An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes
Abstract
1. Introduction
2. Results
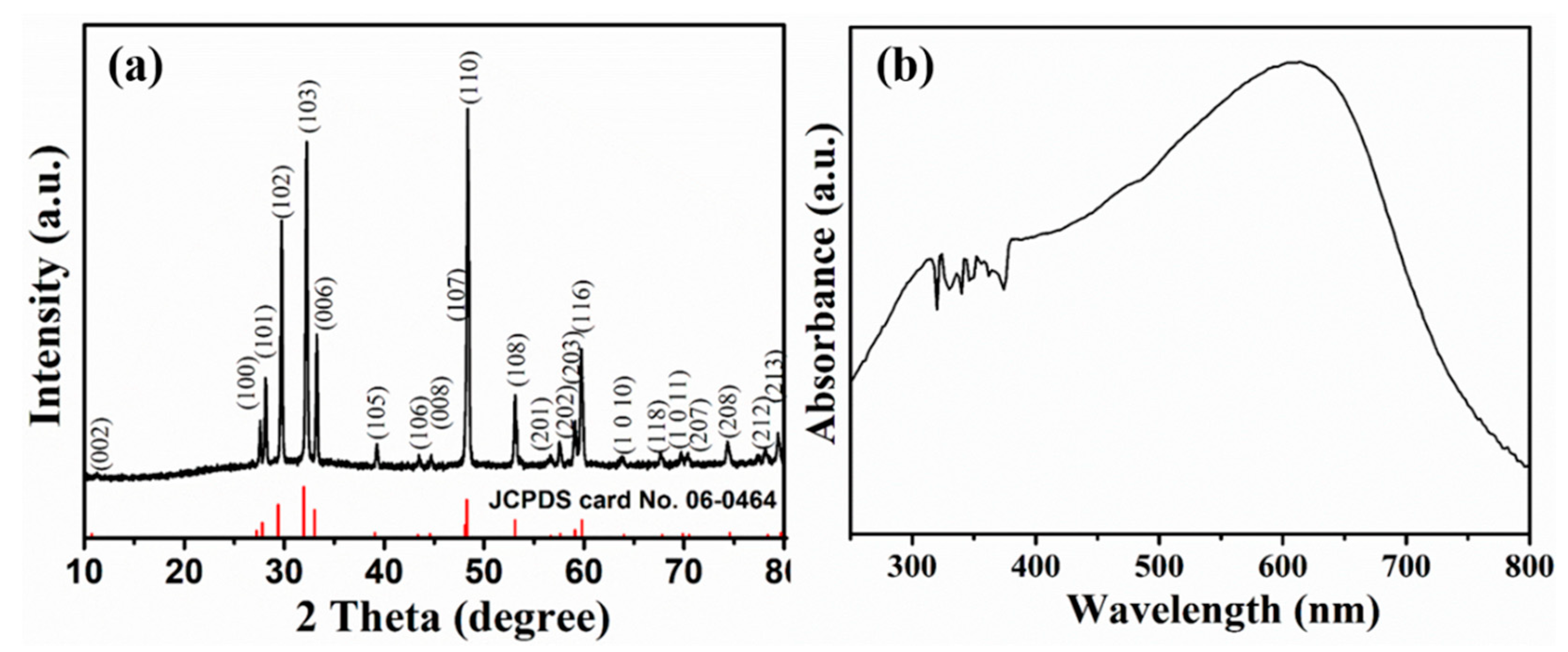
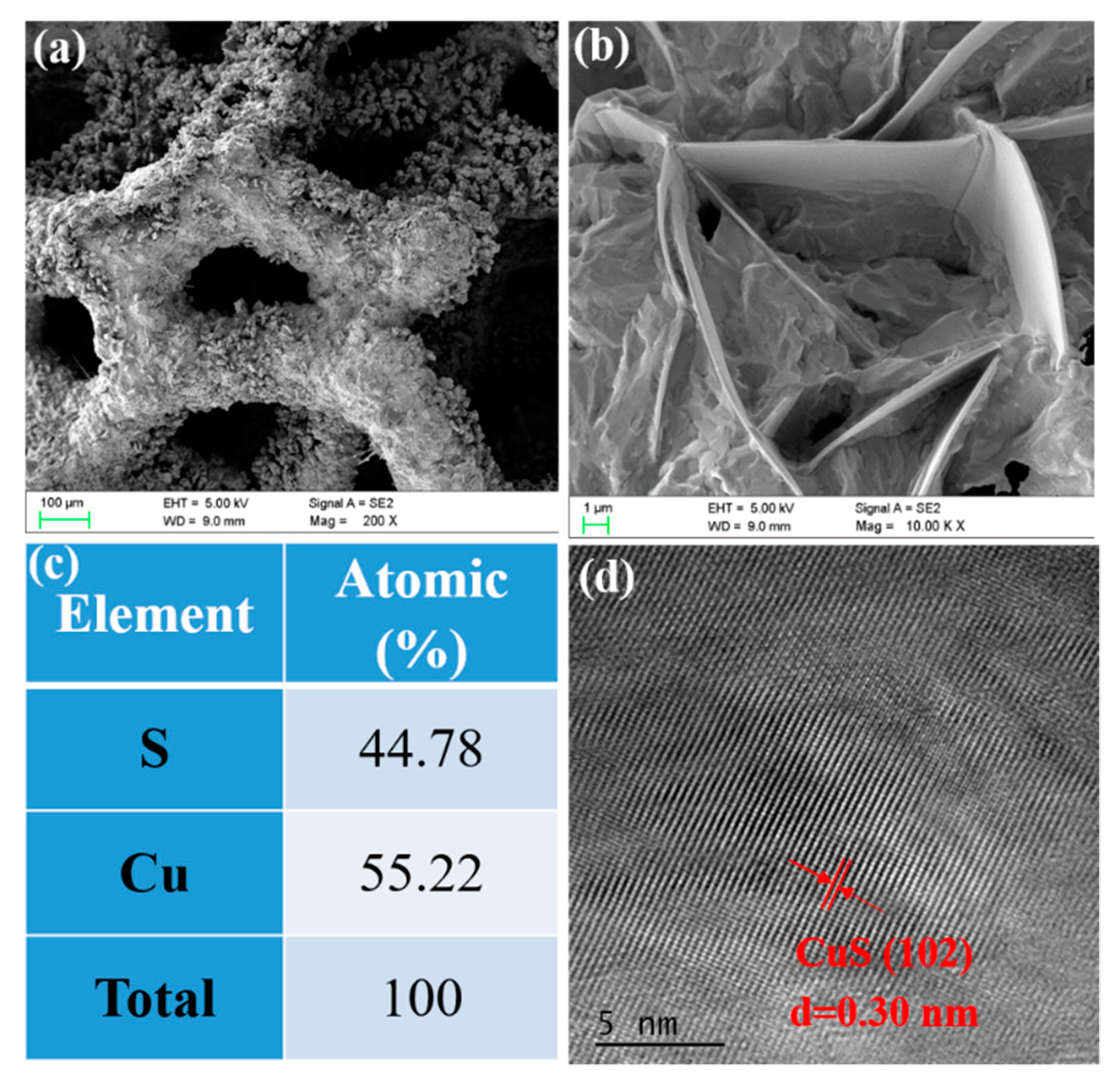
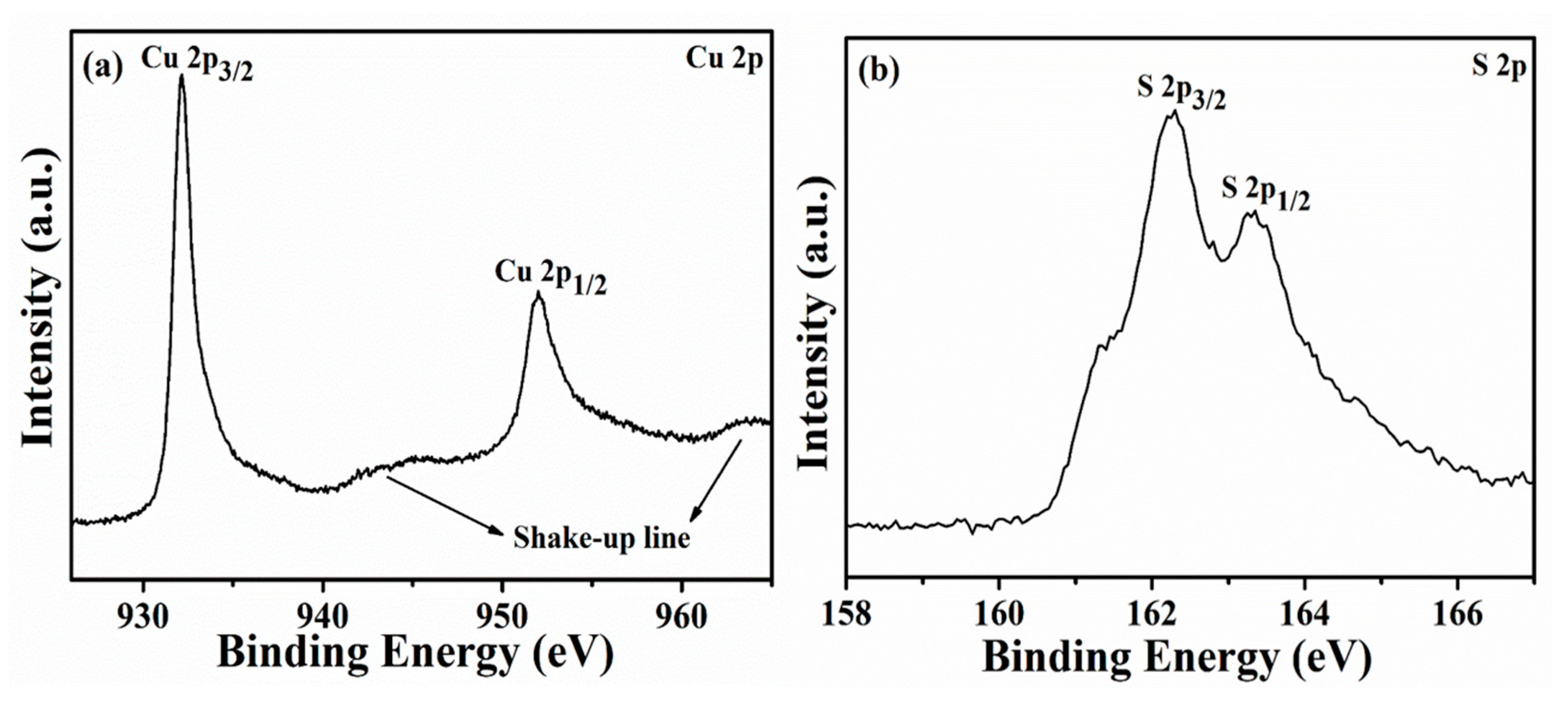
2.1. Characterizations
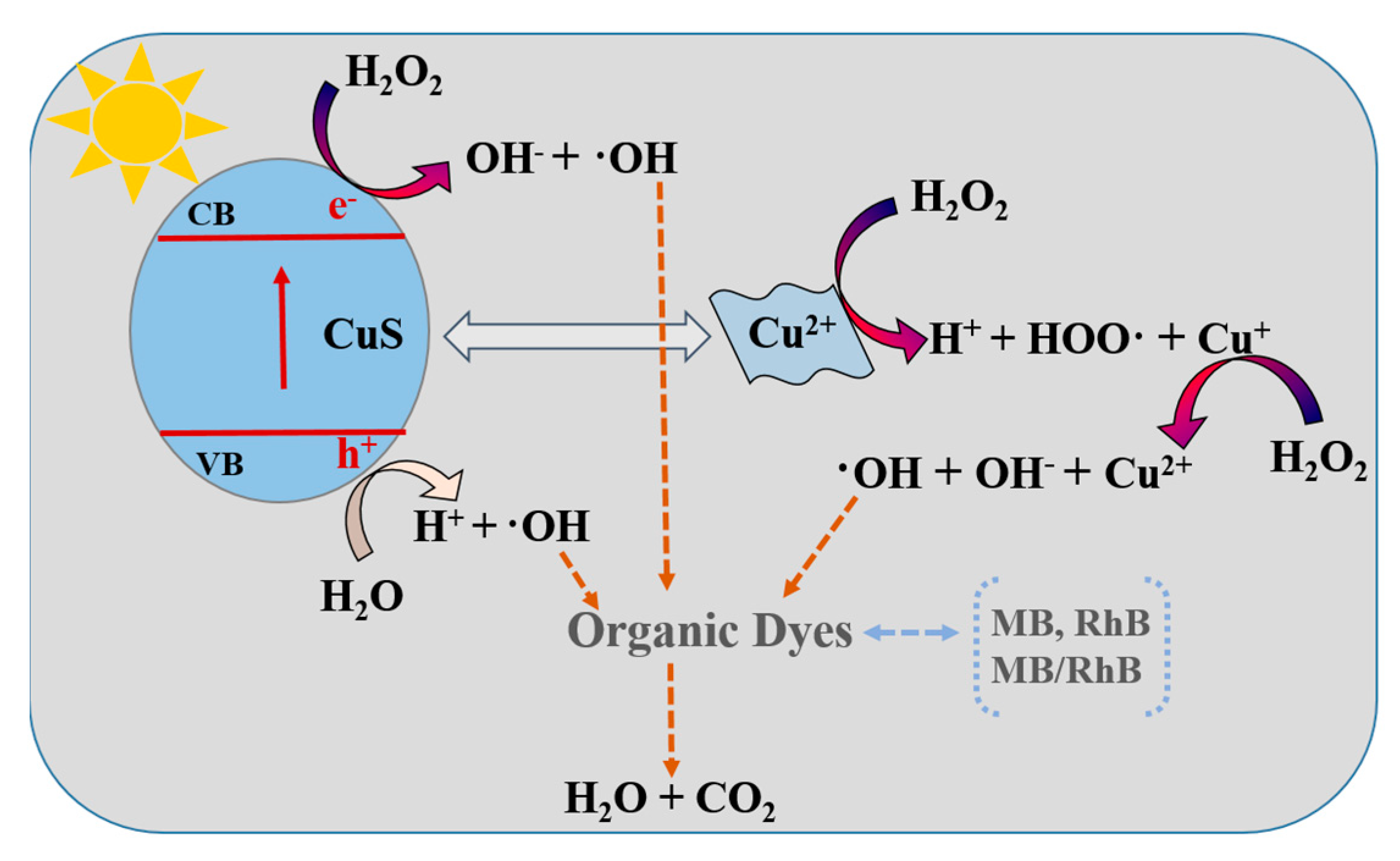
2.2. Photocatalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of 3D Hierarchical CuS Catalysts
3.3. Characterization
3.4. Evaluation of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–53. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Huo, Y.; Zhu, J. Supercritical preparation of a highly active S-doped TiO2 photocatalyst for methylene blue mineralization. Environ. Sci. Technol. 2007, 41, 4410–4414. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Sun, C.; Li, F.; Lu, G.; Cheng, H. Synergistic effects of B/N doping on the visible-light photocatalytic activity of mesoporous TiO2. Angew. Chem. Int. Ed. 2008, 47, 4516–4520. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Cu(II) oxide amorphous nanoclusters grafted Ti3+ self-doped TiO2: An efficient visible light photocatalyst. Chem. Mater. 2011, 23, 5282–5286. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Wang, Y.; Ma, W.; Zhao, J.; Rajh, T.; Zang, L. Enhanced photocatalytic degradation of dye pollutants under visible irradiation on Al(III)-modified TiO2: Structure, interaction, and interfacial electron transfer. Environ. Sci. Technol. 2007, 42, 308–314. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B: Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Qin, N.; Xiong, J.; Liang, R.; Liu, Y.; Zhang, S.; Li, Y.; Li, Z.; Wu, L. Highly efficient photocatalytic H2 evolution over MoS2/CdS-TiO2 nanofibers prepared by an electrospinning mediated photodeposition method. Appl. Catal. B: Environ. 