Abstract
An exclusive trace of CH4 direct carboxylation with CO2 by a stepwise technology was investigated using in-situ FT-IR spectroscopy. The results showed that CH4 was dissociated to atomic hydrogen and M-CHx species on catalyst surface when it was first introduced in the system, then CO2 was inserted into the intermediate to direct carboxylate. Finally, the subsequent adsorption of CH4 provided active hydrogen for the species of previous surface reaction, thus leading to the formation of the product. It was also found that the first introduction of CO2 on the surface of the “clean” catalyst might likely react with surface H species, which had an irreversible effect on the catalytic activity of CH4.
1. Introduction
Chemical conversion of CO2 into value-added fuels and chemicals is regarded as an attractive strategy to simultaneously reduce the CO2 emissions and relieve the shortage of fossil-fuels both in the energy and chemical industries [1]. To date, much attention has been paid to CO2 hydrogenation routes [2]. However, the high energy and H2 consumptions, as well as the rigor operating conditions related to this process, are major challenges.
Instead of using H2, the high H/C ratio and abundant reserves of CH4 make it a viable source of both energy and hydrogen for CO2 conversion. In fact, CH4 is an ideal H supplier to replace H2, while CO2 can provide oxygen for the chemical fixation of CH4. Thus, the simultaneous conversion of CO2 and CH4 to C2+oxygenates (e.g., acetic acid) is an ideal combination of a reduction reaction and an oxygenation reaction. Moreover, due to the stoichiometric ratio of C and O atoms, the co-conversion of CO2 and CH4 into acetic acid (CO2 + CH4→CH3COOH) is 100% atom economy, which can increase the atom utilization and avoid the formation of H2O comparing with CO2 hydrogenation. Unfortunately, the reaction is thermodynamically unfavorable under moderate conditions (△G298K = 71.2 kJ/mol). Many works are devoted to selectively break the C–H bond in CH4 and simultaneously utilize the inertness of CO2 to realize C-C coupling for the synthesis of high-value chemicals [3,4,5,6,7]. Most of these works are based on theoretical calculation and experiments which always require additional energy supply (such as plasma), few in-situ characterizations studies have been conducted. Weng et al. [8] employed in-situ time-resolved FT-IR to investigate the partial oxidation of methane to syngas at 500 °C, which indicated that significant different mechanisms were proceeded over supported Rh and Ru catalysts. Besides, the in-situ FT-IR spectroscopy was also conducted to investigate the surface reaction of CH4 with NOx species [9,10,11,12].
Our group was committed to investigating the direct conversion of CH4 with CO2 to acetic acid by stepwise reaction technology (Scheme 1) that bypassed the thermodynamic limitation and demonstrated the feasible and efficient through initial experiments in micro-reactor units at low temperatures over Cu-Co and Co-Pd catalyst [13,14,15]. However, the possible reaction intermediates in this process had not been elucidated. In addition, to the best of our knowledge, there were no detailed studies focus on direct carboxylation of CH4 to investigate the reaction mechanism over Cu-Co catalyst at a lower temperature using in-situ technologies. Therefore, in this study, an exclusive trace of adsorbed CH4 direct carboxylation with CO2 was performed using the stepwise technology by in-situ FT-IR spectroscopy to gain a deeper understanding of the results of the interaction between CH4 and CO2.
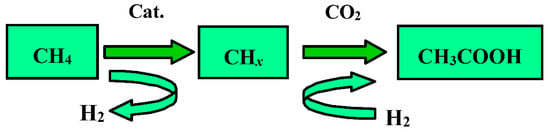
Scheme 1.
Stepwise reaction technology for direct synthesis of CH3COOH from CO2 and CH4.
2. Results
2.1. Blank Experiment
Figure 1a showed the FT-IR spectra of pure SiO2 (diluent in this experiment) exposed in CH4 for the first time after pretreatment in N2 for 1 h. The SiO2 also underwent the same reduction and purging process under N2 atmosphere as that of the catalyst sample. One could see that the intensity of the two main absorption bands at 3015 cm−1 and 1302 cm−1 gradually increased with the introduction of CH4, which was assigned to the adsorption of gas CH4 and weak adsorption CH4, respectively [16,17]. Amplification of 1400 cm−1–2200 cm−1 (see Figure 1e) showed that there were two broad hydroxyl absorption peaks in 1687 cm−1 and 1524 cm−1, which gradually became clear and stable with the increase of contact time.
