DeNOx of Nano-Catalyst of Selective Catalytic Reduction Using Active Carbon Loading MnOx-Cu at Low Temperature
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Characterization of Support
2.2. The Influence of MnOx Loading Amount on Catalytic Activity
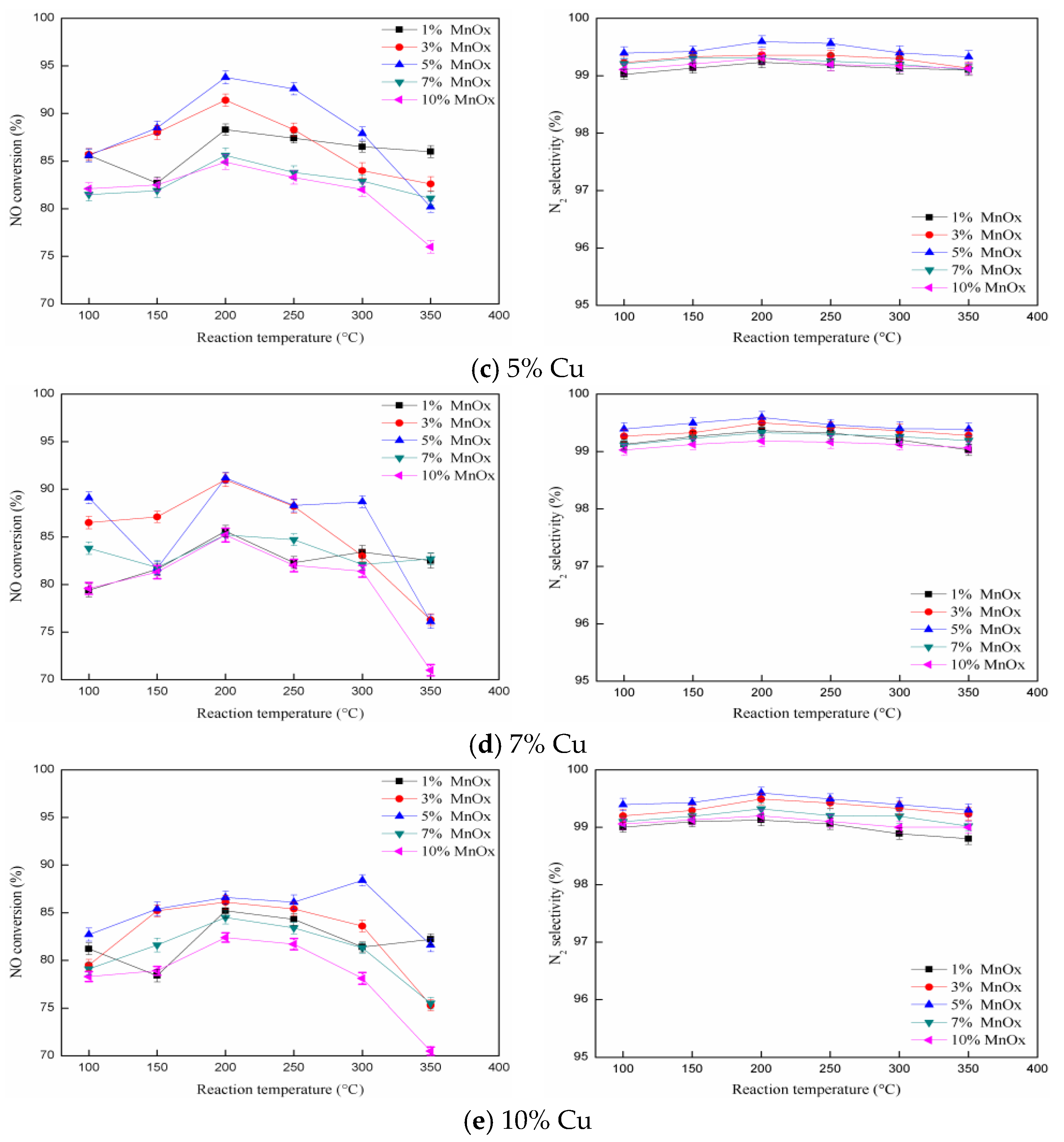
2.3. The Influence of Cu Loading Amount on Catalytic Activity
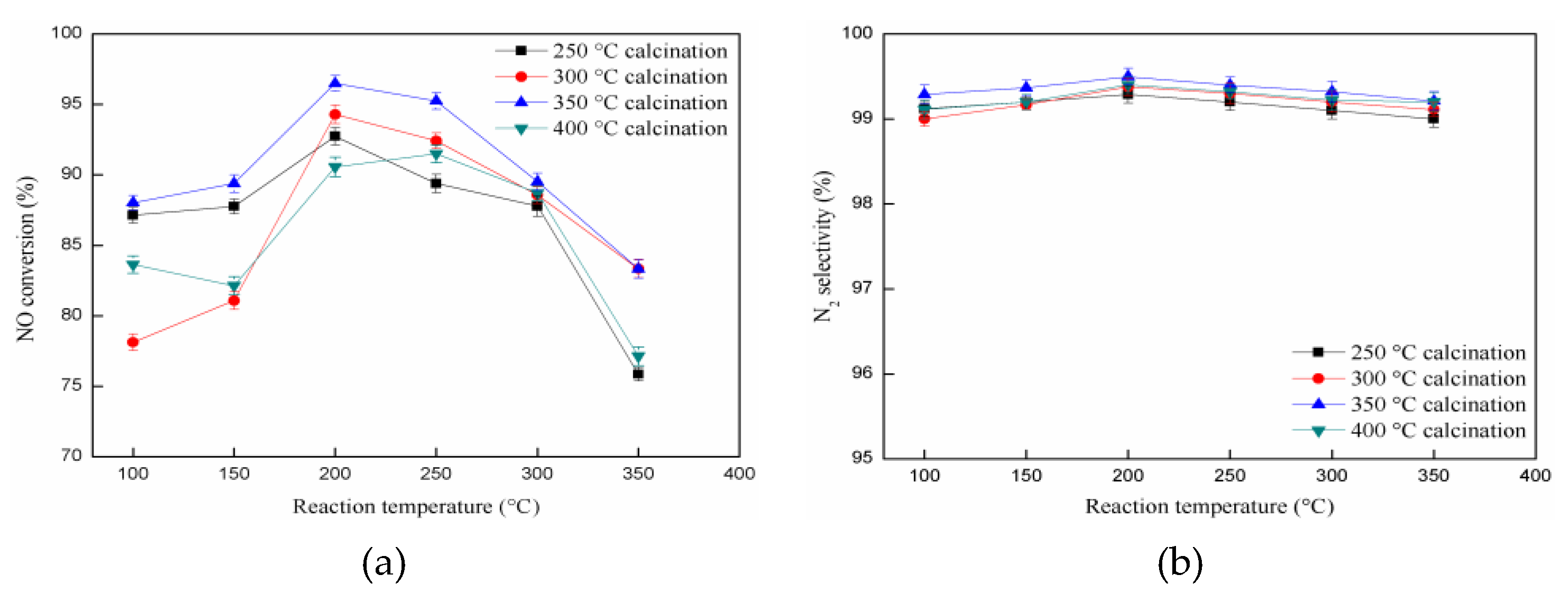
2.4. The Influence of Calcination Temperature on Catalytic Activity
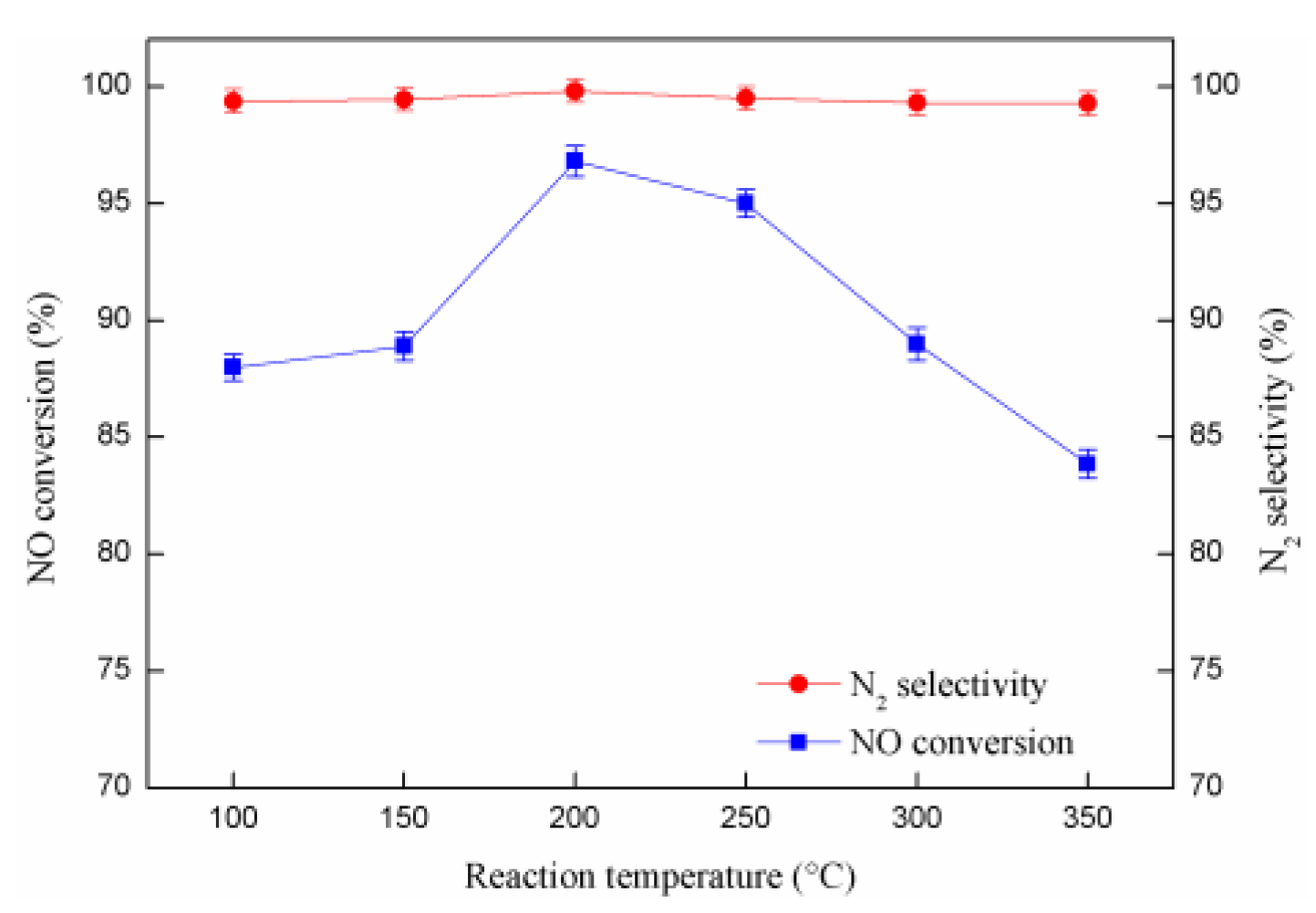
2.5. The Influence of Reaction Temperature on Catalytic Activity
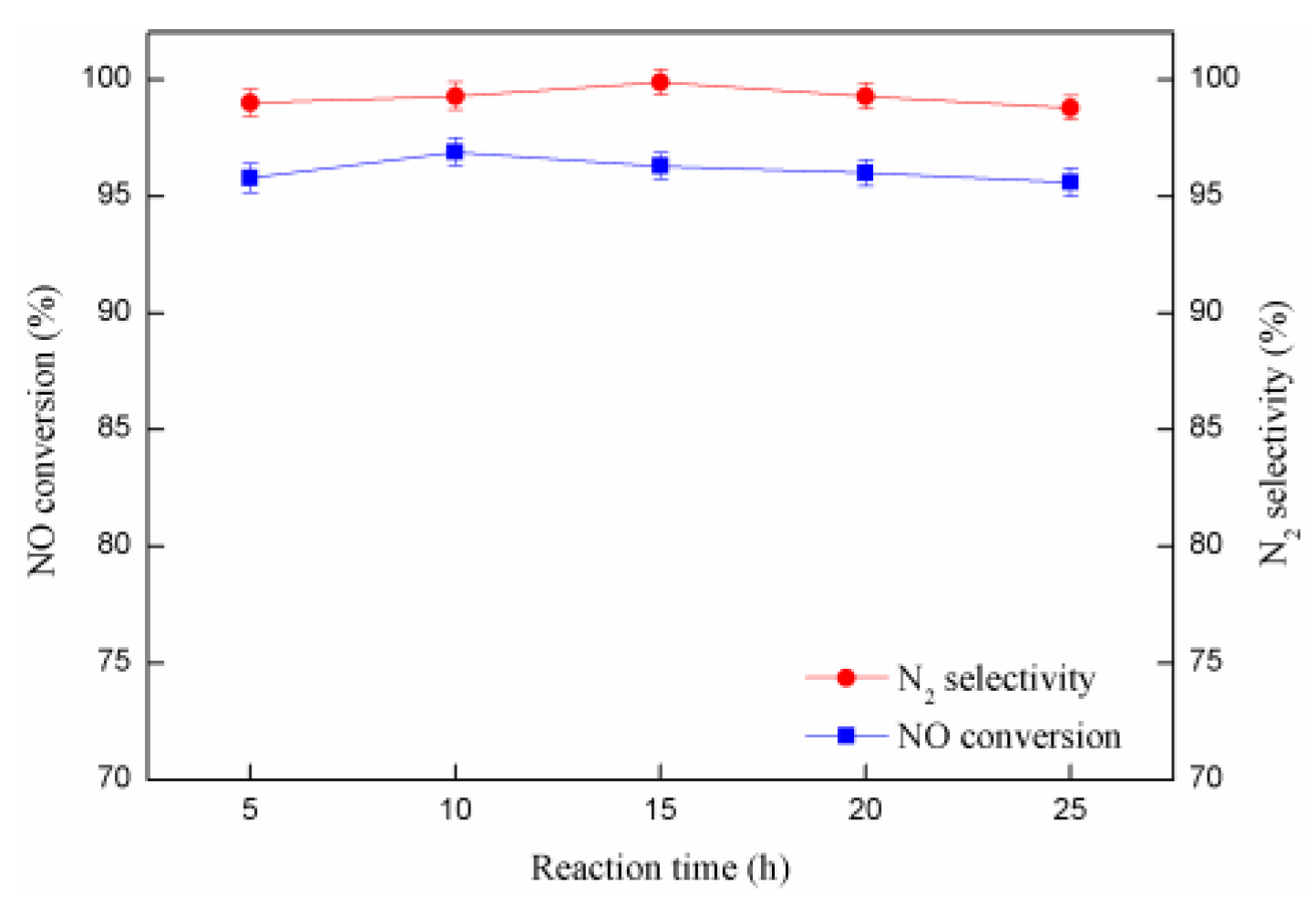
2.6. The Catalytic Stability Test of SCR Catalyst
2.7. The Catalytic Activity Analysis of the SCR Catalyst
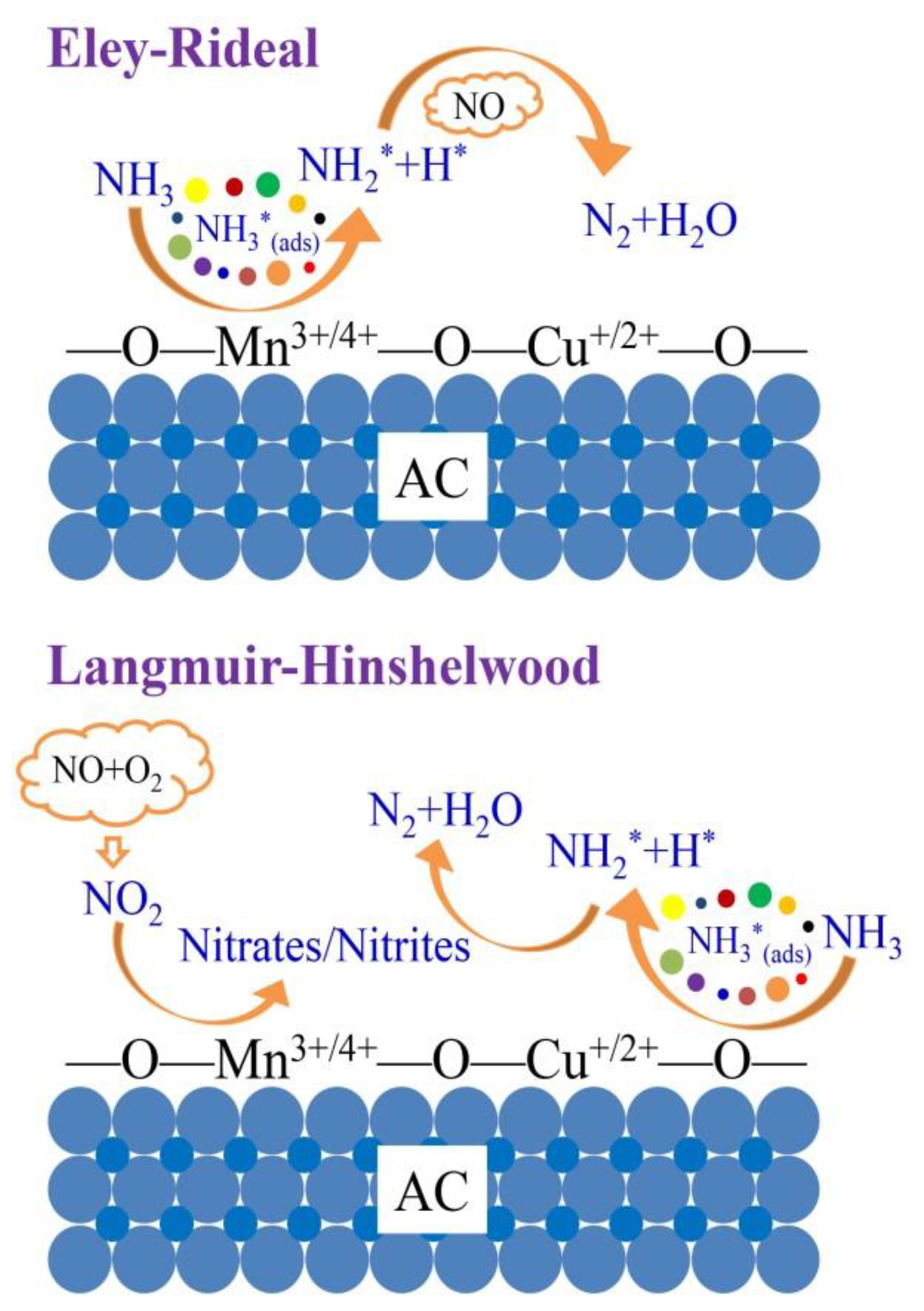
2.8. The Reaction Mechanism of NH3-SCR
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
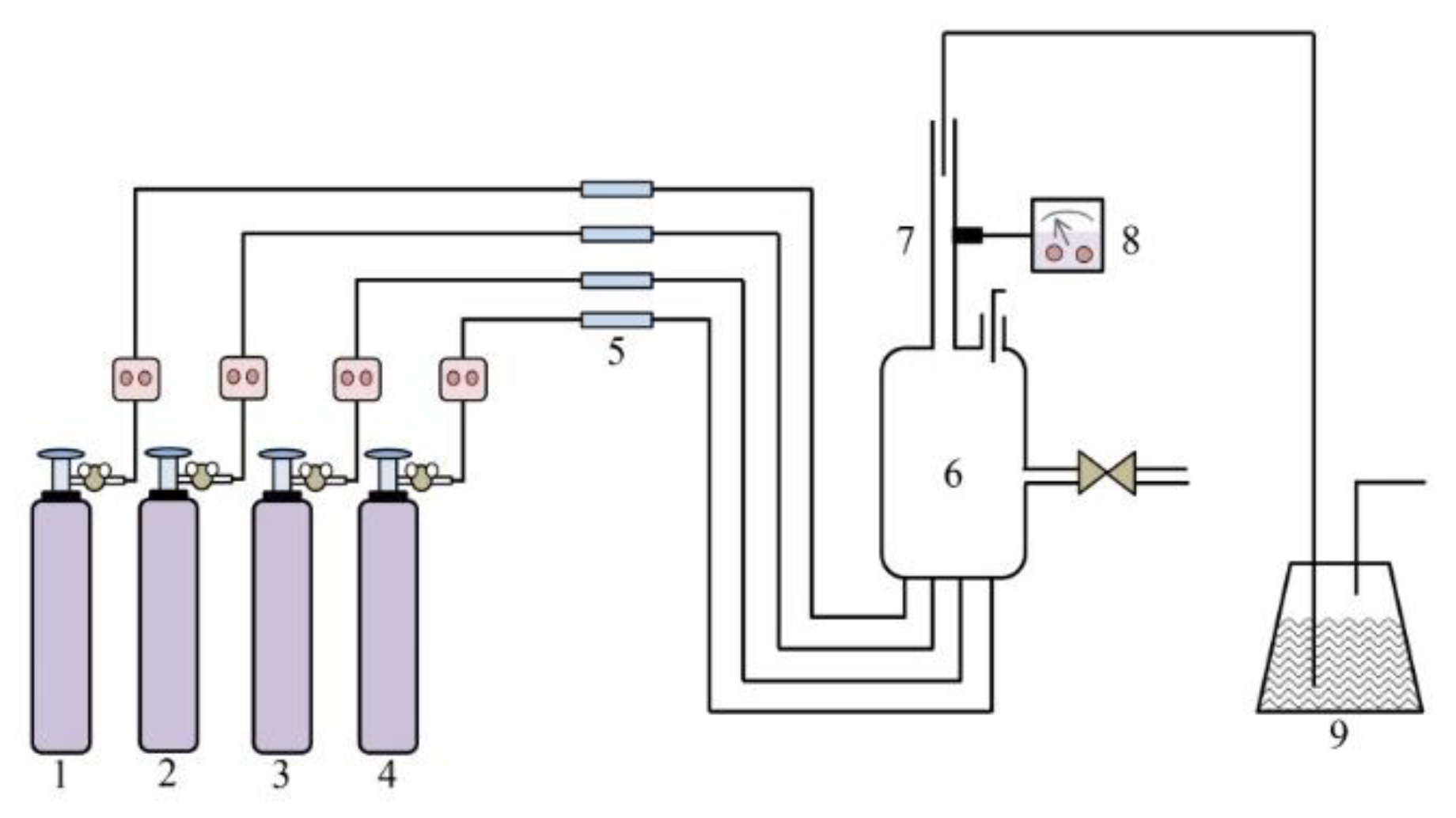
3.3. Catalyst Activity Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, C.J.; Zhang, H.L.; Dong, L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Mosrati, J.; Atia, H.; Eckelt, R.; Lund, H.; Agostini, G.; Bentrup, U.; Rockstroh, N.; Keller, S.; Armbruster, U.; Mhamdi, M. Nb-Modified Ce/Ti Oxide Catalyst for the Selective Catalytic Reduction of NO with NH3 at Low Temperature. Catalysts 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Daood, S.S.; Yelland, T.S.; Nimmo, W. Selective non-catalytic reduction Fe-based additive hybrid technology. Fule 2017, 208, 353–362. [Google Scholar] [CrossRef]
- Yan, Q.H.; Yang, R.Y.; Zhang, Y.L.; Umar, A.; Huang, Z.G.; Wang, Q. A comprehensive review on selective catalytic reduction catalysts for NOx emission abatement from municipal solid waste incinerators. Environ. Prog. Sustain. 2016, 35, 1061–1069. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, H.T.; Luo, X.; Shi, K.Q.; Zhao, H.B.; Wang, W.K.; Chen, Q.H.; Fan, H.; Wu, T. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/gamma-Al2O3 catalysts. Appl. Catal. B Environ. 