Fe-Based Metallic Glasses and Dyes in Fenton-Like Processes: Understanding Their Intrinsic Correlation
Abstract
1. Introduction
2. Results and Discussion
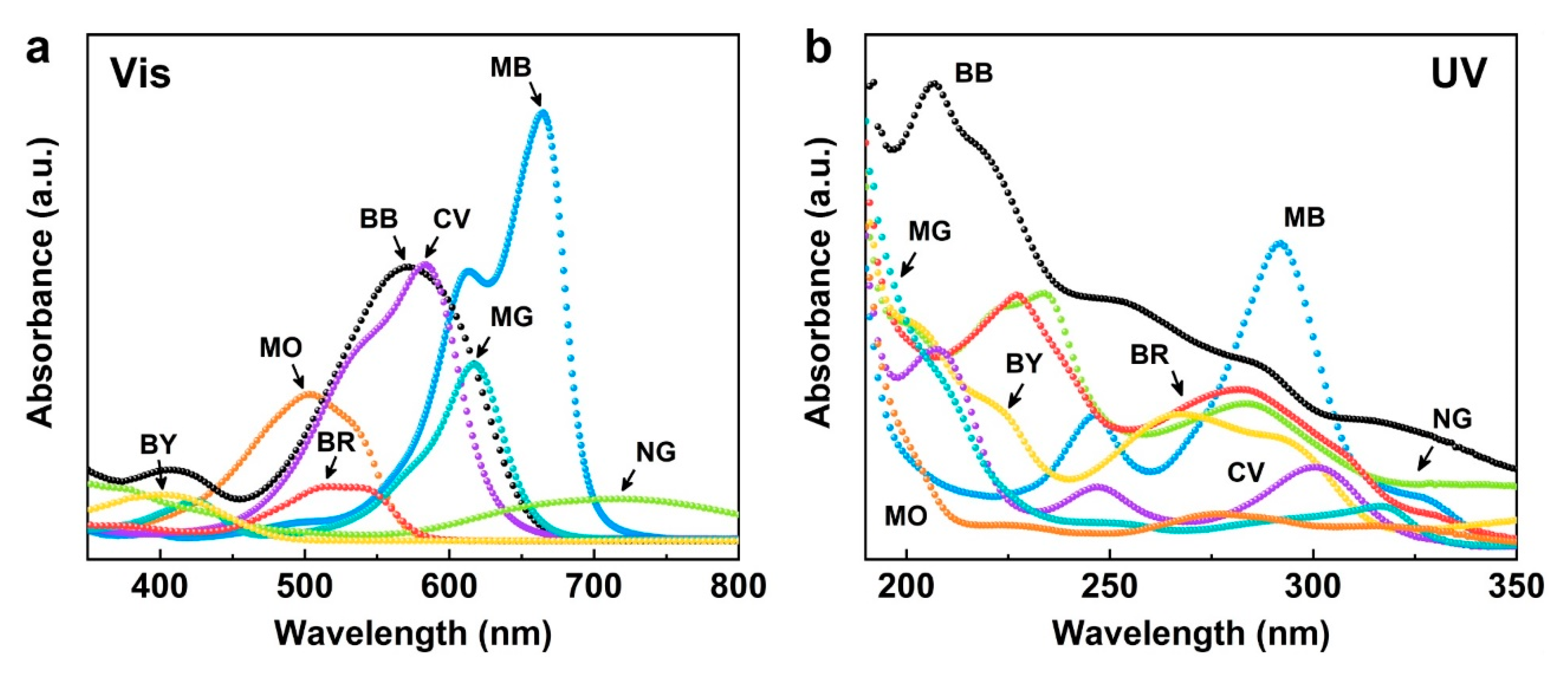
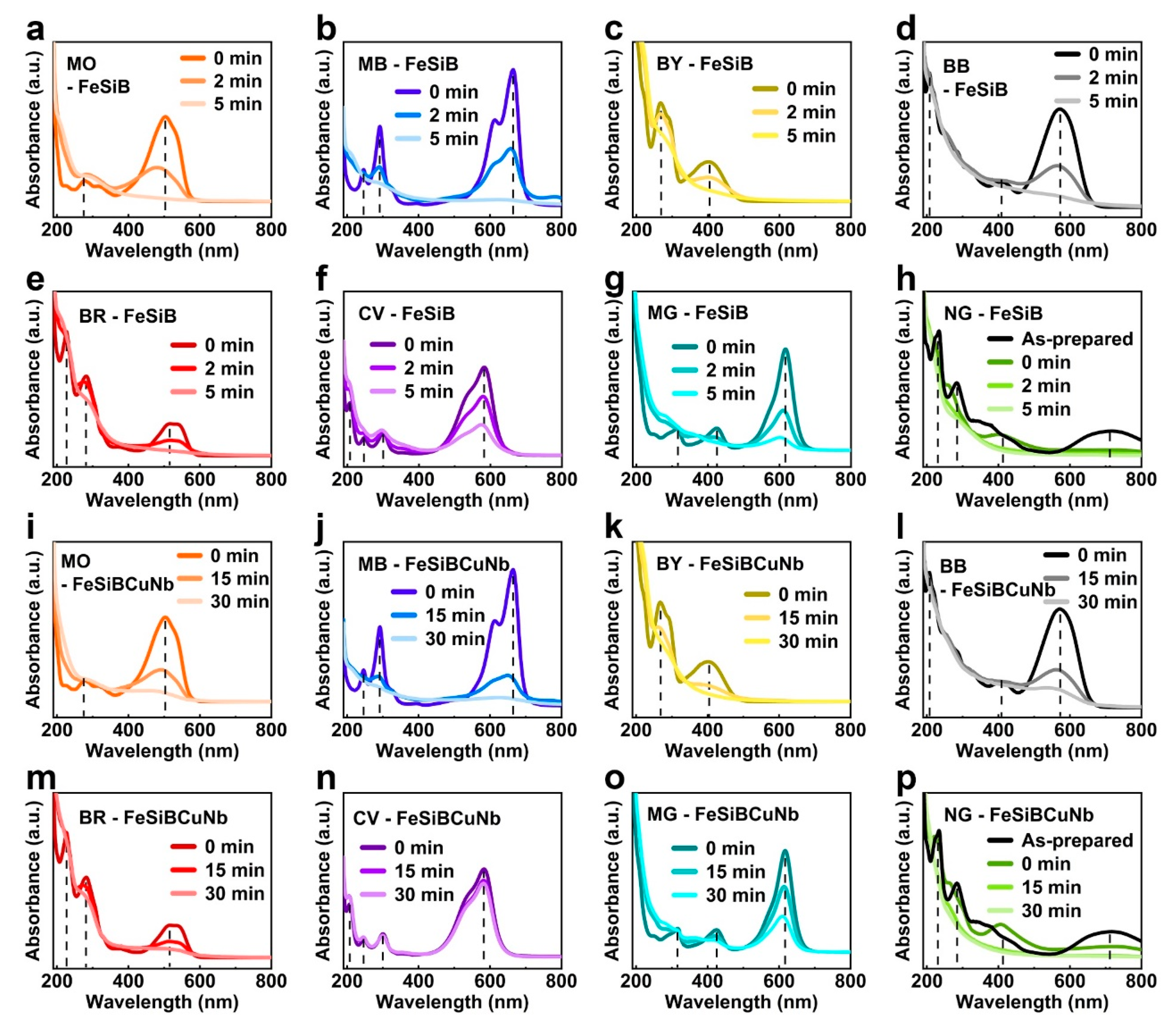
2.1. Dye Characteristics and Properties
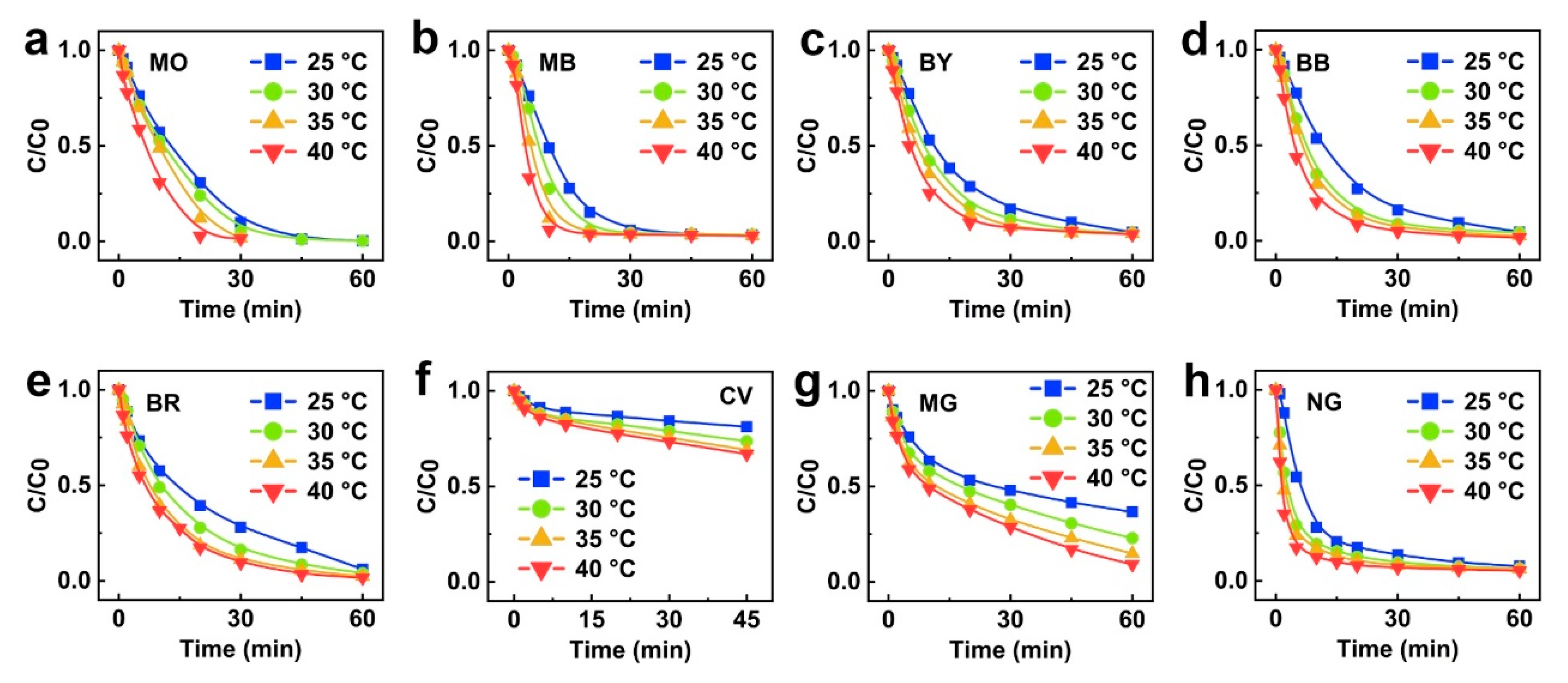