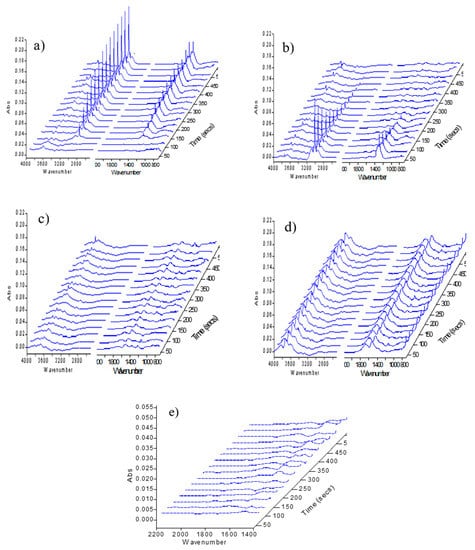
Figure 1.
FT-IR spectra of SiO2 exposed in different atmospheres. (a) Exposed in CH4 for the first time; (b) Exposed in N2 after the first time of CH4 adsorption; (c) Exposed in CO2 for the first time; (d) IR spectra after alternate feeds of CH4 and CO2 three times; (e) Amplification of 1400 cm−1–2200 cm−1 when SiO2 exposed in CH4 for the first time.
Figure 1b was the FT-IR spectra under N2 purging after the first cycle of CH4 adsorption. It could be seen that the peaks of CH4 absorption at 3015 cm−1 and 1302 cm−1 gradually decreased and disappeared, while these OH absorption peaks at 1687 cm−1, 1524 cm−1 and 3200 cm−1–3800 cm−1 slightly increased with the purge of N2. After N2 purging, the C–H absorption could hardly be observed at 2950 cm−1 and 2864 cm−1, indicating that CH4 could not be activated on the “clean” SiO2.
After N2 purging, the pure SiO2 was exposed in CO2 for the first time and the corresponding IR spectra was presented in Figure 1c. It could be seen that few changes of the adsorbed species on the surface were observed except at 1687 cm−1 and 1524 cm−1, indicating that no significant surface reaction had been taken place. In the following series of repetitive cycles, the intensity of peak at 1690 cm−1 and 1530 cm−1 further increased, and two weak peaks at 2950 cm−1 and 2864 cm−1 were found, which might be the result of the interaction between CO2 and CH4 (Figure 1d).
In conclusion, the above experiments demonstrated that the CH4 activation and the co-conversion with CO2 on pure SiO2 were extremely weak under the reaction conditions.
2.2. Cu-Co Catalyst Sample Experiment
Figure 2 displayed the FT-IR spectra of Cu-Co catalyst for the first cycle of CH4 adsorption, N2 purge, and CO2 adsorption. As seen, compared with pure SiO2, obvious C-H absorption peaks could be observed in the region of 2800 cm−1–3000 cm−1 after the adsorption of CH4 (see Figure 2a). Besides, the peaks of 1690 cm−1 and 1530 cm−1 appeared faster and stronger (Figure 2b). Moreover, with the introduction of CH4, negative peaks appeared in the region of 3200 cm−1–3800 cm−1 which belonged to hydroxyl, and the peaks intensity decreased slightly when CO2 was introduced (Figure 2c). These results suggested that CH4 had been activated on Cu-Co catalyst surface, which would be discussed later.
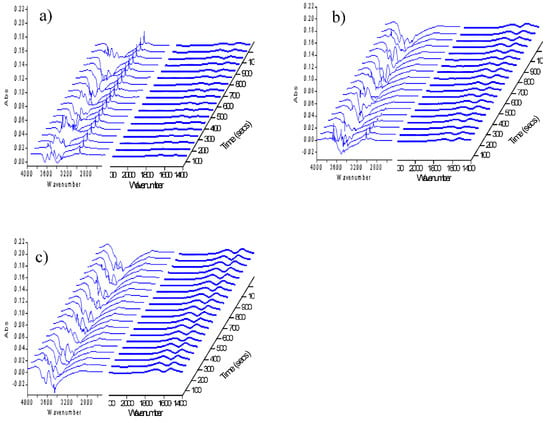
Figure 2.
