Preparation of Quasi-MIL-101(Cr) Loaded Ceria Catalysts for the Selective Catalytic Reduction of NOx at Low Temperature
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of the Catalysts
2.2. Catalytic Performance
2.2.1. NH3-SCR Performance
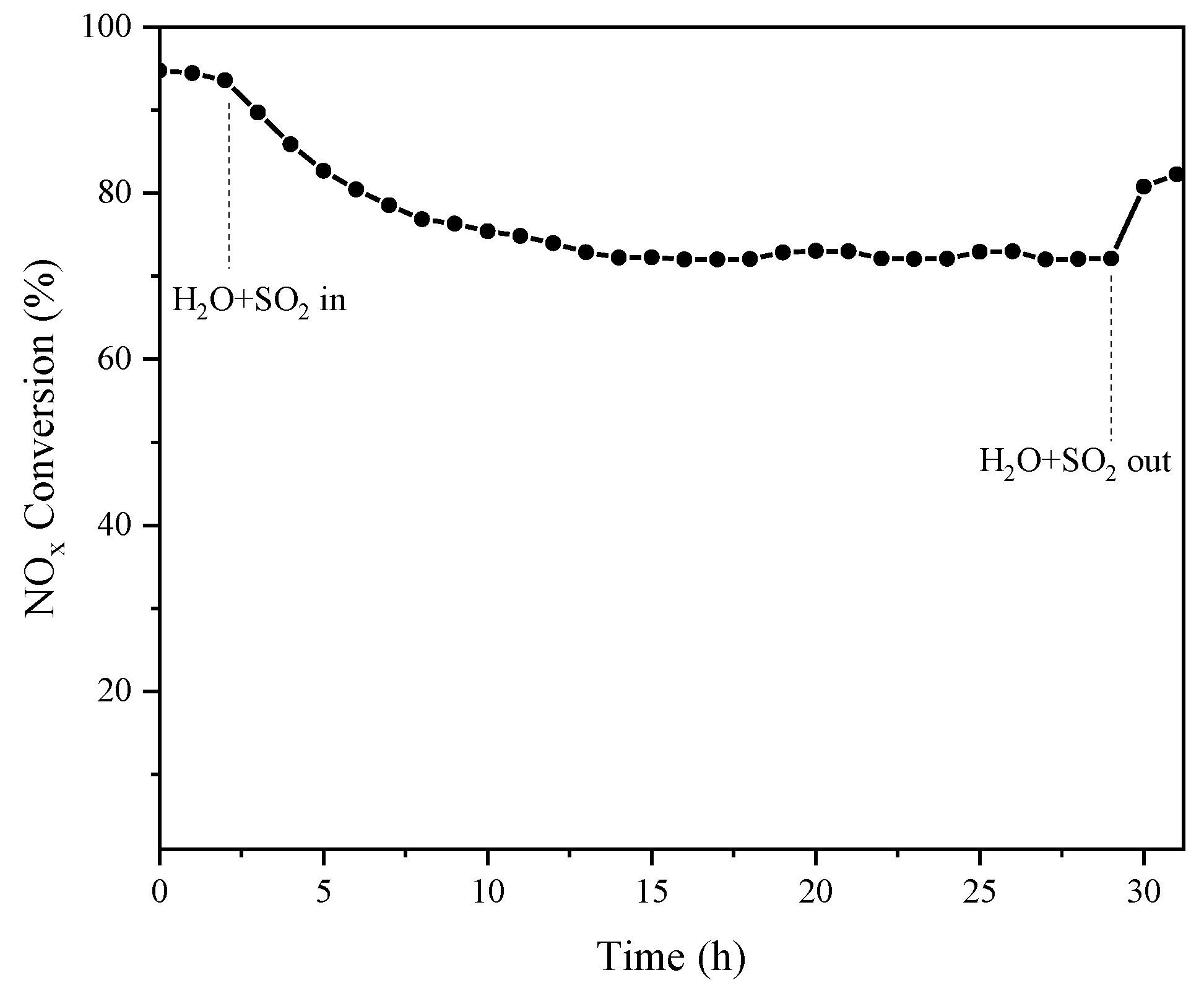
2.2.2. Effect of SO2 and H2O on the SCR Reaction
3. Experimental
3.1. Materials
3.2. Preparation of Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Ihl Woo, S. Recent Advances in Catalytic DeNOx Science and Technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Liang, J.; Yu, H.; Liu, W.; Wang, X.; Peng, H.; Wu, P. Exploring the nano-size effect of mordenite zeolites on their performance in the removal of NOx. Ind. Eng. Chem. Res. 2019, 58, 8625–8635. [Google Scholar]
- Zhang, L.; Wen, X.; Lei, Z.; Gao, L.; Sha, X.L.; Ma, Z.H.; He, H.B.; Wang, Y.S.; Jia, Y.; Li, Y.H. Study on the mechanism of a manganese-based catalyst for catalytic, NOX flue gas denitration. Aip Adv. 2018, 8, 045004. [Google Scholar] [CrossRef]
- Hueso, J.; Cotrino, J.; Caballero, A.; Espinos, J.; Gonzalezelipe, A. Plasma catalysis with perovskite-type catalysts for the removal of NO and CH4 from combustion exhausts. J. Catal. 2007, 247, 288–297. [Google Scholar] [CrossRef]
- Gholami, R.; Stere, C.E.; Goguet, A.; Hardacre, C. Non-thermal-plasma-activated de-NOx catalysis. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, H.; Li, D.; Qin, Y.; Xie, Y.; Wang, S. WO3/CeO2-ZrO2, a promising catalyst for selective catalytic reduction (SCR) of NOx with NH3 in diesel exhaust. Chem. Commun. 2008, 1470–1472. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5–WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.-K.; Tumuluri, U.; Ford, M.E.; Wu, Z.; Wachs, I.E. Reaction Pathways and Kinetics for Selective Catalytic Reduction (SCR) of Acidic NOx Emissions from Power Plants with NH3. ACS Catal. 2017, 7, 8358–8361. [Google Scholar] [CrossRef]