2019, 245, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Sala, R.; Dzida, J.; Krasowski, J. Ammonia Concentration Distribution Measurements on Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Yu, F.; Zhu, M.Y.; Dan, J.M.; Wang, X.G.; Zhang, J.L.; Dai, B. Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3_Vermiculite Monolithic Honeycomb Catalysts. Catalysts 2018, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.H.; Hao, Z.F.; Li, Y.; Liu, G.Q.; Zhang, H.; Wang, H.T.; Xia, Y.G.; Zhan, S.H. Relationship between structure and performance of a novel highly dispersed MnOx on Co-Al layered double oxide for low temperature NH3-SCR. Appl. Catal. B Environ. 2019, 258, 983–992. [Google Scholar] [CrossRef]
- Han, L.P.; Gao, M.; Hasegawa, J.; Li, S.X.; Shen, Y.J.; Li, H.R.; Shi, L.Y.; Zhang, D.S. SO2-tolerant selective catalytic reduction of NOx over meso-TiO2@Fe2O3@Al2O3 metal-based monolith catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef]
- Blanco, J.; Avila, P.; Suárez, S.; Yates, M.; Martin, J.A.; Marzo, L.; Knapp, C. CuO/NiO monolithic catalysts for NOx removal from nitric acid plant flue gas. Chem. Eng. J. 2004, 97, 1–9. [Google Scholar] [CrossRef]
- Lin, T.; Zhang, Q.L.; Li, W.; Gong, M.C.; Xing, Y.X.; Chen, Y.Q. Monolith Manganese-Based Catalyst Supported on ZrO2-TiO2 for NH3-SCR Reaction at Low Temperature. Acta Phys. Chim. Sin. 2008, 24, 1127–1131. [Google Scholar] [CrossRef]
- Brookshear, D.W.; Nam, J.; Nguyen, K.; Toops, T.J.; Binder, A. Impact of sulfation and desulfation on NOx reduction using Cu-chabazite SCR catalysts. Catal. Today 2015, 258, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Sheng, Z.; Yang, L.; Dong, F. Low-temperature selective catalytic reduction of NOx with NH3 over a manganese and cerium oxide/graphene composite prepared by a hydrothermal method. Catal. Sci. Technol. 2016, 6, 1507–1514. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Rafalskalasocha, A.; Majda, D.; Dziembaj, R. Catalytic activity of Co-Mg-Al, Cu-Mg-Al and Cu-Co-Mg-Al mixed oxides derived from hydrotalcites in SCR of NO with ammonia. Appl. Catal. B Environ. 2002, 35, 195–210. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, W.; Tang, Y.; Li, L.; Wang, H.J.; Cui, Y.L.; Gu, J.L.; Li, Y.S.; Shi, J.L. