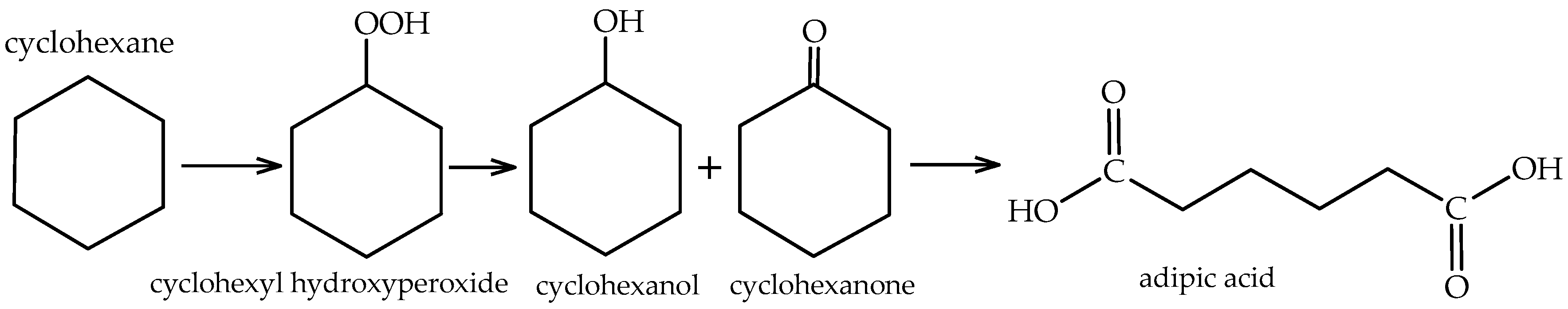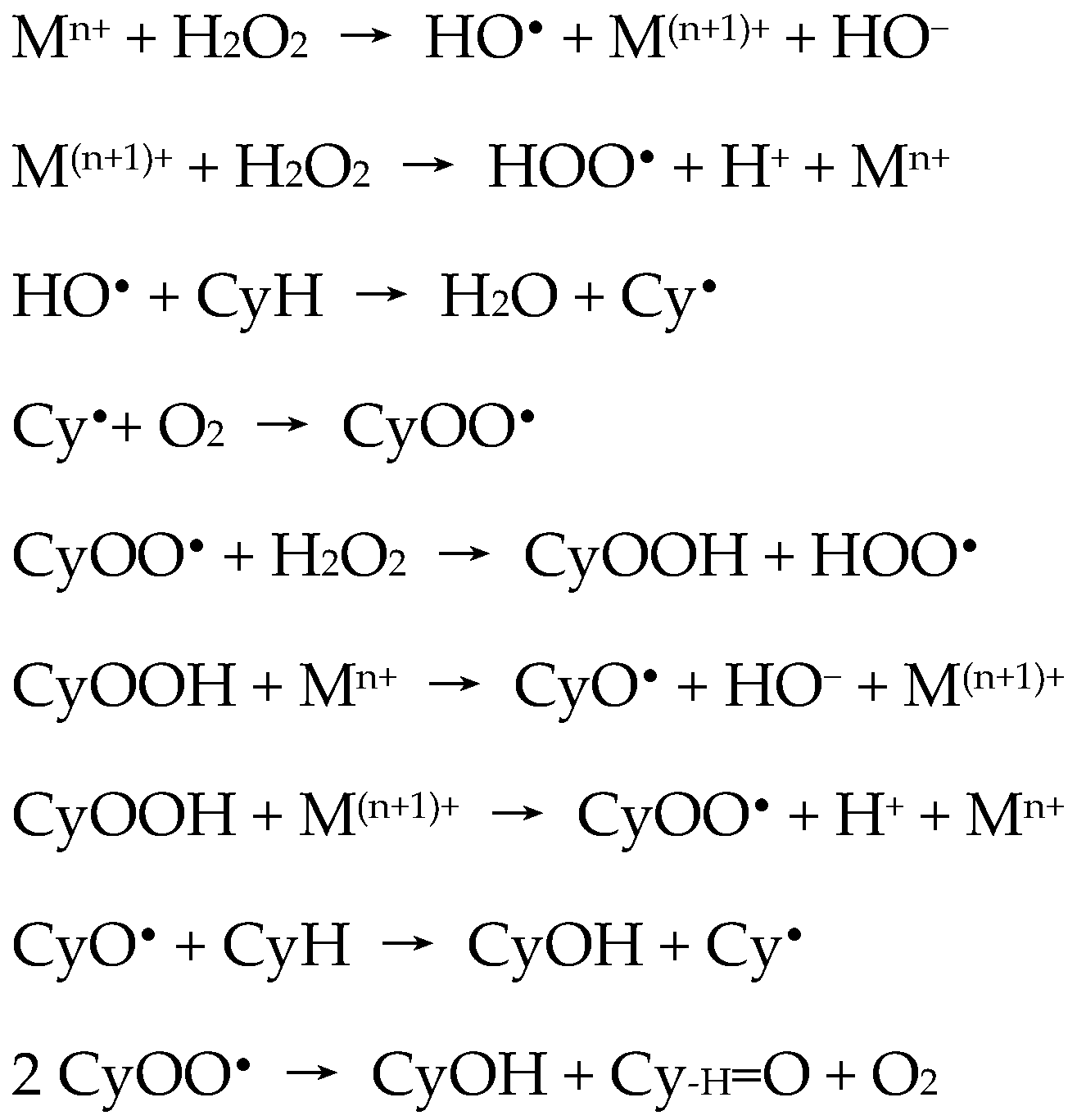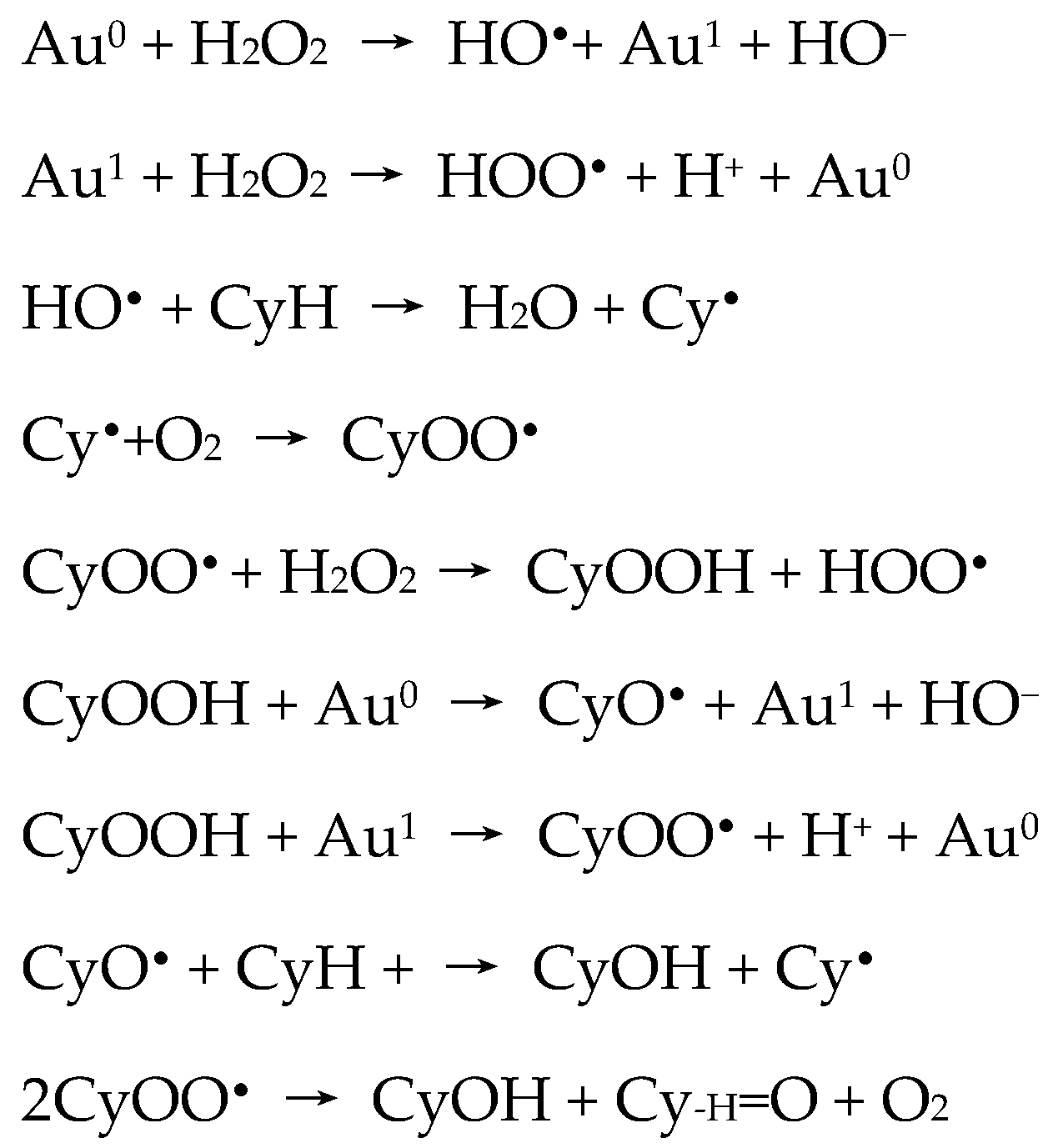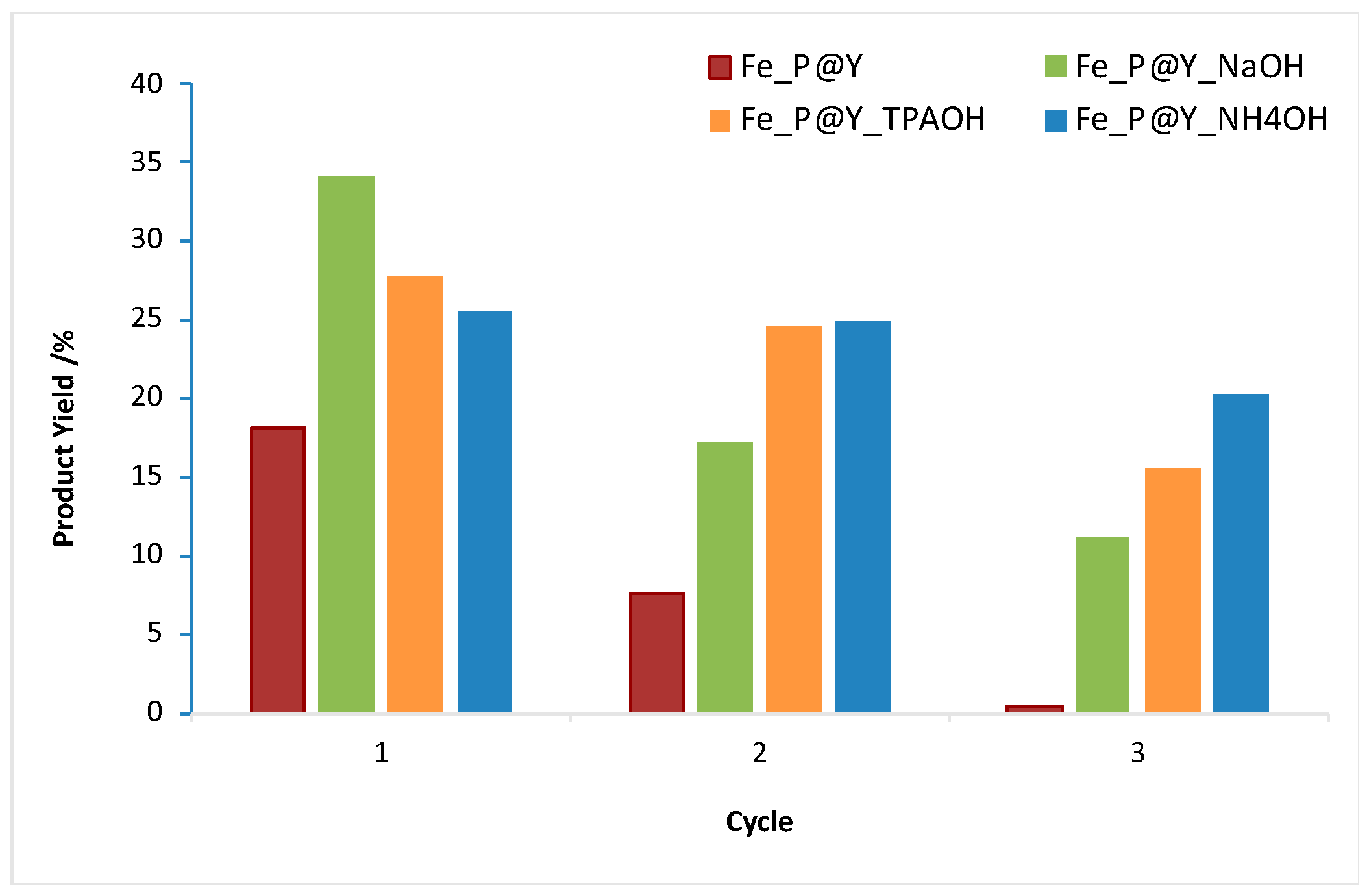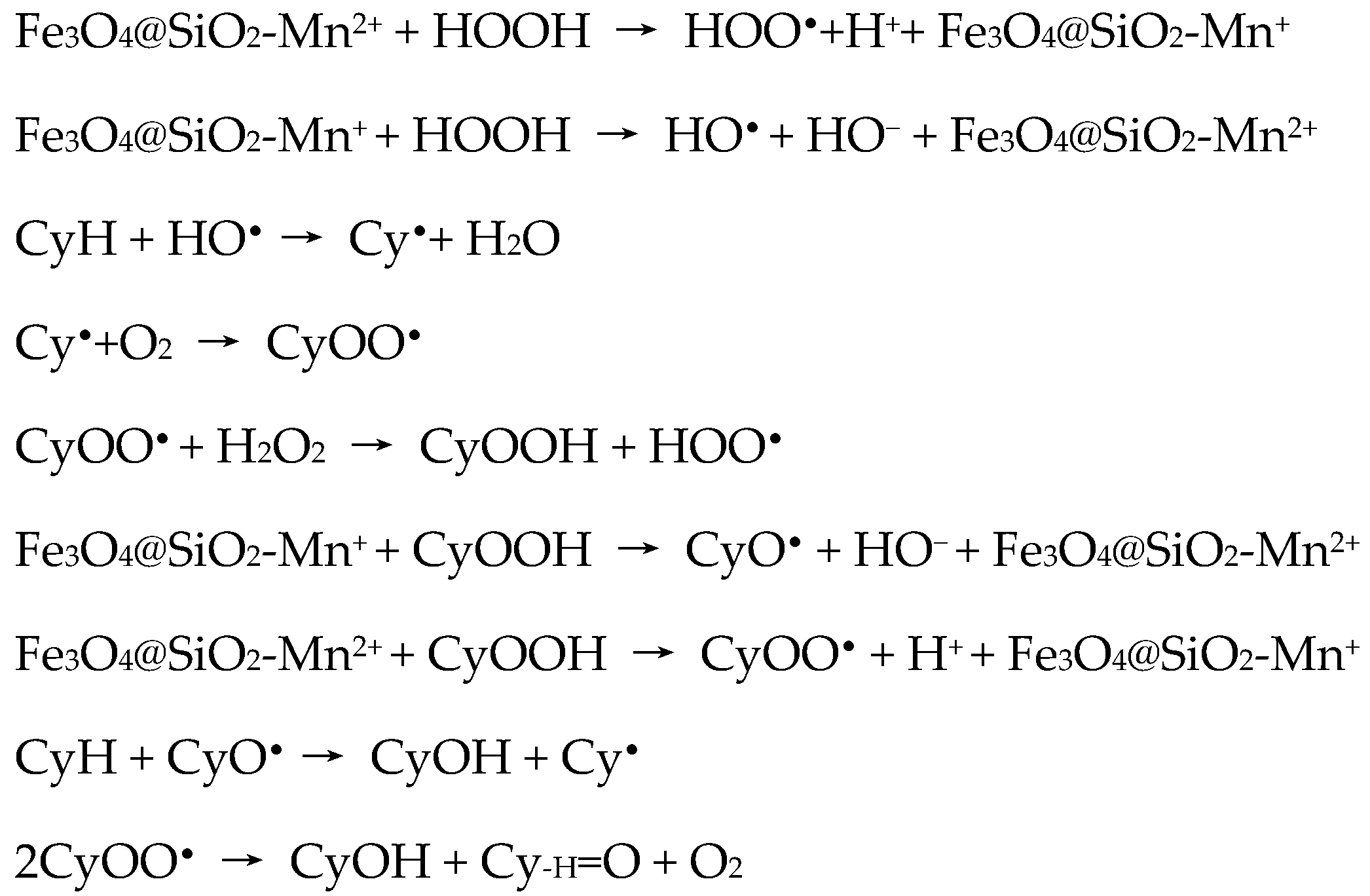5. Carbon as Supports
A large variety of carbon nanomaterials can and have been used as catalyst supports. Given the versatility of carbon, this class includes activated carbon (AC), graphite, fullerenes, carbon nanotubes (CNTs), diamond, graphene, etc., each of them presenting unique characteristics [
12,
16,
20,
21,
22,
23,
24].
