Agro-Waste Derived Biomass Impregnated with TiO2 as a Potential Adsorbent for Removal of As(III) from Water
Abstract
:1. Introduction
2. Results and Discussion
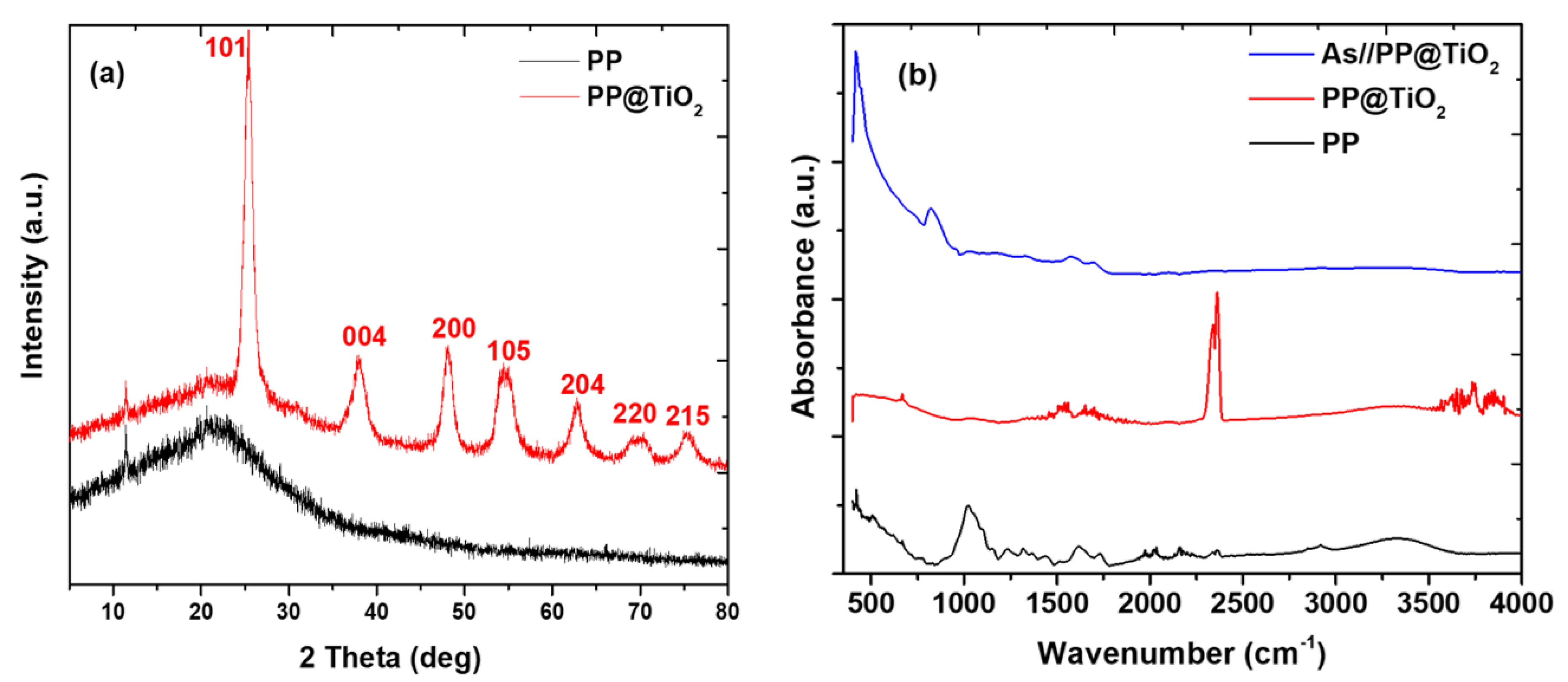
2.1. Adsorbent Characterization
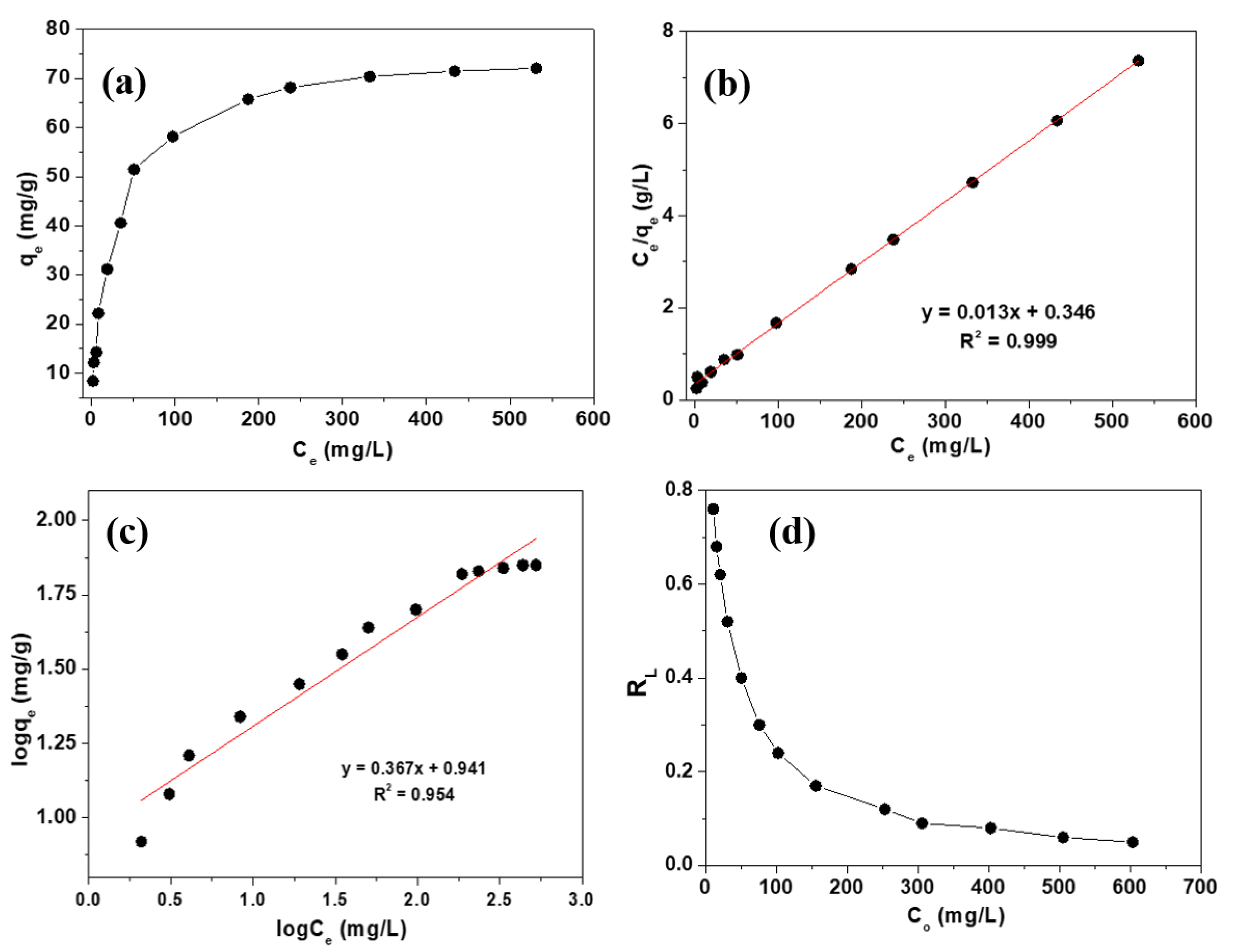
2.2. Adsorption Isotherm
2.3. Effect of pH and As(III) Adsorption Mechanism
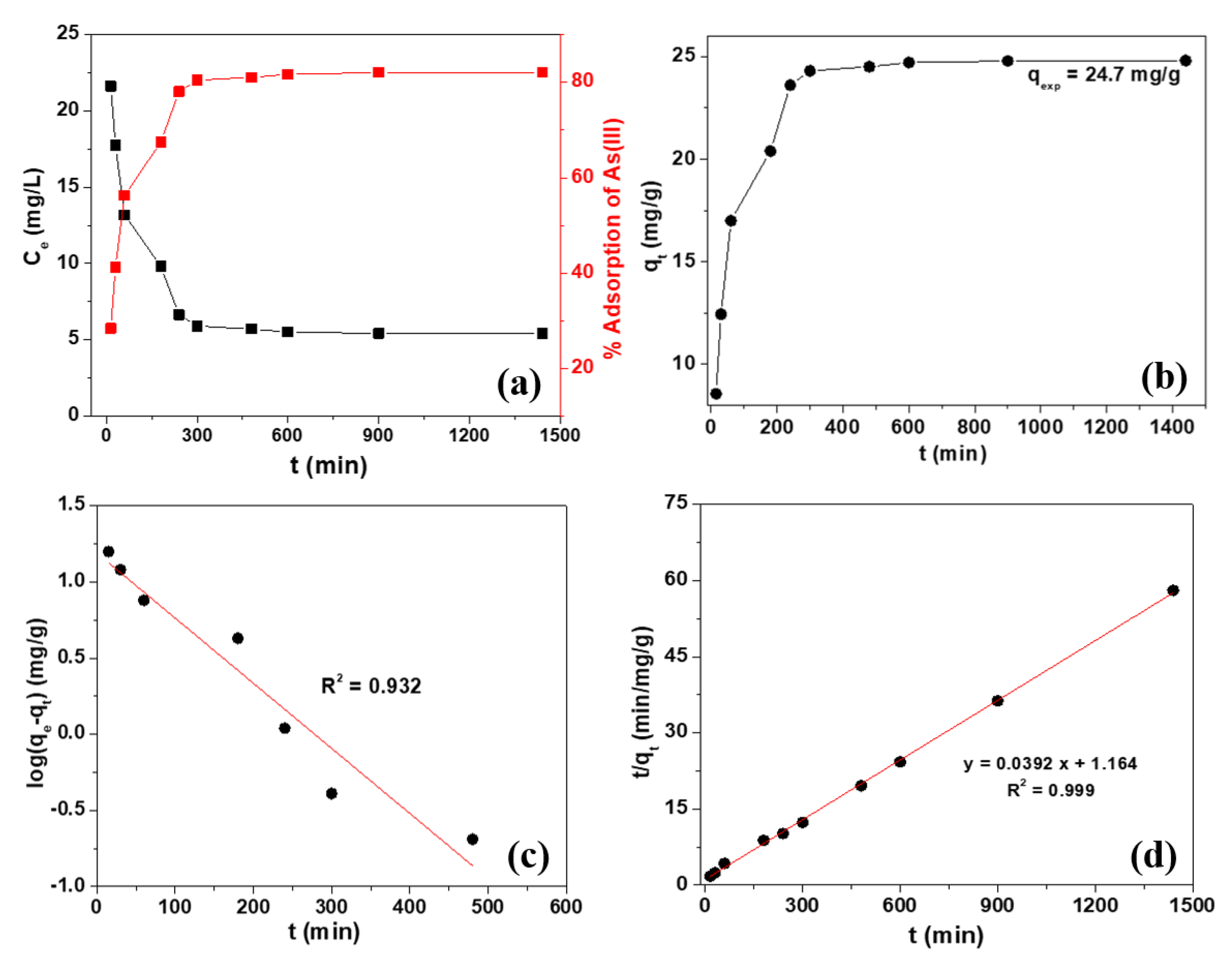
2.4. Adsorption Kinetics
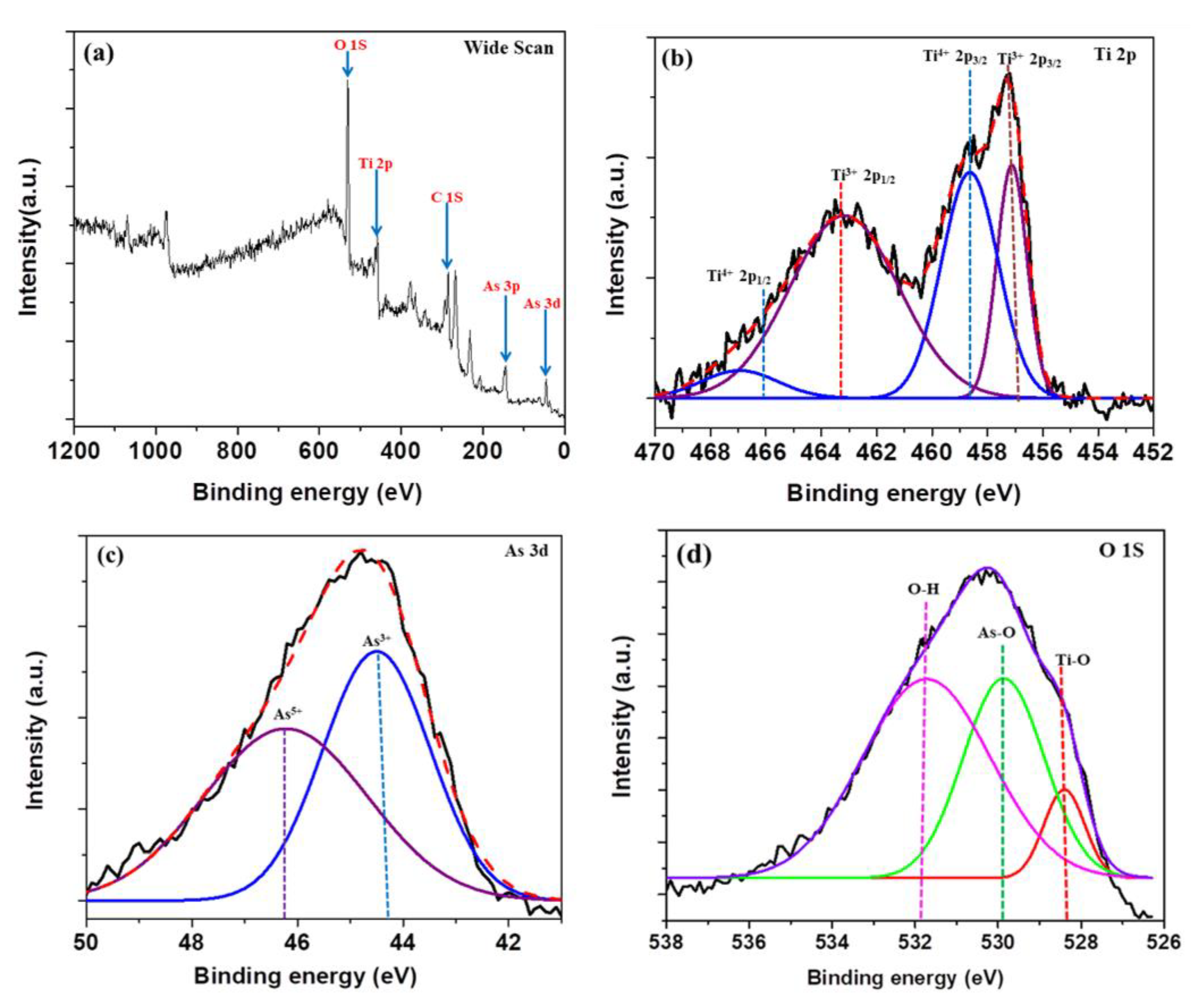
2.5. X-ray Photoelectron Spectroscopy (XPS) Studies
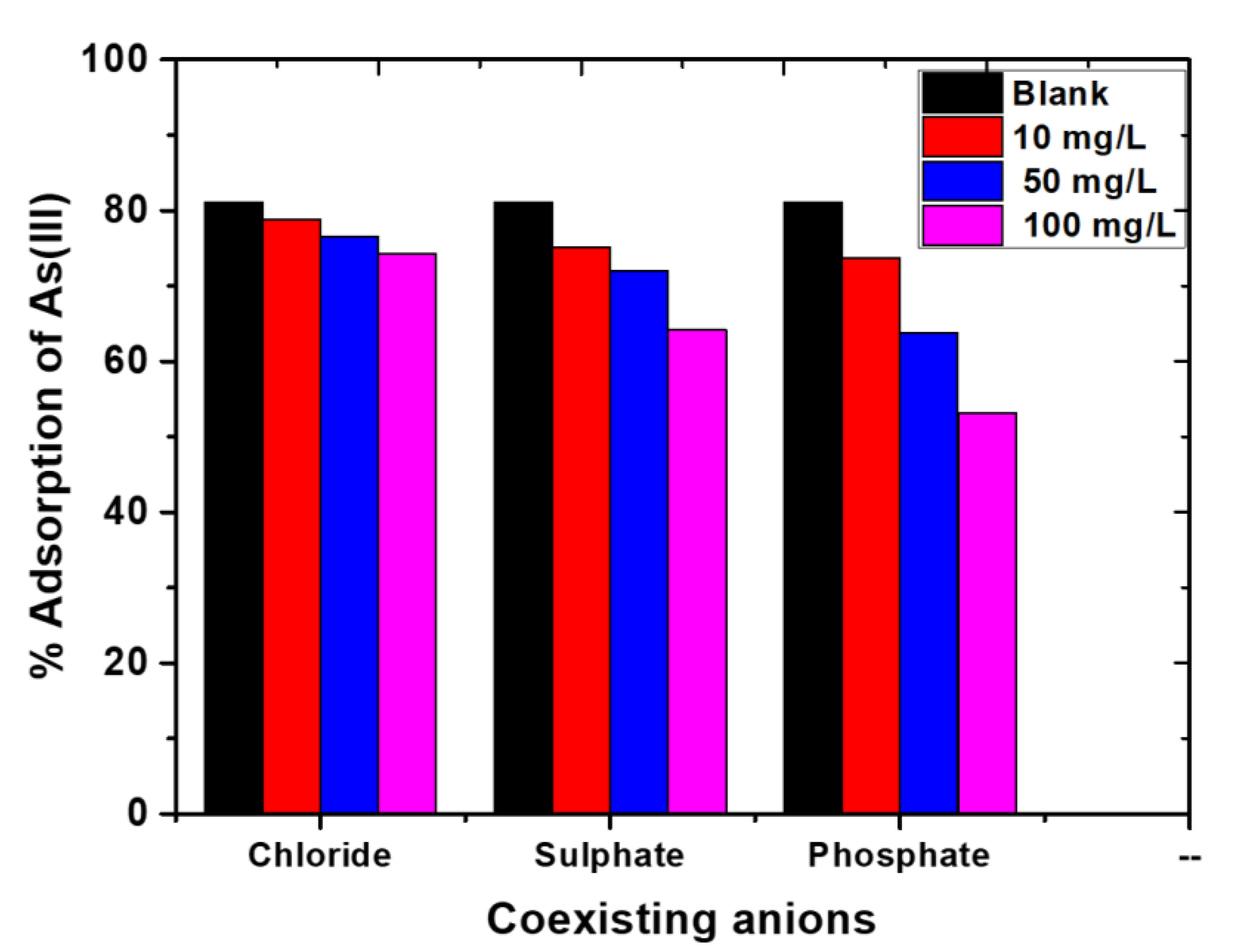
2.6. Effect of Common Coexisting Anions