FT-IR spectra of Cu-Co catalyst exposed in different atmospheres for the first cycle (a) CH4; (b) N2; (c) CO2.
Figure 3 showed the FT-IR spectra of CH4/CO2 repeated feeds. The peaks intensity of 3200 cm−1–3800 cm−1, 2700 cm−1–3000 cm−1, and 1400 cm−1–1700 cm−1 continued to increase with CH4/CO2 repeated feeding into the reaction, indicating that the activation and surface reaction of CH4 and CO2 were further proceeding. Meanwhile, the results demonstrated that the generated species accumulated on the catalyst surface.
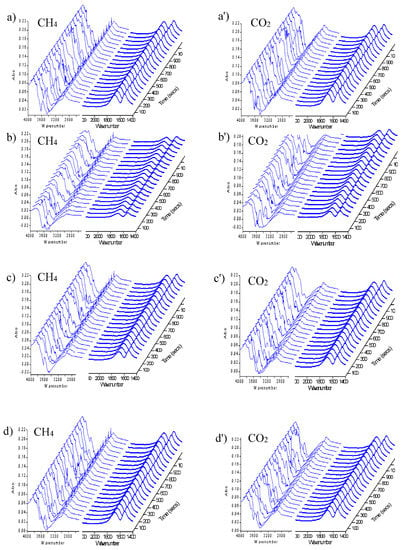
Figure 3.
FT-IR spectra of CH4/CO2 repeated feed over Cu-Co catalyst. (a,a’): the second cycle; (b,b’): the third cycle; (c,c’): the fourth cycle; (d,d’): the fifth cycle.
3. Discussion
3.1. Activation of CH4 on “Clean”Catalyst Surface
Generally, the characteristic absorption of C–H bond occurred in three regions: 3000 cm−1–2700 cm−1, 1475 cm−1–1300 cm−1 and 1000 cm−1–650 cm−1 [18]. Among them, 1000 cm−1–650 cm−1 fell in the fingerprint area, and there were many influence factors that were complex and difficult to give an unequivocal identification. For 1475 cm−1–1300 cm−1, since the SiO2 had a strong absorption and coverage, the variation was difficult to observe. Therefore, this study mainly focused on the C–H bond absorption in the region of 3000 cm−1–2700 cm−1.
In the first cycle, CH4 was introduced after the catalyst was reduced and swept by N2 for 1 h. Thus, it was considered that the interaction between CH4 and the catalyst was carried out on a “clean” surface, reflecting the activation of CH4 by the catalyst itself. Compared with pure SiO2, Figure 2b clearly showed that the bonds in the region of 2800 cm−1–3000 cm−1 increased significantly on the Cu-Co catalyst, indicating that CH4 had been activated by the catalyst.
With the introduction of CH4, a large negative peak appeared in the OH absorption region at 3200 cm−1–3800 cm−1 (Figure 2a,b). The intensity of the peak increased with the increase of contact time, while it decreased slightly after the introduction of CO2. The appearance of negative peaks indicated that OH groups were continuously consumed during the process of CH4 adsorption and activation. According to the literature [16], it was pointed out that the adsorbed methyl groups on metal sites could react with OH groups and then spilled onto the carrier. In this process, the following reactions were most likely to occur. Take Cu/SiO2 as an example:
Si–OH + Cu–CH3 → SiOCH3 + 1/2H2 + Cu.
Therefore, it was suggested that the decrease of surface OH in this study was due to the interaction between surface C-H species and adjacent OH groups, which caused the transition of CH4 activation site to inactive site. Obviously, the spillover of these CHx species on the surface favored to empty the metal active sites and thus activated more amount of CH4.
3.2. Reaction of Adsorbed CH4 and CO2
After the first introduction of CH4, pure N2 was purged to remove the free and weakly adsorbed CH4 for 20 min, CO2 was then introduced to investigate the reaction between CO2 and surface species. Rasko and Solymosi [19,20] studied the reaction of CH3 species (formed by the decomposition of diazomethane) with CO2 on different carriers and metal surfaces. The results showed that the adsorbed CH3 species could react with CO2 to produce CO and H2O under very mild conditions even at room temperature. The reason was that the introduction of CO2 led to the decrease or even disappearance of the CH3 species peak at 2800 cm−1–3000 cm−1, and the generation of CO also could be detected. However, in our study, the introduction of CO2 did not lead to the consumption of adsorbed CHx species. On the contrary, these CHx species increased. The results indicated that, on the one hand, there was no reaction occurred between surface CHx species and CO2 to form CO and H2 under the used catalyst and experimental conditions. On the other hand, it also suggested that CO2 might react with surface H species deriving from CH4 activation, which led to the rise of surface hydroxyl species. The two aspects were consistent with our previous experimental results [13].