Typically, these materials possess high specific surface area and porosity, excellent electron conductivity, and tunable surface chemistry, making them remarkably flexible and suitable for numerous applications, and have brought great development to the areas of energy, environment, and technology [
24,
25,
26,
27,
28]. These materials they can be considered interesting and cheaper alternatives to be used as catalysts or as catalyst supports [
21,
29] given the large surface area and excellent chemical and physical stability that provides unmatched flexibility in tailoring their physical and chemical properties. The presence of surface functional groups on the carbon material is especially relevant for catalysis, since oxygen functional groups are the most important in this perspective (
Figure 2) [
30,
31]. The careful selection of the preparation conditions, the application of secondary treatments, such as oxidation, impregnation, and heat treatments, can easily tune the type and concentration of these groups on the surface [
25,
30].
Unfortunately, porous carbon presents some from limitations arising from the microporous structure, limiting mass transfer of molecules in reactions and the number of anchoring sites for the active phase, leading to the nonuniform dispersion of the active species on the surface of the carbon and additional easy aggregation and leaching [
29,
32]. Hence, carbon materials with tunable porosity and surface chemistry are needed to obtain highly active metal/carbon catalysts that show great industrial prospects.
C-homoscorpionate tris(pyrazol-1-yl)methane metal complexes, or C-scorpionates, are one of the scarce mononuclear class of complexes active and selective enough to catalyze oxidative alkane functionalization under mild reaction conditions. In this way, these catalysts are helpful tools to overcome the drawbacks that have hindered the potential use of alkanes as feedstocks for the synthesis of valuable chemicals [
33,
34]. The following examples illustrate studies dedicated to the support of an iron C-scorpionate complex in several carbon nanomaterials, from activated carbons to carbon nanohorns. In all the cases, the aim was the recycling and reuse of the catalyst in consecutive catalytic cycles.
The fruitful experiments involving scorpionates complexes on the homogeneous catalytic oxidation of cyclohexane [
33,
35,
36] led, in 2013, Martins et al. to initiate the studies in heterogeneous catalysis with these complexes, aiming to enhance their stability and recyclability [
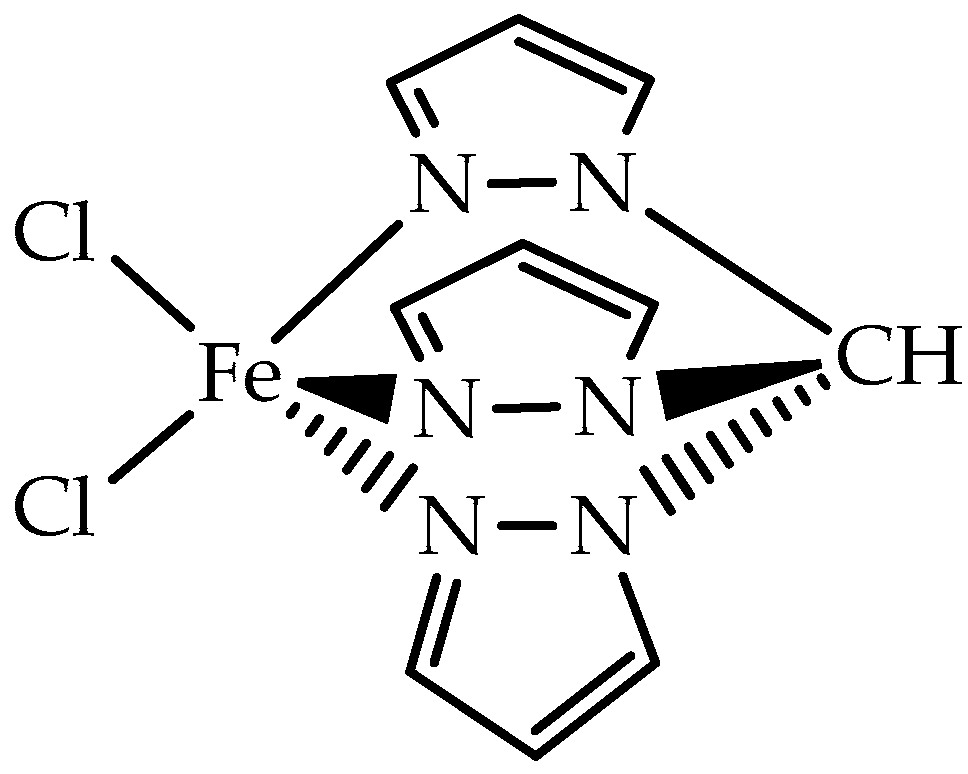
37]. In this sense, the hydrotris(pyrazol-1-yl)methane iron(II) complex [FeCl
2{κ
3-HC(pz)
3}] (pz = pyrazol-1-yl), commonly designated by [FeCl
2Tpm] (Tpm = HC(pz)
3) [
38] (
Figure 3), was immobilized on different carbon materials, as follows: AC, carbon xerogel (CX), and multi-walled carbon nanotubes (MWCNT), original (designated as -ori), treated with nitric acid (designated as -oxi), and treated with nitric acid followed by sodium hydroxide treatment (designated as -oxi-Na).
The different treatments that the original carbon materials were subjected to affected their structure and surface chemistry. In the case of AC, the acid treatment provoked a slight decrease of the surface areas and micropore volumes, probably related to the collapse of some pore wall or concerning the presence of numerous oxygen-containing surface groups that could partially block the access of N2 molecules to the microporosity. On the other hand, the -oxi treatment increased the surface area for CX and CNT. Regarding the additional -Na treatment, a reduction of the surface area for all the materials was observed. The heterogenization process was more efficient for CNT-oxi-Na, with a final Fe loading of 2 wt.%.
The peroxidative cyclohexane oxidation reactions were performed using the catalyst immobilized onto a carbon material in acetonitrile with H2O2 as 30% aqueous solution, stirred for 6 h, at room temperature. An improved catalytic performance was reported for the heterogeneous system FeCl2Tpm@CNT-oxi-Na, with overall turnover numbers (TON, moles of product obtained per mol of catalyst) and yields up to approximately 5.6×103 and 20.8% (in relation to the alkane), respectively. Overall, the achieved TONs were remarkably high, much above those reported for the homogeneous catalyst.
The heterogenized system allowed the simple recovery and reuse of the most active catalyst, that was reused for up to five consecutive catalytic cycles, while 96% of the initial activity was maintained with high selectivity to cyclohexanol and cyclohexanone.
Considering the used supports, the materials with an -oxi-Na treatment provided higher yields, which indicates that the additional surface groups originated by the Na treatment on the -oxi materials improved the activity of these catalysts. The microporosity of the AC sample was pointed out to play a detrimental role towards the catalytic activity, as the mesoporosity of sample CX provided better results. CNT have a cylindrical structure and the pores result from the free space in the bundles, which is to a certain extent equivalent to mesopores.
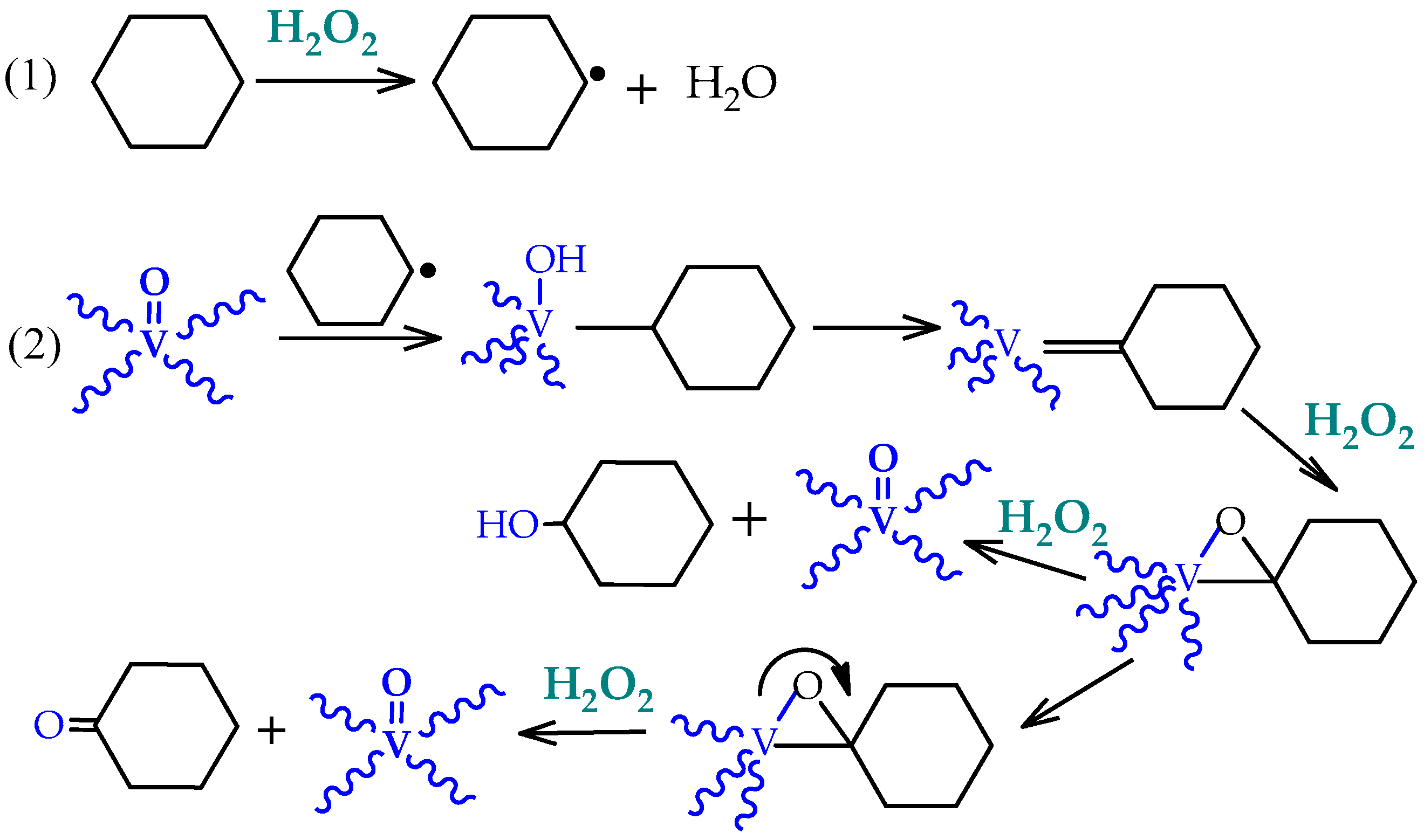
Detailed investigation of the effects of several experimental parameters, the use of radical traps, and kinetic and selectivity studies complemented with theoretical calculations [
39,
40,
41] indicated that the hydroxyl (OH
•) and cyclohexyl (Cy
•) radicals were involved in a radical-type mechanism. The authors proposed a route for the metal-catalyzed decomposition of hydrogen peroxide (Haber–Weiss mechanism) and further reactions of hydroxyl (HO
•) radical, that are represented by the following key steps of
Scheme 2.