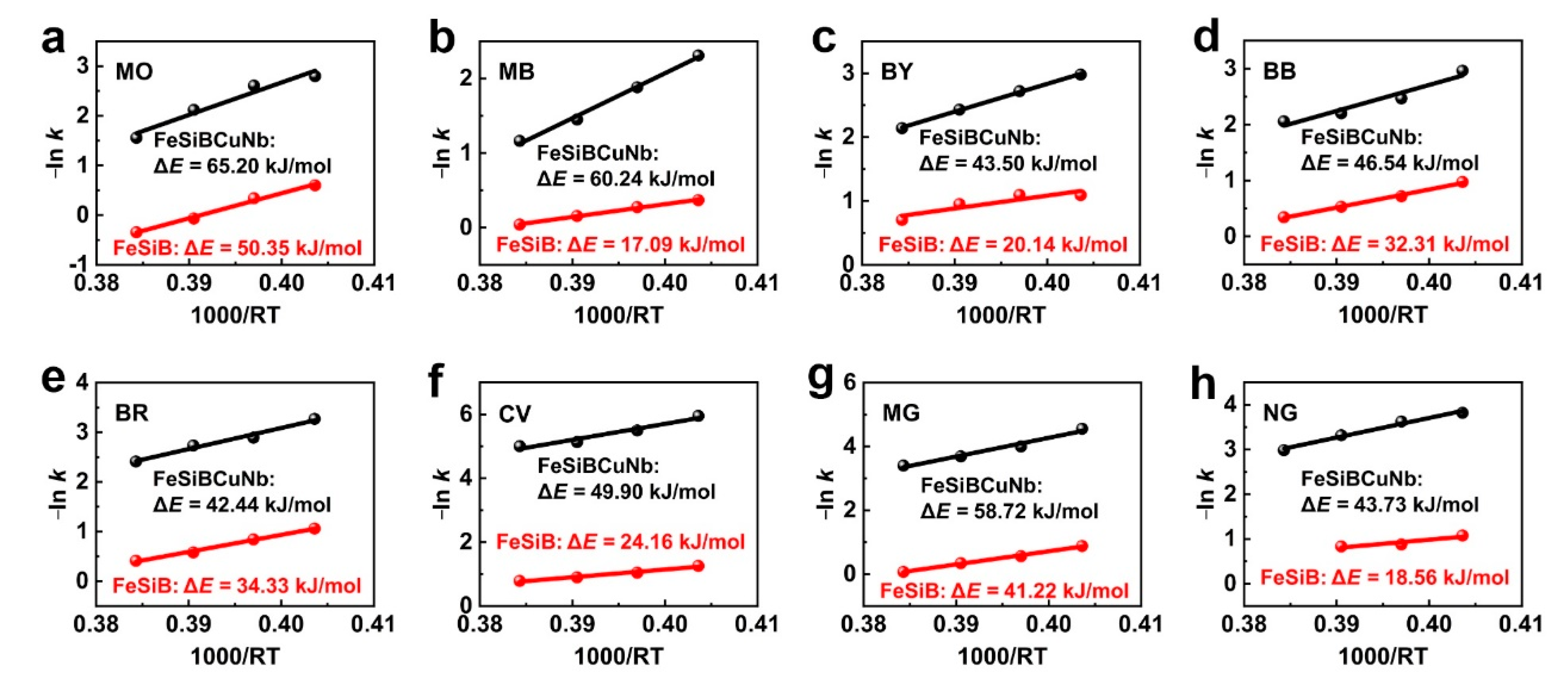
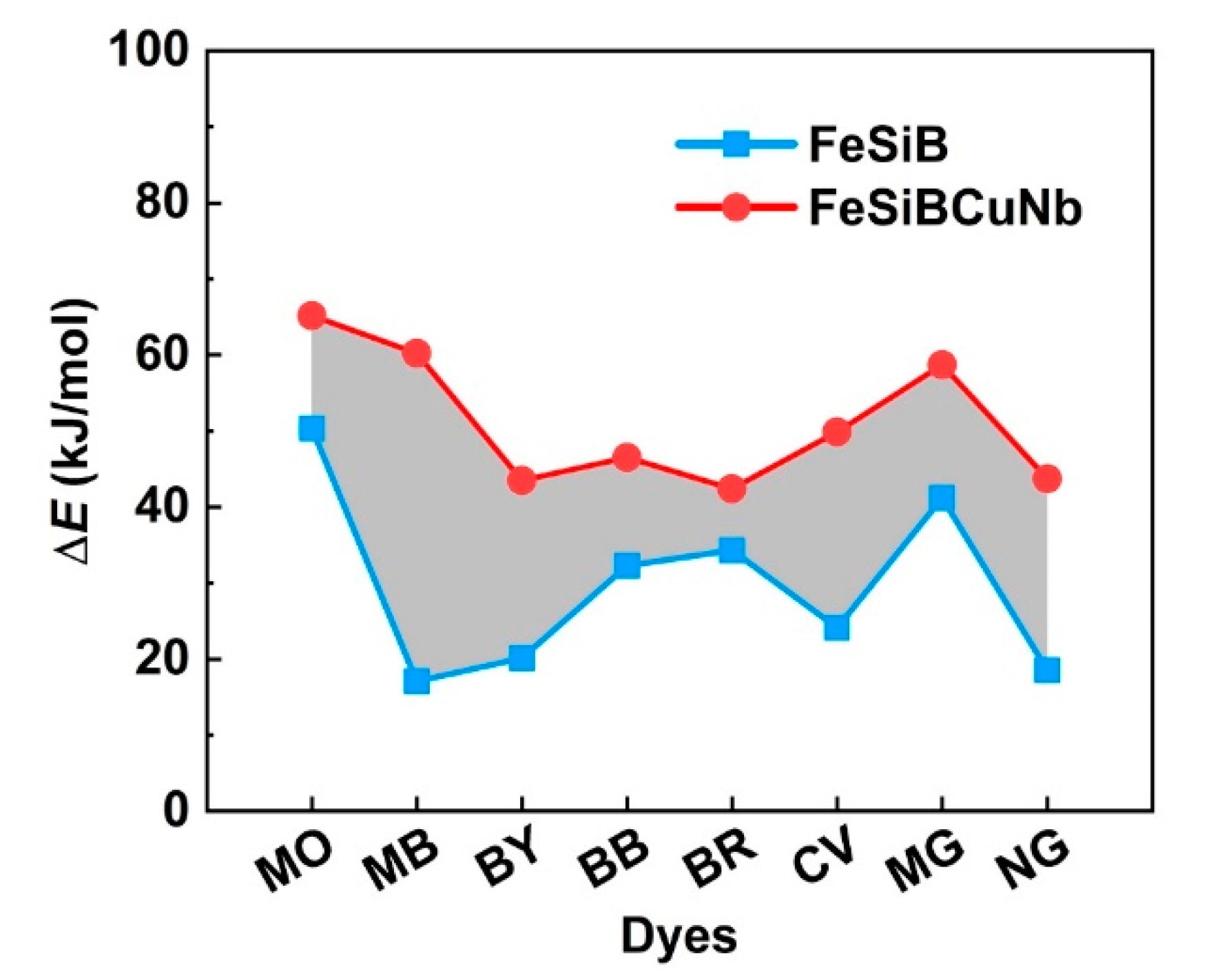
2.2. Thermal Catalytic Behavior Correlation Between Metallic Glasses and Dyes
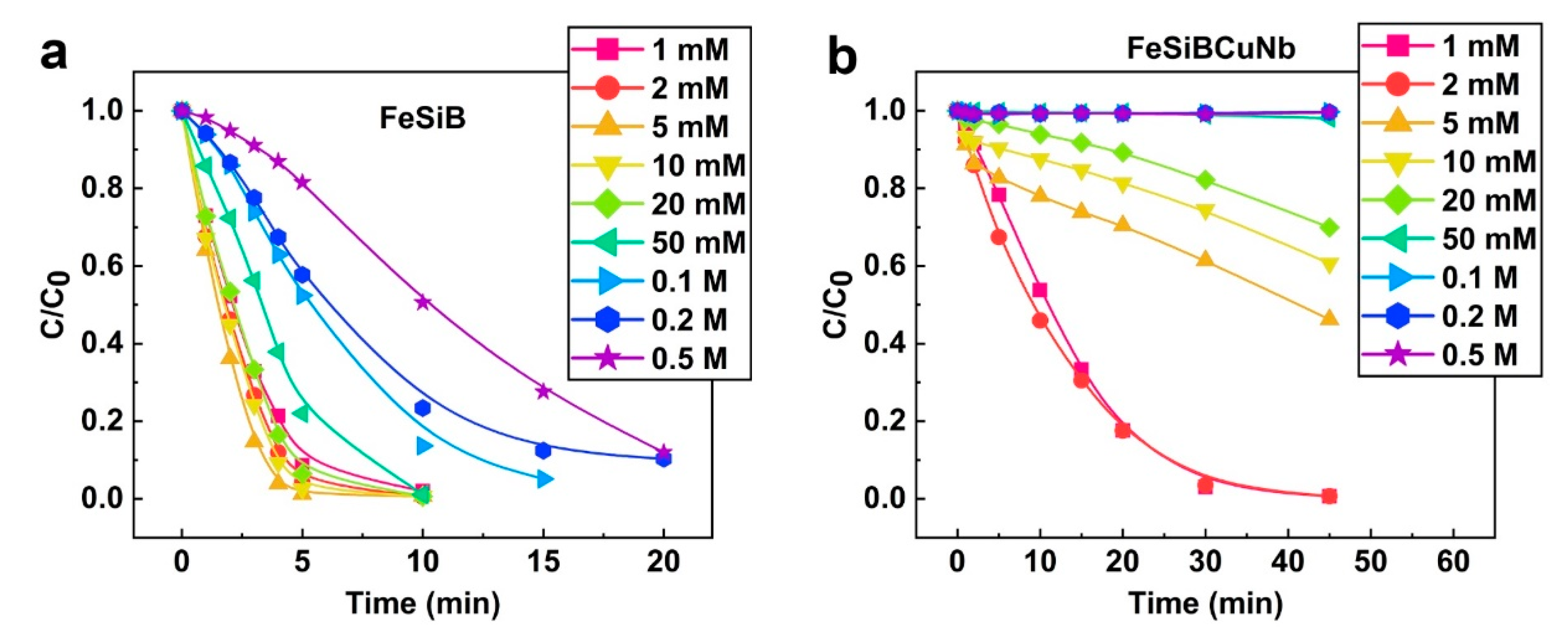
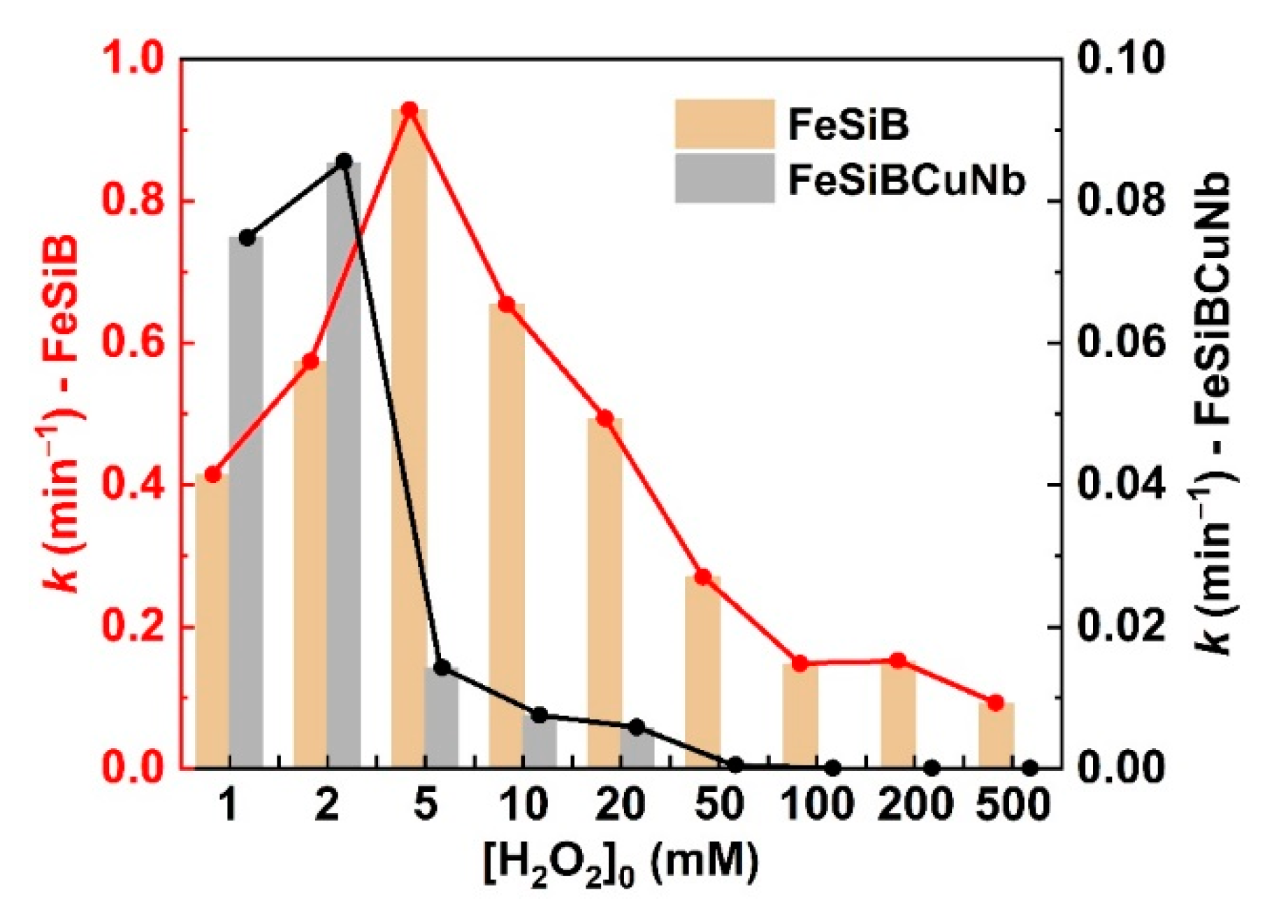
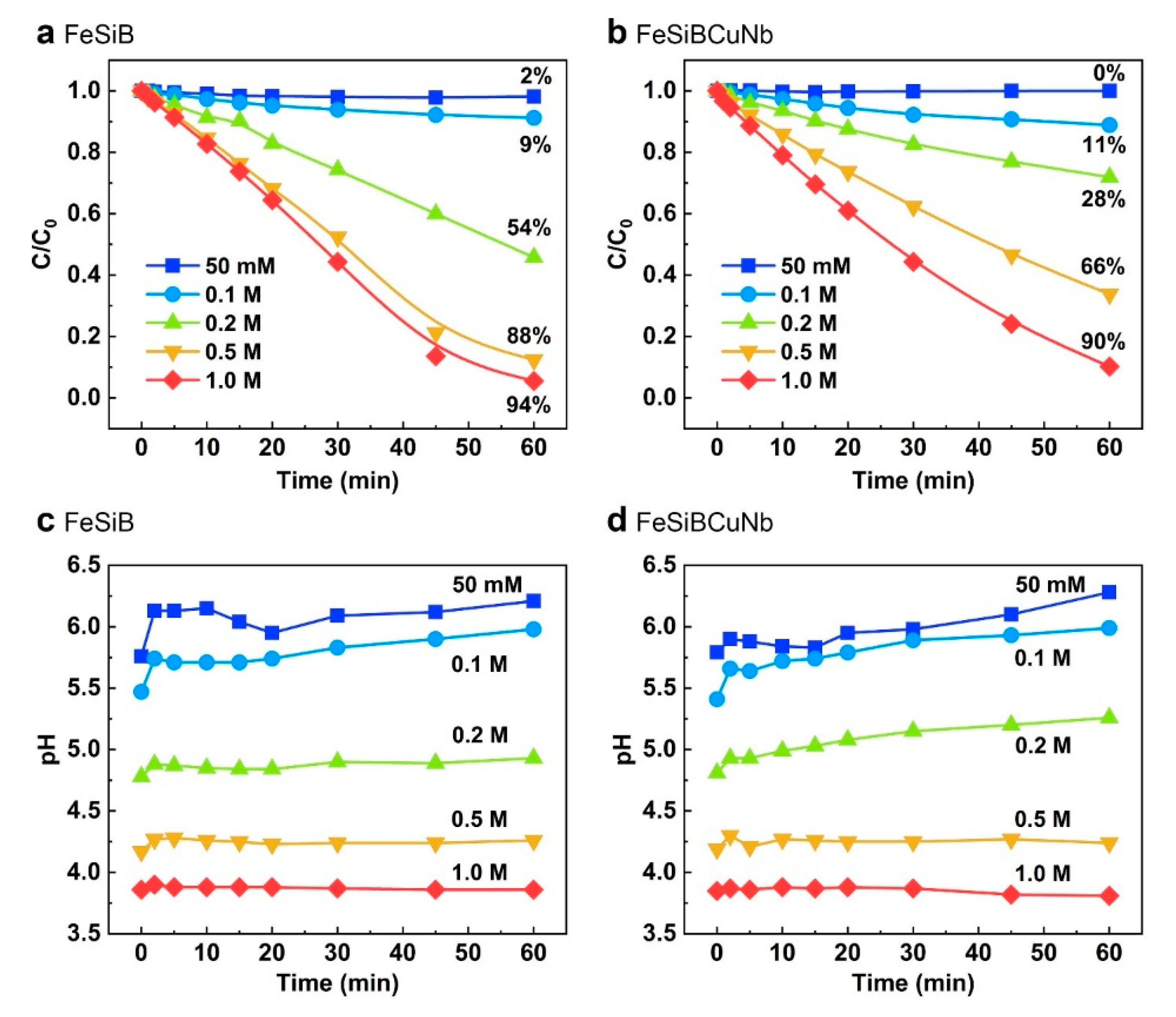