3.3. Activation of CH4 on “Polluted” Catalyst Surface
The adsorption and activation of CH4 on the “polluted” catalyst surface could be divided into two situations: One was that the surface of the reduced “clean” catalyst was first filled with CO2, and then CH4 was introduced after N2 purge for 20 min. Another was that CH4 was re-introduced after a cycle of reaction, at which time the catalyst surface was adsorbed by CO2. Figure 4a was the FT-IR spectra when CO2 was first introduced. It was shown that no CHx species were formed on the surface of “clean” catalysts when CO2 was first introduced, suggesting that there was no adsorbed hydrogen on the catalyst surface after reduction and N2 purging process for 1 h, thus no hydrogenation reaction occurred. When CH4 was introduced, surface CHx species were generated (Figure 4b). Compared with the first introduction of CH4, the intensity and peak type of CHx species changed greatly, while the peak position had negligible changes. Although no strict quantitative determination had been made in this experiment, the ratio of catalyst and diluent in each sample was almost the same. Thus, it was concluded that the decrease in the peak area of CHx species was caused by the first adsorption of CO2. Considering that the position of CH adsorption peak moved from 2939 cm−1 to 2963 cm−1, it was considered that the first introduction of CO2 led to the oxidation of catalyst active components.
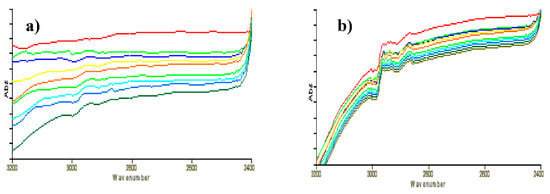
Figure 4.
FT-IR spectra of “clean” catalyst surface (a) first exposed in CO2; (b) first exposed in CO2 and then in CH4.
The peak intensity of CHx species with the introduction of CH4 in different cycles was presented in Figure 5. It could be seen that the peak intensity of CHx species increased linearly in the first cycle, whereas the peak intensity first decreased and then increased for other cycles. This was mainly due to that the first cycles occurred on the “clean” surface of the catalyst, while the adsorption and activation of CH4 were carried out on a “non-clean” surface for others. Obviously, on the “polluted” catalyst surface, the subsequent passing of CH4 provided hydrogen for the hydrogen-deficient species generated in the previous cycle. Meanwhile, one also could see that the degree of reduction increased with the accumulation of CHx species on the surface, which indicated that the desorption of these CHx species occurred on the catalyst surface. These results explained the formation of CH3COOH, HCOOH and other oxygenated products without the condition of H2 supplementation using the stepwise technology. Obviously, it could be inferred that the direct conversion of CH4 and CO2 to CH3COOH by two-step reaction without hydrogen replenishment was due to that the subsequent adsorption of CH4 provided thermodynamic power for the previous step, thus leading to the formation of products, which was also consistent with our previous experimental results [13]. The relevant reaction process could be described as follows:
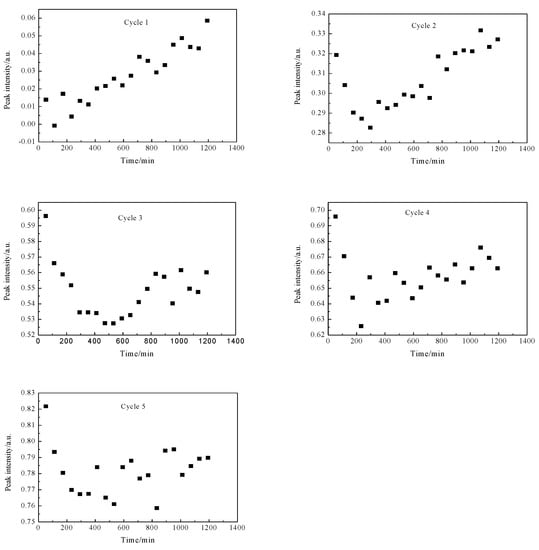
Figure 5.