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B Environ. 2014, 148, 114–122. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Ouzzine, M.; Cifredo, G.A.; Gatica, J.M.; Harti, S.; Chafik, T.; Vidal, H. Original carbon-based honeycomb monoliths as support of Cu or Mn catalysts for low-temperature SCR of NO: Effects of preparation variables. Appl. Catal. A Gen. 2008, 342, 150–158. [Google Scholar] [CrossRef]
- Yao, X.; Kong, T.; Chen, L.; Ding, S.; Yang, F.; Dong, L. Enhanced low-temperature NH3-SCR performance of MnOx/CeO2 catalysts by optimal solvent effect. Appl. Surf. Sci. 2017, 420, 407–415. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.W.; Fan, Z.Y.; Gao, G.; Niu, C.M. Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catalysts 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.C.; Yao, L.; Pu, Y.J.; Yang, L.; Jiang, X.; Jiang, W.J. Low-temperature selective catalytic reduction of NOx with NH3 over an activated carbon-carbon nanotube composite material prepared by in situ method. RSC Adv. 2019, 9, 36658–36663. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.J.; Yu, Q.B.; Wang, B.L.; Qin, Q.; Wei, M.Q.; Fu, Q. Low temperature selective catalytic reduction of nitric oxide with urea over activated carbon supported metal oxide catalysts. Environ. Technol. 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Li, X.; Chen, Y.Q. The effect of the oxidation method of an activated carbon on the selective catalytic reduction of NOx with NH3 over CeO2/activated carbon catalysts. New Carbon Mater. 2018, 33, 237–244. [Google Scholar]
- Samojeden, B.; Grzybek, T. The influence of the promotion of N-modified activated carbon with iron on NO removal by NH3-SCR (Selective catalytic reduction). Energy 2016, 116, 1484–1491. [Google Scholar] [CrossRef]
- Li, Y.R.; Guo, Y.Y.; Xiong, J.; Zhu, T.Y.; Hao, J.K. The Roles of Sulfur-Containing Species in the Selective Catalytic Reduction of NO with NH3 over Activated Carbon. Ind. Eng. Chem. Res. 2016, 55, 12341–12349. [Google Scholar] [CrossRef]
