Structure and Activity of Ni2P/Desilicated Zeolite β Catalysts for Hydrocracking of Pyrolysis Fuel Oil into Benzene, Toluene, and Xylene
Abstract
1. Introduction
2. Results and Discussion
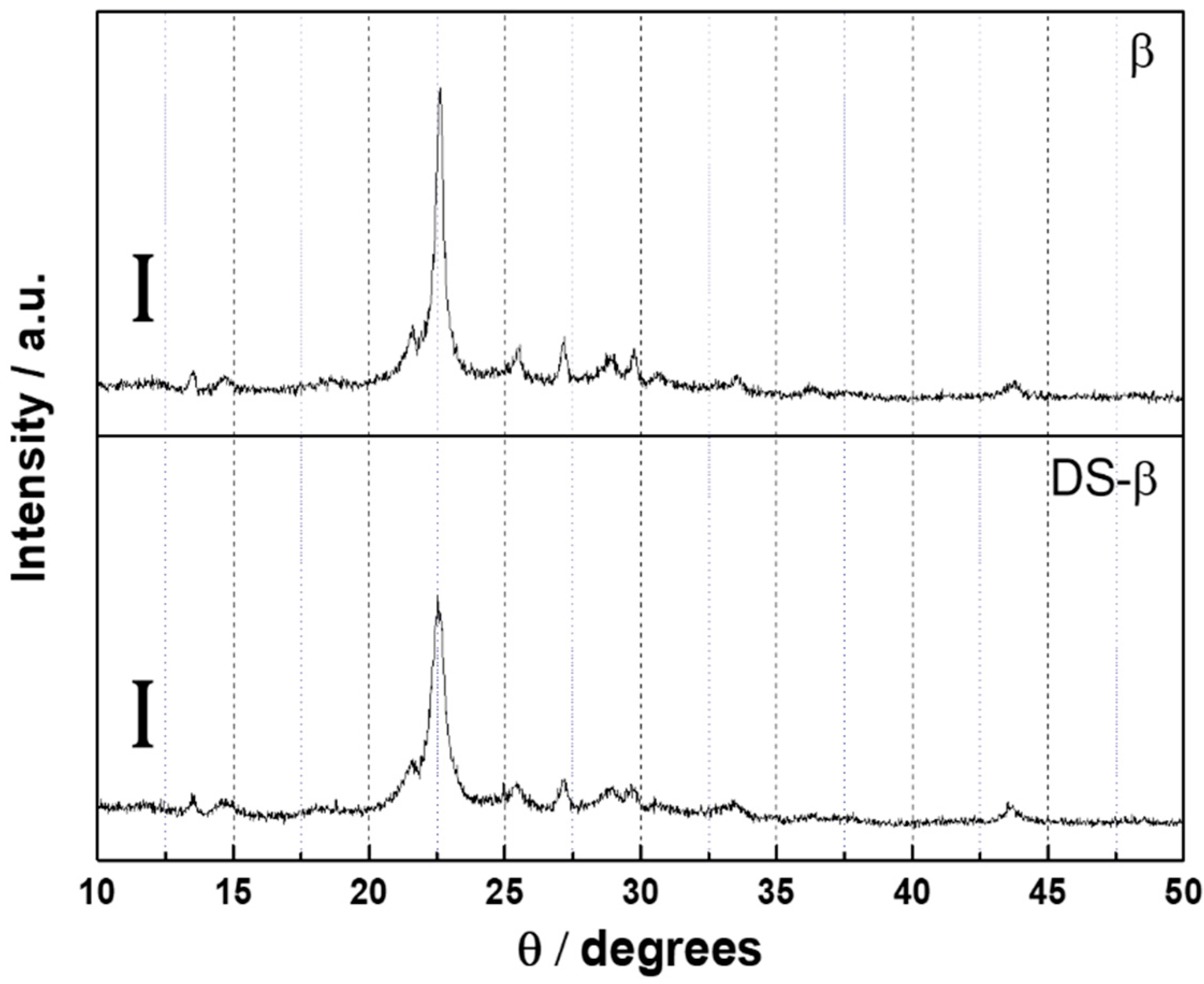
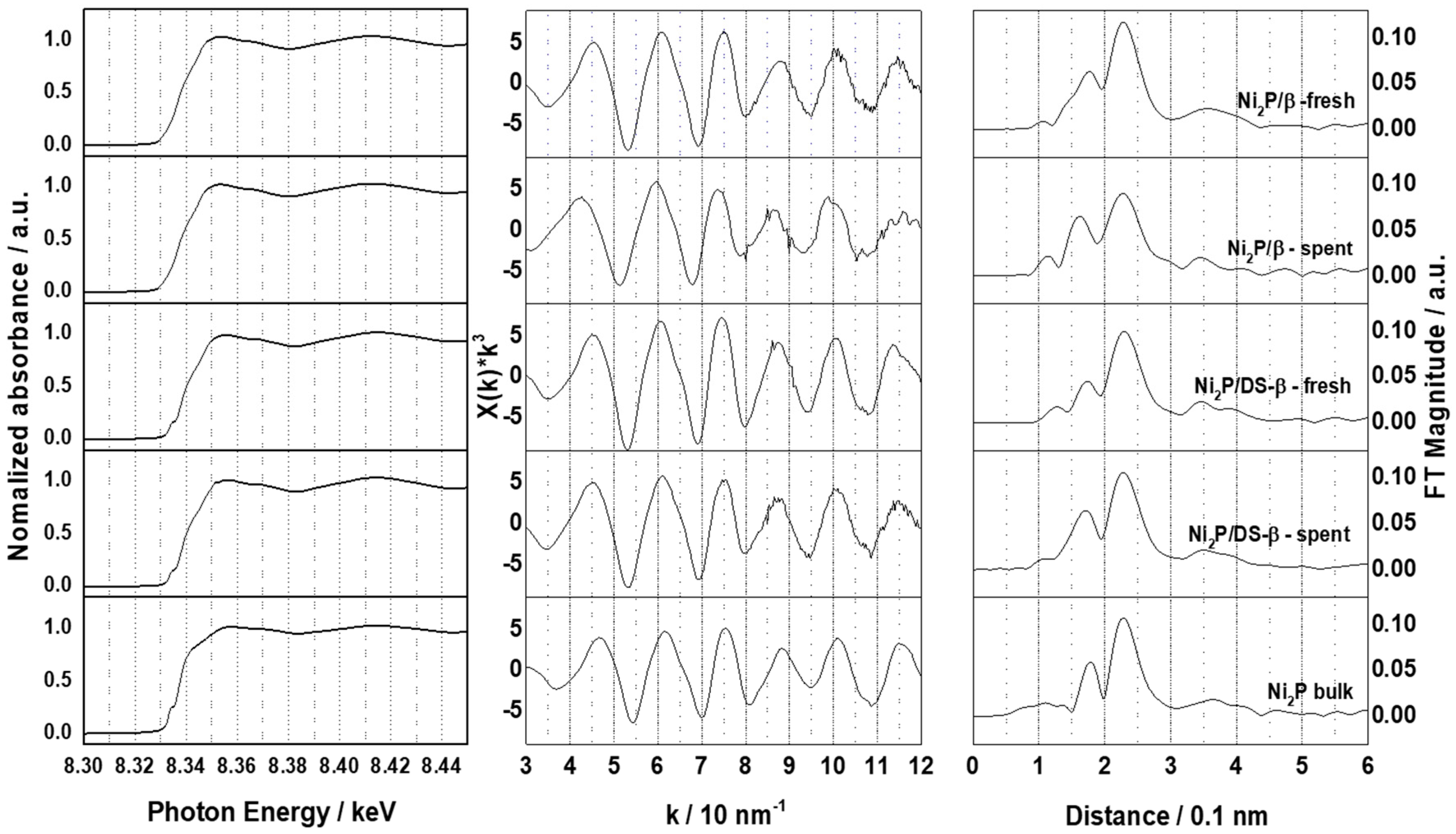
2.1. Characterization of Ni2P Catalysts
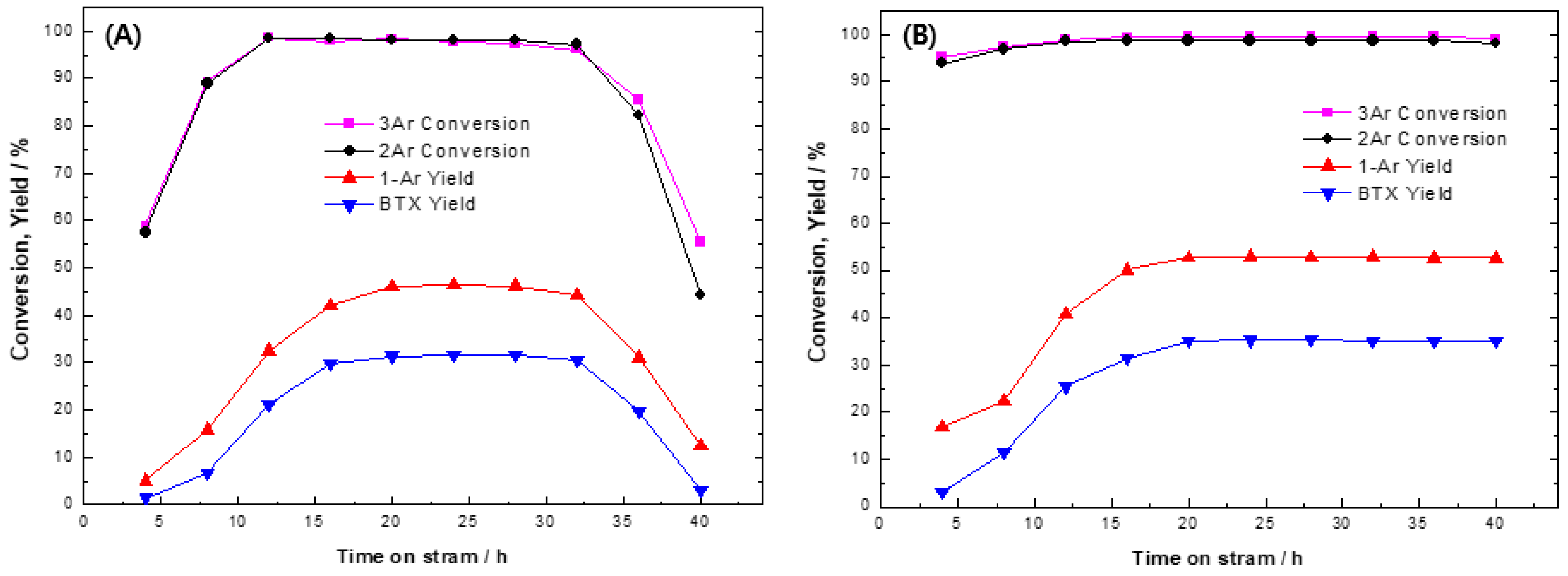
2.2. Hydrocracking Activity of Ni2P Catalysts
3. Experimental
3.1. Materials and Catalysts Preparation
3.2. Catalyst Characterization
3.3. Activity Tests for Hydrocracking of Polycyclic Aromatic Hydrocarbons and PFO
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayani, V.J.; Mayani, S.V.; Lee, Y.; Park, S.-K. A non-chromatographic method for the separation of highly pure naphthalene crystals from pyrolysis fuel oil. Sep. Purif. Technol. 2011, 80, 90–95. [Google Scholar] [CrossRef]
- Khani, M.R.; Guy, E.D.; Gharibi, M.; Shahabi, S.S.; Khosravi, A.; Norouzi, A.A.; Shokri, B. The effects of microwave plasma torch on the cracking of Pyrolysis Fuel Oil feedstock. Chem. Eng. J. 2014, 237, 169–175. [Google Scholar] [CrossRef]
- Kim, J.G.; Liu, F.; Lee, C.-W.; Lee, Y.-S.; Im, J.S. Boron-doped carbon prepared from PFO as a lithium-ion battery anode. Solid State Sci. 2014, 34, 38–42. [Google Scholar] [CrossRef]
- Ishihara, A.; Itoh, T.; Nasu, H.; Hashimoto, T.; Doi, T. Hydrocracking of 1-methylnaphthalene/decahydronaphthalene mixture catalyzed by zeolite-alumina composite supported NiMo catalysts. Fuel Process. Technol. 2013, 116, 222–227. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, J.K.; Miyawaki, J.; Kim, Y.K.; Yoon, S.H.; Mochida, I. Hydro-conversion of 1-methyl naphthalene into (alkyl)benzenes over alumina-coated USY zeolite-supported NiMoS catalysts. Fuel 2011, 90, 182–189. [Google Scholar] [CrossRef]
- Lapinas, A.T.; Klein, M.T.; Gates, B.C.; Macris, A.; Lyons, J.E. Catalytic hydrogenation and hydrocracking of fluorene: Reaction pathways, kinetics, and mechanisms. Ind. Eng. Chem. Res. 1991, 30, 42–50. [Google Scholar] [CrossRef]
