Abstract
Ni-Ce-Zr-Oδ catalysts were prepared via one-pot hydrothermal synthesis. It was found that Ni can be partially incorporated into the Ce-Zr lattice, increasing surface oxygen species. The catalysts possess high surface areas even at high Ni loadings. The catalyst with Ni content of 71.5 wt.% is able to activate CO2 methanation even at a low temperature (200 °C). Its CO2 conversion and methane selectivity were reported at 80% and 100%, respectively. The catalyst was stable for 48 h during the course of CO2 methanation at 300 °C. Catalysts with the addition of medium basic sites were found to have better catalytic activity for CO2 methanation.
1. Introduction
Catalytic hydrogenation of CO2 has drawn considerable attention due to its potential for the production of methane or other useful hydrocarbons. For CO2 methanation, the greenhouse gas (CO2) is consumed, and converted to methane, promoting energy regeneration, and methane can be used as a clean fuel. This reaction (Rxn. 1) occurs competitively with reverse water gas shift reaction (Rxn. 2) and CO methanation reaction (Rxn. 3) as expressed below.
CO2 methanation (CDM):
Reverse water gas shift (RWGS):
CO methanation (CMM):
It was reported that CO2 methanation (CDM) could occur through CO intermediates through which CO2 had initially undergone the reverse water gas shift then was followed by CO methanation (CMM) [1,2]. With weak basic supports such as Ce-Zr oxide supports, CO2 formed carbonates on the surface followed by being hydrogenated to yield formate, and then CH4 [3,4,5]. Due to the exothermic nature of the CDM reaction, CO2 conversion is not favorable at a high-temperature operation; therefore, it would be desirable to make use of a catalyst which possesses remarkably high catalytic activity at low temperatures in the CDM reaction. Typically, Ni and specific noble metals such as Ru, Rh, and Pd are selected as active species on various supports [6,7,8,9,10,11,12,13,14,15,16]. Although Ni-based catalysts were commonly utilized due to their low cost and high activity [17,18,19,20], they were prone to suffer from the deactivation by metal sintering and carbon deposition even at low temperatures [21,22]. The nature of support also affected the state of an active phase involved in the adsorption and catalysis [23]. Apparently, Ce-Zr mixed oxides were demonstrated as good catalyst support potential for methanation because of their advantages, including good redox properties, high thermal stability, as well as resistance to sintering and coke formation [24,25,26]. Moreover, CeO2 was found to improve CO2 adsorption and active metal dispersion resulting in better catalytic activity [3]. Nonetheless, most Ni-based catalysts were not active at low temperatures [18,19,20]. According to the literature, loading a high amount of Ni could improve the catalytic activity by offering more adsorption arenas for the migration of intermediate species [27,28,29,30]; however, high metal loading via the conventional impregnation method often resulted in the low dispersion of the bulk oxide and channel blocking by the formation of bulk metal oxide clusters [31]. Therefore, the method to prepare high nickel content catalysts with good promoting effects of the support is essential.
In this work, the high Ni-loading Ni-Ce-ZrOδ catalysts were prepared via one-pot hydrothermal synthesis for which such high surface area catalysts with Ni loading of up to 71.5 wt.% were achieved. The results of their catalytic activity for CO2 methanation investigated in the temperature range of 200–600 °C were herein elucidated.
2. Results and Discussion
2.1. BET Surface Areas, XRD, XPS, H2-TPR, and CO2-TPD Analyses
The results showed that Brunauer–Emmett–Teller (BET) surface areas of the catalysts synthesized are in the range of ca. 145–189 m2 g−1 (Table 1). Noticeably, there is no directly reciprocal correlation between the surface area and Ni loading. Unlike the conventional impregnation method, the one-pot hydrothermal method provided a considerably high surface area of Ni-Ce-ZrOδ catalysts, especially for the catalysts with such high Ni loadings of 62.5 and 71.5 wt.%, the surface areas of ca. 156 and 158 m2 g−1 could be, respectively, observed. The pore volume of the catalysts still remained high, indicating that there was no channel blocking due to the formation of bulk metal oxide clusters. It was noticed that the pore diameter increases with increasing Ni content. As determined, the pore diameters range from 4.67 to 15.58 nm (Supplementary Figure S1).