- Yang, R.G.; Han, L.; Yang, T. Research progress on new technology of denitrification in coal-fired power plants. J. Northeast Electr. Power Univ. 2010, 30, 17–20. [Google Scholar]
- Locci, C.; Vervisch, L.; Farcy, B.; Domingo, P.; Perret, N. Selective Non-catalytic Reduction (SNCR) of Nitrogen Oxide Emissions: A Perspective from Numerical Modeling. Flow Turbul. Combust. 2017, 100, 301–340. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, N.; Zhang, H.Z.; Cui, S.P.; Zhang, Y.N. Effect of the Cement Raw Meal Rate Value on SNCR deNOx Efficiency with NH3 as Reducing Agent. Mater. Sci. Forum 2019, 944, 1215–1220. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, N.; Liu, X.; Li, L.; Song, L.; Qiu, W.; He, H. The Keggin Structure: An Important Factor in Governing NH3–SCR Activity Over the V2O5–MoO3/TiO2 Catalyst. Catal. Lett. 2018, 148, 1228–1235. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Chang, H.; Wu, Q.; Zhang, T.; Li, J. New Insight into SO2 Poisoning and Regeneration of CeO2-WO3/TiO2 and V2O5-WO3/TiO2 Catalysts for Low-Temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 7064–7071. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Chen, Y.; Zhang, Y.W.; Zhang, Q. Study on Denitration Performance of Fly Ash Supported Vanadium Oxide Low Temperature SCR Catalyst. J. Northeast Electr. Power Univ. 2015, 35, 59–63. [Google Scholar]
- Gao, X.; Jiang, Y.; Fu, Y.; Zhong, Y.; Luo, Z.; Cen, K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 11, 465–469. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Chi Tsang, S. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Withdrawn: Novel Vanadium supported onto mixed Molybdenum-Titanium Pillared Clay catalysts for the low temperature SCR-NO by NH3. Chem. Eng. J. 2017, 356, 598–608. [Google Scholar] [CrossRef]