2.7. Desorption Study and Reusability of PP@TiO2
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2 Impregnated Pomegranate Peels (PP@TiO2)
3.3. Characterizations
3.4. Batch Adsorption Studies
3.5. X-ray Photoelectron Spectroscopy (XPS) Studies
3.6. Desorption Study and Reusability of PP@TiO2
3.7. Analysis of Arsenic Concentration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Rathore, E.; Biswas, K. Selective and ppb level removal of Hg(II) from water: Synergistic role of graphene oxide and SnS2. J. Mater. Chem. A 2018, 6, 13142–13152. [Google Scholar] [CrossRef]
- Raj, K.R.; Kardam, A.; Srivastava, S. Development of polyethyleneimine modified Zea mays as a high capacity biosorbent for the removal of As (III) and As (V) from aqueous system. Int. J. Miner. Process. 2013, 122, 66–70. [Google Scholar] [CrossRef]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Toda, M.; Otani, M.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of arsenic(III) ion from aqueous media using complex nickel-aluminum and nickel-aluminum-zirconium hydroxides. Water 2020, 12, 1697. [Google Scholar] [CrossRef]
- Shehzad, K.; Ahmad, M.; He, J.; Liu, T.; Xu, W.; Liu, J. Synthesis of ultra-large ZrO2 nanosheets as novel adsorbents for fast and efficient removal of As (III) from aqueous solutions. J. Colloid Interface Sci. 2019, 533, 588–597. [Google Scholar] [CrossRef]
- Aryal, M.; Ziagova, M.; Liakopoulou-Kyriakides, M. Study on arsenic biosorption using Fe (III)–treated biomass of Staphylococcus xylosus. Chem. Eng. J. 2010, 162, 178–185. [Google Scholar] [CrossRef]
- WHO. Arsenic in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. In Guidelines for Drinking-water Quality; (No. WHO/SDE/WSH/03.04/75/Rev/1); World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf (accessed on 16 April 2020).
