
- Xu, H.; Ren, B.; Zhao, W.; Xin, X.; Lu, Y.; Pei, Y.; Dong, H.; Pei, Z. Regioselective mono and multiple alkylation of diols and polyols catalyzed by organotin and its applications on the synthesis of value-added carbohydrate intermediates. Tetrahedron 2016, 72, 3490–3499. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q. Scalable Sn-catalyzed regioselective allylation of 1-methyl-L-α-rhamnopyranoside. Org. Proc. Res. Dev. 2017, 21, 1653–1658. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Dong, H.; Lu, Y.; Pei, Y.; Pei, Z. Organotin-catalyzed regioselective benzylation of carbohydrate trans-diols. Tetrahedron Lett. 2017, 58, 4039–4042. [Google Scholar] [CrossRef]
- Gavale, K.S.; Chavan, S.R.; Khan, A.; Joshi, R.; Dhavale, D.D. Azetidine- and N-carboxylic azetidine-iminosugars as amyloglucosidase inhibitors: Synthesis, glycosidase inhibitory activity and molecular docking studies. Org. Biomol. Chem. 2015, 13, 6634–6646. [Google Scholar] [CrossRef]
- Baradzenka, A.G.; Barysau, B.M.; Hurski, A.L.; Zhabinskii, V.N.; Khripach, V.A. Synthesis of brassinosteroids with a keto group in the side chain. Steroids 2015, 101, 90–95. [Google Scholar] [CrossRef]
- Almond, M.; Suleiman, M.G.; Hawkins, M.; Winder, D.; Robshaw, T.; Waddoups, M.; Humphreys, P.N.; Laws, A.P. Developing cellulosic waste products as platform chemicals: Protecting group chemistry of α-glucoisosaccharinic acid. Carbohydr. Res. 2018, 455, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.K.; Yim, C.B.; Jadhav, S.; Verhassel, A.; Tuomela, J.; Solin, O.; Groenroos, T.J.; Virta, P. Synthesis of an alkyne-modified bleomycin disaccharide precursor, conversion to a 18F-labeled radiotracer, and preliminary in vivo-PET imaging studies. Eur. J. Org. Chem. 2019, 156–163. [Google Scholar] [CrossRef]
- Sethi, K.P.; Kartha, K.P.R. Stannylene acetal-mediated solvent-free mechanochemical regioselective alkylation of galactosides and lactosides. Trends Carbohydr. Res. 2016, 8, 29–32. [Google Scholar]
- Traboni, S.; Bedini, E.; Iadonisi, A. Solvent-free one-pot diversified protection of saccharide polyols via regioselective tritylation. ChemistrySelect 2017, 2, 4906–4911. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Iadonisi, A. Orthogonal protection of saccharide polyols through solvent-free one-pot sequences based on regioselective silylations. Beilstein J. Org. Chem. 2016, 12, 2748–2756. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.R.; Karth, K.P.R. Application of ball billing technology to carbohydrate reactions: I. regioselective primary hydroxyl protection of hexosides and nucleoside by planetary ball milling. J. Carbohydr. Chem. 2008, 27, 279–293. [Google Scholar] [CrossRef]
- Jereb, M. Highly atom economical uncatalyzed and I2-catalyzed silylation of phenols, alcohols and carbohydrates, using HMDS under solvent-free reaction conditions. Tetrahedron 2012, 68, 3861–3867. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Giordano, M.; Iadonisi, A. One-pot synthesis of orthogonally protected sugars through sequential base-promoted/acid-catalyzed steps: A solvent-free approach with self-generation of a catalytic species. Tetrahedron Lett. 2019, 60, 1777–1780. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Iadonisi, A. Solvent-free conversion of alcohols to alkyl iodides and one-pot elaborations thereof. ChemistrySelect 2018, 3, 1616–1622. [Google Scholar] [CrossRef]
- Manzo, E.; Gallo, C.; Fioretto, L.; Nuzzo, G.; Barra, G.; Pagano, D.; Krauss, I.R.; Paduano, L.; Ziaco, M.; DellaGreca, M.; et al. Diasteroselective colloidal self-assembly affects the immunological response of the molecular adjuvant sulfavant. ACS Omega 2019, 4, 7807–7814. [Google Scholar] [CrossRef] [Green Version]
- Traboni, S.; Liccardo, F.; Bedini, E.; Giordano, M.; Iadonisi, A. Solvent-free synthesis of glycosyl chlorides based on the triphenyl phosphine/hexachloroacetone system. Tetrahedron Lett. 2017, 58, 1762–1764. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Pedersen, C.M. Catalytic glycosylations in oligosaccharide synthesis. Chem. Rev. 2018, 118, 8285–8358. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.R.; Kartha, K.P.R. Solvent-free mechanochemical synthesis of aryl glycosides. J. Carbohydr. Chem. 2008, 27, 411–419. [Google Scholar] [CrossRef]