Table 1.
Textural and crystal structural properties of Ni-Ce-ZrOδ catalysts.
The X-ray diffraction (XRD) patterns of the catalysts are shown in Figure 1. The results showed a typical cubic fluorite structure of CeO2 indices at 2θ = 28°, 33°, 48°, and 58° for all the catalysts with varied intensity. At lower Ni contents (below ca. 25 wt.%), the absence of anticipated peaks pertaining to Ni species is observed with a maintained peak intensity of the cubic fluorite structure, indicating dissolution of Ni in Ce-Zr mixed oxide structure. However, at higher Ni contents, the appearance of additional peaks at 37°, 43°, 63°, and 75° attributed to NiO phases is observed with a decrease in peak intensity of the cubic fluorite structure inferring the existence of free NiO species on the catalyst surface. In addition, it was found that the incorporation of Ni into the Ce-Zr mixed oxide via one-pot hydrothermal synthesis has a slight influence on the lattice parameter of pure cerium oxides. The CeO2 crystallite size was found to decrease with increasing Ni loading (Table 1). This might be because Ni ionic radius is smaller than the cerium ionic radius [32].
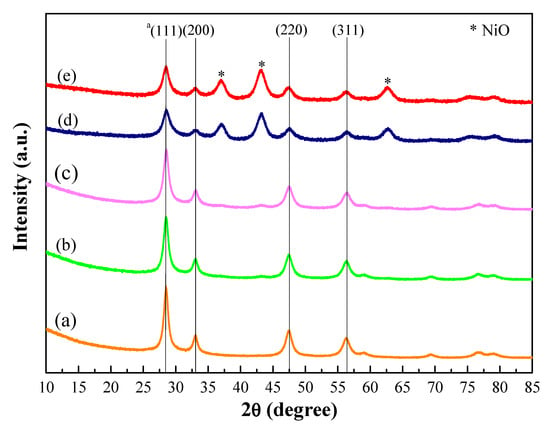
Figure 1.
XRD patterns of Ni-Ce-ZrO2 catalysts; (a) Ni0.15, (b) Ni0.45, (c) Ni0.75, (d) Ni4.0, and (e) Ni6.0; the numerical value after Ni represents the Ni/Ce molar ratio as well as a Cubic phase of CeO2.
Figure 2 shows the reducibility of the catalysts obtained by the temperature-programmed reduction (H2-TPR) technique. The results showed that at Ni loading below 25 wt.%, there was no obvious reduction peak of NiO. A small broaden peak beginning at ca. 300 °C was observed with catalysts having 15.8 (Ni0.45) and 23.8 (Ni0.75) wt.% Ni. This suggested that at lower Ni loadings, most of Ni could be incorporated into the Ce-Zr lattice [33] until it reached saturation, resulting in the increased oxygen surface reduction. Furthermore, the excess nickel might form free small NiO particles well dispersed on the surface of the Ni-Ce-ZrOδ, which cannot be detected by XRD. At higher Ni loadings, there is a reduction peak of NiO centered at ca. 490 °C, indicating that the NiO species formed a larger cluster and strongly interacted with Ce/Zr oxides. Noticeably, the peak was more intense as the Ni loading was increased.
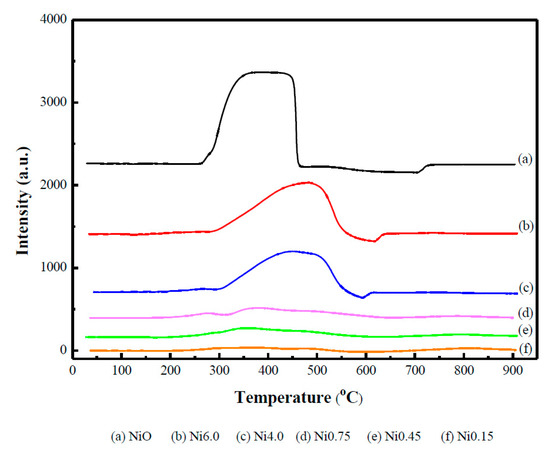
Figure 2.