- Ward, J.W. Hydrocracking processes and catalysts. Fuel Process. Technol. 1993, 35, 55–85. [Google Scholar] [CrossRef]
- Weitkamp, J. Isomerization and hydrocracking of C9 through C16 n-alkanes on Pt/HZSM-5 zeolite. Appl. Catal. 1983, 8, 123–141. [Google Scholar] [CrossRef]
- Martens, J.A.; Jacobs, P.A.; Weitkamp, J. Attempts to rationalize the distribution of hydrocracked products. I. qualitative description of the primary hydrocracking modes of long chain paraffins in open zeolites. Appl. Catal. 1986, 20, 239–281. [Google Scholar] [CrossRef]
- Martens, J.A.; Jacobs, P.A.; Weitkamp, J. Attempts to rationalize the distribution of hydrocracked products. II. Relative rates of primary hydrocracking modes of long chain paraffins in open zeolites. Appl. Catal. 1986, 20, 283–303. [Google Scholar] [CrossRef]
- Dufresne, P.; Quesada, A.; Mignard, S. Influence of nitrogen feed content on the performances of A zeolite hydrocracking catalyst. Stud. Surf. Sci. Catal. 1990, 53, 301–315. [Google Scholar]
- Leglise, J.; Manoli, J.M.; Potvin, C.; Djega-Mariadassou, G.; Cornet, D. The nature of NiMo phases encaged in HY zeolites. J. Catal. 1995, 152, 275–290. [Google Scholar] [CrossRef]
- Cid, R.; Neira, J.; Godoy, J.; Palacios, J.M.; Lopez Agudo, A. Thiophene hydrodesulfurization on sulfided nickel exchanged USY zeolites. Effect of the pH of the catalyst preparation. Appl. Catal. A Gen. 1995, 125, 169–183. [Google Scholar] [CrossRef]
- Welters, W.J.J.; Vorbeck, G.; Zandbergen, H.W.; de Haan, J.W.; de Beer, V.H.J.; van Santen, R.A. HDS activity and characterization of zeolite-supported nickel sulfide catalysts. J. Catal. 1994, 150, 155–169. [Google Scholar] [CrossRef]
- Korányi, T.I. Phosphorus promotion of Ni (Co)-containing Mo-free catalysts in thiophene hydrodesulfurization. Appl. Catal. A Gen. 2003, 239, 253–267. [Google Scholar] [CrossRef]