The peak intensity of CHx species as a function of CH4 introduction in different cycles.
4. Experimental
4.1. Catalyst Preparation
Cu-Co catalyst was synthesized by a co-precipitation method. The Cu:Co atomic ratio in the starting solution was kept at 1:1. Typically, two aqueous solutions, a solution of Cu(II), Co(II) nitrates (analytical reagent, Tianjin, China) and a mixed solution of Na2CO3 (analytical reagent, Tianjin, China) precipitant, were added dropwise to 250 mL of deionized water under vigorous stirring. The pH value was maintained at 6–8. The precipitate was aged at 343 K for 1 h under stirring, and filtered, washed, dried at 393 K for 16 h, and then calcined at 623 K for 6 h. The as-synthesized catalyst was crushed and sieved to particles in the range of 40–60 meshes before use.
4.2. Test of In-Situ FT-IR
The in-situ FT-IR experiments were performed on a Perkin Elmer Spectrum 2000 FT-IR spectrometer (PerkinElmer, USA). The above catalyst was mixed with high purity SiO2 to make thin sheets and placed in in-situ IR cells for the test. Before the experiment, the catalyst was reduced in 10% H2/N2 at a flow rate of 20 mL/min under atmospheric pressure. The reduction temperature was programmed to increase from room temperature to 473 K with a heating rate of 5 K/min and maintained at 473 K for 2 h. After reduction, the reaction system was purged with N2 at 473 K for 1 h. Then CH4 and CO2 were alternately switched to react, during which N2 was purged with 20 min or not. At the same time, the spectral data were recorded using TimeBase software (PerkinElmer company, MA, USA) at the frequency of one graph per minute.
5. Conclusions
In conclusion, a possible reaction mechanism for the direct conversion of CH4 and CO2 to CH3COOH by a step-wise technology had been proposed based on the in-situ FT-IR spectroscopy. Results showed that CH4 was dissociated to atomic hydrogen and M-CHx species on catalyst surface when it was first introduced in the system, then CO2 was inserted into the intermediate to direct carboxylate. Finally, the subsequent CH4 adsorption provided active hydrogen for the species of previous surface reaction, thus leading to the formation of the product. The activated CH4 also could be transferred to unreduced oxides through interaction with adjacent hydroxyl groups, which favored to empty the metal active sites and thus greatly enhanced the catalytic activity of CH4. However, the first introduction of CO2 on the surface of the “clean” catalyst might likely react with surface H species, which had an irreversible effect on the catalytic activity of CH4.
Author Contributions
The experimental work was designed and supported by W.H. Y.L., analyzed the data and write the original article. N.C. and P.J. prepared the catalyst and conduct the experiment. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Project of National Natural Science Foundation of China (21336006), the National Natural Science Foundation of China (21908157), the Natural Science Younger Foundation of Shanxi Province (201801D221076) and the Key R&D Program of Shanxi Province (201803D121043).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, Q.W.; Zhou, Z.H.; He, L.N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Wang, H.; Han, J.Y.; Zhu, X.L.; Ge, Q.F. Active site ensembles enabled C-C coupling of CO2 and CH4 for acetone production. J. Phys. Chem. C 2018, 122, 9570–9577. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Cui, C.N.; Han, J.Y.; Wang, H.; Zhu, X.L.; Ge, Q.F. Direct C-C coupling of CO2 and the methyl group from CH4 activation through facile insertion of CO2 into Zn-CH3 σ‑Bond. J. Am. Chem. Soc. 2016, 138, 10191–10198. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, Y.H.; Wu, C.F.; Guo, H.C.; Tu, X. One-step reforming of CO2 and CH4 into high-value liquid chemicals and fuels at room temperature by plasma-driven catalysis. Angew. Chem. Int. Ed. 2017, 56, 13679–13683. [Google Scholar] [CrossRef] [PubMed]