H2-TPR profiles of Ni-Ce-ZrOδ catalysts.
Temperature-programmed desorption (CO2-TPD) technique was used to investigate the basic strength and basicity of the catalysts. Figure 3 shows two major CO2 desorption peaks located at 100–200 °C and 400 °C indicating weak and moderate Lewis basic sites, respectively [3]. It was found that the amount of CO2 adsorption is related to the amount of Ni loading by which the moderate basic sites were present in the catalysts containing Ni of more than ca. 25 wt.%. According to the X-ray photoelectron spectroscopy (XPS) data, as shown in Figure 4, it was found that with increasing Ni content, the amount of OH− groups and surface oxygen vacancies was found to increase (Figure 4a). The presence of the OH− groups was subjected to the desorption of CO2 at a low temperature [3], while the surface oxygen species responsible for the desorption at medium temperatures were due to the enhancement of the NiOx lattice oxygen dominating the overall composition. As evidenced, the stronger spectrum of Ni2p3/2 (Ni2+) (Figure 4b) was observed with the lessen spectra of Zr3d3/2 (Figure 4c) and Ce3d (Figure 4d). This suggested that the presence of more pronounced surface nickel species resulting from a high Ni loading by one-pot hydrothermal synthesis would give rise to a better activity. Moreover, it was found that the Ni6.0 catalyst has larger amounts of both OH− group and surface oxygen vacancy, which help promote CO2 adsorption. More details related to XPS analysis are presented in Supplementary Table S1.
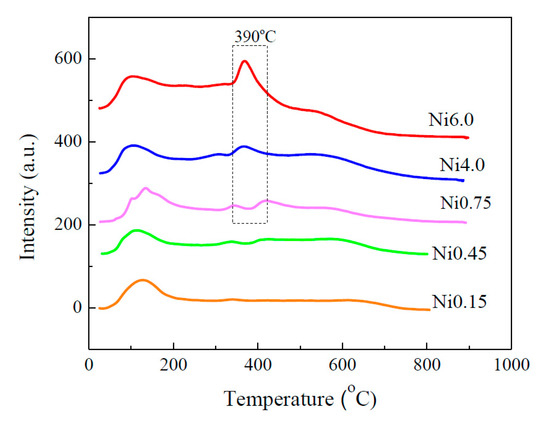
Figure 3.
CO2-TPD profiles of Ni-Ce-ZrOδ catalysts.
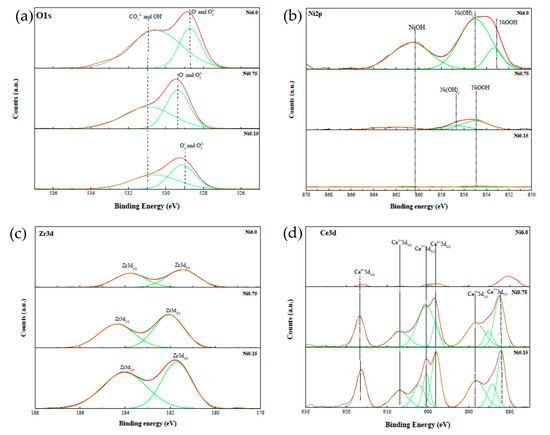
Figure 4.
XPS spectra of Ni-Ce-ZrOδ catalysts; (a) O1s, (b) Ni2p, (c) Zr3d, and (d) Ce3d.