- Qu, L.; Li, C.; Zeng, G.; Zhang, M.; Fu, M.; Ma, J.; Zhan, F.; Luo, D. Support modification for improving the performance of MnOx–CeOy/γ-Al2O3 in selective catalytic reduction of NO by NH3. Chem. Eng. J. 2014, 242, 76–85. [Google Scholar] [CrossRef]
- Selleri, T.; Gramigni, F.; Nova, I.; Tronconi, E. NO oxidation on Fe- and Cu-zeolites mixed with BaO/Al2O3: Free oxidation regime and relevance for the NH3-SCR chemistry at low temperature. Appl. Catal. B Environ. 2018, 225, 324–331. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R.-T.; Li, M.-Y.; Sun, P.; Liu, S.-M.; Pan, W.-G.; Liu, S.-W.; Sun, X. Enhancement of the SO2 resistance of Mn/TiO2 SCR catalyst by Eu modification: A mechanism study. Fuel 2018, 223, 385–393. [Google Scholar] [CrossRef]
- Cao, L.; Wu, X.; Xu, Y.; Lin, Q.; Hu, J.; Chen, Y.; Ran, R.; Weng, D. Ceria-modified WO3-TiO2-SiO2 monolithic catalyst for high-temperature NH3-SCR. Catal. Commun. 2019, 120, 55–58. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, M.; Wu, S.; Ye, B.; Su, Y. Iron based monolithic catalysts supported on Al2O3, SiO2, and TiO2: A comparison for NO reduction with propane. Fuel 2018, 220, 330–338. [Google Scholar] [CrossRef]
- Ren, S.; Li, S.; Su, Z.; Yang, J.; Long, H.; Kong, M.; Yang, J.; Cai, Z. Poisoning effects of KCl and As2O3 on selective catalytic reduction of NO with NH3 over Mn-Ce/AC catalysts at low temperature. Chem. Eng. J. 2018, 351, 540–547. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Li, Q.; Liu, Y.; Huang, Z. Insight into the role of TiO2 modified activated carbon fibers for the enhanced performance in low-temperature NH3-SCR. Fuel 2019, 245, 554–562. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Fan, Z.; Chen, M.; Zhang, Z.; Shangguan, W. Widened Active Temperature Window of a Fe-ZSM-5 Catalyst by an Impregnation Solvent for NH3-SCR of NO. Ind. Eng. Chem. Res. 2018, 57, 13703–13712. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, Z.; Shi, C.; Zhang, W. Insight into the Synergic Effect of Fe-SSZ-13 Zeolite and FeMnTiZrOx Catalyst with Enhanced Reactivity in NH3-SCR of NOx. J. Phys. Chem. C 2019, 123, 2216–2227. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Tang, J.; Yamauchi, Y. Carbon materials: MOF morphologies in control. Nat. Chem. 2016, 8, 638–639. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Woll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Damasceno Borges, D.; Normand, P.; Permiakova, A.; Babarao, R.; Heymans, N.; Galvao, D.S.; Serre, C.; De Weireld, G.; Maurin, G. Gas Adsorption and Separation by the Al-Based Metal-Organic Framework MIL-160. J. Phys. Chem. C 2017, 121, 26822–26832. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weselinski, L.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.H.; et al. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based soc-MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, L.; Yan, Y.; Shi, H.; Wang, B.; Liang, Z.; Li, J. A novel photo- and hydrochromic europium metal-organic framework with good anion sensing properties. J. Mater. Chem. C 2017, 5, 8999–9004. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zhang, N.-N.; Liu, C.-S.; Du, M. Template-directed synthesis of a luminescent Tb-MOF material for highly selective Fe3+ and Al3+ ion detection and VOC vapor sensing. J. Mater. Chem. C 2017, 5, 2311–2317. [Google Scholar] [CrossRef]