A few years later, in 2018, the same C-scorpionate complex [FeCl
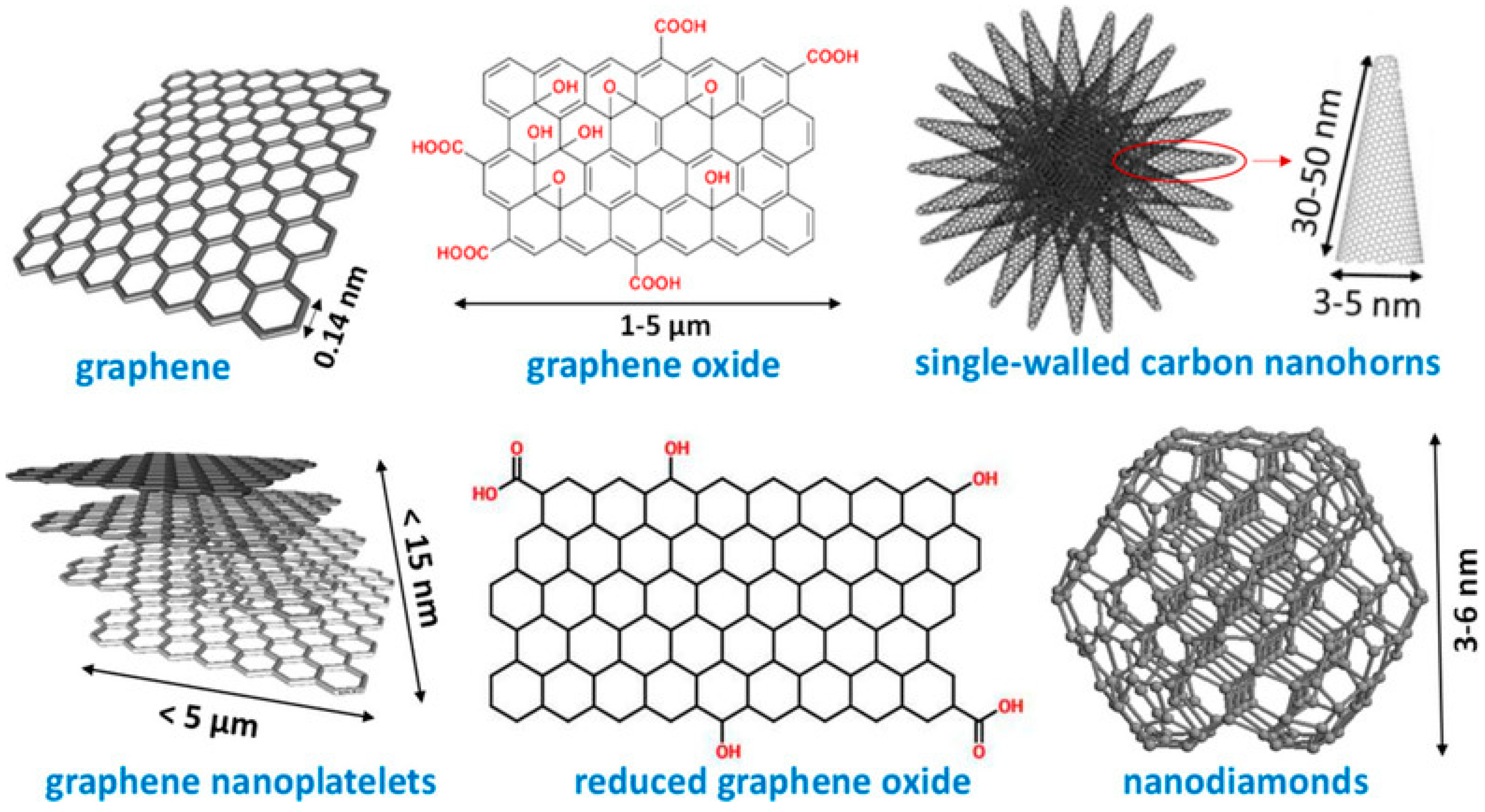
2(Tpm)] was immobilized by Ribeiro and co-workers, yet on a different set of carbon nanostructured materials, including nanodiamonds (ND), graphene nanoplatelets (G-NPL), graphene oxide (GO), reduced graphene oxide (rGO), and for the first time, nanohorns (SWCNH) (
Figure 4) [
42]. The supports disclosed surface areas that varied from 35 m
2g
−1 (G-NPL) up to 450 m
2g
−1 (for both GO and rGO), with ND and SWCNH showing in-between values of 295 and 304 m
2g
−1, respectively.
The analysis by X-ray photoelectron spectroscopy (XPS) of the carbon supports was also performed, revealing some important features of the materials. The amount of C=C bonds was higher in non-oxidized graphene (G-NPL), and not surprisingly, GO and rGO presented a higher number of oxygenated groups. The peak of HO-C=O (carboxylic acids) was larger for NDox, as expected, due to the oxidation treatment. The peaks obtained from the NDs were more intense than those observed for the other materials. SWCNH presented three types of N surface groups: Oxidized, pyrrolic, and pyridinic. G-NPL showed no peaks related to nitrogen, which is evidence of their high purity. Regarding heterogenization efficiency of the complex on the carbon materials, the obtained iron loadings ranged from 0.88% for FeCl2Tpm@rGO to 1.90% for FeCl2Tpm@SWCNH-oxi. Overall, the complex heterogenized better on the oxidized surface, due to the increased number of surface groups on the materials, that provided additional sites for the anchorage of the complexes.
The studied heterogenized systems revealed good activity with rather high selectivity to the formation of KA oil, achieving yields up to 29% for the microwave (MW) assisted oxidation of cyclohexane, using tert-butyl hydroxyperoxide (TBHP) as oxidant, for the catalyst FeCl2Tpm@SWCNH-oxi, with a TON of 288, after 1.5 h of MW irradiation at 50 °C in the presence of the additive pyrazinecarboxylic acid (Hpca). Overall, the best results were observed when using the oxidized materials, which confirmed the importance of the additional surface oxygen groups as anchorage sites.
The easy recovery and reuse of the best catalyst was also accomplished, for five consecutive cycles maintaining 90.3% of the initial activity. The use of MW irradiation was found to be advantageous for the catalytic cyclohexane oxidation, obtaining a more efficient and faster process, at lower temperatures [
43,
44], pointing out enhanced activity, however maintaining the selectivity for KA oil. Blank experiments in the absence of [FeCl
2(Tpm)], only with TBHP, confirmed the crucial role of the iron complex to effectively catalyze the reaction. Furthermore, the different pristine carbon nanomaterials did not demonstrate any catalytic activity for the MW-assisted oxidation reaction of cyclohexane.
Early this year, Andrade and co-workers reported a study on the role of nanoporous carbons as supports for the same iron scorpionate towards the peroxidative cyclohexane oxidation, under MW irradiation [
45]. The C-scorpionate iron (II) complex [FeCl
2(Tpm)] was supported on nanoporous carbon with distinct porosity: Microporous (GL50-ox, wet oxidized GL50 Norit sample, and S, a biomass-derived activated carbon prepared by chemical activation) [
46], and mesoporous (CMK-3) [
47]. The samples were characterized regarding their textural parameters obtained from the N
2 isotherm adsorption data obtained at −196 °C (
Table 1), that revealed the microporous nature of carbon S, with a pore network composed almost exclusively by micropores. Conversely, carbons GL50 and GL50-ox displayed type I+IV isotherms [
48], characteristic of microporous materials with an important contribution of mesoporosity. By analysis of the results, it can be concluded that upon the oxidation treatment, slight decreases in the textural characteristics of the material GL50 were observed.
The configuration of the isotherms remained practically unchanged after immobilization of the iron complex, and as it was expected, a downward deviation was noticed, in respect to the support. The heterogenization efficiency is also presented in
Table 1, revealing a higher Fe content on carbon S and GL50-ox. The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images allowed us to observe the morphology of the carbon materials with the immobilized complex. For sample FeCl
2Tpm@S and FeCl
2Tpm@GL50-ox, we observed a homogeneous dispersion of the complex in the carbons and also large aggregates that were heterogeneously dispersed in the ordered structure of carbon CMK-3.
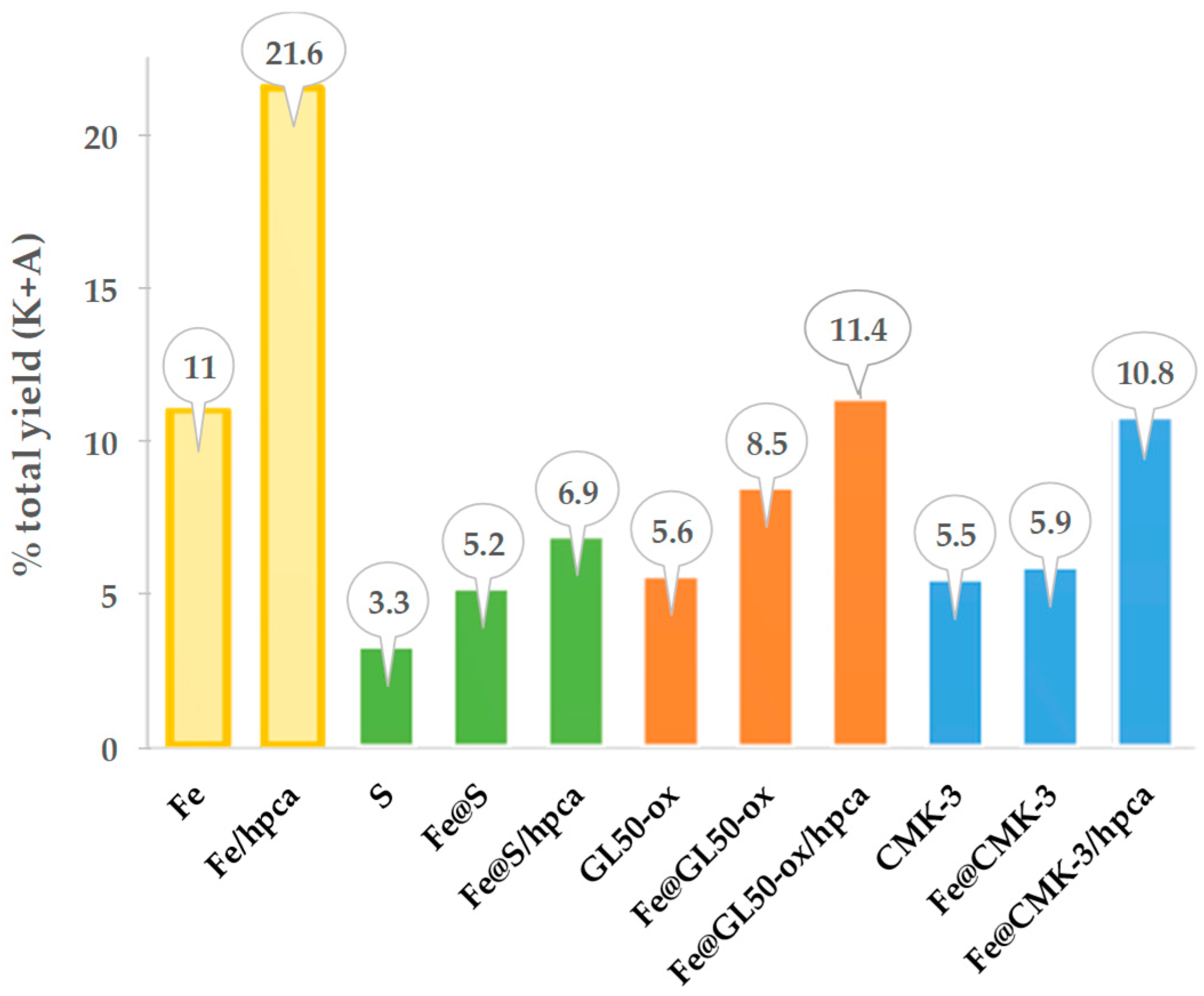
The catalytic systems studied demonstrated a good activity and quite high selectivity to the formation of KA oil from MW-assisted oxidation of cyclohexane (
Figure 5). The best catalytic results were achieved using the iron complex supported at GL50-ox and onto CMK-3, in acidic (Hpca) media, under MW irradiation at 100 °C for 2 h, leading to a yield of KA oil up to 11.4% and 10.8%, respectively. The easy recovery and reuse of the catalysts were performed for four consecutive catalytic cycles. The most important finding of this work was the significant self-catalytic, metal-free activity reported by the authors for the carbon materials GL50-ox and CMK-3, without the presence of the iron complex.
In 2013, gold catalysts were emerging as a “hot topic” of research, given their applications in several reactions of industrial and environmental importance [
48,
49]. By this time, Almeida et al. reported the immobilization of C-scorpionate gold(III) compounds [AuCl
2{κ
2-RC(R’pz)
3}]Cl (R = R’ = H, R = CH
2OH and R’= H, or R = H and R’ = 3,5-Me
2,
Figure 6) on carbon materials (AC, carbon xerogel and multi-walled carbon nanotubes) used for the peroxidative oxidation of cyclohexane to cyclohexanol and cyclohexanone, under mild conditions [
50].
Among the studied materials, the most effective catalyst was [AuCl2(κ2-TpmOH)]Cl supported on CNT-oxi-Na, for that, in a single batch, TONs up to 8 × 102 and 3 × 102, and yields up to 16% and 10%, both under heterogeneous and homogeneous conditions, respectively, were achieved. The systems with CNT-oxi-Na as support were always observed to be the most active. The catalyst was recovered from the reaction mixture performing an easy step of filtration and it was reused without great loss of activity.
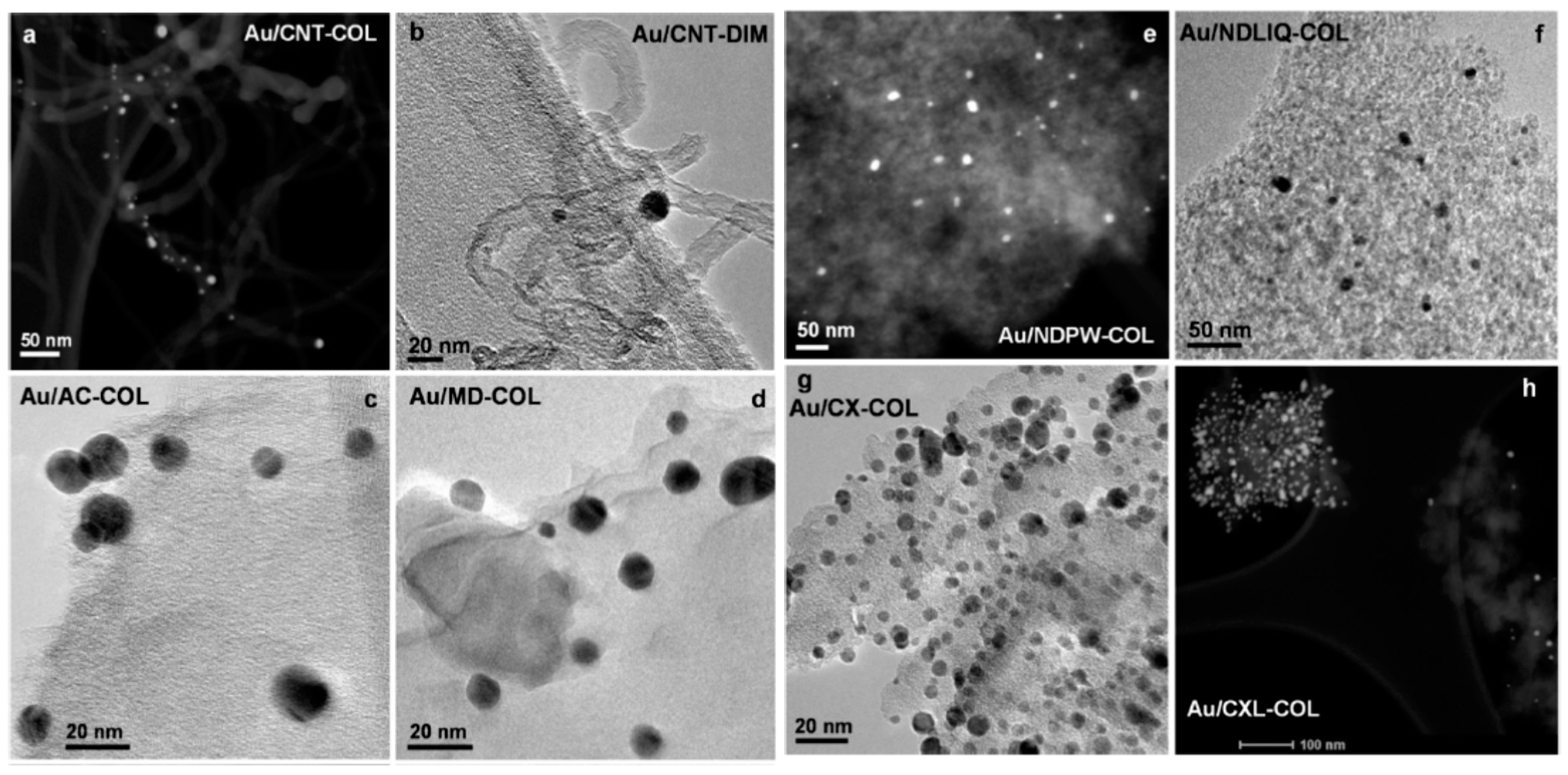
Also in 2013, Carabineiro et al. supported gold (1 wt.%) on multiple different types of carbon materials, AC, polymer-based carbon xerogels, multi-walled carbon nanotubes (MWCNT), microdiamonds, nanodiamonds (NDPW), silicon carbide, and graphite [
50], using two different methodologies, colloidal method (COL) and double impregnation (DIM).