2.2. CO2 Methanation
Figure 5 illustrates the CO2 conversion and methane selectivity for Ni-Ce-ZrOδ catalysts in the CO2 methanation. Of most catalysts, CO2 conversion is increased with increasing reaction temperature until its maximum conversion is reached, then, followed by the slight decline approaching the equilibrium conversion due to the thermodynamic limitations of this reaction. However, the maximum CO2 conversion cannot be observed with the lowest Ni loading catalyst (Ni0.15). A similar trend for CH4 selectivity can be obtained for all the catalysts as they deviated from the maximum value in an adverse exponential manner when the temperature was raised over 300 °C. Noteworthy, each of the catalysts yields different minimum CH4 selectivity at the same reaction temperature of 600 °C. At a given temperature, Ni-Ce-ZrOδ catalysts with high Ni content demonstrate better performance for CO2 methanation by which the catalytic activity is increased in the order of Ni loading: Ni0.15 < Ni0.45 < Ni0.75 < Ni4.0 < Ni6.0. Interestingly, the catalyst with 71.5 wt.% Ni loading (Ni6.0) gave the highest activity even at a low temperature (200 °C) with a turnover frequency (TOF) of 0.66 h−1 (Table 2), whereas the other catalysts yielded a minute activity at such a temperature. This is because the Ni6.0 catalyst possesses the highest amounts of OH− group and surface oxygen species, as evidenced by CO2-TPD and XPS results (Figure 3 and Figure 4). Since OH− group and oxygen species adsorbed CO2 to form bidentate formate and monodentate formate intermediates, respectively [3], the formation of these intermediate species could effectively induce the catalytic hydrogenation of CO2 molecules by consuming active H species existing on the Ni-Ce-Zr surfaces to form a C-H bond [32], thus, significantly enhancing the CO2 conversion. Moreover, increasing Ni content also provides more adsorption arenas for the migration of intermediate species, and hence, leading to a high activity [34]. Similar findings on the other supports were reported elsewhere [28,29,30,35]. According to the obtained results and analyses, it can be postulated that a decrease in the methanation activity of the catalyst would be related to the consumption of active H species. For low Ni content catalysts, this is attributed to low reaction temperature, which is not conducive to the activation of reactant H2 molecules to form active H species for the CO2 methanation reaction. Moreover, the formation of monodentate formate was nearly unattainable for these catalysts due to the lack of medium basic sites. However, these catalysts achieve high CH4 selectivity. The CH4 selectivity was attained with ca. 100% for all the catalysts at a low temperature of 200 °C. However, it was found to decrease drastically at the temperature above 500 °C because of a reverse water-gas shift reaction. It should be pointed out that at the temperature above 300 °C, low Ni content catalysts seem to promote a reverse water-gas shift reaction. Nevertheless, this could be explained that at low Ni concentrations, Ni can be incorporated into Ce-Zr lattice generating more oxygen vacancies on the surface. Since the amounts of active H species are less with low Ni content on the surface, some intermediates are desorbed as CO without the formation of C-H bonds [32]. In addition, the Ni6.0 catalyst showed no sign of deactivation during CO2 methanation at 300 °C for 48 h (Supplementary Figure S2). The amount of carbon deposition on the spent catalyst was detected by TG analysis at less than 1 wt.% (not shown), and there was also no clear evidence of carbon deposition observed by transmission electron microscope (TEM) (Supplementary Figure S3).
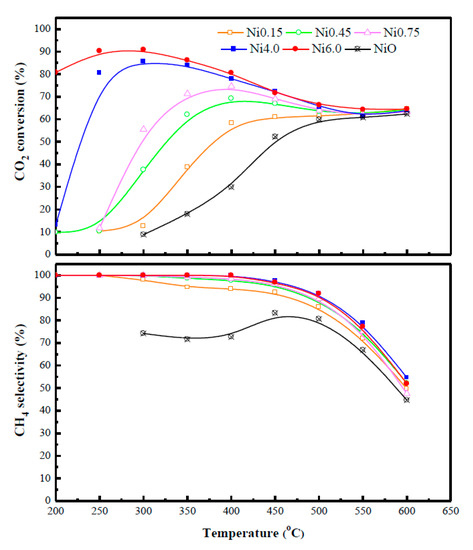
Figure 5.
CO2 conversion and CH4 selectivity of Ni-Ce-ZrOδ catalysts for the methanation of CO2.

Table 2.
Summary of the reaction results of CO2 methanation.