- USEPA. Drinking Water Requirements for States and Public Water System. 2017. Available online: https://www.epa.gov/dwreginfo/chemical–contaminant–rules (accessed on 16 April 2020).
- Bureau of Indian Standard (BIS). Indian Standards, Drinking Water Specification, 2nd ed.; Bureau of Indian Standards: New Delhi, India, 2012.
- National Drinking Water Quality Standards (NDWQS), Nepal. Nepal Gazette (B.S. 2063–03–12); Government of Nepal/Ministry of Physical Planning: Kathmandu, Nepal, 2006.
- Sanjoy, K.M.; Anjali, P.; Trasankar, P. Arsenic removal from real-life groundwater by adsorption on laterite soil. J. Hazard. Mater. 2008, 153, 811–820. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano–porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of arsenic (III) from aqueous solution using metal-organic framework-graphene oxide nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, S.; Pokhrel, M.R. Removal of As(III) from aqueous solution using Fe(III) loaded pomegranate waste. J. Nepal Chem. Soc. 2012, 30, 29–36. [Google Scholar] [CrossRef]
- Tamayo, R.; Espinoza-González, R.; Gracia, F.; Rodrigues-Filho, U.P.; Flores, M.; Sacari, E. As(III) removal from aqueous solution by calcium titanate nanoparticles prepared by the sol-gel method. Nanomaterials 2019, 9, 733. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.K.; Inoue, J.I.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Adsorptive removal of As (V) and As (III) from water by a Zr (IV)-loaded orange waste gel. J. Hazard. Mater. 2008, 154, 1066–1074. [Google Scholar] [CrossRef]
- Ghimire, K.N.; Inoue, K.; Makino, K.; Miyajima, T. Adsorptive removal of arsenic using orange juice residue. Sep. Sci. Technol. 2002, 37, 2785–2799. [Google Scholar] [CrossRef]
- Yu, L.; Peng, X.; Ni, F.; Li, J.; Wang, D.; Luan, Z. Arsenite removal from aqueous solutions by γ-Fe2O3-TiO2 magnetic nanoparticles through simultaneous photocatalytic oxidation and adsorption. J. Hazard. Mater. 2013, 246, 10–17. [Google Scholar] [CrossRef]
- Ortega, A.; Oliva, I.; Contreras, K.E.; González, I.; Cruz-Díaz, M.R.; Rivero, E.P. Arsenic removal from water by hybrid electro–regenerated anion exchange resin/electrodialysis process. Sep. Purif. Technol. 2017, 184, 319–326. [Google Scholar] [CrossRef]
- Wickramasinghe, S.R.; Han, B.; Zimbron, J.; Shen, Z.; Karim, M.N. Arsenic removal by coagulation and filtration: Comparison of groundwaters from the United States and Bangladesh. Desalination 2004, 169, 231–244. [Google Scholar] [CrossRef]
- Litter, M.I.; Morgada, M.E.; Bundschuh, J. Possible treatments for arsenic removal in Latin American waters for human consumption. Environ. Pollut. 2010, 158, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, M.R.; Poudel, B.R.; Aryal, R.L.; Paudyal, H.; Ghimire, K.N. Removal and recovery of phosphate from water and wastewater using metal–loaded agricultural waste-based adsorbents: A review. JIST 2019, 24, 77–89. [Google Scholar] [CrossRef]
- Amin, M.N.; Kaneco, S.; Kitagawa, T.; Begum, A.; Katsumata, H.; Suzuki, T.; Ohta, K. Removal of arsenic in aqueous solutions by adsorption onto waste rice husk. Ind. Eng. Chem. Res. 2006, 45, 8105–8110. [Google Scholar] [CrossRef]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. J. Environ. Health Sci. Eng. 2014, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Bissen, M.; Vieillard-Baron, M.M.; Schindelin, A.J.; Frimmel, F.H. TiO2-catalyzed photooxidation of arsenite to arsenate in aqueous samples. Chemosphere 2001, 44, 751–757. [Google Scholar] [CrossRef]
- Guan, X.H.; Du, J.S.; Meng, X.G.; Sun, Y.K.; Sun, B.; Hu, Q.H. Application of titanium dioxide in arsenic removal from water: A review. J. Hazard. Mater. 2012, 215, 1–16. [Google Scholar] [CrossRef]