- Stinner, C.; Tang, Z.; Haouas, M.; Weber, T.; Prins, R. Preparation and P-31 NMR characterization of nickel phosphides on silica. J. Catal. 2002, 208, 456–466. [Google Scholar] [CrossRef]
- Deliy, I.V.; Shamanaev, I.V.; Aleksandrov, P.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Yakovlev, I.V.; Lapina, O.B.; Bukhtiyarova, G.A. Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate. Catalysts 2018, 8, 515. [Google Scholar] [CrossRef]
- Jang, J.-G.; Lee, Y.-K. Promotional Effect of Ga for Ni2P Catalyst on Hydrodesulfurization of 4,6-DMDBT. Appl. Catal. B Environ. 2019, 250, 181–188. [Google Scholar] [CrossRef]
- Yun, G.-N.; Cho, K.-S.; Kim, Y.-S.; Lee, Y.-K. A New Approach to Deep Desulfurization of Light Cycle Oil over Ni2P Catalysts: Combined Selective Oxidation and Hydrotreating. Catalysts 2018, 8, 102. [Google Scholar]
- Yun, G.-N.; Lee, Y.-K. Dispersion Effects of Ni2P Catalysts on Hydrotreating of Light Cycle Oil. Appl. Catal. B Environ. 2014, 150, 647–655. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Oyama, S.T. Sulfur Resistant Nature of Ni2P Catalyst in Deep Hydrodesulfurization. Appl. Catal. A Gen. 2017, 548, 103–113. [Google Scholar] [CrossRef]
- Cho, K.-S.; Seo, H.-R.; Lee, Y.-K. A New Synthesis of Highly Active Ni2P/Al2O3 Catalyst by Liquid Phase Phosphidation for Deep Hydrodesulfurization. Catal. Commun. 2011, 12, 470–474. [Google Scholar] [CrossRef]
- Cho, K.-S.; Kim, S.-H.; Lee, Y.-K. XAFS Studies on Highly Dispersed Ni2P/SiO2 Catalysts for Hydrodesulfurization of 4,6-Dimethyldibenzothiophene. Nucl. Instrum. Methods Phys. Res. A 2010, 621, 690–694. [Google Scholar] [CrossRef]
- Cho, K.-S.; Seo, H.-R.; Kim, S.-H.; Lee, Y.-K. Nickel Phosphide Catalysts Supported on SBA-15 for the Hydrodesulfurization of 4,6-DMDBT. J. Jpn. Petrol. Inst. 2010, 53, 173–177. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Zhao, H.; Lee, Y.-K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Shu, Y.; Oyama, S.T. Active Phase of a Nickel Phosphide (Ni2P) Catalyst Supported on KUSY Zeolite for the Hydrodesulfurization of 4,6-DMDBT. Appl. Catal. A Gen. 2007, 322, 191. [Google Scholar] [CrossRef]