The textural characterization of the carbon supports obtained from N
2 adsorption data revealed surface areas from 974 m
2g
−1 for AC (microporous), of ca. 600 m
2g
−1 for CX (mainly external area), of about 300 m
2g
−1 for NDPW and 257 for CNT (mainly external area), being the areas of the materials negligible. Gold deposition on CNT by COL led to a higher dispersion and lower particle size of the nanoparticles (
Table 2). The different mesoporosity of the materials does not seem to influence the gold deposition process or gold distribution. The many defects in the structure of the support seems to be beneficial for gold deposition, acting as anchoring sites, and thus, limiting the increase in nanoparticle size that happens during further agglomeration, before the gold nanoparticles attach onto the support [
51]. A larger gold nanoparticle size was obtained on the case of the microporous AC. Hence, mesoporosity seems to be more essential for the formation of relatively small gold nanoparticle sizes. The different sizes obtained for ND might be explained by a strong in situ agglomeration of the core particles upon gold formation.
Figure 7 shows high-resolution transmission electron microscopy (HRTEM) and high-angle annular dark field (HAADF) with energy-dispersive X-ray spectroscopy (EDS). The first technique allows the detection of the gold particles through the Z-contrast micrographs that are seen as bright spots, while on HRTEM images the particles can be seen as darker areas. The presence of gold was confirmed by EDS.
The most active catalyst of the series towards the catalytic oxidation of cyclohexane with aqueous H2O2 was Au@CNT-COL, achieving an overall TON of ca. 171 along with an overall yield of 3.6% for 6 h of reaction time, being comparable to the industrial process, although obtained at a much lower temperature and with rather considerable low loads of catalyst. Furthermore, a very high selectivity towards the formation of KA oil was obtained, as no traces of by-products were detected by GC-MS. The promoting effect of the additive Hpca was also observed in this work.
The authors explained the differences in activity in terms of the textural properties of the carbon support and the gold nanoparticle size. The lower sizes and mesoporosity characteristics of the support generally showed improved activity. Seemingly, the existence of surface groups was not pointed out as playing a determining role in the peroxidative oxidation of cyclohexane. Nonetheless, using the microporous AC as support also provided good results, although this material presents a large amount of surface groups. The authors stated that the peroxidative oxidation of cyclohexane should most likely proceed through a radical chain mechanism (
Scheme 3).
The recyclability of the system Au@CNT-COL was tested for six catalytic cycles, without decrease of the original level of activity after several reaction cycles with a rather high selectivity to KA oil and without catalyst leaching, excluded by atomic absorption spectroscopy analysis of the filtrate relative to the presence of Au. Additionally, when it was tested in a new reaction (by addition of fresh reagents) no oxidation products were detected.
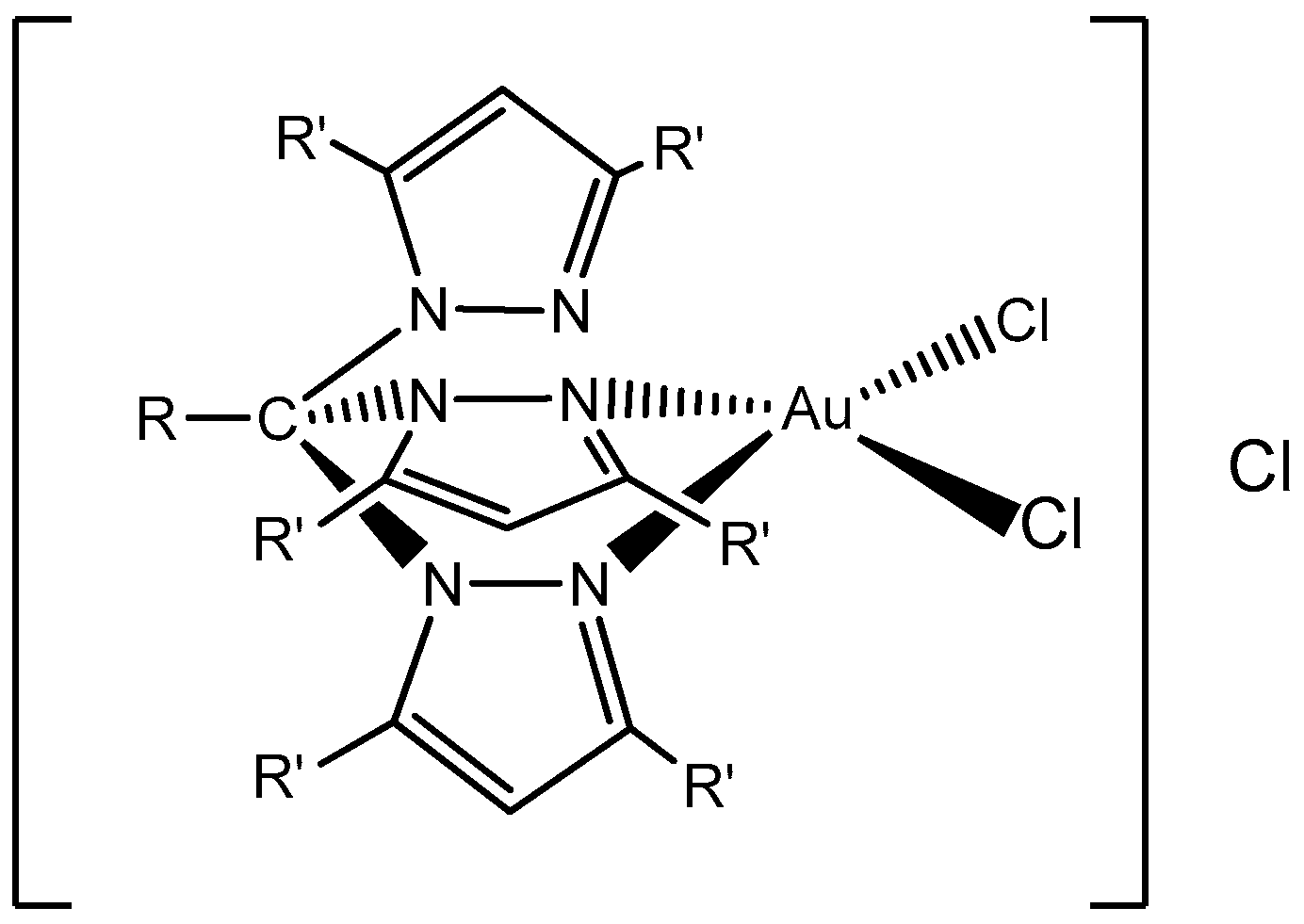
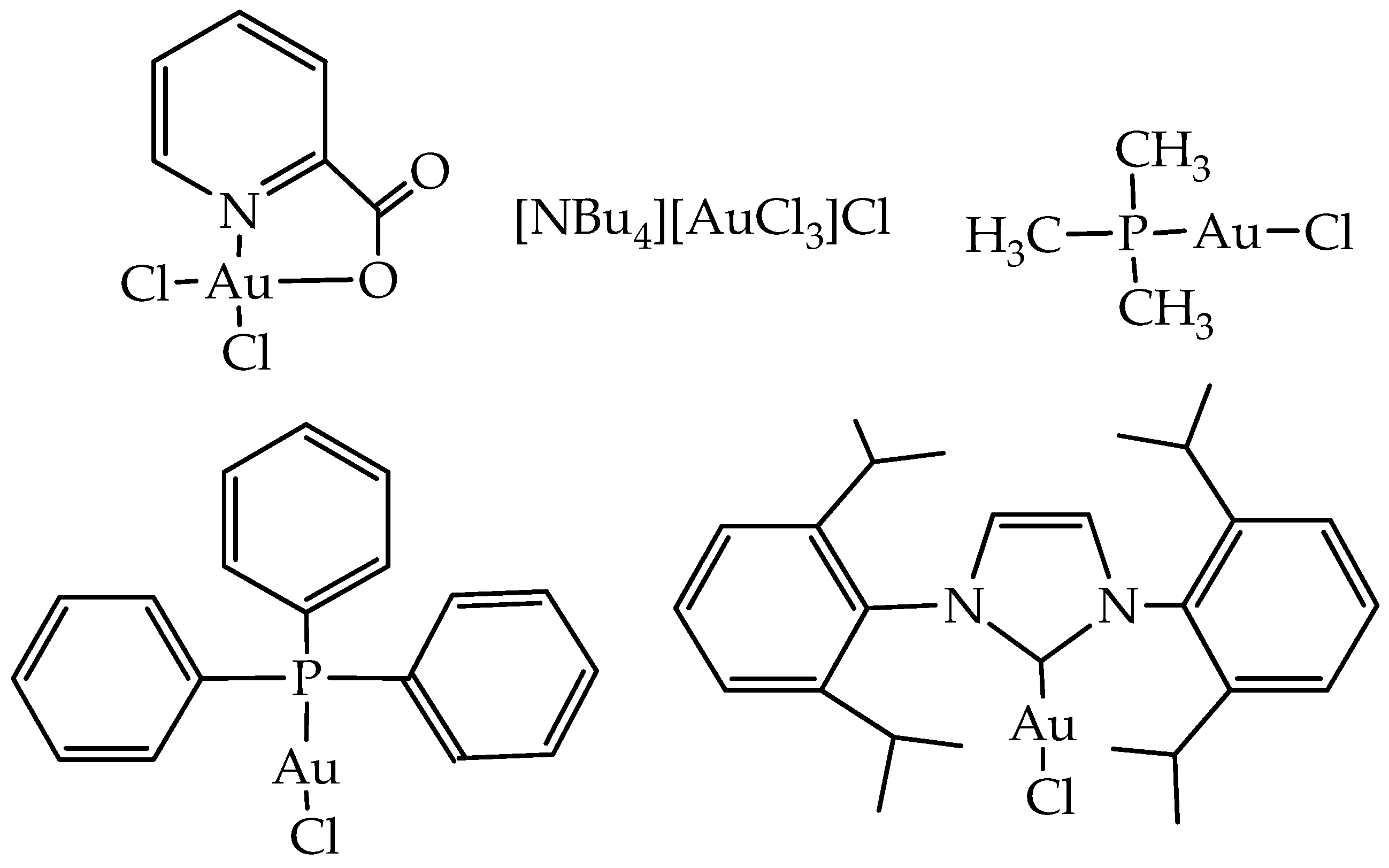
Further on, in 2018, Carabineiro and co-workers reported the heterogenization of five Au(I) and Au(III) complexes (
Figure 8) on different types of carbon materials (AC, CX, and CNT), with three different surface treatments [
52] as a new strategy for single-pot efficient oxidative functionalization of cyclohexane. In general, Au compounds heterogenized better on -ox-Na or -ox samples than on the bare supports. Regarding the textural characterization of the pristine materials, AC presented the largest surface area with an abundance of micropores, CX was mesoporous, and the porosity of CNT was due to the free space in the bundles of their cylindrical structure, presenting lower surface areas.
According to the Temperature Programmed Desorption studies, the -ox treatment greatly increased the CO and CO2 amounts. The peaks observed for the -ox-Na samples were sharper, more intense, and with a maximum at a higher temperature than the -ox materials, suggesting the conversion of phenol groups into phenolates, which are more stable. The CO2 desorption profiles of the -ox and -ox-Na supports indicates a significant increase of the carboxylic acids compared with the bare materials. The peaks of -ox-Na supports were, in general, narrower with centers slightly shifted to higher temperatures, suggesting a possible conversion of the carboxylic acids into carboxylates, increasing the stability.
The supported materials were tested for the oxidation of cyclohexane using TBHP (70% aqueous solution) as oxidant. The activity of all gold heterogenized systems were higher than that of the homogenous counterparts, regardless of the type of support and surface chemistry. In addition, the activities of supported gold complexes were also higher than that observed for gold nanoparticles (NP) on carbon supports [
50], and for those reported when using Au nanoparticles supported on different oxides (with yields varying from 1.3% to 13.5%), even though the comparison was not that easy, since H
2O
2 was also used in these systems instead of TBHP. The authors refer as positive effects of the immobilization of complexes on solid supports to confinement effect, site-isolation, cooperative effect of support, and prevention of dimerization, among others. The largest increases observed were of about 10-fold (comparison between the homogenous catalyst with that supported on CNT-ox-Na), with regard to the unsupported gold complexes. All materials were very selective to the production of KA oil and no trace of by-products were detected.
6. Zeolites as Supports
Zeolites are a class of compounds widely employed in adsorption, separation processes, and heterogeneous catalysis [
14,
53,
54]. These crystalline materials have quite unique properties, such as high surface area, intrinsic acidity ion, shape selectivity, exchange capacity, and high mechanical stability. Some advantages of zeolites over other supports rely on their mechanical stability, high porosity, and controlled acidity, making them particularly appealing to be used as hosts for encapsulation of metal complexes. However, their purely microporous character can be a drawback in certain applications, owing to diffusion limitations due to the restricted access of those molecules to the porous structure. Zeolites are not, therefore, widely used as supports for large active species when compared with other mesoporous. The immobilization is then typically confined to the external surface, given its microporosity resulting in limiting protection of the guest molecule and uptake.
To tackle this disadvantage, several approaches have been attempted to enhance accessibility and molecular transport in these materials [
55]. These methodologies include the use of zeolites with hierarchical porosity, with both micro- and mesoporosity, efficiently combining the intrinsic properties of zeolites with the presence of mesopores; post-synthesis treatments, like dealumination and desilication, result in the generation of mesoporosity in the zeolite, and also in the enlargement of the existent micropores, that in this way can be interpreted as a porous structure with a certain hierarchy [
56]. In the last years, the number of publications concerning the characterization and potential applications of desilicated zeolites has increased [
57], although the application of hierarchical zeolites as support materials for transition metal complexes for oxidation reactions is somewhat less described.