- Yao, S.; Jia, Y.; Shi, Z.; Zhao, S. Photocatalytic oxidation of arsenite by a composite of titanium dioxide and activated carbon fiber. Photochem. Photobiol. 2010, 86, 1215–1221. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W. Photocatalytic oxidation of arsenite in TiO2 suspension: Kinetics and mechanisms. Environ. Sci. Technol. 2002, 36, 3872–3878. [Google Scholar] [CrossRef]
- Ferguson, M.A.; Hoffmann, M.R.; Hering, J.G. TiO2-photocatalyzed As (III) oxidation in aqueous suspensions: Reaction kinetics and effects of adsorption. Environ. Sci. Technol. 2005, 39, 1880–1886. [Google Scholar] [CrossRef]
- Dutta, P.K.; Pehkonen, S.O.; Sharma, V.K.; Ray, A.K. Photocatalytic oxidation of arsenic (III): Evidence of hydroxyl radicals. Environ. Sci. Technol. 2005, 39, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Itoh, H. Photocatalytic oxidation and removal of arsenite from water using slag–iron oxide-TiO2 adsorbent. Chemosphere 2006, 65, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Patel, M.; Lippincott, L.; Meng, X. Removal of arsenic from groundwater by granular titanium dioxide adsorbent. Chemosphere 2005, 60, 389–397. [Google Scholar] [CrossRef] [PubMed]
- López Paraguay, M.Z.; Cortes, J.A.; Pérez-Robles, J.F.; Alarcón-Herrera, M.T. Adsorption of arsenite from groundwater using titanium dioxide. Clean (Weinh.) 2014, 42, 713–721. [Google Scholar] [CrossRef]
- Pena, M.E.; Korfiatis, G.P.; Patel, M.; Lippincott, L.; Meng, X.G. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 2006, 39, 2327–2337. [Google Scholar] [CrossRef]
- Pirila, M.; Martikainen, M.; Ainassaari, K.; Kuokkanen, T.; Keiski, R.L. Removal of aqueous As(III) and As(V) by hydrous titanium dioxide. J. Colloid Interface Sci. 2011, 353, 257–262. [Google Scholar] [CrossRef]
- Wei, Z.G.; Liang, K.; Wu, Y.; Zou, Y.D.; Zuo, J.H.; Arriagada, D.C.; Pan, Z.C.; Hu, G.H. The effect of pH on the adsorption of arsenic(III) and arsenic(V) at the TiO2 anatase [1 0 1] surface. J. Colloid Interface Sci. 2016, 562, 252–259. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Sharif, F.; Bashir, S.; Shaheen, M.S.; Wang, H.; Ok, Y.S.; Rinklebe, J. Arsenic removal by natural and chemically modified watermelon rind in aqueous solutions and groundwater. Sci. Total Environ. 2018, 645, 1444–1455. [Google Scholar] [CrossRef]
- Pehlivan, E.; Tran, H.T.; Ouédraogo, W.K.I.; Schmidt, C.; Zachmann, D.; Bahadir, M. Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As (V) from aqueous solutions. Food Chem. 2013, 138, 133–138. [Google Scholar] [CrossRef]
- Gupta, A.; Vidyarthi, S.R.; Sankararamakrishnan, N. Concurrent removal of As (III) and As (V) using green low cost functionalized biosorbent-Saccharum officinarum bagasse. J. Environ. Chem. Eng. 2015, 3, 113–121. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Gozdecka, A.; Jurak, M. Physicochemical characteristics of chitosan-TiO2 biomaterial. 1. Stability and swelling properties. Ind. Eng. Chem. Res. 2018, 57, 1859–1870. [Google Scholar] [CrossRef]
- Li, Q.; Su, H.; Tan, T. Synthesis of ion-imprinted chitosan–TiO2 adsorbent and its multi-functional performances. Biochem. Eng. J. 2008, 38, 212–218. [Google Scholar] [CrossRef]
- Wu, S.; Kan, J.; Dai, X.; Shen, X.; Zhang, K.; Zhu, M. Ternary carboxymethyl chitosan-hemicellulose-nanosized TiO2 composite as effective adsorbent for removal of heavy metal contaminants from water. Fibers Polym. 2017, 18, 22–32. [Google Scholar] [CrossRef]
- Tao, Y.; Ye, L.; Pan, J.; Wang, Y.; Tang, B. Removal of Pb (II) from aqueous solution on chitosan/TiO2 hybrid film. J. Hazard. Mater. 2009, 161, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Araújo, J.; Castro, F. Alternative low-cost adsorbent for water and wastewater decontamination derived from eggshell waste: An overview. Waste Biomass Valor. 2011, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Özsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Kang, Y.; Yin, W.; Fan, J.; Guo, Z. Removal of antibiotics from aqueous solutions by a carbon adsorbent derived from protein-waste-doped biomass. ACS Omega 2020. [Google Scholar] [CrossRef]