- Oyama, S.T.; Lee, Y.-K. Mechanism of Hydrodenitrogenation on Phosphides and Sulfides. J. Phys. Chem. B 2005, 109, 2109–2119. [Google Scholar] [CrossRef]
- Oyama, S.T.; Wang, X.; Lee, Y.-K.; Bando, K.; Requejo, F.G. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and Other Techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Yun, G.-N.; Lee, Y.-K. Novel Ni2P/zeolite catalysts for naphthalene hydrocracking to BTX. Catal. Commun. 2014, 45, 133–138. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Cho, K.-S.; Lee, Y.-K. Morphology effect of β-zeolite supports for Ni2P catalysts on the hydrocracking of polycyclic aromatic hydrocarbons to benzene, toluene, and xylene. J. Catal. 2017, 351, 67–78. [Google Scholar] [CrossRef]
- You, S.J.; Park, E.D. Effects of dealumination and desilication of H-ZSM-5 on xylose dehydration. Micropor. Mesopor. Mater. 2014, 186, 121–129. [Google Scholar] [CrossRef]
- Rac, V.; Rakic, V.; Stosic, D.; Otaman, O.; Auroux, A. Hierarchical ZSM-5, Beta and USY zeolites: Acidity assessment by gas and aqueous phase calorimetry and catalytic activity in fructose dehydration reaction. Micropor. Mesopor. Mater. 2014, 194, 126–134. [Google Scholar] [CrossRef]
- Mlekodaj, K.; Tarach, K.; Datka, J.; Gora-Marek, K.; Makowski, W. Porosity and accessibility of acid sites in desilicated ZSM-5 zeolites studied using adsorption of probe molecules. Micropor. Mesopor. Mater. 2014, 183, 54–61. [Google Scholar] [CrossRef]
- Sadowska, K.; Gora-Marek, K.; Datka, J. Hierarchic zeolites studied by IR spectroscopy: Acid properties of zeolite ZSM-5 desilicated with NaOH and NaOH/tetrabutylamine hydroxide. Vib. Spectrosc. 2012, 63, 418–425. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Tailoring the mesopore structure of HZSM-5 to control product distribution in the conversion of propanal. J. Catal. 2010, 271, 88–98. [Google Scholar] [CrossRef]