- Valdés-Sols, T.; Marbán, G.; Fuertes, A.B. Low-temperature SCR of NOx with NH3 over carbon-ceramic supported catalysts. Appl. Catal. B Environ. 2003, 46, 261–271. [Google Scholar] [CrossRef]
- García-bordejé, E.; Calvillo, L.; Lázaro, M.J.; Moliner, R. Vanadium supported on carbon-coated monoliths for the SCR of NO at low temperature: Effect of pore structure. Appl. Catal. B Environ. 2004, 50, 235–242. [Google Scholar] [CrossRef]
- Wang, Y.L.; Huang, Z.G.; Liu, Z.Y.; Liu, Q.Y. A novel activated carbon honeycomb catalyst for simultaneous SO2 and NO removal at low temperatures. Carbon 2004, 42, 423–460. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Liu, Z.Y.; Niu, H.X.; Liu, S.J.; Hu, T.D.; Liu, T.; Xie, Y.N. Mechanism of SO2 Promotion for NO Reduction with NH3 over Activated Carbon-Supported Vanadium Oxide Catalyst. J. Catal. 2001, 197, 6–16. [Google Scholar] [CrossRef]
- Ye, T.; Chen, D.L.; Yin, Y.S.; Liu, J.; Zeng, X. Experimental Research of an Active Solution for Modeling In Situ Activating Selective Catalytic Reduction Catalyst. Catalysts 2018, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Flura, A.; Can, F.; Courtois, X.; Royer, S.; Duprez, D. High-surface-area zinc aluminate supported silver catalysts for low-temperature SCR of NO with ethanol. Appl. Catal. B Environ. 2012, 126, 275–289. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, L.M.; Jiang, B.Q.; Cheng, M.; Zhang, J.W.; Hu, Y.J. Stability of Cu-Mn bimetal catalysts based on different zeolites for NOx removal from diesel engine exhaust. Chin. J. Catal. 2018, 39, 800–809. [Google Scholar] [CrossRef]
- Jiang, H.X.; Zhao, J.; Jiang, D.Y.; Zhang, M.H. Hollow MnOx–CeO2 nanospheres prepared by a green route: A novel low-temperature NH3-SCR catalyst. Catal. Lett. 2014, 144, 325–332. [Google Scholar] [CrossRef]
- Xu, L.T.; Niu, S.L.; Lu, C.M.; Zhang, Q.; Li, J. Influence of calcination temperature on Fe0.8Mg0.2Oz catalyst for selective catalytic reduction of NOx with NH3. Fuel 2018, 219, 248–258. [Google Scholar] [CrossRef]
- Huang, L.; Zong, Y.H.; Wang, H.; Li, Q.; Chen, T.; Dong, L.; Zou, W.X.; Guo, K. Influence of calcination temperature on the plate-type V2O5-MoO3/TiO2 catalyst for selective catalytic reduction of NO. React. Kinet. Mech. Catal. 2018, 124, 603–617. [Google Scholar] [CrossRef]