- Tyagi, M.; Khurana, D.; Kartha, K.P.R. Solvent-free mechanochemical glycosylation in ball mill. Carbohydr. Res. 2013, 379, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sethi, K.P.; Kartha, K.P.R. Solvent-free bismuth oxycarbonate-mediated mechanochemical glycosylation: A simple greener alternative to access O-/S-glycosides efficiently. Carbohydr. Res. 2016, 434, 132–135. [Google Scholar] [CrossRef]
- Sarala, G.; Kartha, K.P.R. Solvent -free mechanochemical synthesis of glycosylated curcumin. Trends Carboh. Res. 2016, 8, 19–28. [Google Scholar]
- Patil, P.R.; Kartha, K.P.R. Solvent-free synthesis of thioglycosides by ball milling. Green Chem. 2009, 11, 953–956. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, N.; Kartha, K.P.R. In(III) triflate-catalyzed detritylation and glycosylation by solvent-free ball milling. Carbohydr. Res. 2014, 397, 18–26. [Google Scholar] [CrossRef]
- Kumar, V.; Taxak, N.; Jangir, R.; Bharatam, P.V.; Kartha, K.P.R. In(III) triflate-mediated solvent-free synthesis and activation of thioglycosides by ball milling and structural analysis of long chain alkyl thioglycosides by TEM and quantum chemical methods. J. Org. Chem. 2014, 79, 3427–3439. [Google Scholar] [CrossRef]
- Aich, U.; Loganathan, D. Zeolite-catalyzed Helferich-type glycosylation of long-chain alcohols. Synthesis of acetylated alkyl 1,2-trans glycopyranosides and alkyl 1,2-cis C2-hydroxy-glycopyranosides. Carbohydr. Res. 2007, 342, 704–709. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Jadhav, A.R.; Kim, H. Polyvinyl trisulfonate ethylamine based solid acid catalyst for the efficient glycosylation of sugars under solvent free conditions. RSC Adv. 2015, 5, 104715–104724. [Google Scholar] [CrossRef]
- Thombal, R.S.; Jadha, V.H. Sulfonated graphene oxide as highly efficient catalyst for glycosylation. J. Carbohydr. Chem. 2016, 35, 57–68. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Hendriks, K.B.; Stick, R.V.; James, K.J. Halide ion catalyzed glycosidation reactions. Syntheses of alpha-linked disaccharides. Am. Chem. Soc. 1975, 97, 4056–4062. [Google Scholar] [CrossRef]
- Traboni, S.; Vessella, G.; Bedini, E.; Iadonisi, A. Solvent-free, under air selective synthesis of α-glycosides adopting glycosyl chlorides as donors. Org. Biomol. Chem. 2020, 18, 5157–5163. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Liu, Y.P.; Lam, S.; Lin, S.C.; Wang, C.C. TMSBr-mediated solvent- and work-up-free synthesis of α-2-deoxyglycosides from glycals. Beilstein J. Org. Chem. 2016, 12, 1758–1764. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.U. The Huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, M.; Taxak, N.; Bharatam, P.V.; Nandanwar, H.; Kartha, K.P.R. Mechanochemical click reaction as a tool for making carbohydrate-based triazole-linked self-assembling materials (CTSAMs). Carbohydr. Res. 2015, 407, 137–147. [Google Scholar] [CrossRef]
- Tyagi, M.; Kartha, K.P.R. Synthesis of glycotriazololipids and observations on their self-assembly properties. Carbohydr. Res. 2015, 413, 85–92. [Google Scholar] [CrossRef]
- Mahajan, N.; Giri, S.K.; Kartha, K.P.R. Solvent-free mechanochemical synthesis of triazole-sugar conjugates as potential glycosidase inhibitors. Trends Carbohydr. Res. 2019, 11, 33–43. [Google Scholar]
- Kumar, V.; Giri, S.K.; Venugopalan, P.; Kartha, K.P.R. Synthesis of cross-linked glycopeptides and ureas by a mechanochemical, solvent-free reaction and determination of their structural properties by TEM and X-ray crystallography. ChemPlusChem 2014, 79, 1605–1613. [Google Scholar] [CrossRef]
- Mugunthan, G.; Kartha, K.P.R. Application of ball milling technology to carbohydrate reactions-II. Solvent-free mechanochemical synthesis of glycosyl azides. J. Carbohydr. Chem. 2008, 27, 294–299. [Google Scholar] [CrossRef]
- Lingome, C.E.; Pourceau, G.; Gobert-Deveauxb, V.; Wadouachi, A. Efficient synthesis of glycosylamines in solventless conditions promoted by mechanical milling. RSC Adv. 2014, 4, 36350. [Google Scholar] [CrossRef]
- Russ, C.; Ilgen, F.; Reil, C.; Luff, C.; Haji, B.A.; Koenig, B. Efficient preparation of β-D-glucosyl and β-D-mannosyl ureas and other N-glucosides in carbohydrate melts. Green Chem. 2011, 13, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Cheetham, N.W.H.; Tran, T.D. Direct formation and isolation of unprotected α-and β-D-ribopyranosyl urea, α-and β-D-ribofuranosyl urea, and a ribosyl-1,2-cyclic carbamate in carbohydrate melts. Carbohydr. Res. 2020, 492, 108021. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.T.; Khan, M.M. Bromodimethylsulfonium bromide (BDMS) mediated dithioacetalization of carbohydrates under solvent-free conditions. Carbohydr. Res. 2010, 345, 2139–2145. [Google Scholar] [CrossRef]