In 2008, Salavati-Niasari et al. reported the preparation of complexes [M(salophen)] and [M(H
4salophen)], (salophen=N,N-bis-(salycilidene)-1,2-phenylenediamine; H
2[H
4salophen]=2-({2-[(2-hydroxybenzyl)amino]anilino}methyl)phenol), (M = Mn(II), Co(II), Ni(II) and Cu(II)) encapsulated in the nanopores of zeolite-Y [
58]. The synthesis involved the exchange of transition metal(II) ions with sodium of NaY followed by reaction of metal-exchanged zeolite-Y (M(II)-NaY) with H
2salophen and H
2[H
4salophen] in the molten state. The new nanocomposite materials were characterized by several techniques, including spectroscopic methods as DRS, FTIR and UV/VIS, BET technique, and conductometric and magnetic measurements. The encapsulation of the metal complex resulted in a lower surface area and adsorption capacity of the zeolite. The catalytic activity of the zeolite complexes was evaluated for the oxidation of cyclohexane with H
2O
2. Zeolite-encapsulated tetrahydro-salophen copper(II) complexes were found to be more active than the corresponding cobalt(II), manganese (II), and nickel(II) complexes for this reaction. The catalytic properties of the complexes seemed to be influenced by their geometry and by the steric environment of the active sites. The encapsulated catalyst systems, [M(H
4salophen)]-NaY, were more active than the corresponding neat complexes, [M(H
4salophen)]. The activity of cyclohexane oxidation decreased in the series [Cu(H
4salophen)]-NaY > [Co(H
4salophen)]-NaY > [Mn(H
4salophen)]-NaY > [Ni(H
4salophen)]-NaY. The best result was reported for [Cu(H
4salophen)]-NaY, with a conversion of 53.8% and high selectivity to KA oil. The increase of cyclohexane conversion from 10.6% to 53.8% compared to Cu(II)-NaY with [Cu(H
4salophen)]-NaY points out that the existence of ligand increased the catalytic activity by a factor of 5. The recycle ability of [Cu(H
4salophen)]-NaY was tested in the catalytic oxidation of cyclohexane. The material proved to be stable enough to be reused without loss in catalytic activity, therefore indicating the presence of the complex in the nanopores of the zeolite-Y. No evidence for leaching of metal or decomposition of the catalyst complex was observed during the catalytic reaction and no metal could be detected by atomic absorption spectroscopic measurement of the liquid reaction mixture after each catalytic reaction. The IR spectrum of the solid catalyst after reuse was also identical to the fresh catalyst.
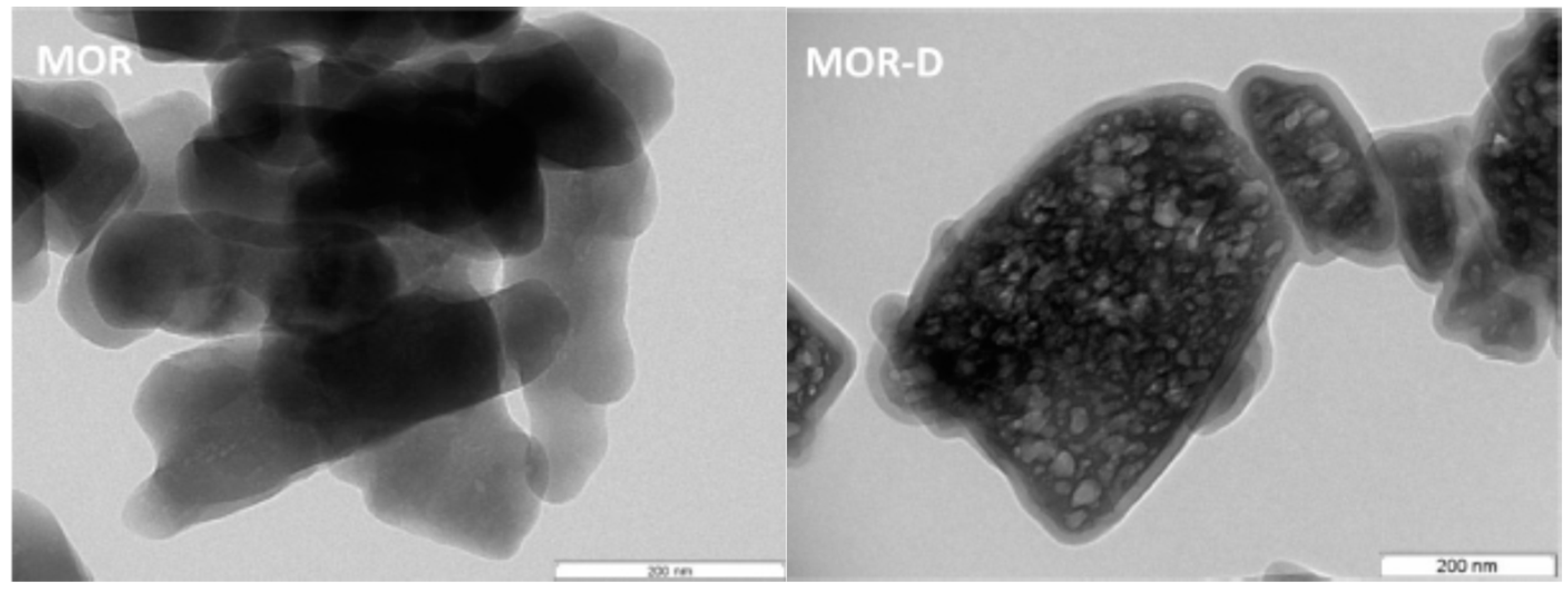
In 2013, Martins et al. immobilized the scorpionate complex [FeCl
2Tpm] on a commercial (MOR) or desilicated (MOR-D) zeolite [
59], for the catalytic oxidation of cyclohexane with hydrogen peroxide. The metal content of the immobilized complexes was quantified by ICP, being of 0.28% of on FeCl
2Tpm@MOR-D MOR-D support, whereas in FeCl
2Tpm@MOR was shown to be of 0.40%. The alkaline treatment performed on zeolites led to some loss of surface groups, affecting the interaction of the metal complex with the zeolite, with a decrease in crystallinity, as was the case of the conditions used to prepare MOR-D. TEM micrographs for MOR and MOR-D samples are presented in
Figure 9. The original MOR zeolite shows the presence of solid dense crystals, whereas corroded areas at the crystal external surface and also lighter zones, due to the perforation of the crystals, can be observed as a consequence of the basic treatment.
Regarding the textural parameters, the desilication of MOR promoted an accentuated development of mesoporosity along with a small decrease of the volume correspondent to micropores (
Table 3). This fact shows that the mesoporosity resulted mainly from the corrosion of the external surface of the crystals. In addition, the decrease of the microporous volume also indicates the occurrence of intracrystalline mesoporosity. A considerable decrease on all the textural parameters was observed as a consequence of the complex immobilization on the zeolitic supports.
FeCl2Tpm@MOR-D provided a remarkable catalytic activity (TON up to 2.90 × 103) with an overall yield of 38% for the oxidation of cyclohexane using hydrogen peroxide (30% aqueous solution) as the oxidizing agent, in a slightly acidic medium (in the presence of Hpca), at room temperature and 10 h of reaction.
A rather high selectivity towards the formation of KA oil was observed by the systems under study, since no traces of by-products were detected by GC-MS analysis of the final reaction mixtures. Furthermore, in the absence of Fe or hydrogen peroxide, no alkane oxidation products were obtained. The authors correlated the high catalytic activity of FeCl2Tpm@MOR-D with the textural parameters of the support, given that, after immobilization, the Fe complex must be in the intercrystalline mesoporosity of the modified zeolite. The desilication procedure originated a strong enhancement of the accessibility of the reactant molecules, which consequently led to a higher catalytic activity.
MOR-D/Fe could be easily recovered and reused, exhibiting 54% of its initial activity, while maintaining the selectivity. After the first catalytic run, a decrease of about half of the initial content of Fe was determined for FeCl2Tpm@MOR-D, which indicated the occurrence of leaching during the catalytic reaction or catalyst recovery process, also pointing out a weak type of electrostatic interaction between the complex and the support.
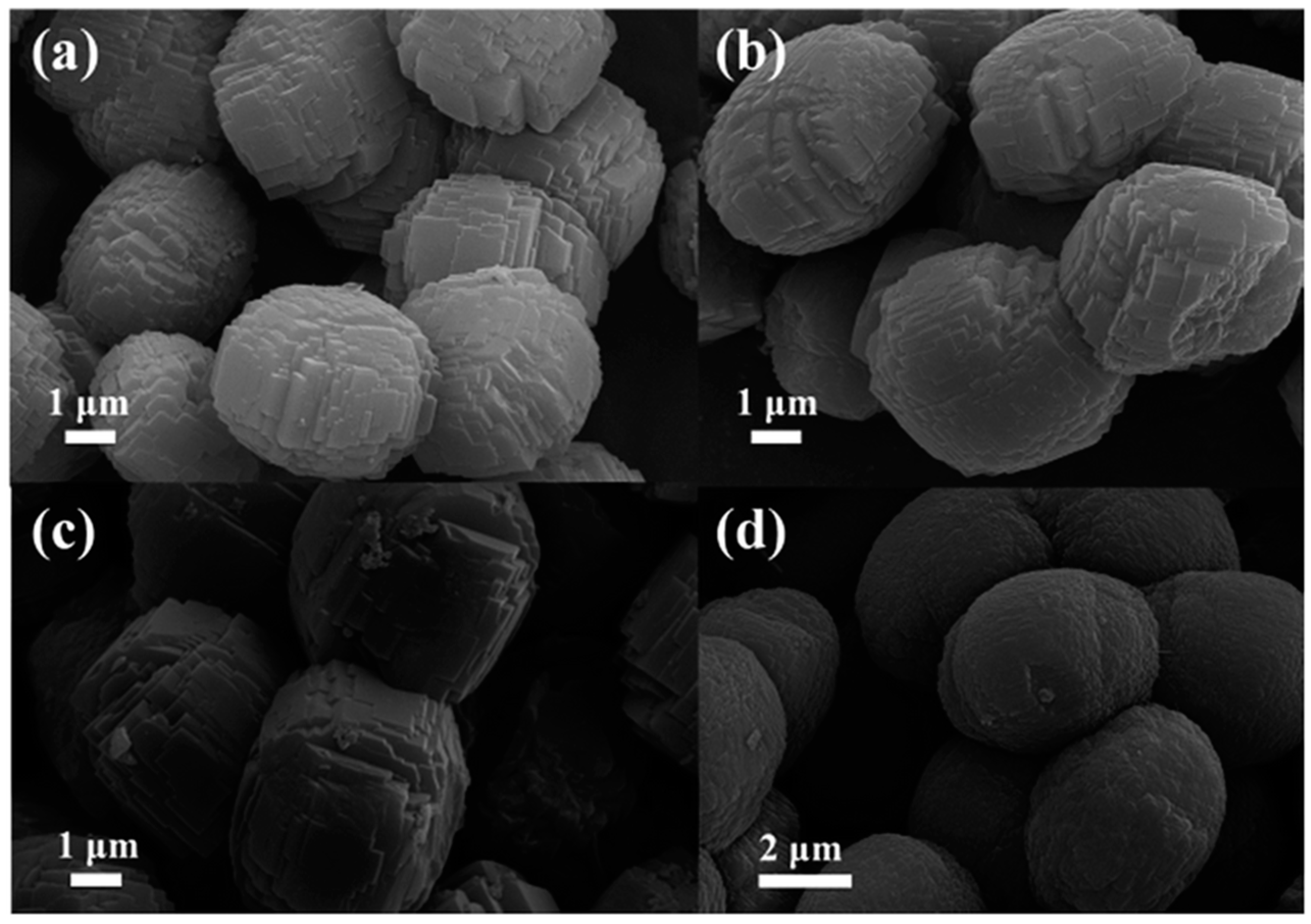
In 2017, Niu et al. reported the preparation of Mn-containing ZSM-5 materials that performed as active and selective catalysts for the mild oxidation of cyclohexane. The authors synthesized a series of ZSM-5 zeolites functionalized in situ with different amounts of Mn via a one-pot hydrothermal approach followed by calcination for the removal of the structure directing agent, tetrapropylammonium hydroxide, TPAOH [
60]. From the analysis of the SEM images (
Figure 10), it is possible to observe that the morphology of the pristine synthesized ZSM-5 samples consisted of microellipse spheres formed of irregular blocks. As it can be observed, the sizes of the Mn-ZSM-5 samples become larger (4–6 mm) with the increasing content of Mn, resulting in the growth acceleration of the parent ZSM-5 via impurities (Mn ions).
The catalytic results obtained for Mn-ZSM-5 towards the mild oxidation of cyclohexane with hydrogen peroxide in liquid phase varied in terms of conversions from 19.53% to 30.66% and regarding selectivities to KA oil, from 94.11% to 97.41%, according to different amounts of Mn4+ ions incorporated in the structure. The best results were obtained for the 2% Mn4+ doped ZSM-5, while the pristine ZSM-5 revealed little catalytic activity towards cyclohexane oxidation.
The stability of the as-prepared Mn-ZSM-5 was demonstrated through the performance of recycling catalytic tests up to 6 times. The conversion of cyclohexane and selectivity of KA oil remained above 20% and 92% for Mn-doped ZSM-5, without leaching, which seems to confirm, according to the authors, the successful substitution of Al3+ by Mn4+ in the inner structure of ZSM-5, that occurred during the hydrothermal synthesis. The decrease of the catalyst activity in the fifth usage cycle was attributed to the n(CN) stretching frequencies of the adsorbed complexes associated with the bridging hydroxyls on Mn-ZSM-5 that most likely led to the blocking of the active sites. The calcination of the catalyst at 550 °C for 6 hours allowed it to recover its best performance, as observed on the first cycle. Furthermore, the stability of Mn-ZSM-5 for the oxidation of cyclohexane with H2O2 in acetonitrile was confirmed by FTIR, since the characteristic peaks of the material were maintained, even after several cycles.
Later, in 2018, Van-Dúnem et al. accomplished the immobilization of the complex [FeCl
2(Tpm)] in the parent and hierarchical zeolite Y [
61]. The authors used the incipient wetness impregnation method and also a modification treatment through a surfactant intermediated methodology using various bases (NH
4OH, NaOH, or TPAOH). This methodology consisted of providing different methods with the aim to control hierarchical porosity as the combination, for instance, of a base with a surfactant.
The selection of the experimental conditions (concentration, pH, time, and temperature) led to local silica dissolution and to a rearrangement of the zeolite subunits that were released into an ordered mesostructured, resulting in an appearance of ordered mesopores within the zeolite crystalline structure. Regarding the textural parameters of the samples, upon alkaline treatment, it was possible to observe that the microporosity was preserved to a large extent (
Table 4), in agreement with the high crystallinity values obtained by XRD data. On the other hand, the development of larger porosity appeared to be dependent on the base used in the modification procedure. The immobilization of the complex led in all the cases to a small decrease of the characteristic microporosity of the zeolitic structure. When considering the effect of the immobilization on larger porosity the authors observed that for samples FeCl
2Tpm@_Y and Fe@Y_NaOH the values of porosity were practically identical to those of the supports, indicating that the complex must be dispersed on the outer surface of the crystals, thus blocking some pore openings. In the case of the other catalysts, FeCl
2Tpm@Y_NH
4OH and FeCl
2Tpm@Y_TPAOH, the results indicate that at least a fraction of the complex will be in the supermicropores and narrow mesopores.