- Ren, B.N. Low-temperature selective catalytic reduction of NO with NH3 over CuOx/CNTs Catalyst. Mat. Sci. Eng. R. 2017, 281, 12006. [Google Scholar] [CrossRef]
- Jiang, L.J.; Liu, Q.C.; Zhao, Q.; Ren, S.; Kong, M.; Yao, L.; Meng, F. Promotional effect of Ce on the SCR of NO with NH3 at low temperature over MnOx supported by nitric acid-modified activated carbon. Res. Chem. Intermed. 2018, 44, 1729–1744. [Google Scholar] [CrossRef]
- Tang, X.L.; Hao, J.M.; Xu, W.G.; Li, J.H. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2006, 8, 329–334. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.Y.; Liu, J.; Liu, H.Z.; Wang, Y.; Sun, B.M. Effects of temperature on NOx removal with Mn-Cu/ZSM5 catalysts assisted by plasma. Appl. Therm. Eng. 2018, 130, 1224–1232. [Google Scholar] [CrossRef]
- Gao, X.; Liu, S.J.; Zhang, Y.; Luo, Z.Y.; Cen, K.F. Physicochemical properties of metal-doped activated carbons and relationship with their performance in the removal of SO2 and NO. J. Hazard. Mater. 2011, 188, 58–66. [Google Scholar] [CrossRef]
- Xin, Y.; Li, H.; Zhang, N.N.; Li, Q.; Zhang, Z.L.; Cao, X.M.; Hu, P.; Zheng, L.R.; Anderson, J.A. Molecular-level insight into selective catalytic reduction of NOx with NH3 to N2 over a highly efficient bifunctional V-alpha-MnOx catalyst at low temperature. ACS Catal. 2018, 8, 4937–4949. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Guo, Y.X.; Yang, Y.J.; Huang, H.; Qu, D.L. MnOx-CeOx Nanoparticles supported on graphene aerogel for selective catalytic reduction of nitric oxides. J. Nanomater. 2019, 4239764. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Hong, S.C. MnOx/CeO2-TiO2 mixed oxide catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chem. Eng. J. 2012, 195, 323–331. [Google Scholar] [CrossRef]
- Fang, D.; Xie, J.L.; Mei, D.; Zhang, Y.M.; He, F.; Liu, X.Q.; Li, Y.M. Effect of CuMn2O4 spinel in Cu–Mn oxide catalysts on selective catalytic reduction of NOx with NH3 at low temperature. RSC Adv. 2014, 4, 25540–25551. [Google Scholar] [CrossRef]