2.3. Correlation between Metallic Glasses and H2O2 in Different pH
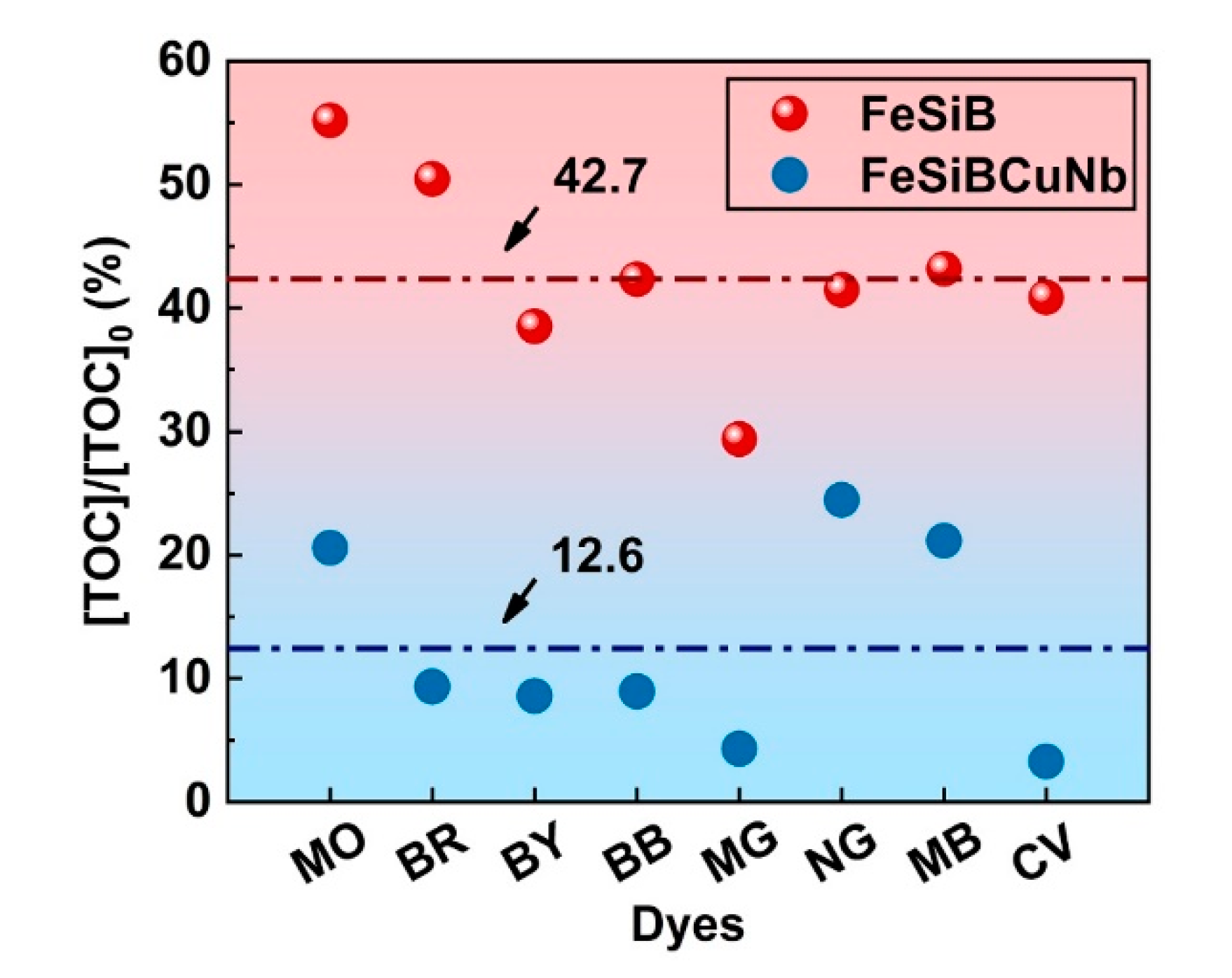
2.4. Correlation between Decolorization and Mineralization
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Characterization
3.3. Catalytic Analysis
4. Conclusions
- The correlation of the thermal catalytic behavior between metallic glasses and dyes has been revealed, where Fe78Si9B13 metallic glass ribbons with active catalytic ability have a lower activation energy (ΔE) than that of Fe73.5Si13.5B9Cu1Nb3 in all dye degradations, and the ΔE of the metallic glass depends on the reaction environment (dyes).
- Under acidic conditions, a high H2O2 concentration is unfavorable to catalytic activity. However, under neutral conditions, a photo-enhanced Fenton-like process catalyzed by Fe-based metallic glasses facilitates dye degradation at higher concentrations of H2O2 due to the decrease of pH and enhancement of irradiation.