- Tarach, K.; Góra-Marek, K.; Tekla, J.; Brylewska, K.; Datka, J.; Mlekodaj, K.; Makowski, W.; Igualada López, M.C.; Martínez Triguero, J.; Rey, F. Catalytic cracking performance of alkaline-treated zeolite Beta in the terms of acid sites properties and their accessibility. J. Catal. 2014, 312, 46–57. [Google Scholar] [CrossRef]
- Kenmogne, R.; Finiels, A.; Cammarano, C.; Hulea, V.; Fajula, F. Hydroconversion of n-hexadecane over bifunctional microporous and mesoporous model catalysts. Influence of pore architecture on selectivity. J. Catal. 2015, 329, 348–354. [Google Scholar] [CrossRef]
- Gackowski, M.; Taracz, K.; Kuterasiński, L.; Podobiński, J.; Sulikowski, B.; Datka, J. Spectroscopic IR and NMR studies of hierarchical zeolites obtained by desilication of zeolite Y: Optimization of the desilication route. Micropor. Mesopor. Mater. 2019, 281, 134–141. [Google Scholar] [CrossRef]
- Haouas, M.; Taulelle, F.; Martineau, C. Recent advances in application of 27Al NMR spectroscopy to materials science. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 94–95, 11–36. [Google Scholar] [CrossRef]
- Sazama, P.; Tabor, E.; Klein, P.; Wichterlova, B.; Sklenak, S.; Mokrzycki, L.; Pashkkova, V.; Ogura, M.; Dedecek, J. Al-rich beta zeolites. Distribution of Al atoms in the framework and related protonic and metal-ion species. J. Catal. 2016, 333, 102–114. [Google Scholar] [CrossRef]
- Gorte, R.J. What do we know about the acidity of solid acids? Catal. Lett. 1999, 62, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]



| Samples | Conditions | BET Surface Area (m2g−1) | Pore Volume (cm3g−1) | CO Uptake (μmol g−1) | Coke Formation d (wt.%) | Si/Al Raio e | |||
|---|---|---|---|---|---|---|---|---|---|
| Stotal | Smicro a | Smeso b | Vmicro a | Vmeso c | |||||
| β | As calcined | 609.6 | 484.2 | 125.3 | 0.24 | 0.39 | - | - | 12.66 |