- Zhang, M.H.; Cao, H.B.; Chen, Y.F.; Jiang, H.X. Role of Mn: Promotion of Fast-SCR for Cu-SAPO-34 in Low-Temperature Selective Catalytic Reduction with Ammonia. Catal. Surv. Asia 2019, 23, 245–255. [Google Scholar] [CrossRef]
- Cai, S.X.; Zhang, D.S.; Zhang, L.; Huang, L.; Li, H.R.; Gao, R.H.; Shi, L.Y.; Zhang, J.P. Comparative study of 3D ordered macroporous Ce0.75Zr0.2M0.05O2-delta (M = Fe, Cu, Mn, Co) for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2014, 4, 93–101. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.Y.; Sun, N.N.; Wang, H.; Zhong, L.S.; He, C.; Wei, W.; Sun, Y.H. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Yao, Z.; Qu, D.L.; Guo, Y.X.; Yang, Y.J.; Huang, H. Fabrication and Characteristics of Mn@ Cu3(BTC)2 for Low-Temperature Catalytic Reduction of NOx with NH3. Adv. Mater. Sci. Eng. 2019, 2019, 2935942. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Zhang, F.; Wang, H.C. Manganese-cerium mixed oxides supported on rice husk based activated carbon with high sulfur tolerance for low-temperature selective catalytic reduction of nitrogen oxides with ammonia. RSC Adv. 2019, 9, 23964–23972. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.L.; Chen, F.; Dong, L.F.; Liu, X.Q.; Huang, B.C.; Wang, X.N.; Zhong, S.B. Manganese-rich MnSAPO-34 molecular sieves as an efficient catalyst for the selective catalytic reduction of NOx with NH3: One-pot synthesis, catalytic performance, and characterization. Environ. Sci. Pollut. R. 2017, 24, 7499–7510. [Google Scholar] [CrossRef]
- Luo, S.P.; Zhou, W.T.; Xie, A.J.; Wu, F.Q.; Yao, C.; Li, X.Z.; Zuo, S.X.; Liu, T.H. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2-Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Yan, Q.H.; Chen, S.N.; Zhang, C.; O’Hare, D.; Wang, Q. Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. J. Colloid Interf. Sci. 2018, 526, 63–74. [Google Scholar] [CrossRef]
- Bennici, S.; Carniti, P.; Gervasini, A. Bulk and surface properties of dispersed CuO phases in relation with activity of NOx reduction. Catal. Lett. 2004, 98, 187–194. [Google Scholar] [CrossRef]