- Fe78Si9B13 metallic glass ribbons achieve an average TOC removal rate of 42.7% dye degradation in 15 min, which is higher than Fe73.5Si13.5B9Cu1Nb3 (12.6%). The decolorization rate is closely related to the mineralization rate.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Qiao, J.C.; Wang, Q.; Pelletier, J.M.; Kato, H.; Casalini, R.; Crespo, D.; Pineda, E.; Yao, Y.; Yang, Y. Structural heterogeneities and mechanical behavior of amorphous alloys. Prog. Mater. Sci. 2019, 104, 250–329. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Excellent capability in degrading azo dyes by MgZn-Based metallic glass powders. Sci. Rep. 2012, 2, 418. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.C.; Wang, Y.J.; Pelletier, J.M.; Keer, L.M.; Fine, M.E.; Yao, Y. Characteristics of stress relaxation kinetics of La60Ni15Al25 bulk metallic glass. Acta Mater. 2015, 98, 43–50. [Google Scholar] [CrossRef]
- Hu, F.; Zhu, S.; Chen, S.; Li, Y.; Ma, L.; Wu, T.; Zhang, Y.; Wang, C.; Liu, C.; Yang, X.; et al. Amorphous metallic NiFeP: A conductive bulk material achieving high activity for oxygen evolution reaction in both alkaline and acidic media. Adv. Mater. 2017, 29, 1606570. [Google Scholar] [CrossRef]
- Jia, Z.; Duan, X.; Qin, P.; Zhang, W.; Wang, W.; Yang, C.; Sun, H.; Wang, S.; Zhang, L.C. Disordered atomic packing structure of metallic glass: Toward ultrafast hydroxyl radicals production rate and strong electron transfer ability in catalytic performance. Adv. Funct. Mater. 2017, 27, 1702258. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid degradation of azo dye by Fe-based metallic glass powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, Y.Z.; Su, R.; Cao, C.R.; Li, F.; Sun, C.W.; Yang, Y.; Guan, P.F.; Ding, D.W.; Wang, Z.L.; et al. A highly efficient and self-stabilizing metallic-glass catalyst for electrochemical hydrogen generation. Adv. Mater. 2016, 28, 10293–10297. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Q.; Sun, L.; Wang, Q.; Zhang, L.C.; Wu, G.; Luan, J.H.; Jiao, Z.B.; Wang, A.; Liang, S.X.; et al. Attractive in situ self-reconstructed hierarchical gradient structure of metallic glass for high efficiency and remarkable stability in catalytic performance. Adv. Funct. Mater. 2019, 29, 1807857. [Google Scholar] [CrossRef]
- Miao, F.; Wang, Q.Q.; Zeng, Q.S.; Hou, L.; Liang, T.; Cui, Z.Q.; Shen, B.L. Excellent reusability of FeBC amorphous ribbons induced by progressive formation of through-pore structure during acid orange 7 degradation. J. Mater. Sci. Technol. 2020, 38, 107–118. [Google Scholar] [CrossRef]
- Lu, S.; Lian, J.; Zhang, F.; Jiang, W.; Hu, Q.; Li, D.; Zhang, B. Fe40Co40Se20 glassy films supported on carbon fiber paper as electrocatalysts in the oxygen evolution reaction. J. Electrochem. Soc. 2019, 166, F620–F626. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, F.; Wang, H.; Tian, Y.; Hirata, A.; Fujita, T.; Chen, M. Noble-metal-free metallic glass as a highly active and stable bifunctional electrocatalyst for water splitting. Adv. Mater. Interfaces 2017, 4, 1601086. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, J.; Jiang, W.; Hu, Q.; Zhang, B. New and efficient electrocatalyst for hydrogen production from water splitting: Inexpensive, robust metallic glassy ribbons based on iron and cobalt. ACS Appl. Mater. Interfaces 2017, 9, 31340–31344. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Yan, C.; Chen, G.; Ding, Y.; Sun, J.; Zhou, Y.; Yu, G. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed. 2018, 57, 6073–6076. [Google Scholar] [CrossRef]
- Zhang, L.C.; Liang, S.X. Fe-based metallic glasses in functional catalytic applications. Chem. Asian J. 2018, 13, 3575–3592. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Li, X.F.; Wang, W.M.; Lin, H.C.; Zhang, L.C. Ultrafast activation efficiency of three peroxides by Fe78Si9B13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Appl. Catal. B 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Silva, G.C.; Ciminelli, V.S.T.; Ferreira, A.M.; Pissolati, N.C.; Paiva, P.R.P.; López, J.L. A facile synthesis of Mn3O4/Fe3O4 superparamagnetic nanocomposites by chemical precipitation: Characterization and application in dye degradation. Mater. Res. Bull. 2014, 49, 544–551. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002, 36, 4717–4724. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, T.; Lu, S.; Wang, Z.X.; Chen, S.; Zhang, L. Improved hardness and wear resistance of plasma sprayed nanostructured NiCrBSi coating via short-time heat treatment. Surf. Coat. Technol. 2018, 350, 436–444. [Google Scholar] [CrossRef]
- Lindsey, M.E.; Tarr, M.A. Quantitation of hydroxyl radical during Fenton oxidation following a single addition of iron and peroxide. Chemosphere 2000, 41, 409–417. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Liang, S.X.; Zhang, W.; Wang, W.; Jia, G.; Yang, W.; Zhang, L.C. Surface reactivation of FeNiPC metallic glass: A strategy for highly enhanced catalytic behavior. J. Phys. Chem. Solids 2019, 132, 89–98. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Chen, M.X.; Lin, P.H.; Cui, Z.Q.; Chu, C.L.; Shen, B.L. Investigation of FePC amorphous alloys with self-renewing behaviour for highly efficient decolorization of methylene blue. J. Mater. Chem. A 2018, 6, 10686–10699. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Chen, M.X.; Shao, L.L.; Ge, Y.W.; Lin, P.H.; Chu, C.L.; Shen, B.L. Effects of structural relaxation on the dye degradation ability of FePC amorphous alloys. J. Non-Cryst. Solids 2019, 525, 119671. [Google Scholar] [CrossRef]