3. Experimental Section
3.1. Catalyst Preparation
Ni-Ce-ZrOδ catalysts were prepared via one-pot hydrothermal synthesis adopted from what reported elsewhere [32]. The molar ratio of Ce/Zr was maintained at 3:1 with the alteration of the Ni/Ce molar ratio. Typically, 0.1 M of metal salt solutions were premixed to the desired ratio of Ni/Ce/Zr. The resultant solution was then mixed with a 0.4 M of urea solution at the metal-to-urea ratio of 2:1. Then, the solution was transferred to a Teflon lining autoclave and kept at 105 °C for 50 h. The precipitate was washed with ethanol and dried at 105 °C prior to calcination at 500 °C for 4 h.
3.2. Catalyst Characterization
The catalysts were characterized for Brunauer–Emmett–Teller (BET) surface areas using a Micromeritics ASAP 2460 apparatus (Norcross, GA, U.S.A.). To ensure the accuracy of the data, the samples were outgassed at 350 °C for 6 h before being subjected to N2 adsorption. X-Ray diffraction (XRD) patterns were attained and analyzed using a Rigaku Smart Lab X-ray powder diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation. The XRD patterns were recorded with 2θ ranged from 10° to 90°. Temperature-programmed reduction (H2-TPR) and temperature-programmed desorption (CO2-TPD) were carried out using in-house equipment. The catalyst was pretreated under a flow of N2 at 400 °C for 30 min prior to running the TPR and TPD experiments and then cooled down to room temperature. For H2-TPR, a 5% H2/N2 gas was used as a reducing gas. The sample temperature was raised at a constant rate of 10 °C min−1 from room temperature to 950 °C. For CO2-TPD, CO2 as a reactant gas was introduced with a flow rate of 30 mL min−1 into a sample cell at room temperature for 1 h, then Ar with a flow rate of 30 mL min−1 was introduced for the desorption experiment using the same heating rate from room temperature to 800 °C. The amounts of H2 consumption and CO2 desorbed were determined from a TCD signal validated by appropriate calibrations. X-ray photoelectron spectroscopy (XPS) measurements were carried out on an Axis Supra (Kratos Analytical Ltd., Wharfside, Manchester, U.K.) using a monochromatic AlKα source. The surface charging effects were corrected with the C1′s binding energy value of 284.6 eV.
3.3. CO2 Methanation
CO2 methanation was carried out in a continuous flow packed bed reactor (inner diameter (i.d.) 0.6 mm) placed in a tubular furnace equipped with temperature controllers. Typically, 0.05 g of catalyst was packed between layers of quartz wool. Prior to the reaction, the catalyst was reduced in situ using H2with a flow rate of 50 mL min−1 at 500 °C for 2 h, and then the temperature was cooled to 150 °C in Ar. The CO2 methanation was carried out in the temperature range of 200–600 °C with a GHSV (gas hourly space velocity) of 10,000 h−1. The H2/CO2 molar ratio was 4:1 with a total flow rate of 50 mL min−1. The water in the product stream was condensed, and the permanent gases were analyzed using a Shimadzu GC14B gas chromatograph (Shimadzu Corp., Kyoto, Japan) equipped with a TCD (thermal conductivity detector) and installed with Alltech CTR I and Supelco Carboxen columns. The CO2 conversion and methane selectivity were calculated by the following equations.
4. Conclusions
In conclusion, Ni-Ce-ZrOδ catalysts were successfully prepared via one-pot hydrothermal synthesis with the maximum Ni loading of up to 71.5 wt.%. The obtained catalysts possess high surface areas even at high loading Ni contents and provide good catalytic activities and CH4 selectivity for CO2 methanation. It was found that Ni6.0 was the most active catalyst achieving the low-temperature CO2 conversion of ca. 80% at 200 °C and 100% CH4 selectivity. This is believed to owe to the two main basic sites, which facilitate the formation of the intermediates and the ability to activate the active H species at a low temperature. The presence of OH− groups enhances the catalytic activity at low temperatures while that of surface oxygen vacancies promotes the catalytic activity at moderate temperatures.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/1/32/s1, Supplemental Figure S1: Isotherm and pore size distribution (inset) of Ni-Ce-ZrO2 catalysts, Supplemental Table S1: XPS core level electron binding energy of Ni-Ce-ZrOδ catalysts for different Ni content, Supplemental Figure S2: The stability of Ni6.0 catalyst at 300 °C for 48 h, Supplemental Figure S3: TEM images of (a) fresh and (b) spent Ni6.0 catalysts. Reaction conditions: GHSV = 10,000 h-1, H2/CO2 = 4, reaction temperature = 300 °C and reaction time 30 min.