- Bhadra, M.; Sasmal, H.S.; Basu, A.; Midya, S.P.; Kandambeth, S.; Pachfule, P.; Balaraman, E.; Banerjee, R. Predesigned Metal-Anchored Building Block for In Situ Generation of Pd Nanoparticles in Porous Covalent Organic Framework: Application in Heterogeneous Tandem Catalysis. ACS Appl. Mater. Interfaces 2017, 9, 13785–13792. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. Engl. 2016, 55, 5414–5445. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Sennu, P.; Aravindan, V.; Lee, Y.-S. High energy asymmetric supercapacitor with 1D@2D structured NiCo2O4@Co3O4 and jackfruit derived high surface area porous carbon. J. Power Sources 2016, 306, 248–257. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Tan, B.; Luo, Y.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Mixed-Solvothermal Synthesis of MIL-101(Cr) and Its Water Adsorption/Desorption Performance. Ind. Eng. Chem. Res. 2019, 58, 2983–2990. [Google Scholar] [CrossRef]
- Hupp, J.T.; Poeppelmeier, K.R. Chemistry. Better living through nanopore chemistry. Science 2005, 309, 2008–2009. [Google Scholar] [CrossRef]
- Zhao, T.; Jeremias, F.; Boldog, I.; Nguyen, B.; Henninger, S.K.; Janiak, C. High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 2015, 44, 16791–16801. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, H.; Sun, H.; Yu, H.; Chen, S.; Quan, X. Porous metal–organic framework MIL-100(Fe) as an efficient catalyst for the selective catalytic reduction of NOx with NH3. RSC Adv. 2014, 4, 48912–48919. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, B.; Jiang, H.; Chen, Y. Metal-organic framework loaded manganese oxides as efficient catalysts for low-temperature selective catalytic reduction of NO with NH3. Front. Chem. Sci. Eng. 2017, 11, 594–602. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, J.; Wang, C.; Li, Y.; Chen, Y.; Zhang, M. Effect of Cosolvent and Temperature on the Structures and Properties of Cu-MOF-74 in Low-temperature NH3-SCR. Ind. Eng. Chem. Res. 2017, 56, 3542–3550. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Wang, H.; Chen, Y.; Zhang, M. MOF-74 as an Efficient Catalyst for the Low-Temperature Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2016, 8, 26817–26826. [Google Scholar] [CrossRef]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef]