- Liang, S.X.; Zhang, W.; Zhang, L.; Wang, W.; Zhang, L.C. Remediation of industrial contaminated water with arsenic and nitrate by mass-produced Fe-based metallic glass: Toward potential industrial applications. Sustain. Mater. Technol. 2019, 22, e00126. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, S.; Ding, Y.; Sun, C.; Zhang, A.; Wang, L. Microwave-assisted rapid photocatalytic degradation of malachite green in TiO2 suspensions: Mechanism and pathways. J. Phys. Chem. A 2008, 112, 11172–11177. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Oliveira, E.; Bértolo, E.; Núñez, C.; Pilla, V.; Santos, H.M.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Djafari, J.; Capelo, J.L.; Lodeiro, C. Green and red fluorescent dyes for translational applications in imaging and sensing analytes: A dual-color flag. ChemistryOpen 2018, 7, 9–52. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl. Catal. B 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Bafana, A.; Saravana Devi, S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–370. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, W.C.; Wang, W.M.; Habibi, D.; Zhang, L.C. Amorphous Fe78Si9B13 alloy: An efficient and reusable photo-enhanced Fenton-like catalyst in degradation of cibacron brilliant red 3B-A dye under UV–vis light. Appl. Catal. B 2016, 192, 46–56. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, C.; Liu, L. Excellent degradation performance of 3D hierarchical nanoporous structures of copper towards organic pollutants. J. Mater. Chem. A 2018, 6, 20992–21002. [Google Scholar] [CrossRef]
- Paciullo, C.A.; Horner, D.M.; Hatton, K.W.; Flynn, J.D. Methylene blue for the treatment of septic shock. Pharmacotherapy 2010, 30, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lyu, F.; Zhang, L.C.; Zeng, S.; Liang, S.X.; Li, Y.Y.; Lu, J. Pt nanoparticles decorated heterostructured g-C3N4/Bi2MoO6 microplates with highly enhanced photocatalytic activities under visible light. Sci. Rep. 2019, 9, 7636. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, Q.Q.; Fan, X.D.; Miao, F.; Yang, W.M.; Shen, B.L. Effect of Co addition on catalytic activity of FePCCu amorphous alloy for methylene blue degradation. New J. Chem. 2019, 43, 6126–6135. [Google Scholar] [CrossRef]
- Yang, W.M.; Wang, Q.Q.; Li, W.Y.; Xue, L.; Liu, H.S.; Zhou, J.; Mo, J.Y.; Shen, B.L. A novel thermal-tuning Fe-based amorphous alloy for automatically recycled methylene blue degradation. Mater. Des. 2019, 161, 136–146. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, W.C.; Chiou, M.R.; Chen, S.W.; Chen, Y.Y.; Fan, H.J. Degradation of crystal violet by an FeGAC/H2O2 process. J. Hazard. Mater. 2011, 196, 420–425. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Wang, W.M.; Zhang, L.C. Rapid malachite green degradation using Fe73.5Si13.5B9Cu1Nb3 metallic glass for activation of persulfate under UV-vis light. Mater. Des. 2017, 119, 244–253. [Google Scholar] [CrossRef]
- Kusic, H.; Peternel, I.; Ukic, S.; Koprivanac, N.; Bolanca, T.; Papic, S.; Bozic, A.L. Modeling of iron activated persulfate oxidation treating reactive azo dye in water matrix. Chem. Eng. J. 2011, 172, 109–121. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.; Lee, S.; Choi, W. Dye decolorization test for the activity assessment of visible light photocatalysts: Realities and limitations. Catal. Today 2014, 224, 21–28. [Google Scholar] [CrossRef]
- Wang, J.C.; Liang, S.X.; Jia, Z.; Zhang, W.C.; Wang, W.M.; Liu, Y.J.; Lu, J.; Zhang, L. Chemically dealloyed Fe-based metallic glass with void channels-like architecture for highly enhanced peroxymonosulfate activation in catalysis. J. Alloys Compd. 2019, 785, 642–650. [Google Scholar] [CrossRef]
- Jia, Z.; La, L.B.T.; Zhang, W.C.; Liang, S.X.; Jiang, B.; Xie, S.K.; Habibi, D.; Zhang, L.C. Strong enhancement on dye photocatalytic degradation by ball-milled TiO2: A study of cationic and anionic dyes. J. Mater. Sci. Technol. 2017, 33, 856–863. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, J.C.; Liang, S.X.; Zhang, W.C.; Wang, W.M.; Zhang, L. Activation of peroxymonosulfate by Fe78Si9B13 metallic glass: The influence of crystallization. J. Alloys Compd. 2017, 728, 525–533. [Google Scholar] [CrossRef]
- Jia, Z.; Miao, J.; Lu, H.B.; Habibi, D.; Zhang, W.C.; Zhang, L.C. Photocatalytic degradation and absorption kinetics of cibacron brilliant yellow 3G-P by nanosized ZnO catalyst under simulated solar light. J. Taiwan Inst. Chem. Eng. 2016, 60, 267–274. [Google Scholar] [CrossRef]
- Jia, Z.; Liang, S.X.; Zhang, W.C.; Wang, W.M.; Yang, C.; Zhang, L. Heterogeneous photo Fenton-like degradation of cibacron brilliant red 3b-A dye using amorphous Fe78Si9B13 and Fe73.5Si13.5B9Cu1Nb3 alloys: The influence of adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 128–136. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Xu, D.; Zhang, L. Microwave-assisted catalytic degradation of crystal violet with barium ferrite nanomaterial. Ind. Eng. Chem. Res. 2016, 55, 11869–11877. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, S.; Ding, Y.; Sun, C.; Gu, C.; He, Z.; Qin, C.; He, H.; Xu, B. Microwave-enhanced H2O2-based process for treating aqueous malachite green solutions: Intermediates and degradation mechanism. J. Hazard. Mater. 2009, 171, 123–132. [Google Scholar] [CrossRef]
- Li, X.F.; Liang, S.X.; Xi, X.W.; Jia, Z.; Xie, S.K.; Lin, H.C.; Hu, J.P.; Zhang, L. Excellent performance of Fe78Si9B13 metallic glass for activating peroxymonosulfate in degradation of naphthol green B. Metals 2017, 7, 273. [Google Scholar] [CrossRef]