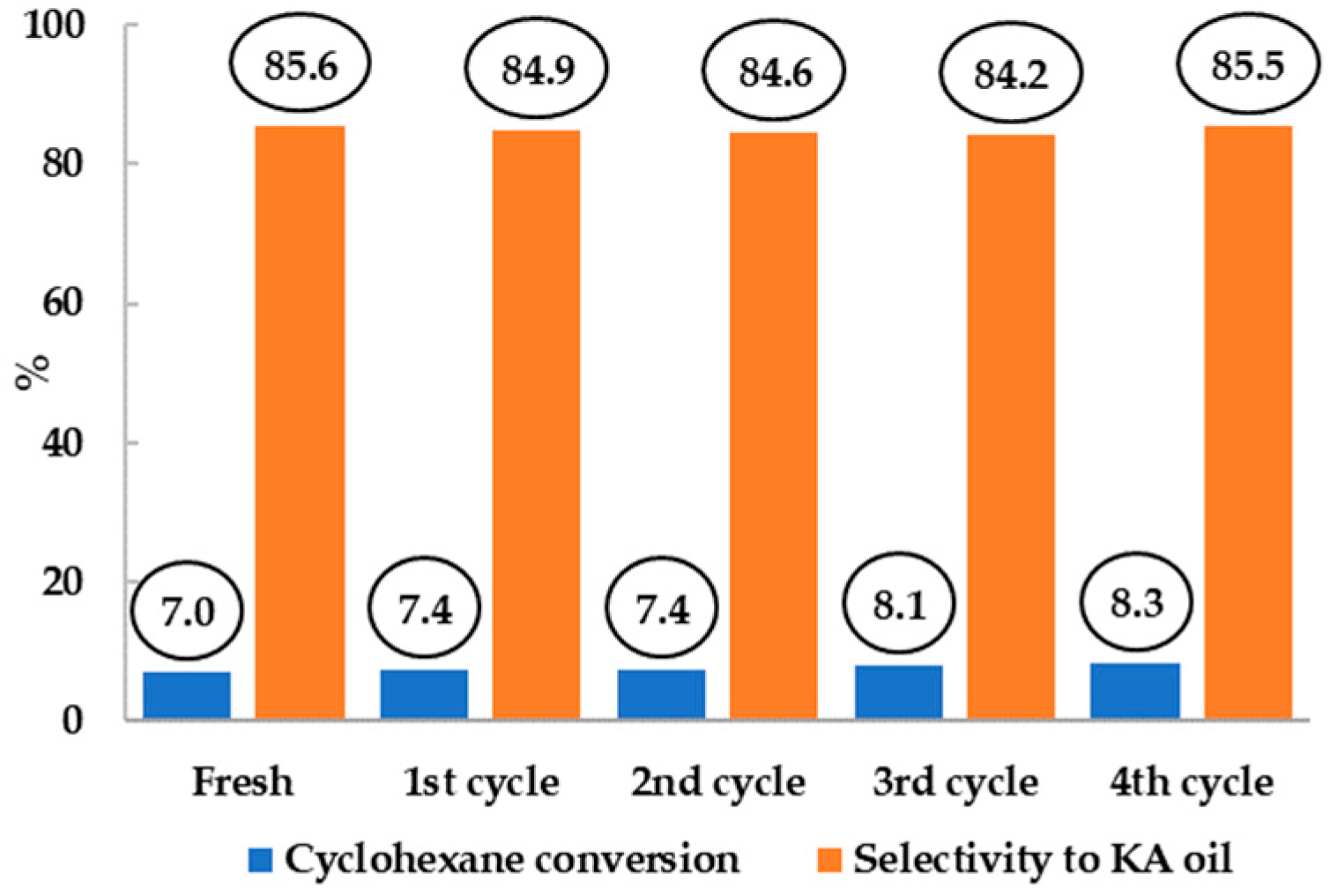
The catalytic performance and recyclability of the immobilized Fe complex was evaluated towards the oxidation of cyclohexane with hydrogen peroxide. The hybrid materials have led to a maximum KA oil yield of 34% and presenting turnover numbers (TONs) up to 271. According to the authors, the use of hydrogen peroxide was preferred to TBHP, as the latter affords much lower KA oil yields (13.8% for Fe@Y_NaOH) with low selectivity (diols detected by GC-MS analysis) under identical conditions.
The catalysts were recovered and recycled in three consecutive cycles (
Figure 11). The recycling behavior of the catalysts seemed to be related to the porosity differences observed in the modified mediated basic treatment samples. When using zeolite Y and Y_NaOH, complex anchorage occurred predominantly at the outer surface of the supports, resulting in high leaching after the first catalytic cycle. On the other hand, when NH
4OH and TPAOH were used during the zeolite treatments it resulted in a higher recyclability, especially in the case of NH
4OH-treated zeolite.
XPS analysis revealed an important increase of carbon and nitrogen content as well as a significant decrease of silicon and oxygen contents after reuse for Fe@Y_NH4OH, most likely due to the presence of adsorbed species on the catalysts surface. Regarding the content of Fe, the data obtained for the fresh catalyst showed that there was not a homogenous dispersion of the complex in the solid matrix, and that it is preferentially situated in the external surface of the crystals. The reuse of the sample in the catalytic oxidation reaction led only to a small decrease of the surface Fe content, after the third catalytic cycle. Fe 2p XPS spectra were performed to gain a deeper insight on the iron oxidation states. The predominance of the Fe2+ oxidation state was observed, which is in accordance with the regeneration of the initial oxidation state of the catalysts through the well-known redox Weiss reactions that are involved in the mechanism of cyclohexane oxidation, thus, explaining the catalytic performance of this material.
In the present year, another work covering this topic was reported, involving the immobilization of a B-scorpionate complex, the hydrotris(3,5-dimethylpyrazol-1-yl)borate dioxido-vanadium(V) complex [VO
2{HB(3,5-Me
2pz)
3}] (pz=pyrazol-1-yl) on a modified hierarchical MOR zeolite through a surfactant mediated technology [
62], obtaining three catalysts designated as V@MOR (the parent zeolite, V@MOR_NaOH, and V@MOR_TPAOH).
X-ray diffraction patterns obtained for the pristine and modified zeolitic supports revealed that no important structural changes occurred as a consequence of the applied treatments. The alkaline + surfactant treatments promoted some loss of crystallinity when compared with the parent material. No significant changes were detected on the X-ray diffraction patterns after the complex immobilization when compared with the respective supports. Regarding the textural characterization, the parent MOR sample exhibited type I isotherm in accordance with its microporous nature. As expected, the alkaline treatments promoted the development of a mesopore network, resulting in type I+IV isotherms, revealing a significant change in the textural characteristics of the samples. After immobilization, the isotherms of the samples obtained did not present any changes with respect to those before immobilization of the complex.
The catalytic activity of V@MOR_NaOH was evaluated for the oxidation of cyclohexane to KA oil using as oxidant aqueous TBHP, at room temperature, in a slightly acidic (Hpca) medium. The V loaded zeolitic material performed as an efficient catalyst for this reaction, and a maximum KA oil yield of 52% was obtained with TONs up to 6.2 × 102. The KA oil yield obtained in this study was considerably higher than that achieved by the C-scorpionate iron(II) complex [FeCl2{κ3−HC(pz)3}] discussed above. The stability of V@MOR_NaOH allowed its recovery at the end of the catalytic cycle and reuse in consecutive ones, and also a significant improvement (from 24.1% to 51.8%) of the KA oil yield was achieved. Recycling studies of this catalyst were performed up to four cycles, being the first decrease (18%) on the oxidation yield observed, without considerable leaching of the V complex. This decrease was most likely due to some adsorption phenomena that can cause diffusional constraints, restricting the access of the reactants to the complex anchored at the mesoporosity of the material.
7. Mesoporous Silicas as Supports
Silica-based mesoporous materials, such as SBA-15, ZSM-5, MCM-41, MCM-48, or mesoporous SiO
2, and the hexagonal mesoporous silica (HMS) have been widely used as catalyst supports [
4,
18,
19,
63]. The potentialities of these mesoporous materials to be used as supports in reactions like hydrogenation, alkylation, and oxidation of numerous substrates have been recognized [
64]. The major advantages of these hybrid mesoporous materials rely on features such as their extremely high surface areas and free accessibility of their porosity networks [
64,
65].
Hamdy and co-workers synthesized cobalt-containing mesoporous TUD-1 with different Si/Co ratios through a direct hydrothermal treatment method, Co-TUD-1, and evaluated its catalytic performance in the liquid-phase oxidation of cyclohexane, using TBHP, in solvent-free conditions [
66]. TUD-1 is a well-defined mesoporous (siliceous) oxide, presenting a sponge-like structure with tunable porosity that delivers a high substrate accessibility. Moreover, the synthesis of TUD-1 is cost-effective and environmentally friendly, as it is surfactant-free [
67]. This mesoporous material was thoroughly characterized regarding its structural and textural features. XRD patterns indicated that Co-TUD-1 is a mesostructured material, and a shift to large angles at higher Co-loading was observed, with a slight decrease in the peak intensity. Elemental analysis indicated that most of the Co cations were incorporated into the final solid product. Concerning texture, type IV adsorption isotherms were reported, confirming their mesostructured character with a narrow pore size distribution. Upon Co loading, the apparent surface areas did not vary significantly, although a decrease in the volume and pore size diameters was observed. This finding also confirmed that Co was incorporated into the framework of TUD-1. The characteristics of the Co-TUD-1 samples, resulting from the analysis of several techniques, are displayed in
Table 5.
The catalytic performance of the Co-doped samples revealed higher catalytic efficiency in samples with low Co-loading and, therefore, most likely isolated Co(II) active sites than in samples with high Co content and nanoparticles or bulk Co3O4 integrated in the TUD-1 silica. The conversion of both cyclohexane and cyclohexanol decreases as the cobalt concentration in TUD-1 increases. The selectivity towards cyclohexanone decreased with an increased loading of Co, being possibly related to the increasing aggregation of Co or Co oxide at higher cobalt concentrations. The agglomeration of Co reduced the accessibility of the individual cobalt atoms and, therefore, of the number of active sites. At lower conversion levels, all these catalysts behaved in a similar manner, while at a moderate conversion of 5% the selectivities of the catalysts showed significant differences, with Co-1 being clearly the most selective catalyst (Co-1 > Co-2 > Co-5 > Co-10). In this work, the reuse of the catalysts was not performed.
In 2015, Fu and co-workers prepared a Ce-doped MCM-48 (Ce-MCM-48) mesoporous molecular sieve through a hydrothermal procedure and modified its surface using organic groups or fluorine [
68]. These mesoporous silicas are of great interest as catalyst supports due to their large surface area and uniform pore size distribution, nanoscale periodicity, which facilitates mass transfer, and the active sites of macromolecular compounds. Moreover, the abundance of Si-OH groups on their surface makes them excellent substrates for surface modification.
The results indicated a high dispersion of Ce species on the MCM-48 materials, with a Ce content of 0.51% in the sample Ce-MCM-48 (
Table 6). The post-functionalization of the Ce-MCM-48 sample improved the surface hydrophobicity through the immobilization of organic groups or fluorine. Also, the cerium content was kept after post-functionalization in the Ce-MCM-48 sample.
The analysis of the textural features of the samples revealed the typical IV-type isotherms of mesoporous materials. The textural parameters, such as surface area, porous volume, and pore size, of Ce-MCM-48 sample decreased after post-functionalization; the increase in the number of organic groups or fluorine brought about a decrease of the porosity, due to the graft on the inner surface of the mesopores. The silica channels and also the absence of agglomerated cerium species outside the mesoporous structure could be clearly observed, in HRTEM images (
Figure 12) of Ce-MCM-48 and post-functionalized samples.
The catalytic activity testing for the oxidation of cyclohexane in a solvent-free system with molecular oxygen showed that post-functionalized Ce-MCM-48 exhibited the higher conversion of cyclohexane and selectivity to cyclohexanol and cyclohexanone, achieving 8.9% cyclohexane conversion with 91.2% selectivity (
Table 7). This feature could be achieved with the Ce-MCM-48 with F-modified catalyst being attributed to the modification of their hydrophobicity and polarity properties. The Fluor-modified catalyst also presented excellent reusability, and no obvious deterioration of its catalytic performance was reported, after five cycles.
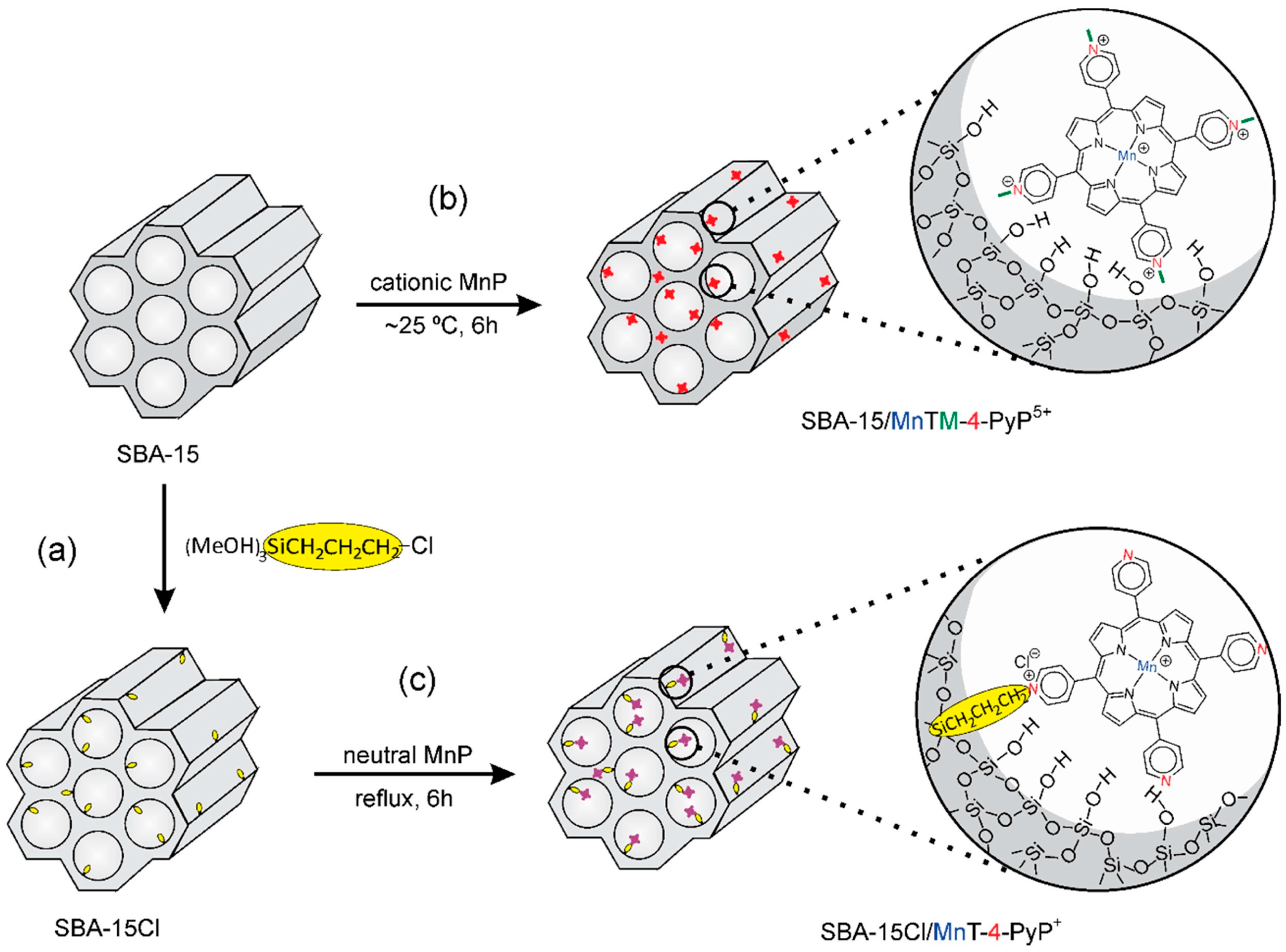
In a work published in 2016 by Pinto et al. the use of ordered mesoporous silica SBA-15 and its correspondent chloropropyl-functionalized silica SBA-15Cl as supports for immobilization of different neutral and charged Mn porphyrins (MnP) was investigated (
Figure 13) [
69]. The resulting MnP-supported materials with MnP loadings of 0.3% w/w were obtained in these materials and the heterogenized systems SBA-15/MnTM-X-PyPCl5 and SBA-15Cl/MnT-X-PyPCl (X = 2, 3, 4), and non-immobilized neutral MnP isomers were evaluated as catalysts for cyclohexane oxidation using iodosylbenzene (PhIO) as oxygen donor.