2017, 202, 374–380. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Jiang, W.-J.; Zhang, Y.; Zhang, X.; Wan, L.-J.; Hu, J.-S. MoS2/CdS nanosheets-on-nanorod heterostructure for highly efficient photocatalytic H2 generation under visible light irradiation. ACS Appl. Mater. Interfaces 2016, 8, 15258–15266. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Khannam, M.; Vinothkumar, N.; De, M.; Qureshi, M. Hierarchical 3D NiO–CdS heteroarchitecture for efficient visible light photocatalytic hydrogen generation. J. Mater. Chem. 2012, 22, 12090–12095. [Google Scholar] [CrossRef]
- Gogoi, G.; Keene, S.; Patra, A.S.; Sahu, T.K.; Ardo, S.; Qureshi, M. Hybrid of g-C3N4 and MoS2 integrated onto Cd0.5Zn0.5S: Rational design with efficient charge transfer for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 6718–6729. [Google Scholar] [CrossRef]
- Yin, X.; Liu, J.; Jiang, W.; Zhang, X.; Hu, J.; Wan, L. Urchin-Like Au@CdS/WO3 Micro/Nano Heterostructure as a Visible-Light Driven Photocatalyst for Efficient Hydrogen Generation. Chem. Commun. 2015, 51, 13842–13845. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Pal, T. Evolution of hierarchical hexagonal stacked plates of CuS from liquid−liquid interface and its photocatalytic application for oxidative degradation of different dyes under indoor lighting. Environ. Sci. Technol. 2010, 44, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, S.; Wang, Q.; Geng, B. A facile solution chemical route to self-assembly of CuS ball-flowers and their application as an efficient photocatalyst. CrystEngComm 2010, 12, 144–149. [Google Scholar] [CrossRef]