Author Contributions
Conceptualization, V.M., and C.L.; Methodology, V.M., C.L., and T.R.; Validation, V.M., C.L., and T.R.; Formal Analysis, V.M., N.P. (Noppadol Panchan) and N.P. (Nat Phongprueksathat); Investigation, V.M., N.P. (Nat Phongprueksathat), N.P. (Noppadol Panchan), X.G. and A.T.; Resources, V.M. and C.L.; Data Curation, V.M., N.P. (Noppadol Panchan) and T.R.; Writing—Original Draft Preparation, V.M.; Writing—Review and Editing, V.M. and T.R.; Supervision, V.M. Project Administration, V.M. and C.L.; Funding Acquisition, V.M. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Thailand (NRCT) Thai-Chinese joint project (2559-152) and NSFC-NRCT (51661145012).
Acknowledgments
V. Meeyoo would like to thank Chinese Academy of Sciences for his visiting fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tada, S.; Kikuchi, R. Mechanistic study and catalyst development for selective carbon monoxide methanation. Catal. Sci. Technol. 2015, 5, 3061–3070. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B Environ. 2012, 113, 237–249. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- De Leitenburg, C.; Trovarelli, A.; Kašpar, J. A Temperature-programmed and transient kinetic study of CO2 activation and methanation over CeO2 supported noble metals. J. Catal. 1997, 166, 98–107. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Schild, C.; Wokaun, A.; Koeppel, R.A.; Baiker, A. Carbon dioxide hydrogenation over nickel/zirconia catalysts from amorphous precursors: On the mechanism of methane formation. J. Phys. Chem. 1991, 95, 6341–6346. [Google Scholar] [CrossRef]
- Upham, D.C.; Derk, A.R.; Sharma, S.; McFarland, E.W.; Metiu, H. CO2 methanation by Ru-doped ceria: The role of the oxidation state of the surface. Catal. Sci. Technol. 2015, 5, 1783–1791. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Duan, H.; Hou, B.; Lin, Q.; Liu, X.; Pan, X.; Pei, G.; Geng, H.; Huang, Y.; et al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Ovalles, C. Catalytic carbon dioxide methanation by alumina-supported mono- and polynuclear ruthenium carbonyls. Inorg. Chem. 1986, 25, 1603–1609. [Google Scholar] [CrossRef]
- Deleitenburg, C.; Trovarelli, A. Metal-support interactions in Rh/CeO2, Rh/TiO2, and Rh/Nb2O5 catalysts as inferred from CO2 methanation activity. J. Catal. 1995, 156, 171–174. [Google Scholar] [CrossRef]
- Trovarelli, A.; Deleitenburg, C.; Dolcetti, G.; Lorca, J. CO2 methanation under transient and steady-state conditions over Rh/CeO2 and CeO2-promoted Rh/SiO2: The role of surface and bulk ceria. J. Catal. 1995, 151, 111–124. [Google Scholar] [CrossRef]
- Park, J.-N.; McFarland, E.W. A highly dispersed Pd–Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Schild, C.; Wokaun, A.; Baiker, A. Surface species in CO2 methanation over amorphous palladium/zirconia catalysts. J. Mol. Catal. 1991, 69, 347–357. [Google Scholar] [CrossRef]
- Ocampo, M.; Louis, B.; Roger, A.C. Methanation of carbon dioxide over nickel-based Ce0.72Zr0.28O2 mixed oxide catalysts prepared by sol-gel method. Appl. Catal. A 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Zhao, J.; Chou, L. Methanation of carbon dioxide over a highly dispersed Ni/La2O3 catalyst. Chin. J. Catal. 2010, 31, 21–23. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Tada, S.; Ikeda, S.; Shimoda, N.; Honma, T.; Takahashi, M.; Nariyuki, A.; Satokawa, S. Sponge Ni catalyst with high activity in CO2 methanation. Int. J. Hydrog. Energy 2017, 42, 30126–30134. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeCO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Abate, S.; Perathoner, S.; Chen, S.; Centi, G. CO2 methanation over Ni catalysts based on ternary and quaternary mixed oxide: A comparison and analysis of the structure-activity relationships. Catal. Today 2018, 304, 181–189. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Agnelli, M.; Kolb, M.; Mirodatos, C. CO hydrogenation on a nickel catalyst: I. kinetics and modeling of a low-temperature sintering process. J. Catal. 1994, 148, 9–21. [Google Scholar] [CrossRef]
- Agnelli, M.; Swaan, H.; Marquez-Alvarez, C.; Martin, G.; Mirodatos, C. CO hydrogenation on a nickel catalyst. J. Catal. 1998, 175, 117–128. [Google Scholar] [CrossRef]
- Vance, C.K.; Bartholomew, C.H. Hydrogenation of carbon dioxide on group viii metals: III. effects of support on activity/selectivity and adsorption properties of nickel. Appl. Catal. 1983, 7, 169–177. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Kim, J.H.; Seo, S.-J.; Yoo, Y.D.; Prasad, P.S.S.; Lim, H.J.; Byun, C.D. Co-methanation of CO and CO2 on the Nix-Fe1−x/Al2O3 catalysts: Effect of Fe contents. Korean J. Chem. Eng. 2011, 28, 2282–2286. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Razzaq, R.; Zhu, H.; Jiang, L.; Muhammad, U.; Li, C.; Zhang, S. Catalytic methanation of CO and CO2 in coke oven gas over Ni–Co/ZrO2–CeO2. Ind. Eng. Chem. Res. 2013, 52, 2247–2256. [Google Scholar] [CrossRef]
- Graça, I.; Gonzalez, L.; Bacariza, C.; Fernandes, A.; Henriques, C.; Lopes, J.; Ribeiro, M.F. CO2 hydrogenation into CH4 on NiHNaUSY zeolites. Appl. Catal. B Environ. 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nanocrystalline γ-Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 1346–1352. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Saad, M. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2015, 260, 757–764. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Kim, M.S.; Park, E.D. A highly loaded Ni@SiO2 core–shell catalyst for CO methanation. Appl. Catal. A Gen. 2016, 513, 98–105. [Google Scholar] [CrossRef]
- Sun, F.M.; Yan, C.F.; Wang, Z.D.; Guo, C.Q.; Huang, S.L. Ni/Ce–Zr–O catalyst for high CO2 conversion during reverse water gas shift reaction (RWGS). Int. J. Hydrog. Energy 2015, 40, 15985–15993. [Google Scholar] [CrossRef]
- Thammachart, M.; Meeyoo, V.; Risksomboon, T.; Osuwan, S. Catalytic activity of CeO2–ZrO2 mixed oxide catalysts prepared via sol–gel technique: CO oxidation. Catal. Today 2001, 68, 53–61. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Bacariza, M.; Graça, I.; Ribeiro, M.F.; Lopes, J.; Henriques, C. Insight into CO2 methanation mechanism over NiUSY zeolites: An operando IR study. Appl. Catal. B Environ. 2015, 174, 120–125. [Google Scholar] [CrossRef]
- Nie, W.; Zou, X.; Chen, C.; Wang, X.; Ding, W.; Lu, X. Methanation of carbon dioxide over Ni-Ce-Zr oxides prepared by one-pot hydrolysis of metal nitrides with ammonium carbonate. Catalysts 2017, 7, 104. [Google Scholar] [CrossRef]
- Atzori, L.; Rombi, E.; Meloni, D.; Sini, M.F.; Monaci, R.; Cutrufello, M.G. CO and CO2 Co-Methanation on Ni/CeO2-ZrO2 soft-templated catalysts. Catalysts 2019, 9, 415. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Helden, J.W.A.; Parastaev, A.; Groeneveld, E.; Pidko, E.A.; Hensen, E.J.M. Ceria-ziconia encapsulated Ni nanoparticles for CO2 methanation. Catal. Sci. Technol. 2019, 9, 5001–5010. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).