- Cruz, G.J.F.; Mondal, D.; Rimaycuna, J.; Soukup, K.; Gomez, M.M.; Solis, J.L.; Lang, J. Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J. Environ. Chem. Eng. 2020, 8, 103800. [Google Scholar] [CrossRef]
- Pincus, L.N.; Melnikov, F.; Yamani, J.S.; Zimmerman, J.B. Multifunctional photoactive and selective adsorbent for arsenite and arsenate: Evaluation of nano titanium dioxide–enabled chitosan cross-linked with copper. J. Hazard. Mater. 2018, 358, 145–154. [Google Scholar] [CrossRef]
- Fausey, C.L.; Zucker, I.; Shaulsky, E.; Zimmerman, J.B.; Elimelech, M. Removal of arsenic with reduced graphene oxide-TiO2-enabled nanofibrous mats. Chem. Eng. J. 2019, 375, 122040. [Google Scholar] [CrossRef]
- Liu, H.; Zuo, K.; Vecitis, C.D. Titanium dioxide-coated carbon nanotube network filter for rapid and effective arsenic sorption. Environ. Sci. Technol. 2014, 48, 13871–13879. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Park, M. Fe1−xS modified TiO2 NPs embedded carbon nanofiber composite via electrospinning: A potential electrode material for supercapacitors. Molecules 2020, 25, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, B.; Pant, H.R.; Park, M.; Liu, Y.; Choi, J.W.; Barakat, N.A.M.; Kim, H.Y. Electrospun CdS-TiO2 doped carbon nanofibers for visible-light-induced photocatalytic hydrolysis of ammonia borane. Cata. Commun. 2014, 50, 63–68. [Google Scholar] [CrossRef]
- Choi, C.; Hwang, K.J.; Kim, Y.J.; Kim, G.; Park, J.Y.; Jin, S. Rice-straw-derived hybrid TiO2–SiO2 structures with enhanced photocatalytic properties for removal of hazardous dye in aqueous solutions. Nano Energy 2016, 20, 76–83. [Google Scholar] [CrossRef]
- Gallios, G.P.; Tolkou, A.K.; Katsoyiannis, I.A.; Stefusova, K.; Vaclavikova, M.; Deliyanni, E.A. Adsorption of arsenate by nanoscaled activated carbon modified by iron and manganese oxides. Sustainability 2017, 9, 1684. [Google Scholar] [CrossRef] [Green Version]
- Barkat, M.; Nibou, D.; Chegrouche, S.; Mellah, A. Kinetics and thermodynamics studies of chromium(VI) ions adsorption onto activated carbon from aqueous solutions. Chem. Eng. Process. Process Intensif. 2009, 48, 38–47. [Google Scholar] [CrossRef]
- Yantasee, W.; Warner, C.L.; Sangvanich, T.; Addleman, R.S.; Carter, T.G.; Wiacek, R.J.; Fryxell, G.E.; Timchalk, C.; Warner, M.G. Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ. Sci. Technol. 2007, 41, 5114–5119. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. JACS 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Uber die adsorption in losungen. J. Phys. Chem. 1916, 57, 385–470. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high–surface–area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Parette, R.; Zou, J.; Cannon, F.S.; Dempsey, B.A. Arsenic removal by iron modified activated carbon. Water Res. 2007, 41, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Lenoble, V.; Bouras, O.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenic adsorption onto pillared clays and iron oxides. J. Colloid Interface Sci. 2002, 255, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ao, H.; Xiong, X.; Xiao, J.; Liu, J. Arsenic removal from water by iron–modified bamboo charcoal. Water Air Soil Pollut. 2012, 223, 1033–1044. [Google Scholar] [CrossRef]
- Setyono, D.; Valiyaveettil, S. Chemically modified sawdust as renewable adsorbent for arsenic removal from water. ACS Sustain. Chem. Eng. 2014, 2, 2722–2729. [Google Scholar] [CrossRef]
- Manju, G.N.; Raji, C.; Anirudhan, T.S. Evaluation of coconut husk carbon for the removal of arsenic from water. Water Res. 1998, 32, 3062–3070. [Google Scholar] [CrossRef]