- Tanveer, M.; Cao, C.; Ali, Z.; Aslam, I.; Idrees, F.; Khan, W.S.; But, F.K.; Tahir, M.; Mahmood, N. Template free synthesis of CuS nanosheet-based hierarchical microspheres: An efficient natural light driven photocatalyst. CrystEngComm 2014, 16, 5290–5300. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Li, H.; Wang, L.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Biomolecule-assisted, environmentally friendly, one-pot synthesis of CuS/reduced graphene oxide nanocomposites with enhanced photocatalytic performance. Langmuir 2012, 28, 12893–12900. [Google Scholar] [CrossRef]
- Li, Y.-H.; Qi, M.-Y.; Li, J.-Y.; Tang, Z.-R.; Xu, Y.-J. Noble metal free CdS@CuS-NixP hybrid with modulated charge transfer for enhanced photocatalytic performance. Appl. Catal. B: Environ. 2019, 257, 117934. [Google Scholar] [CrossRef]
- Li, Z.; Mi, L.; Chen, W.; Hou, H.; Liu, C.; Wang, H.; Zheng, Z.; Shen, C. Three-dimensional CuS hierarchical architectures as recyclable catalysts for dye decolorization. CrystEngComm 2012, 14, 3965–3971. [Google Scholar] [CrossRef]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified copper sulfide (Cu2-xS) micro-/nanostructures: A comprehensive review on synthesis, modifications and applications. Nanoscale 2017, 9, 11357–11404. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, F.; Li, W.; Chen, M.; Zhao, Y. Preparation of various kinds of copper sulfides in a facile way and the enhanced catalytic activity by visible light. J. Mater. Chem. A 2013, 1, 8616–8621. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, L.; Sun, C.; Gu, Y.; Wen, W.; Fang, X. Rose-like CuS microflowers and their enhanced visible-light photocatalytic performance. CrystEngComm 2018, 20, 6529–6537. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Y.; Liu, X.; Meng, J.; Zhang, P.; Liu, H.; Yang, G.; Li, G.; Jiang, L.; Wan, L.-J.; et al. Hierarchical nanowire arrays as three-dimensional fractal nanobiointerfaces for high efficient capture of cancer cells. Nano Lett. 2016, 16, 766–772. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Hu, F.; Wang, Q. Urchin-like CoP nanocrystals as hydrogen evolution reaction and oxygen reduction reaction dual-electrocatalyst with superior stability. Nano Lett. 2015, 15, 7616–7620. [Google Scholar] [CrossRef]
- Wei, W.; Ma, G.-H.; Hu, G.; Yu, D.; Mcleish, T.; Su, Z.-G.; Shen, Z.-Y. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. J. Am. Chem. Soc. 2008, 130, 15808–15810. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, C.; Wang, L.; Zhao, C.; Li, W.; Fung, K.K.; Ma, T.; Hagfeldt, A.; Wang, N. Ultrarapid sonochemical synthesis of ZnO hierarchical structures: From fundamental research to high efficiencies up to 6.42% for quasi-solid dye-sensitized solar cells. Chem. Mater. 2013, 25, 1000–1012. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A.L. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem. Mater. 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Cui, S.; Wei, W.; Chen, W.; Mi, L. Consecutive reaction to construct hierarchical nanocrystalline CuS “branch” with tunable catalysis propertie. Sci. Rep. 2016, 6, 30604. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jing, Y.; Park, N.; Li, C.; Bando, Y.; Wang, D. Solution synthesis of large-scale, high-sensitivity ZnO/Si hierarchical nanoheterostructure photodetectors. J. Am. Chem. Soc. 2010, 132, 15465–15467. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; He, D.; Yang, J.; Zhu, K.; Feng, X. Oriented assembled TiO2 hierarchical nanowire arrays with fast electron transport properties. Nano Lett. 2014, 14, 1848–1852. [Google Scholar] [CrossRef]
- Nagarathinam, M.; Chen, J.; Vittal, J.J. From self-assembled Cu(II) coordination polymer to shape-controlled CuS nanocrystals. Cryst. Growth Des. 2009, 9, 2457–2463. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Wu, D. Fast synthesis, formation mechanism, and control of shell thickness of CuS hollow spheres. Inorg. Chem. 2009, 48, 7099–7104. [Google Scholar] [CrossRef]
- Kundu, J.; Pradhan, D. Controlled synthesis and catalytic activity of copper sulfide nanostructured assemblies with different morphologies. ACS Appl. Mater. Interfaces 2014, 6, 1823–1834. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, L.; Zhang, G.; Dionysiou, D.D.; Li, J.; Du, X. One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr (VI). Appl. Catal. B: Environ. 2014, 144, 730–738. [Google Scholar] [CrossRef]
- Xu, X.; Lu, R.; Zhao, X.; Zhu, Y.; Xu, S.; Zhang, F. Novel mesoporous ZnxCd1-xS nanoparticles as highly efficient photocatalysts. Appl. Catal. B: Environ. 2012, 125, 11–20. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Liu, S. Ion-exchange synthesis and enhanced visible-light photoactivity of CuS/ZnS nanocomposite hollow spheres. J. Phys. Chem. C 2010, 114, 13642–13649. [Google Scholar] [CrossRef]