- Tsumori, N.; Chen, L.; Wang, Q.; Zhu, Q.-L.; Kitta, M.; Xu, Q. Quasi-MOF: Exposing Inorganic Nodes to Guest Metal Nanoparticles for Drastically Enhanced Catalytic Activity. Chem 2018, 4, 845–856. [Google Scholar] [CrossRef]
- Wang, P.; Sun, H.; Quan, X.; Chen, S. Enhanced catalytic activity over MIL-100(Fe) loaded ceria catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. J Hazard. Mater. 2016, 301, 512–521. [Google Scholar] [CrossRef]
- Xu, W.; Yu, Y.; Zhang, C.; He, H. Selective catalytic reduction of NO by NH3 over a Ce/TiO2 catalyst. Catal. Commun. 2008, 9, 1453–1457. [Google Scholar] [CrossRef]
- Qiu, L.; Meng, J.; Pang, D.; Zhang, C.; Ouyang, F. Reaction and Characterization of Co and Ce Doped Mn/TiO2 Catalysts for Low-Temperature SCR of NO with NH3. Catal. Lett. 2015, 145, 1500–1509. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cai, W.; Zhong, Q. The characterization of CrCe-doped on TiO2-pillared clay nanocomposites for NO oxidation and the promotion effect of CeOx. Appl. Surf. Sci. 2013, 268, 535–540. [Google Scholar] [CrossRef]
- Ferey, G. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Shan, W.; Yang, S.; Liu, F. W-Modified Mn-Ti Mixed Oxide Catalyst for the Selective Catalytic Reduction of NO with NH3. Ind. Eng. Chem. Res. 2018, 57, 9112–9119. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.; Niu, C. Rationally Designed Porous MnOx-FeOx Nanoneedles for Low-Temperature Selective Catalytic Reduction of NOx by NH3. ACS Appl. Mater. Interfaces 2017, 9, 16117. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.-W.; Liu, C.; Gao, C.; Fan, Z.; Niu, C. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Jiang, D.; Fang, G.; Tong, Y.; Wu, X.; Wang, Y.; Hong, D.; Leng, W.; Liang, Z.; Tu, P.; Liu, L.; et al. Multifunctional Pd@UiO-66 Catalysts for Continuous Catalytic Upgrading of Ethanol to n-Butanol. ACS Catal. 2018, 8, 11973–11978. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. Studies on the redox properties of chromite perovskite catalysts for soot combustion. J. Catal. 2005, 229, 459–469. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, G.; Wang, H.; Wang, H.; Zhang, C.; Cui, Y.; Huang, J.; Shu, Y. CeO2/TiO2 monolith catalyst for the selective catalytic reduction of NOx with NH3: Influence of H2O and SO2. Chem. Res. Chin. Univ. 2016, 32, 461–467. [Google Scholar] [CrossRef]
- Yang, R.; Huang, H.; Chen, Y.; Zhang, X.; Lu, H. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2015, 36, 1256–1262. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H. Chemistry: Oxygen Vacancies and Catalysis on Ceria Surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-Temperature Selective Catalytic Reduction (SCR) of NO with NH3 by Using Mn, Cr, and Cu Oxides Supported on Hombikat TiO2. Chembiochem. 2010, 32, 2479–2482. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Li, B.; Xiong, S.; Liao, Y.; Xiao, X.; Huang, N.; Geng, Y.; Zou, S.; Yang, S. Why the Low Temperature SCR Performance of Cr/TiO2 Much Less than That of Mn/TiO2: A Mechanism Study. J. Phys. Chem. C 2016, 120, 23511–23522. [Google Scholar] [CrossRef]
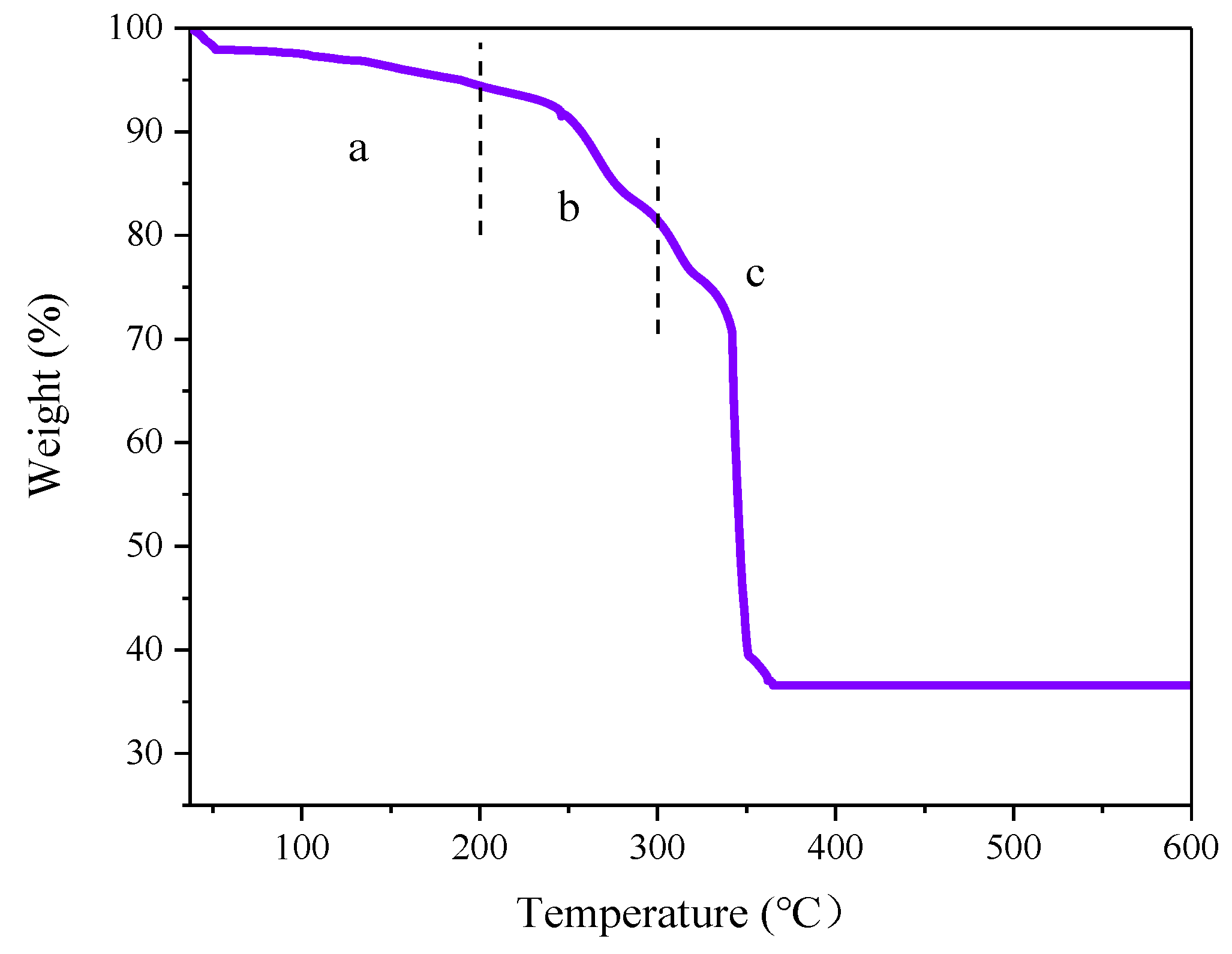
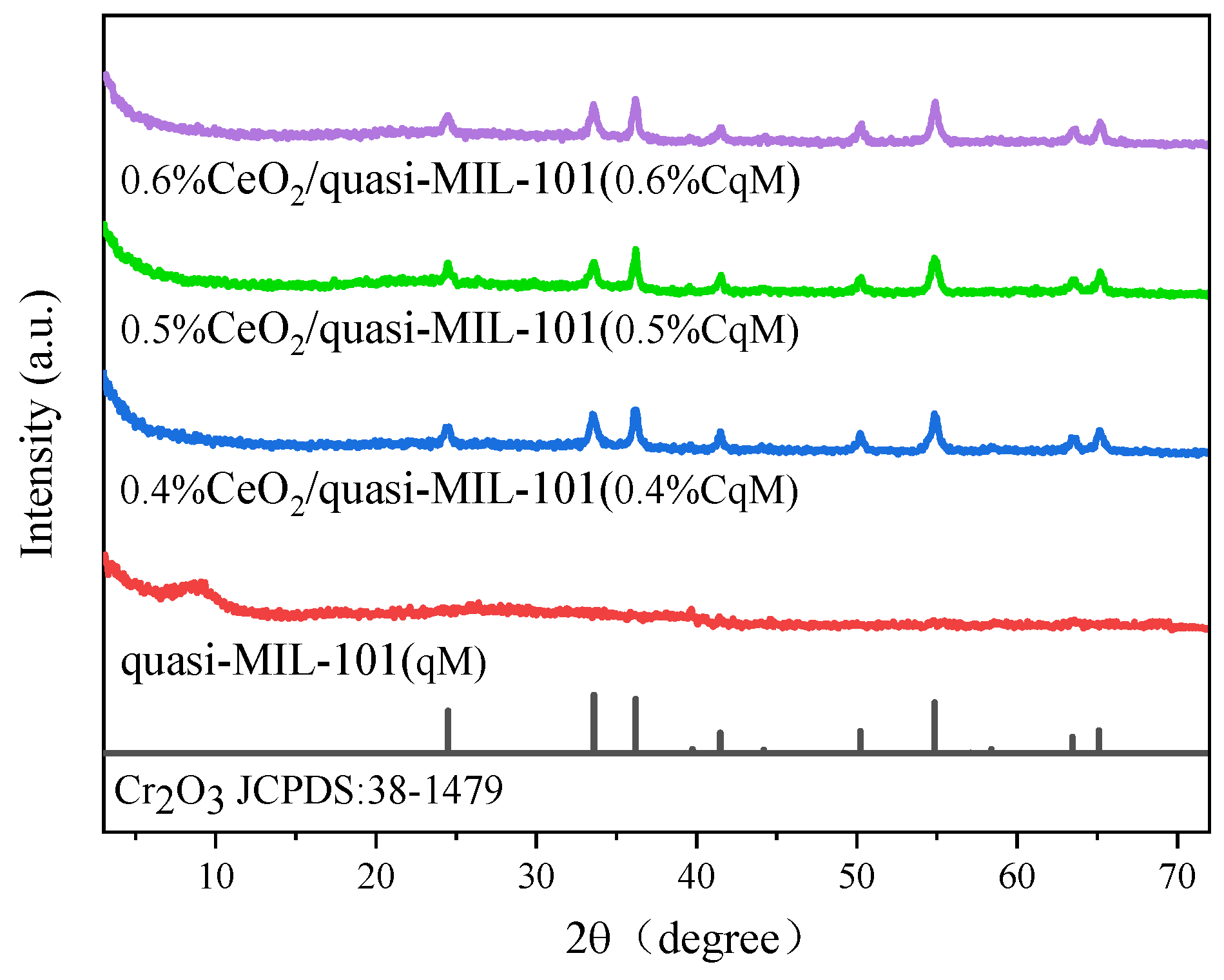
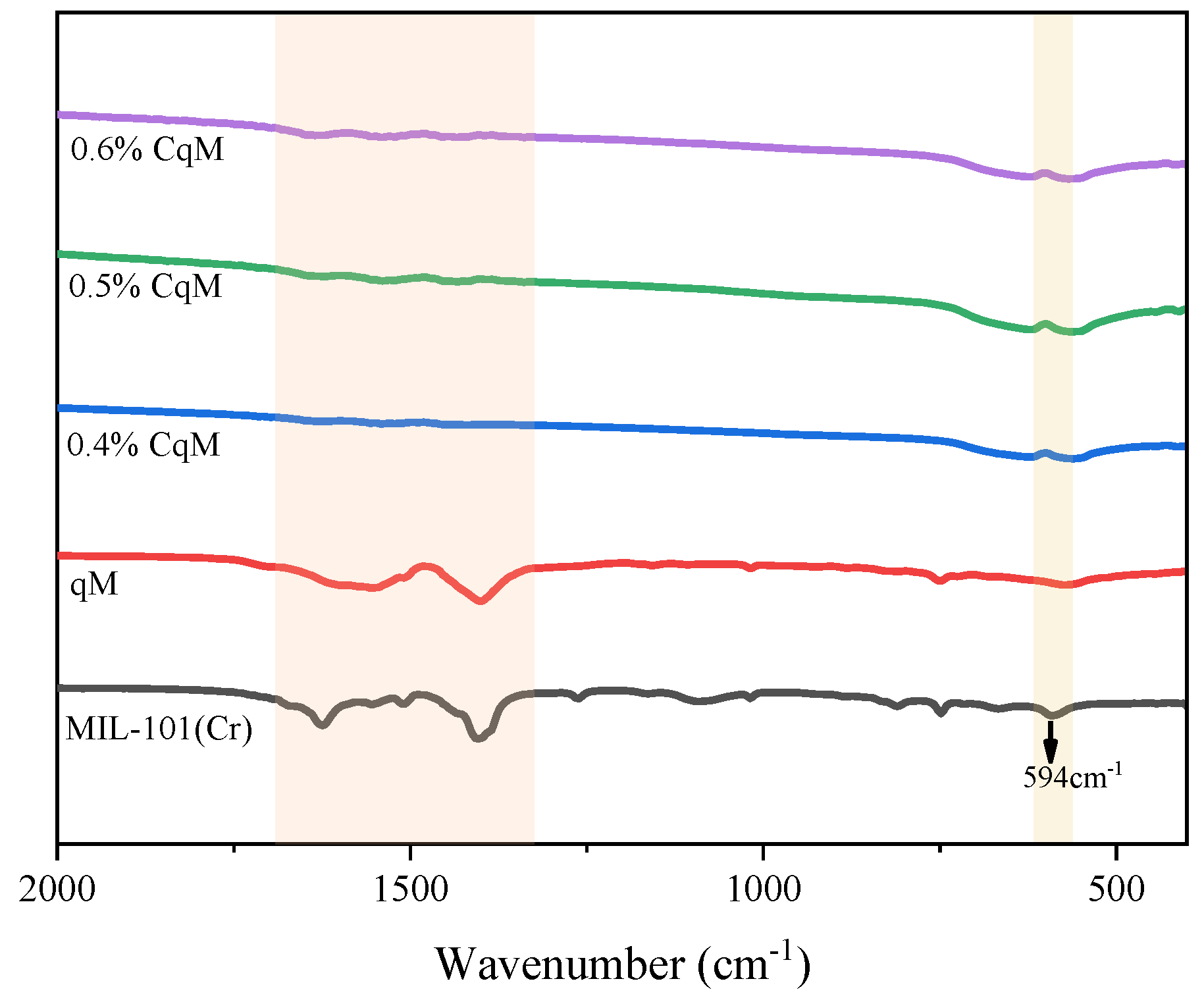
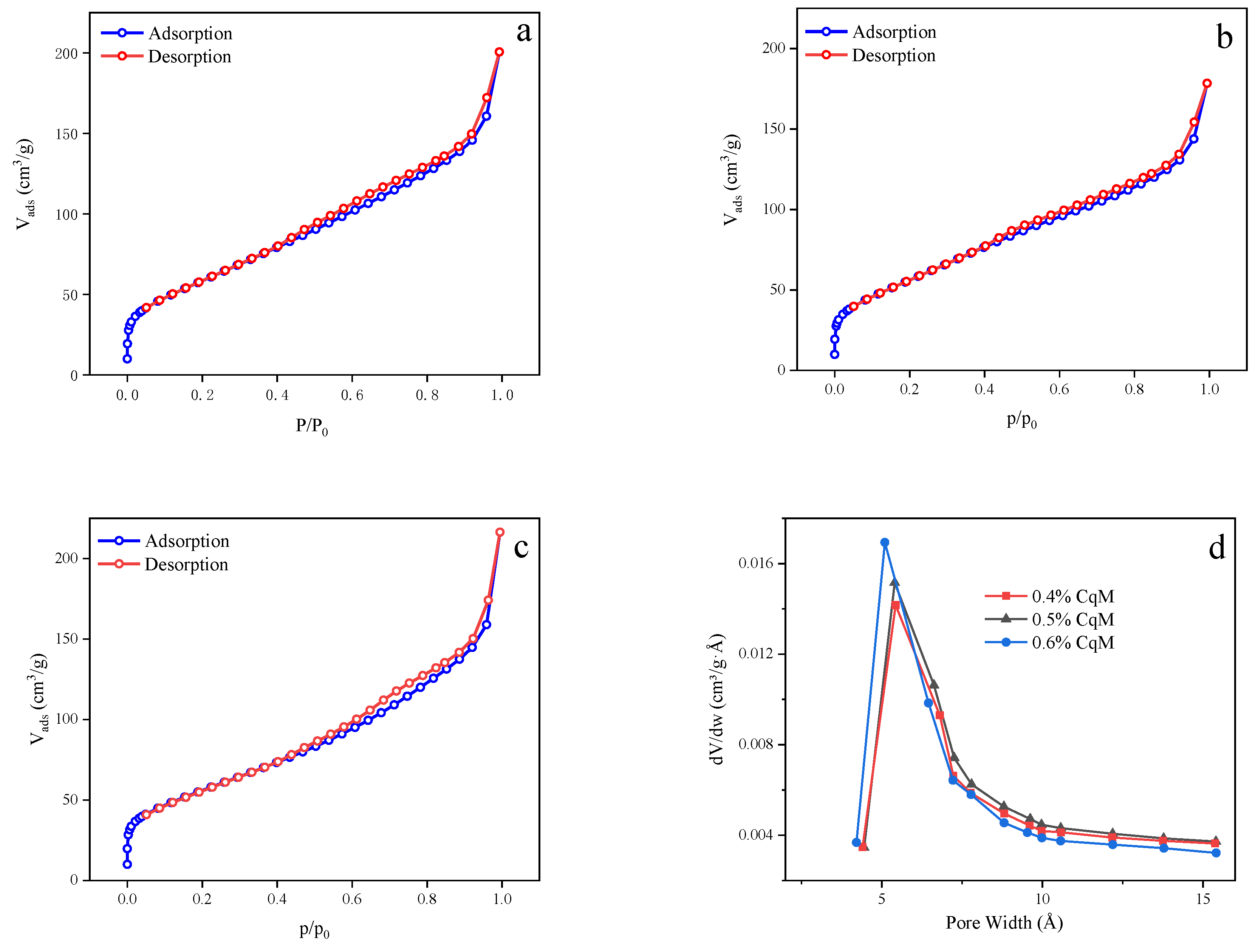
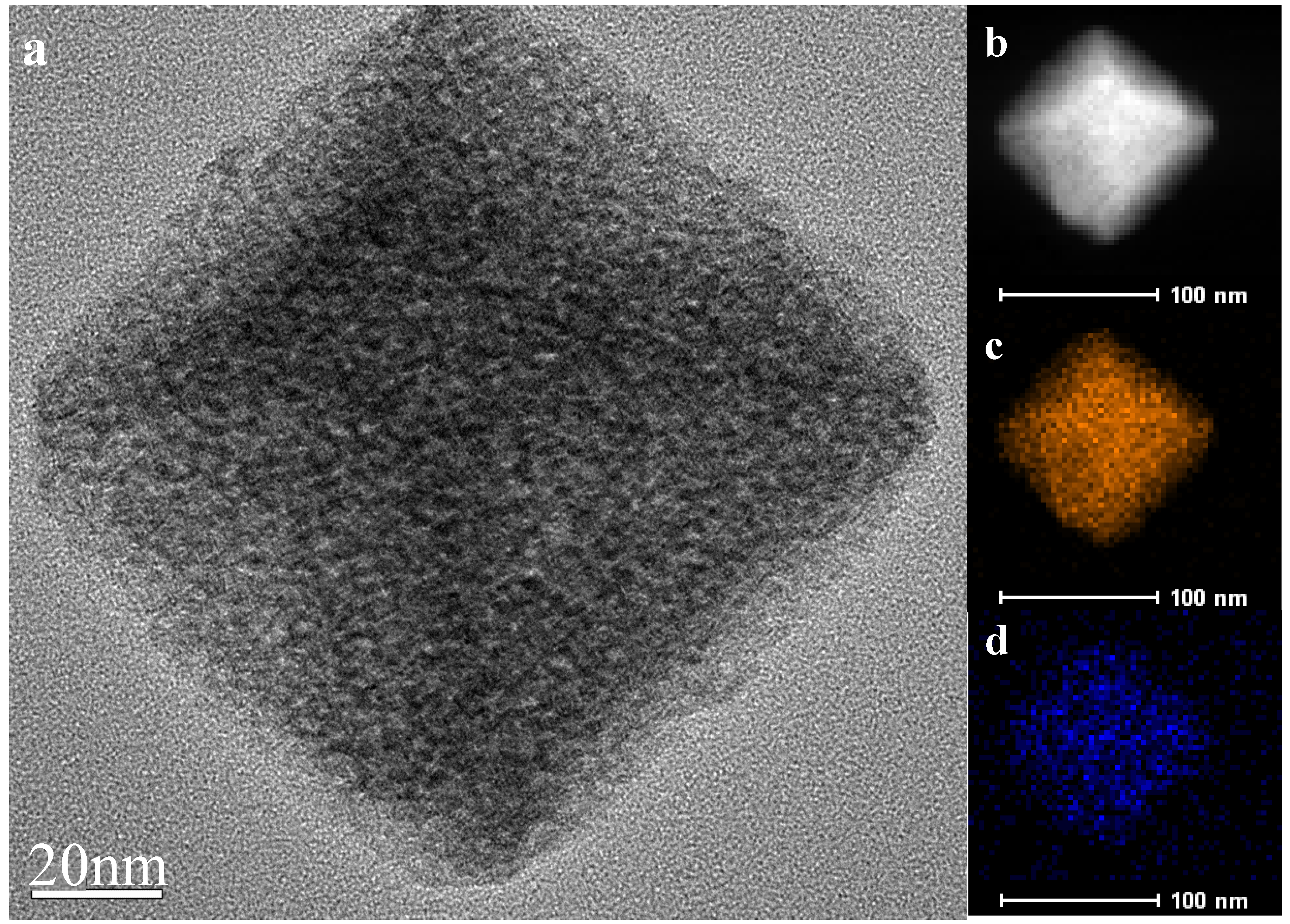



| Sample | Ce 1 (wt%) | SBET (m2g1) | Median Pore Width(Å) |
|---|---|---|---|
| MIL-101(Cr) | — | 1767 | 6.726 |