- Wang, J.C.; Jia, Z.; Liang, S.X.; Qin, P.; Zhang, W.C.; Wang, W.M.; Sercombe, T.B.; Zhang, L. Fe73.5Si13.5B9Cu1Nb3 metallic glass: Rapid activation of peroxymonosulfate towards ultrafast eosin Y degradation. Mater. Des. 2018, 140, 73–84. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Wu, Y.; Chan, K.C.; Lu, Z.P. Flexible glassy grid structure for rapid degradation of azo dye. Mater. Des. 2018, 155, 346–351. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Liu, Y.J.; Zhang, W.; Wang, W.; Lu, J.; Zhang, L.C. Compelling rejuvenated catalytic performance in metallic glasses. Adv. Mater. 2018, 30, 1802764. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Duan, X.; Zhang, W.; Wang, W.; Sun, H.; Wang, S.; Zhang, L.C. Ultra-sustainable Fe78Si9B13 metallic glass as a catalyst for activation of persulfate on methylene blue degradation under UV-vis light. Sci. Rep. 2016, 6, 38520. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Q.; Liu, K. From adsorption to reductive degradation: Different decolorization properties of metallic glasses based on different iron-group elements. J. Alloys Compd. 2018, 741, 1040–1047. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Zhu, Z.; Wu, J. Efficient degradation of rhodamine B using Fe-based metallic glass catalyst by Fenton-like process. Chemosphere 2014, 117, 638–643. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Yun, L.; Chen, M.X.; Xu, D.D.; Cui, Z.Q.; Zeng, Q.S.; Lin, P.H.; Chu, C.L.; Shen, B.L. Competitive effects of structural heterogeneity and surface chemical states on catalytic efficiency of FeSiBPCu amorphous and nanocrystalline alloys. ACS Appl. Nano Mater. 2019, 2, 214–227. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Özcan, A.; Oturan, M.A.; Oturan, N.; Şahin, Y. Removal of acid orange 7 from water by electrochemically generated Fenton’s reagent. J. Hazard. Mater. 2009, 163, 1213–1220. [Google Scholar] [CrossRef]
- Cheng, M.; Ma, W.; Li, J.; Huang, Y.; Zhao, J.; Wen, Y.X.; Xu, Y. Visible-light-assisted degradation of dye pollutants over Fe(III)-loaded resin in the presence of H2O2 at neutral pH values. Environ. Sci. Technol. 2004, 38, 1569–1575. [Google Scholar] [CrossRef]
- Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Kumar, S.G.; Reddy, K.M.; Munikrishnappa, C. Photo degradation of methyl orange an azo dye by advanced Fenton process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009, 164, 459–467. [Google Scholar] [CrossRef] [PubMed]


| Dye | Classification | Empirical Formula | Molecular Weight (g/mol) | λmax (nm) | Structure | Ref. |
|---|---|---|---|---|---|---|
| Methyl Orange (MO) | Azo-anionic | C14H14N3NaO3S | 327.33 | 498 | 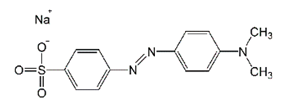 | [9,22,42] |
| Methylene Blue (MB) | Thiazine-cationic | C16H18ClN3S | 319.85 | 664 | 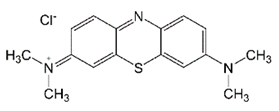 | [24,43,44,45] |
| Bright Black BN (BB) | Azo-anionic | C28H17N5Na4O14S4 | 867.68 | 570 | 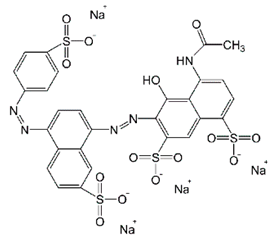 | [23] |
| Cibacron Brilliant Yellow 3G-P (BY) | Azo-anionic | C25H19Cl3N9NaO10S3 | 831.02 | 404 | 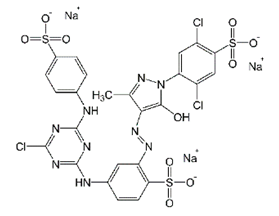 | [46] |
| Cibacron Brilliant Red 3B-A (BR) | Azo-anionic | C32H24ClN8Na4O14S4 | 1000.25 | 517 | 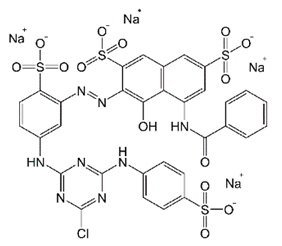 | [33,47] |
| Crystal Violet (CV) | Triarylmethane-cationic | C25N3H30Cl | 407.98 | 582 | 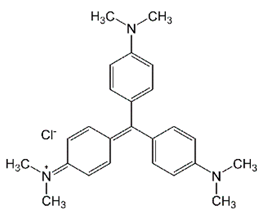 | [16,48] |
| Malachite Green (MG) | Triarylmethane-cationic | C23H25ClN2 | 364.91 | 618 |  | [27,40,49] |
| Naphthol Green B (NG) | Nitroso-anionic | C30H15FeN3Na3O15S3 | 878.45 | 714 | 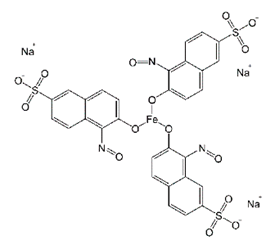 | [50] |
| Catalyst | Reaction Temperature (°C) | Dye Reaction Rate Constant k (min−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MO | MB | BY | BB | BR | CV | MG | NG | ||
| Fe78Si9B13 | 25 | 0.548 | 0.692 | 0.336 | 0.377 | 0.346 | 0.285 | 0.413 | 0.341 |
| 30 | 0.711 | 0.760 | 0.335 | 0.487 | 0.430 | 0.352 | 0.569 | 0.416 | |
| 35 | 1.070 | 0.856 | 0.386 | 0.588 | 0.560 | 0.407 | 0.709 | 0.434 | |
| 40 | 1.410 | 0.961 | 0.494 | 0.709 | 0.662 | 0.455 | 0.931 | - | |
| Fe73.5Si13.5B9Cu1Nb3 | 25 | 0.061 | 0.099 | 0.051 | 0.051 | 0.038 | 0.003 | 0.010 | 0.022 |
| 30 | 0.074 | 0.152 | 0.066 | 0.085 | 0.055 | 0.004 | 0.018 | 0.026 | |
| 35 | 0.120 | 0.235 | 0.088 | 0.110 | 0.065 | 0.006 | 0.025 | 0.036 | |
| 40 | 0.211 | 0.313 | 0.118 | 0.128 | 0.090 | 0.007 | 0.033 | 0.051 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Q.; Liang, S.-X.; Jia, Z.; Zhang, W.; Wang, W.; Zhang, L.-C. Fe-Based Metallic Glasses and Dyes in Fenton-Like Processes: Understanding Their Intrinsic Correlation. Catalysts 2020, 10, 48. https://doi.org/10.3390/catal10010048
Wang X, Zhang Q, Liang S-X, Jia Z, Zhang W, Wang W, Zhang L-C. Fe-Based Metallic Glasses and Dyes in Fenton-Like Processes: Understanding Their Intrinsic Correlation. Catalysts. 2020; 10(1):48. https://doi.org/10.3390/catal10010048
Chicago/Turabian StyleWang, Xueqing, Qiaoyue Zhang, Shun-Xing Liang, Zhe Jia, Wenchang Zhang, Weimin Wang, and Lai-Chang Zhang. 2020. "Fe-Based Metallic Glasses and Dyes in Fenton-Like Processes: Understanding Their Intrinsic Correlation" Catalysts 10, no. 1: 48. https://doi.org/10.3390/catal10010048
APA StyleWang, X., Zhang, Q., Liang, S.-X., Jia, Z., Zhang, W., Wang, W., & Zhang, L.-C. (2020). Fe-Based Metallic Glasses and Dyes in Fenton-Like Processes: Understanding Their Intrinsic Correlation. Catalysts, 10(1), 48. https://doi.org/10.3390/catal10010048