- Song, C.; Wang, X.; Zhang, J.; Chen, X.; Li, C. Enhanced performance of direct Z-scheme CuS-WO3 system towards photocatalytic decomposition of organic pollutants under visible light. Appl. Surf. Sci. 2017, 425, 788–795. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J. Visible light photocatalytic H2-production activity of CuS/ZnS porous nanosheets based on photoinduced interfacial charge transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef]
- Shu, Q.; Lan, J.; Gao, M.; Wang, J.; Huang, C. Controlled synthesis of CuS caved superstructures and their application to the catalysis of organic dye degradation in the absence of light. CrystEngComm 2015, 17, 1374–1380. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Hu, Y.; Jin, G.; Jiang, B.; Huang, Y. Photothermal-conversion-enhanced photocatalytic activity of flower-like CuS superparticles under solar light irradiation. Sol. Energy 2018, 170, 586–593. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zhang, B.-P.; Ge, Z.-H.; Zhu, L.-F.; Li, Y. Preparation by solvothermal synthesis, growth mechanism, and photocatalytic performance of CuS nanopowders. Eur. J. Inorg. Chem. 2014, 2014, 2368–2375. [Google Scholar] [CrossRef]
- Tanveer, M.; Cao, C.; Aslam, I.; Ali, Z.; Idrees, F.; Khan, W.S.; Tahir, M.; Khalid, S.; Nabi, G.; Mahmood, A. Synthesis of CuS flowers exhibiting versatile photocatalyst response. New J. Chem. 2015, 39, 1459–1468. [Google Scholar] [CrossRef]
- Cai, L.; Sun, Y.; Li, W.; Zhang, W.; Liu, X.; Ding, D.; Xu, N. CuS hierarchical hollow microcubes with improved visible-light photocatalytic performance. RSC Adv. 2015, 5, 98136–98143. [Google Scholar] [CrossRef]