| DS-β | As calcined | 588.3 | 347.4 | 240.9 | 0.15 | 0.77 | - | - | 10.47 |
| Ni2P/β | Fresh | 407.4 | 307.3 | 100.3 | 0.17 | 0.30 | 35.6 | - | - |
| Spent | 110.7 | 34.8 | 75.9 | 0.01 | 0.25 | 10.7 | 14.2 | - | |
| Ni2P/DS-β | Fresh | 392.1 | 205.3 | 186.8 | 0.12 | 0.61 | 41.3 | - | - |
| Spent | 261.5 | 87.1 | 174.4 | 0.06 | 0.58 | 32.8 | 5.7 | - | |
| Samples | Amount of Total Acid Sites a (μmol g−1) | Amount of Brønsted Acid Sites b (μmol g−1) | Fraction of Brønsted Acid Site c (%) |
|---|---|---|---|
| β | 710.0 | 315.0 | 48 |
| DS-β | 630.6 | 283.7 | 45 |
| Ni2P/β | 380.6 | 67.9 | 18 |
| Ni2P/DS-β | 517.2 | 113.0 | 22 |
| Physical Properties | PFO | |
|---|---|---|
| C (wt.%) | 87.5 | |
| H (wt.%) | 9.0 | |
| S/ppm | 70 | |
| N/ppm | - | |
| Aromatics/wt.% | Total | 77.6 |
| Mono | 31.1 | |
| Di | 43.0 | |
| Tri+ | 3.5 | |
| Distillation/K | IBP/5/10 30/40/50 60/90/95/EP | 343/388/408/ 448/488/538/ 593/848/873/- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Cho, K.-S.; Lee, Y.-K. Structure and Activity of Ni2P/Desilicated Zeolite β Catalysts for Hydrocracking of Pyrolysis Fuel Oil into Benzene, Toluene, and Xylene. Catalysts 2020, 10, 47. https://doi.org/10.3390/catal10010047
Kim Y-S, Cho K-S, Lee Y-K. Structure and Activity of Ni2P/Desilicated Zeolite β Catalysts for Hydrocracking of Pyrolysis Fuel Oil into Benzene, Toluene, and Xylene. Catalysts. 2020; 10(1):47. https://doi.org/10.3390/catal10010047
Chicago/Turabian StyleKim, Yong-Su, Kye-Sung Cho, and Yong-Kul Lee. 2020. "Structure and Activity of Ni2P/Desilicated Zeolite β Catalysts for Hydrocracking of Pyrolysis Fuel Oil into Benzene, Toluene, and Xylene" Catalysts 10, no. 1: 47. https://doi.org/10.3390/catal10010047
APA StyleKim, Y.-S., Cho, K.-S., & Lee, Y.-K. (2020). Structure and Activity of Ni2P/Desilicated Zeolite β Catalysts for Hydrocracking of Pyrolysis Fuel Oil into Benzene, Toluene, and Xylene. Catalysts, 10(1), 47. https://doi.org/10.3390/catal10010047