- Montejo-Valencia, B.D.; Pagan-Torres, Y.J.; Martínez-Inesta, M.M.; Curet-Arana, M.C. Density functional theory (DFT) study to unravel the catalytic properties of M-exchanged MFI, (M = Be, Co, Cu, Mg, Mn, Zn) for the conversion of methane and carbon dioxide to acetic acid. ACS Catal. 2017, 7, 6719–6728. [Google Scholar] [CrossRef]
- Shavi, R.; Ko, J.; Cho, A.; Han, J.W.; Seo, J.G. Mechanistic insight into the quantitative synthesis of acetic acid by direct conversion of CH4 and CO2: An experimental and theoretical approach. Appl. Catal. B Environ. 2018, 229, 237–248. [Google Scholar] [CrossRef]
- Weng, W.Z.; Chen, M.S.; Yan, Q.G.; Wu, T.H.; Chao, Z.S.; Liao, Y.Y.; Wan, H.L. Mechanistic study of partial oxidation of methane to synthesis gas over supported rhodium and ruthenium catalysts using in situ time-resolved FTIR spectroscopy. Catal. Today 2000, 63, 317–326. [Google Scholar] [CrossRef]
- Lobree, L.J.; Aylor, A.W.; Reimer, J.A.; Bell, A.T. NO reduction by CH4 in the presence of O2 over Pd-H-ZSM-5. J. Catal. 1999, 181, 189–204. [Google Scholar] [CrossRef]
- Kantcheva, M.; Cayirtepe, I. FT-IR spectroscopic investigation of the surface reaction of CH4 with NOx species adsorbed on Pd/WO3-ZrO2 catalyst. Catal. Lett. 2007, 115, 148–162. [Google Scholar] [CrossRef]
- Kantcheva, M.; Vakkasoglu, A.S. Cobalt supported on zirconia and sulfated zirconia I.: FT-IR spectroscopic characterization of the NOx species formed upon NO adsorption and NO/O2 coadsorption. J. Catal. 2004, 223, 352–363. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Averska, V.; Kolev, H.; Marinova, T.; Klissurski, D.; Hadjiivanov, K. FT-IR study of the nature and reactivity of surface NOx compounds formed after NO adsorption and NO + O2 coadsorption on zirconia- and sulfated zirconia-supported cobalt. J. Mol. Catal. A 2003, 193, 139–149. [Google Scholar] [CrossRef]
- Huang, W.; Xie, K.C.; Wang, J.P.; Gao, Z.H.; Yin, L.H.; Zhu, Q.M. Possibility of direct conversion of CH4 and CO2 to high-value products. J. Catal. 2001, 201, 100–104. [Google Scholar] [CrossRef]
- Huang, W.; Sun, W.Z.; Li, F. Efficient synthesis of ethanol and acetic acid from methane and carbon dioxide with a continuous, stepwise reactor. AIChE J. 2010, 56, 1279–1284. [Google Scholar] [CrossRef]
- Ding, Y.H.; Huang, W.; Wang, Y.G. Direct synthesis of acetic acid from CH4 and CO2 by a step-wise route over Pd/SiO2 and Rh/SiO2 catalysts. Fuel Process. Technol. 2007, 88, 319–324. [Google Scholar] [CrossRef]
- Driessen, M.D.; Grassian, V.H. Methyl spillover on silica-supported copper catalysts from the dissociative adsorption of methyl halides. J. Catal. 1996, 161, 810–818. [Google Scholar] [CrossRef]
- Wu, T.H.; Lin, D.M.; Wu, Y.; Zhou, X.P.; Yan, Q.G.; Weng, W.Z.; Wan, H.L. In-situ FT-IR investigation of partial oxidation of methane to syngas over Rh/SiO2 catalyst. J. Nat. Gas Chem. 2007, 16, 316–321. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pandey, A.K.; Jain, S.N. FT-IR spectroscopy, intra-molecular CHO interactions, HOMO, LUMO, MESP analysis and biological activity of two natural products, triclisine and rufescine: DFT and QTAIM approaches. Misra Spectrochim. Acta A 2015, 136, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Raskó, J.; Solymosi, F. Reactions of adsorbed CH3 species with CO2 on Rh/SiO2 catalyst. Catal. Lett. 1997, 46, 153–157. [Google Scholar] [CrossRef]
- Raskó, J.; Solymosi, F. Adsorption of CH3 and its reactions with CO2 over TiO2. Catal. Lett. 1998, 54, 49–54. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).