- Ghosh, A.; Mondal, A. A simple electrochemical route to deposit Cu7S4 thin films and their photocatalytic properties. Appl. Surf. Sci. 2015, 328, 63–70. [Google Scholar] [CrossRef]
- Más, A.A.; Wei, D. Photoelectrochemical properties of graphene and its derivatives. Nanomaterials 2013, 3, 325–356. [Google Scholar]



| Photocatalysts | Reaction Solution | Time (min) | Photodegradation Degree (%) | Reference |
|---|---|---|---|---|
| CuS spherical nanoflowers | 2.5 mg L−1 RhB + 1.5 mL H2O2 | 30.0 | 90.0 | [45] |
| CuS hierarchical microflowers | 12.0 mg L−1 RhB + 1.5 mL H2O2 12.0 mg L−1 MB + 1.5 mL H2O2 | 55.0 45.0 | 95.0 94.2 | [46] |
| CuS hierarchical hollow microcubes | 10.0 mg L−1 MB + 1.3 mL H2O2 | 30.0 | 95.9 | [47] |
| 3D hierarchical CuS network | 10.0 mg L−1 RhB + 40.0 μL H2O2 10.0 mg L−1 MB + 40.0 μL H2O2 | 25.0 25.0 | ≈100.0 ≈100.0 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, N.; Wei, W.; Huang, C.; Mi, L. An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes. Catalysts 2020, 10, 40. https://doi.org/10.3390/catal10010040
Qin N, Wei W, Huang C, Mi L. An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes. Catalysts. 2020; 10(1):40. https://doi.org/10.3390/catal10010040
Chicago/Turabian StyleQin, Na, Wutao Wei, Chao Huang, and Liwei Mi. 2020. "An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes" Catalysts 10, no. 1: 40. https://doi.org/10.3390/catal10010040
APA StyleQin, N., Wei, W., Huang, C., & Mi, L. (2020). An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes. Catalysts, 10(1), 40. https://doi.org/10.3390/catal10010040