Control reactions presented negligible yields, suggesting that neither PhIO nor the supports alone can catalyze the cyclohexane oxidation. Reaction of non-immobilized MnP isomers under homogeneous conditions provided a good catalytic efficiency although poor selectivity to the alcohol was observed. Additionally, for the para-isomer, the selectivity was inverted, the ketone being the major product in detriment of the alcohol. A discreet decreased trend in the total yield was observed following the order: Ortho-, meta-, and para-isomers. The catalytic performance of the SBA-15Cl/MnT-X-PyPCl (X = 2, 3, 4) solids was as good as that observed for homogeneous systems, pointing out that catalyst deactivation did not occur upon the immobilization process. In addition, the total yield trend observed for the SBA-15Cl/MnT-X-PyPCl (X = 2, 3, 4) series followed the order para > meta > ortho, which was the opposite of that observed under homogeneous conditions with the corresponding non-immobilized MnP isomers.
When comparing the homogeneous versus heterogeneous catalytic reactions, a change in selectivity was observed, as an increase of ∼10% of the selectivity to the alcohol was reported with the immobilization of MnP, indicating an effective participation of the inorganic matrix. This influence of the support was difficult to understand; however, as the support (SBA-15Cl) had a lipophilic character due to the presence of the carbon chain, the approximation of nonpolar substrate (cyclohexane) should be favored, justifying the increase in the production of the alcohol. Additionally, the hydrophobic nature of the pendant carbon chain may prevent the approach of cyclohexanol product (more polar compound than cyclohexane) and its further oxidation to ketone. This fact can justify the decrease of cyclohexanone yield in heterogeneous catalysis in comparison to homogeneous catalysis, being more discrepant for the ortho isomer, which showed a decrease of 35% (catalyst in homogeneous medium) to 18% (heterogeneous catalysis). The decrease of ketone resulted in a decrease in total yield (K + A) of 71% to 54%. Nonetheless, cyclohexanone may, however, be formed via other mechanisms, e.g., radical reactions, which cannot be ruled out at this point. The immobilization of MnT-X-PyPCl (X = 2, 3, 4) on the SBA-15Cl support probably also contributed toward the increase of oxidative stability of MnP, since neutral MnP in homogeneous medium showed high percentage of destruction, of ca. 70%.
This improvement in MnP oxidative stability appeared to be more evident in the case of the para-isomer, for which the total yield was higher for heterogeneous catalysis as compared to the corresponding homogeneous catalysis. The catalytic yields for the cationic MnP MnTM-X-PyPCl5 (X = 2, 3, 4) were lower than those of the corresponding neutral species. This can be attributed to the poor solubility of the cationic MnP in the reaction solvent mixture (chloroform-acetonitrile); in both solid systems, the efficiency and selectivity remained essentially unaffected by the immobilized MnP isomer. The strong interaction between MnP and the mesoporous silica, in both SBA-15 and SBA-15Cl systems, was verified by the low-leaching of MnP from the materials even after extensive washings. The solid catalysts showed high resistance against oxidative destruction upon recovering and reuse in consecutive recycling studies with no significant changes in the efficiency of the catalyst.
In 2017, the activity and selectivity of vanadium phosphate (VPO) catalysts supported on KIT-6, a novel type of mesoporous silica [
70] for the oxidation of cyclohexane using hydrogen peroxide was reported [
71]. VPO catalysts have been recognized as efficient catalysts for n-butane partial oxidation to maleic anhydride, with high conversion with good selectivity, and were recently used for the oxidation of alcohols in liquid phase [
72]. KIT-6 possesses a unique structure, with 3D interpenetrating bi-continuous networks of channels, offering a higher number of active sites and resistance to clustering, allowing more reacting molecules. This catalyst exhibits remarkable features like high surface area, large pore volume, high chemical stability, and adjustable pore diameter.
Different amounts of vanadium and phosphorous were loaded on the surface of highly ordered mesoporous silica KIT-6. Nitrogen adsorption isotherms obtained for the loaded KIT-6 and VPK-6 displayed type-IV isotherms, revealing the mesoporous structure of the material, with uniform pore size and wide channel-like mesopores with a narrow pore size distribution. A considerable decrease in surface area and pore volume was observed upon VP loading, suggesting that these species are located inside the pores of KIT-6 covering the inner walls of the matrix. The pore diameter increased, probably caused by the filling of the micropores of KIT-6 support by VP. The XRD analysis showed the loaded VPO material existed almost exclusively as VOHPO40.5H2O phases, and that calcination converted it mostly to the form of (VO)2P2O7 phase on KIT-6.
Under the optimum conditions for cyclohexane conversion and selectivity towards KA oil over vanadium containing catalyst, using 27 wt.% catalyst VP-loading at 65 °C with 1:4 (cyclohexane: H2O2) molar ratio, 19.3% of cyclohexane conversion and 69.9% selectivity for KA oil were observed after 4 h of reaction time. The recyclability of the catalyst 27 wt.% VPK-6 was investigated through five consecutive catalytic cycles and no significant change regarding the conversion and selectivity was observed in the first three runs. In the two following cycles a loss of activity and lower conversion of cyclohexane (without loss of KA oil selectivity) was observed. The stability of the VPK-6 catalyst could be ascribed to the high dispersion of vanadium and phosphorous in the VPK-6, as observed by SEM. N2 adsorption isotherms revealed that the nature of isotherm of the catalyst after 5 times reused remained unchanged. Although the surface area value and pore volume presented a downward deviation, that may be attributed to the blocking of the pores of the support.
The authors postulated the possible mechanism for reaction pathway over the VPK-6 catalyst, that is presented in
Scheme 4.
In 2018, Unnarkat and co-workers carried out the liquid phase cyclohexane oxidation, using molecular oxygen and a cobalt molybdenum oxide (CoMoO4) as catalyst [
73] supported on three mesoporous silica supports (SBA-15, KIT-6, and FDU-12) [
74]. The catalysts were characterized by several techniques, such as surface area analysis, XRD, and TEM (
Figure 14). The adsorption isotherms obtained for the supports and respective loaded catalysts correspond to type-IV isotherm that revealed the mesoporous nature of the materials [
48]. The surface area obtained for the supports (SBA-15 and KIT-6) was above 700 m
2g
−1 and above 400 m
2g
−1 for the loaded catalyst, while for FDU-12 it was 250 m
2g
−1 and for its resultant loaded catalyst, less than 10 m
2g
−1. TEM images show the dispersion of the catalyst particles in the mesopores of the catalyst, and the typical channel structure of SBA-15, cubical pore structure for KIT-6, and a hexagonal pore structure for FDU-12 could be observed (
Figure 14). The particles were not uniform but were found to be well dispersed over the catalyst area.
For each support, the authors studied the catalyst activity as a function of different parameters, such as loading, calcination temperature, and pore size. The catalytic performance of oxide supported on FDU-12, SBA-15, and KIT-6 was evaluated at 150 °C and 1.0 MPa oxygen pressure at 800 rpm stirring. Among the supported catalysts, CoMo/LPFDU showed the highest activity. The catalysts with lower loading, pore size of support, and calcination temperature presented higher activity, but a lower selectivity was observed. Overall, the studied catalysts presented about 7–8% conversion, and selectivities of 85% for cyclohexanone and cyclohexanol. The oxide on silica supports showed deactivation, as previously observed by the authors in the case of the unsupported oxide, presumably due to the adsorption of the products of reaction. The deactivated catalysts were successfully regenerated by recalcination between runs, retaining their activity and selectivity over consecutive cycles (
Figure 15). The kinetic model proposed for the unsupported catalyst was the same followed for the supported catalyst as well.
In 2019, Martins and co-workers developed an interesting approach towards cyclohexane oxidation [
75] by making use of the exceptional properties of the magnetized silica-coated catalysts, as the recyclability and reuse of the materials by easy magnetic separation (
Scheme 5) [
76,
77]. Although a mesoporous silica was not used in this case, we will briefly discuss it in this section.
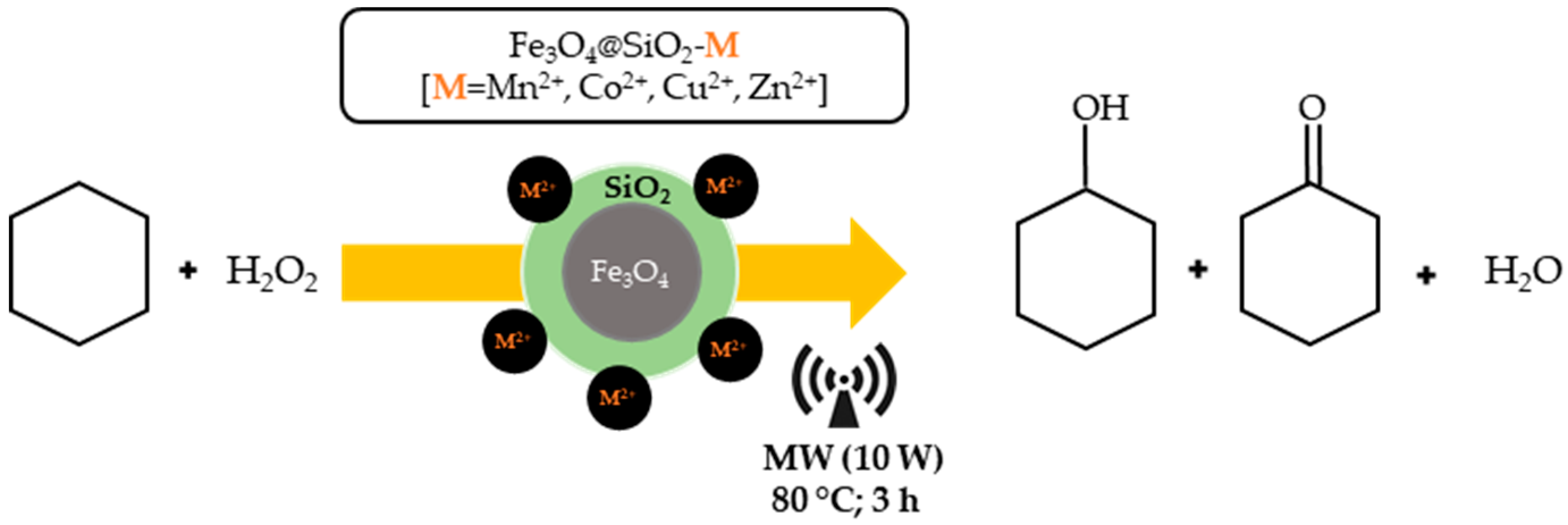
The aim of the authors was to propose a more sustainable synthetic protocol, through the use of low-cost starting materials, as late first-row transition-metals, in absence of any organic co-catalyst, and in solvent-free conditions. In this sense, the catalytic activity of first-row transition-metal silica coated magnetite nanoparticles (MNPs), Fe
3O
4@SiO
2-M
2+ [M = Mn
2+, Co
2+, Cu
2+ or Zn
2+], for the selective oxidation of cyclohexane to cyclohexanone and cyclohexanol was evaluated by the authors, under mild conditions, using MW heating. All tested metals (manganese, cobalt, and copper) species present in the MNPs revealed an effective catalytic performance to selectively produce KA oil from cyclohexane oxidation (
Table 8), using the optimal conditions of 2 h of reaction time at 80 °C, using hydrogen peroxide as oxidant, with exception to zinc.
The manganese and copper surface enriched silica coated MNPs led to the highest yield of KA oil (24% and 21%, respectively). These modified MNPs were readily isolated from the reaction mixture by using an external magnet and no significant loss of activity was reported when the catalysts were reused up to five consecutive catalytic runs.
The authors proposed a mechanism for the Fe
3O
4@SiO
2-M
2+ catalytic system of free radical nature (
Scheme 6), involving the production of hydroperoxyl and hydroxyl radicals from the interaction of the active sites at the surface of the silica-coated MNPs with hydrogen peroxide.
8. Metal-Organic Frameworks as Supports
Metal-organic frameworks (MOFs) are a class of hybrid porous materials, assembled with metal ions/clusters and organic linkers, that feature crystalline porous structures, displaying microporosity and in some cases mesoporosity [
78,
79]. MOFs are currently of great interest due to their potential to tackle some key applications such as hydrogen storage, carbon dioxide sequestration, catalysis, smart sensors, and drug delivery. The already considerable field of MOF chemistry continues to expand at a remarkable pace, in part because of the seemingly limitless number of potential structures, but also because of the myriad of potential applications.
MOF-derived porous materials are very promising materials for catalytic applications owing to their unique attributes, such as large surface area, high porosity, and excellent tailoring ability. This class of functional solid-state materials combines both the virtues of heterogeneous and homogeneous catalysis, such as high efficiency and selectivity, recyclability, well-defined active sites, etc. The use of MOFs in heterogeneous catalysis due to their high surface area, porosity, and chemical tunability has been recently explored, especially for their role as supports for several metal (e.g., Pd, Au, Ru, and Pt) nanoparticles [
80,
81]. MOFs promise catalysis-friendly features such as large internal surface areas, extensive micro- and/or mesoporosity, and well-defined cavities and portals of molecular dimensions. One attractive approach to the construction of catalytic MOFs, therefore, is to incorporate molecules that have already been proven effective as catalysts in homogeneous solution environments.