- Dong, G.J.; Li, Y.M.; Wang, Y.G.; Zhang, J.; Duan, R.R. DeNOx performance of Cu-Mn composite catalysts prepared by the slurry coating method. React. Kinet. Mech. Cat. 2014, 111, 235–245. [Google Scholar] [CrossRef]
- Yan, Q.H.; Gao, Y.S.; Li, Y.R.; Vasiliades, M.A.; Chen, S.N.; Zhang, C.; Gui, R.R.; Wang, Q.; Zhu, T.Y.; Efstathiou, A.M. Promotional effect of Ce doping in Cu4Al1Ox-LDO catalyst for low-T practical NH3-SCR: Steady-state and transient kinetics studies. Appl. Catal. B Environ. 2019, 255, 117749. [Google Scholar] [CrossRef]
- Zhu, B.Z.; Li, G.B.; Sun, Y.L.; Yin, S.L.; Fang, Q.L.; Zi, Z.H.; Zhu, Z.C.; Li, J.X.; Mao, K.K. De-NOx Performance and Mechanism of Mn-Based Low-Temperature SCR Catalysts Supported on Foamed Metal Nickel. J. Brazil. Chem. Soc. 2018, 29, 1680–1689. [Google Scholar] [CrossRef]
- Efstathiou, A.M.; Fliatoura, K. Selective catalytic reduction of nitric oxide with ammonia over V2O5/TiO2 catalyst: A steady-state and transient kinetic study. Appl. Catal. B Environ. 1995, 6, 35–59. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Belessi, V.C.; Costa, C.N.; Neophytides, S.; Falaras, P.; Efstathiou, A.M.; Pomonis, P.J. Catalytic activity of high surface area mesoporous Mn-based mixed oxides for the deep oxidation of methane and lean-NOx reduction. Stud. Surf. Sci. Catal. 2000, 130, 1529–1534. [Google Scholar]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Zhao, H.W.; Haller, G.; Li, Y.D. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Gao, F.; Peden, C.H.F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. [Google Scholar]
- Wang, S.X.; Guo, R.T.; Pan, W.G.; Chen, Q.L.; Sun, P.; Li, M.Y.; Liu, S.M. The deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by alkali metals: TPD and DRIFT studies. Catal. Commun. 2017, 89, 143–147. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; Bliek, A. Kinetics of the selective catalytic reduction of NO with NH3 over MnOx/Al2O3 catalysts at low temperature. Catal. Today 1999, 50, 133–140. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, S.M.; Kim, S.S.; Kwon, D.W.; Hong, S.C. Reversibility of Mn Valence State in MnOx/TiO2 Catalysts for Low-temperature Selective Catalytic Reduction for NO with NH3. Catal. Lett. 2013, 143, 246–253. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, J.; Liu, F.; Wang, Z.; Ding, J.Y.; Huang, H. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst. Chem. Eng. J. 2019, 361, 578–587. [Google Scholar] [CrossRef]






| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| Before processing | 638 ± 1.96 | 0.29 ± 0.01 | 0.26 ± 0.02 | 10.27 ± 0.03 |
| After processing | 696 ± 2.28 | 0.30 ± 0.02 | 0.31 ± 0.03 | 8.56 ± 0.02 |
| SCR Catalysts | Mn2+ (eV) | Mn3+ (eV) | Mn4+ (eV) | N(Mn3+ + Mn4+)/n(Mnx+) |
|---|---|---|---|---|
| 5% MnOx-3% Cu | 642.3 | 643.4 | 644.7 | 0.86 |
| 5% MnOx-5% Cu | 642.3 | 643.4 | 644.8 | 0.85 |
| 3% MnOx-5% Cu | 642.1 | 643.2 | 644.8 | 0.82 |
| 3% MnOx-3% Cu | 640.2 | 641.8 | 643.5 | 0.78 |
| SCR Catalysts | Peak Area | H2 Consumption (μmol/g) |
|---|---|---|
| 5% MnOx-3% Cu | 7197 | 828 |
| 5% MnOx-5% Cu | 6883 | 791 |
| 3% MnOx-5% Cu | 6790 | 780 |
| 3% MnOx-3% Cu | 6612 | 759 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Zhang, X.; Bian, W.; Han, Y.; Liu, T.; Liu, H. DeNOx of Nano-Catalyst of Selective Catalytic Reduction Using Active Carbon Loading MnOx-Cu at Low Temperature. Catalysts 2020, 10, 135. https://doi.org/10.3390/catal10010135
Zhu T, Zhang X, Bian W, Han Y, Liu T, Liu H. DeNOx of Nano-Catalyst of Selective Catalytic Reduction Using Active Carbon Loading MnOx-Cu at Low Temperature. Catalysts. 2020; 10(1):135. https://doi.org/10.3390/catal10010135
Chicago/Turabian StyleZhu, Tao, Xing Zhang, Wenjing Bian, Yiwei Han, Tongshen Liu, and Haibing Liu. 2020. "DeNOx of Nano-Catalyst of Selective Catalytic Reduction Using Active Carbon Loading MnOx-Cu at Low Temperature" Catalysts 10, no. 1: 135. https://doi.org/10.3390/catal10010135
APA StyleZhu, T., Zhang, X., Bian, W., Han, Y., Liu, T., & Liu, H. (2020). DeNOx of Nano-Catalyst of Selective Catalytic Reduction Using Active Carbon Loading MnOx-Cu at Low Temperature. Catalysts, 10(1), 135. https://doi.org/10.3390/catal10010135