- Ranoux, A.; Lemiegre, L.; Benoit, M.; Guegan, J.-P.; Benvegnu, T. Horner-Wadsworth-Emmons reaction of unprotected sugars in water or in the absence of any solvent: One-step access to C-glycoside amphiphiles. Eur. J. Org. Chem. 2010, 1314–1323. [Google Scholar] [CrossRef]
- Richel, A.; Nicks, F.; Laurent, P.; Wathelet, B.; Wathelet, J.-P.; Paquot, M. Efficient microwave-promoted synthesis of glucuronic and galacturonic acid derivatives using sulfuric acid impregnated on silica. Green Chem. Lett. Rev. 2012, 5, 179–186. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Némeththe, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 506–613. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef]
- Tomaszewska, J.; Bielinski, D.; Binczarski, M.; Berlowska, J.; Dziugan, P.; Piotrowski, J.; Stanishevskye, A.; Witonska, I.A. Products of sugar beet processing as raw materials for chemicals and biodegradable polymers. RSC Adv. 2018, 8, 3161–3177. [Google Scholar] [CrossRef] [Green Version]
- Benoit, M.; Rodrigues, A.; Zhang, Q.; Fourrè, E.; Vigier, K.O.; Tatibou, J.-M.; Jerome, F. Depolymerization of cellulose assisted by a nonthermal atmospheric plasma. Angew. Chem. Int. Ed. 2011, 50, 8964–8967. [Google Scholar] [CrossRef] [PubMed]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Furusato, S.; Takagaki, A.; Hayashi, S.; Miyazato, A.; Kikuchi, R.; Oyama, S.T. Mechanochemical decomposition of crystalline cellulose in the presence of protonated layered niobium molybdate solid acid catalyst. ChemSusChem 2018, 11, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Meine, N.; Rinaldi, R.; Schüth, F. Solvent-free catalytic depolymerization of cellulose to water-soluble oligosaccharides. ChemSusChem 2012, 5, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.; De Oliveira Vigier, K.; Marinkovic, S.; Estrine, B.; Oldani, C.; Jerome, F. Conversion of cellulose into amphiphilic alkyl glycosides catalyzed by aquivion, a perfluorosulfonic acid polymer. ChemSusChem 2017, 10, 3604–3610. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Solid acid-catalyzed depolymerization of barley straw driven by ball milling. Bioresour. Technol. 2016, 206, 204–210. [Google Scholar] [CrossRef]
- Dong, Y.; Schneider, L.; Hu, T.; Jaakkola, M.; Holm, J.; Leveque, J.M.; Lassi, U. Direct acid-catalysed mechanical depolymerisation of fibre sludge to reducing sugars using planetary milling. Biomass Bioenerg. 2016, 86, 36–42. [Google Scholar] [CrossRef]
- Dong, Y.; Haverinen, J.; Tuuttila, T.; Jaakkola, M.; Holm, J.; Leveque, J.M.; Lassi, U. Rapid one-step solvent-free acid-catalyzed mechanical depolymerization of pine sawdust to high-yield water-soluble sugars. Biomass Bioenerg. 2017, 102, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Lempiainen, H.; Lappalainen, K.; Haverinen, J.; Tuuttila, T.; Hu, T.; Jakkola, M.; Lassi, U. The effect of mechanocatalytic pretreatment on the structure and depolymerization of willow. Catalysts 2020, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Li, L.Y.; Yan, L.L.; Shen, F.; Qiu, M.; Qi, X.H. Mechanocatalytic production of lactic acid from glucose by ball milling. Catalysts 2017, 7, 170. [Google Scholar] [CrossRef]
- Zang, H.J.; Wang, K.; Zhang, M.C.; Xie, R.R.; Wang, L.; Chen, E.Y.X. Catalytic coupling of biomass-derived aldehydes into intermediates for biofuels and materials. Catal. Sci. Technol. 2018, 8, 1777–1798. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traboni, S.; Bedini, E.; Vessella, G.; Iadonisi, A. Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity. Catalysts 2020, 10, 1142. https://doi.org/10.3390/catal10101142
Traboni S, Bedini E, Vessella G, Iadonisi A. Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity. Catalysts. 2020; 10(10):1142. https://doi.org/10.3390/catal10101142
Chicago/Turabian StyleTraboni, Serena, Emiliano Bedini, Giulia Vessella, and Alfonso Iadonisi. 2020. "Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity" Catalysts 10, no. 10: 1142. https://doi.org/10.3390/catal10101142
APA StyleTraboni, S., Bedini, E., Vessella, G., & Iadonisi, A. (2020). Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity. Catalysts, 10(10), 1142. https://doi.org/10.3390/catal10101142