Maksimchuk and co-workers reported in 2012 the use of Cr- and Fe-MIL-101 for the solvent-free selective oxidation of cyclohexane with molecular oxygen and/or
tert-butyl hydroperoxide [
82]. The presence of mesopores in MIL-101 and its superior stability make it an ideal platform for the encapsulation of molecular catalysts. The MIL-101 framework consists of Cr(III) (or Fe(III)) carboxylate trimeric clusters with terminal water molecules that can be easily removed, thus providing catalytically active coordinatively unsaturated sites [
78,
83,
84]. The mesoporous Cr- and Fe-MIL-101, display a rigid zeotype crystal structure, consisting of quasi-spherical cages accessible through windows of ca. 1.2 and 1.6 nm, with high surface areas (3200–3900 m
2g
−1) and pore volumes (1.4–2.1 cm
3g
−1), revealing good resistance to common solvents and high temperature (
Figure 16).
The studied systems could catalyze the oxidation of cyclohexane with TBHP, but the product distribution is strongly dependent on the nature of the active metal. The Cr-MIL-101/TBHP system produced KA-oil with 92% selectivity at 25% substrate conversion, with the predominance of cyclohexanone, while Fe-MIL-101/TBHP/O2 gave a mixture of cyclohexyl peroxide, A and K with 99% total selectivity at 38% conversion. Cr-containing catalysts are known to produce a high yield of ketone in cyclohexane oxidation, since they favor dehydration of cyclohexyl hydroperoxide and oxidation of the alcohol to the ketone. The ability of Cr-MIL-101 to catalyze the transformation of A to K using TBHP as oxidant was confirmed in a separate experiment. The materials Fe- and Cr-MIL-101 were also reused at least five times, without significant loss of the catalytic activity.
In 2013, Long et al. reported the use of a Au−Pd nanoparticles catalyst deposited on the same MIL-101, for the liquid-phase aerobic oxidation of cyclohexane [
85]. Several techniques were used for the characterization of the prepared catalysts, such as XRD, N
2 physical adsorption, atomic absorption spectroscopy, TEM, EDS, and X-ray photoelectron spectroscopy. Au and Pd were found to be mostly in the form of bimetallic alloys on the MIL-101 support. The structure of MIL-101 was mostly maintained after preparation of the materials, and the stability of its porosity after metal doping was also confirmed by the N
2 adsorption results at -196 °C. The significant decrease in the textural parameters was attributed to the occlusion of cavities of MIL-101 by the metal nanoparticles found in the porosity or at the surface of the MIL-101 framework. TEM images revealed a homogeneous dispersion of the Au−Pd nanoparticles in the materials, with particle sizes typically ranging from 2 to 3 nm, and no signs of significant formation of particle aggregation (
Figure 17).
The Au−Pd/MIL-101 was active and selective in the liquid-phase oxidation of cyclohexane, with conversions exceeding 40%, and selectivity to KA oil > 80% under solvent-free conditions. Furthermore, the authors observed a synergy effect between Au and Pd that led to a higher reactivity than their pure metal counterparts and also than an Au + Pd physical mixture. The enhancement of the performance of cyclohexane aerobic oxidation, high activity, and selectivity of Au−Pd/MIL-101 in cyclohexane aerobic oxidation may be correlated to the synergistic alloying effect of bimetallic Au−Pd nanoparticles. The reusability of the Au−Pd catalyst was also investigated to confirm that the highly active catalyst was stable and can be reused. After the cyclohexane oxidation reaction, the catalyst was separated from the reaction mixture, washed, dried, and reused in subsequent runs under identical reaction conditions. Results have revealed no appreciable loss of conversion and selectivity in the oxidation of cyclohexane up to four runs. The crystalline structure of MIL-101 was mostly retained and only traces of metal (<1%) were detected in the liquid phase during the reaction.
In the same year, the immobilization of metalloporphyrin sites into porous mixed metal-organic framework (M’MOF) materials by a metallo-ligand approach was developed by Xie et al., mimicking cytochrome P450 monooxygenases in biological systems [
86]. The porous structure of [Zn
2(MnOH–TCPP)-(DPNI)]·0.5DMF·EtOH·5.5H
2O, (CZJ-1, CZJ is the Chemistry Department of Zhejiang University) TCPP=tetrakis(4-carboxyphenyl)porphyrin); DPNI =
N,N’-di(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide) presents a doubly interpenetrated cubic α-Po topology, in that the basic Zn
2-(COO)4 clusters are bridged by metalloporphyrin forming two-dimensional sheets that are further bridged by the organic pillar linker DPNI to form a three-dimensional porous structure.
CZJ-1 exhibited significantly high catalytic oxidation of cyclohexane with conversions up to 94% at room temperature and about 100% selectivity toward KA oil. The molecular components MnOHH4TCPP and Mn–TPP have led to much lower conversions of 40% and 47%, respectively. The authors speculated that the catalytically active species were porphyrin–MnV=O formed in situ, as in the case of their homogeneous Mn–porphyrin catalysts. The confinement effect provided by Mn–porphyrin sites within the CZJ-1 framework prevents μ-oxo dimerization, commonly observed in homogeneous molecular catalysts, but might also collaboratively work together to enhance their catalytic activity, given the fact that the neighboring Mn–porphyrin sites were quite close and flexible with Mn⋯Mn distances of about 13.9 Ᾰ. The pores in this flexible M’MOF CZJ-1 can readily encapsulate the substrate cyclohexane, while possibly excluding the larger molecules of cyclohexanone and cyclohexanol, thus facilitating the product release and their chemical transformations. Even after recycling six times, the recovered CZJ-1 still displayed quite high catalytic activity, achieving a conversion of 79% and a TON up to 2668.
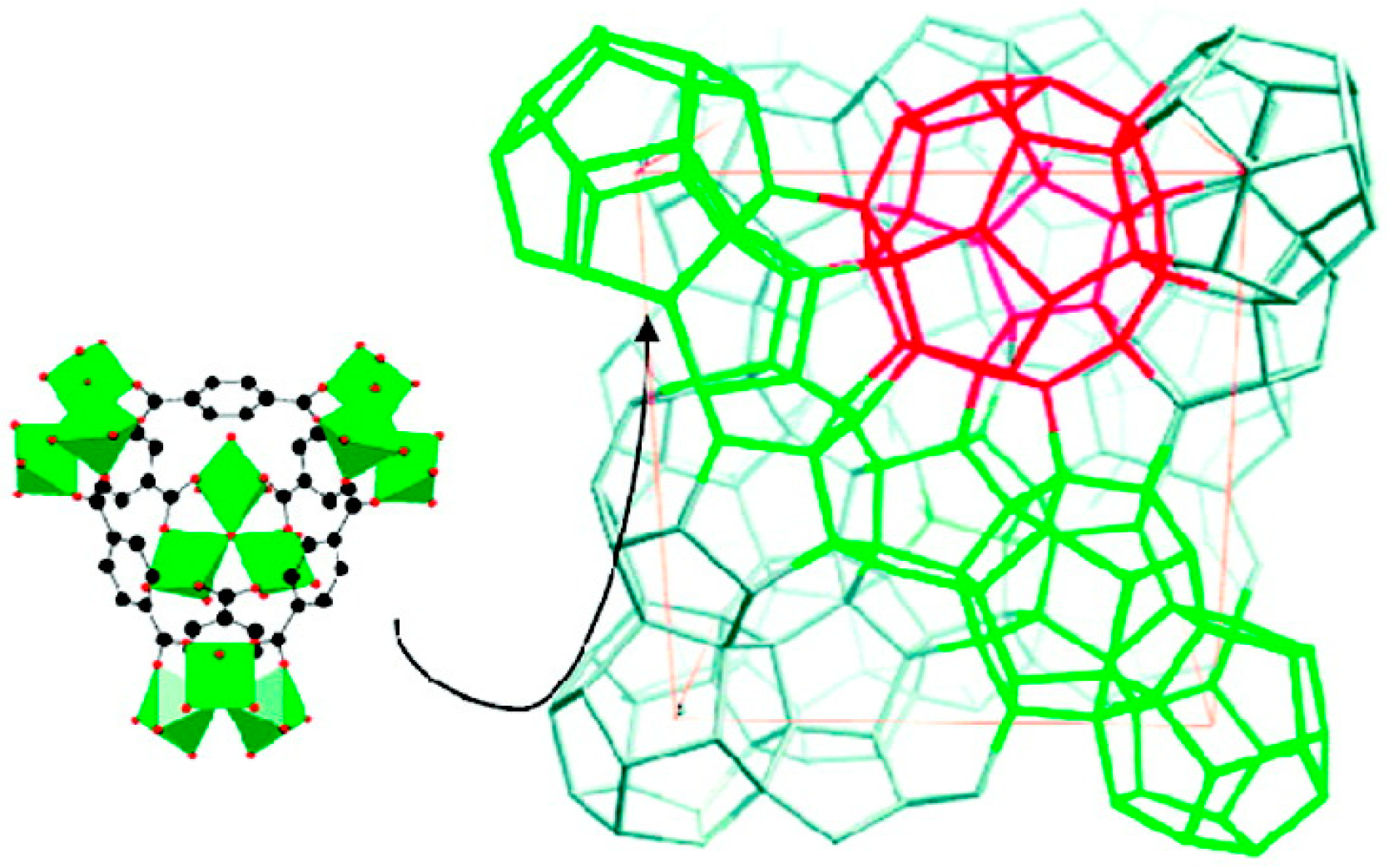
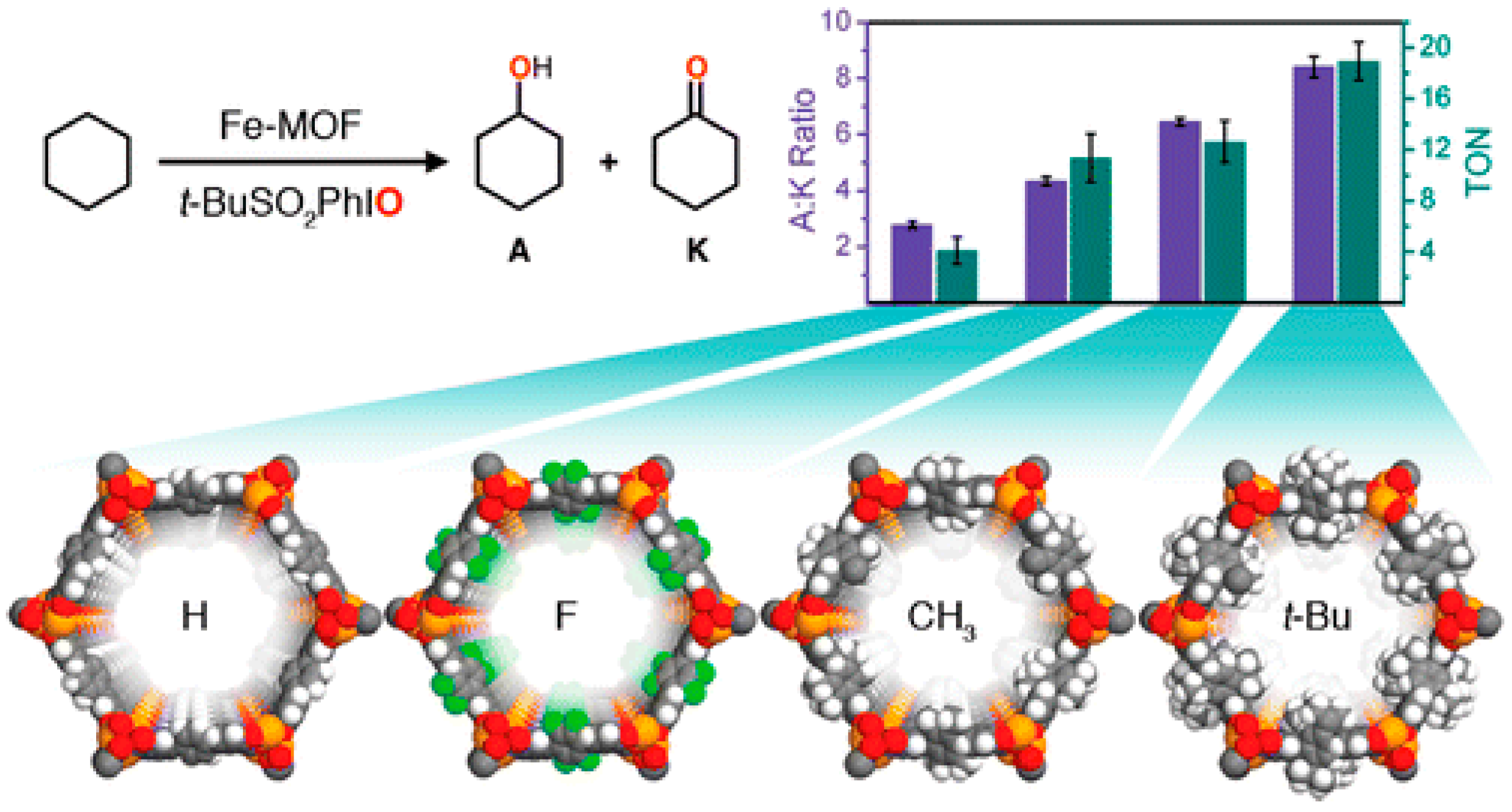
In 2016, the effect of a hydrophobic pore environment on the product selectivity of cyclohexane oxidation reaction and catalyst stability in a series of iron-based frameworks was investigated by Xiao and co-workers [
87]. In that sense, the authors modified a MOF containing exposed iron(II) sites, Fe
2(dotpdc) (H
4dotpdc=4,4”-dihydroxy-[1,1’:4’,1”-terphenyl]-3,3”-dicarboxylic acid), also known as Fe-MOF-74, using several functional groups (R = F, CH
3, and
tBu) (
Figure 18). A decrease of the textural parameters was observed, corresponding to the increasing size of the substituents. Nevertheless, the highest difference was of only ∼10%, between the Fe
2(dotpdc) and the most sterically crowded derivative, Fe
2(dotpdctBu), and did not result in diffusional constrains. A significant enhancement of the A:K ratio was reported for the five MOF materials, and also a remarkable increase in the TON were attained by simply altering the pore diameter of the framework and through the incorporation of nonpolar functional groups near the iron site.
As the textural and unit cell parameters of the four Fe2(dotpdcR) frameworks were quite similar, their A:K selectivities and TONs were rather different, increasing in the order H < F < CH3 < tBu. The variations in the A:K selectivities (increase from 2.8:1 to 8.4:1) was related to the different cyclohexane adsorption enthalpies of these frameworks, which tended to be more favorable as the number of hydrophobic residues increased, promoting the increase of van der Waals interactions. Gas adsorption measurements, kinetic isotope effect, and Mössbauer spectroscopy revealed that hydrophobic moieties interacted favorably with cyclohexane to increase the local concentration of the substrate toward the active iron center, thereby increasing both the activity and selectivity. In every case, the removal of the solid stopped catalysis, without any loss in framework crystallinity. The gradual oxidation of framework iron(II) sites, rather than framework collapse or ligand degradation, led to catalyst deactivation.
To summarize, we presented an overview of the most important outcomes of the catalytic performance of supported catalysts in the contributions discussed here (
Table 9). Nevertheless, it is important to stress that comparison between results presents quite some limitations, as the experimental conditions and obtained parameters were quite diverse in each case.