- Xia, C.L.; Jing, Y.; Jia, Y.Z.; Yue, D.Y.; Ma, J.; Yin, X.J. Adsorption properties of congo red from aqueous solution on modified hectorite: Kinetic and thermodynamic studies. Desalination 2011, 265, 81–87. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hajati, S.; Karimi, F.; Barazesh, B.; Ghezelbash, G. Equilibrium, kinetic and isotherm of some metal ion biosorption. J. Ind. Eng. Chem. 2013, 19, 987–992. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Yan, Z.; Tan, X.; Liu, S.; Zeng, G.; Gu, Y.; Hu, X.; Jiang, L. Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J. Chem. Technol. Biotechnol. 2018, 93, 783–791. [Google Scholar] [CrossRef]
- Ajith, N.; Bhattacharyya, K.; Ipte, P.R.; Satpati, A.K.; Tripathi, A.K.; Verma, R.; Swain, K.K. Interaction of arsenic (III) and arsenic (V) on manganese dioxide: XPS and electrochemical investigations. J. Environ. Sci. Health A 2019, 54, 277–285. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lv, X.; Zhang, Z.; Yu, J.; Wang, T. Arsenic removal from water by photocatalytic functional Fe2O3–TiO2 porous ceramic. J. Porous Mater. 2017, 24, 1227–1235. [Google Scholar] [CrossRef]
- Paudyal, H.; Ohto, K.; Kawakita, H.; Inoue, K. Recovery of fluoride from water through adsorption using orange–waste gel, followed by desorption using saturated lime water. J. Matter. Cycles Waste Manag. 2020, 22, 1484–1491. [Google Scholar] [CrossRef]
- Paudyal, H.; Pangeni, B.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K.; Alam, S. Adsorptive removal of trace concentration of fluoride using orange waste treated using concentrated sulfuric acid. IJMSA 2017, 6, 212. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X. Treatment of wastewater containing reactive brilliant blue KN-R using TiO2/BC composite as heterogeneous photocatalyst and adsorbent. Chemosphere 2018, 206, 777–783. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]



| Adsorbate | Adsorbent | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | KF (mg/g) | n | R2 | ||
| As(III) | PP@TiO2 | 76.92 | 0.03 | 0.999 | 8.72 | 2.72 | 0.954 |
| Adsorbent | Optimum pH | qm (mg/g) | Reference |
|---|---|---|---|
| Orange juice residue | 10.0 | 68.16 | [21] |
| Watermelon rind | 8.2 | 3.40 | [42] |
| Thiol functionalized sugarcane bagasse | 7 | 28.57 | [44] |
| Granular titanium dioxide | 7 | 32.4 | [37] |
| Fe3O4 nanoparticles | 7 | 46.06 | [65] |
| Iron–modified activated carbon | 7.6–8.0 | 38.8 | [66] |
| Amorphous iron hydroxide | 6–8 | 28.0 | [67] |
| Fe3O4/sugarcane bagasse activated carbon composite | 8 | 6.69 | [16] |
| ZrO2 nanosheets | 6 | 74.9 | [9] |
| Iron modified bamboo charcoal | 4–5 | 7.23 | [68] |
| Fe(III) loaded pomegranate waste | 9 | 50.0 | [18] |
| Al-based MOF graphene–oxide nanocomposite | 6.1 | 65.0 | [17] |
| ZrO2–sawdust | 7 | 29.0 | [69] |
| Copper–impregnated coconut husk carbon | 6.5 | 20.35 | [70] |
| TiO2 impregnated pomegranate peels (PP@TiO2) | 7 | 76.92 | This study |
| Order | Adsorbate | R2 | qe (exp) (mg/g) | qe (cal) (mg/g) | k1 (min–1) | k2 (mg/g/min) |
|---|---|---|---|---|---|---|
| Pseudo–2nd | As(III) | 0.999 | 24.7 | 25.51 | – | 1.32 × 10−3 |
| Pseudo–1st | As(III) | 0.932 | 24.7 | 15.45 | 9.85 × 10−3 | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, B.R.; Aryal, R.L.; Bhattarai, S.; Koirala, A.R.; Gautam, S.K.; Ghimire, K.N.; Pant, B.; Park, M.; Paudyal, H.; Pokhrel, M.R. Agro-Waste Derived Biomass Impregnated with TiO2 as a Potential Adsorbent for Removal of As(III) from Water. Catalysts 2020, 10, 1125. https://doi.org/10.3390/catal10101125
Poudel BR, Aryal RL, Bhattarai S, Koirala AR, Gautam SK, Ghimire KN, Pant B, Park M, Paudyal H, Pokhrel MR. Agro-Waste Derived Biomass Impregnated with TiO2 as a Potential Adsorbent for Removal of As(III) from Water. Catalysts. 2020; 10(10):1125. https://doi.org/10.3390/catal10101125
Chicago/Turabian StylePoudel, Bhoj Raj, Ram Lochan Aryal, Sitaram Bhattarai, Agni Raj Koirala, Surendra Kumar Gautam, Kedar Nath Ghimire, Bishweshwar Pant, Mira Park, Hari Paudyal, and Megh Raj Pokhrel. 2020. "Agro-Waste Derived Biomass Impregnated with TiO2 as a Potential Adsorbent for Removal of As(III) from Water" Catalysts 10, no. 10: 1125. https://doi.org/10.3390/catal10101125