| quasi-MIL-101(Cr) | — | 1146 | 6.225 |
| 0.4%CeO2/quasi-MIL-101 | 0.4 | 218 | 6.508 |
| 0.5%CeO2/quasi-MIL-101 | 0.5 | 211 | 6.506 |
| 0.6%CeO2/quasi-MIL-101 | 0.6 | 202 | 6.088 |
| XPS Spectra | Element Valence | 0.4%CqM | 0.5%CqM | 0.6%CqM | |||
|---|---|---|---|---|---|---|---|
| Binding Energies, eV | Percent of Valence State, % | Binding Energies, eV | Percent of Valence State, % | Binding Energies, eV | Percent of Valence State, % | ||
| Cr 2p | Cr2+ | 575.77 | 13.95 | 575.71 | 13.99 | 575.92 | 19.17 |
| Cr3+ | 576.87 | 46.14 | 576.83 | 42.71 | 577.05 | 36.84 | |
| Cr5+ | 578.48 | 39.91 | 578.58 | 43.30 | 578.55 | 43.99 | |
| O 1s | OLatt | 530.35 | 49.47 | 530.30 | 54.40 | 530.41 | 56.73 |
| OAds | 531.50 | 50.53 | 531.30 | 45.60 | 531.37 | 43.27 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Hou, H.; Wei, C.; Guan, X.; Wei, W.; Wang, G.-S. Preparation of Quasi-MIL-101(Cr) Loaded Ceria Catalysts for the Selective Catalytic Reduction of NOx at Low Temperature. Catalysts 2020, 10, 140. https://doi.org/10.3390/catal10010140
Lu M, Hou H, Wei C, Guan X, Wei W, Wang G-S. Preparation of Quasi-MIL-101(Cr) Loaded Ceria Catalysts for the Selective Catalytic Reduction of NOx at Low Temperature. Catalysts. 2020; 10(1):140. https://doi.org/10.3390/catal10010140
Chicago/Turabian StyleLu, Min, Haili Hou, Chuanying Wei, Xiaohui Guan, Wei Wei, and Guang-Sheng Wang. 2020. "Preparation of Quasi-MIL-101(Cr) Loaded Ceria Catalysts for the Selective Catalytic Reduction of NOx at Low Temperature" Catalysts 10, no. 1: 140. https://doi.org/10.3390/catal10010140
APA StyleLu, M., Hou, H., Wei, C., Guan, X., Wei, W., & Wang, G.-S. (2020). Preparation of Quasi-MIL-101(Cr) Loaded Ceria Catalysts for the Selective Catalytic Reduction of NOx at Low Temperature. Catalysts, 10(1), 140. https://doi.org/10.3390/catal10010140






