Structural Evolution of Ni-Based Co-Catalysts on [Ca2Nb3O10]− Nanosheets during Heating and Their Photocatalytic Properties
Abstract
:1. Introduction
2. Results and Discussion
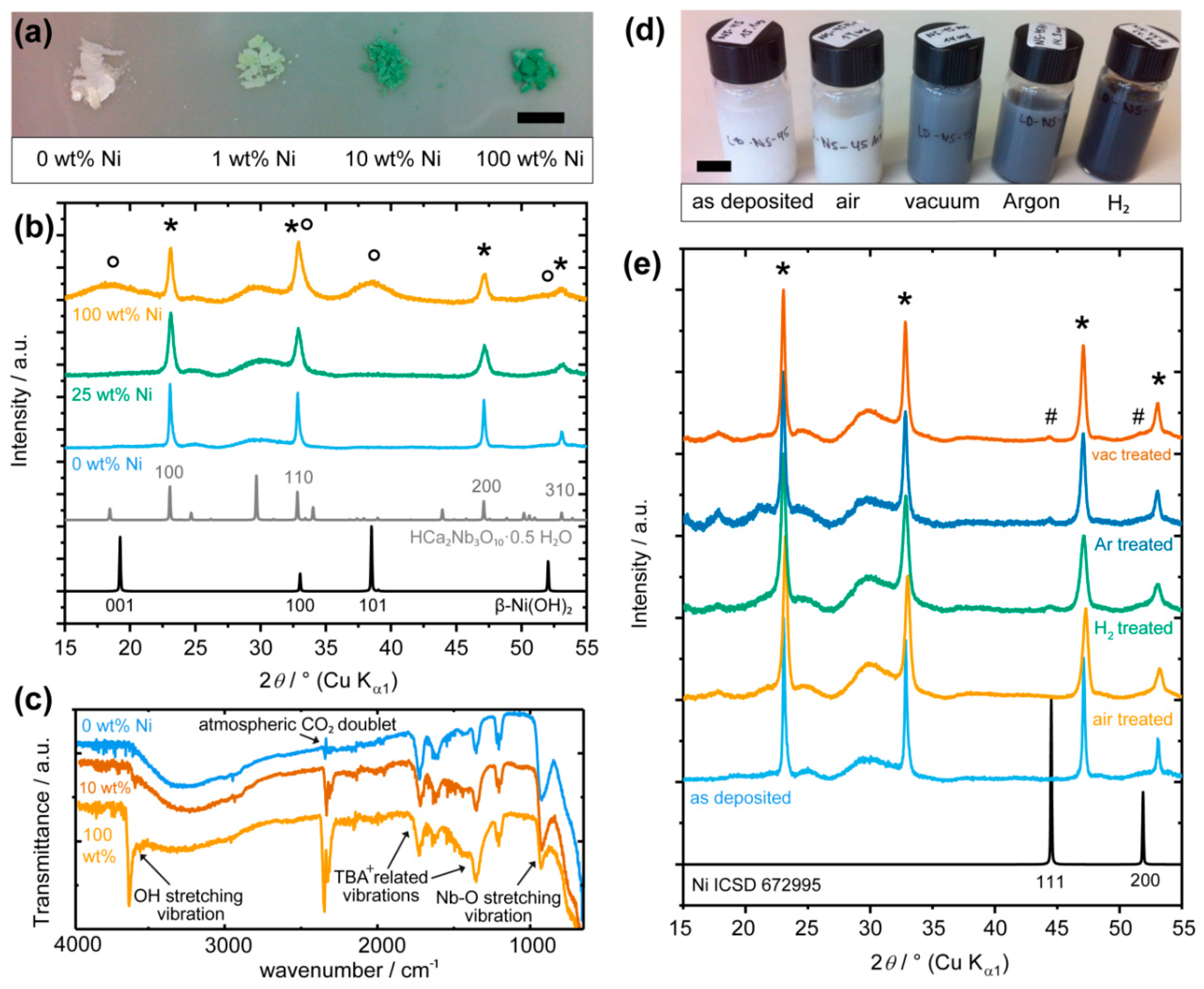
2.1. Loading of Ni on [Ca2Nb3O10]− Nanosheets
2.2. Morphology of Ni(OH)2 and Ni Nanoparticles on [Ca2Nb3O10]− Nanosheets
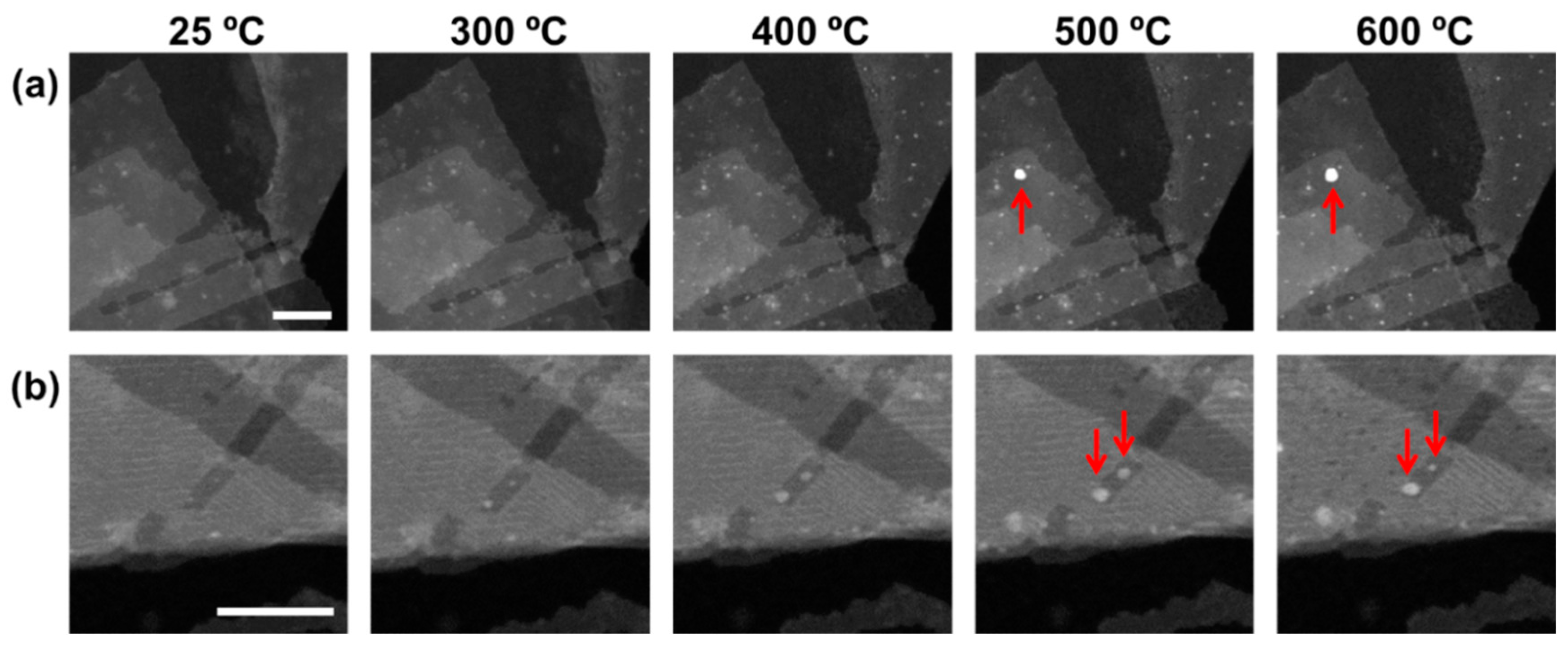
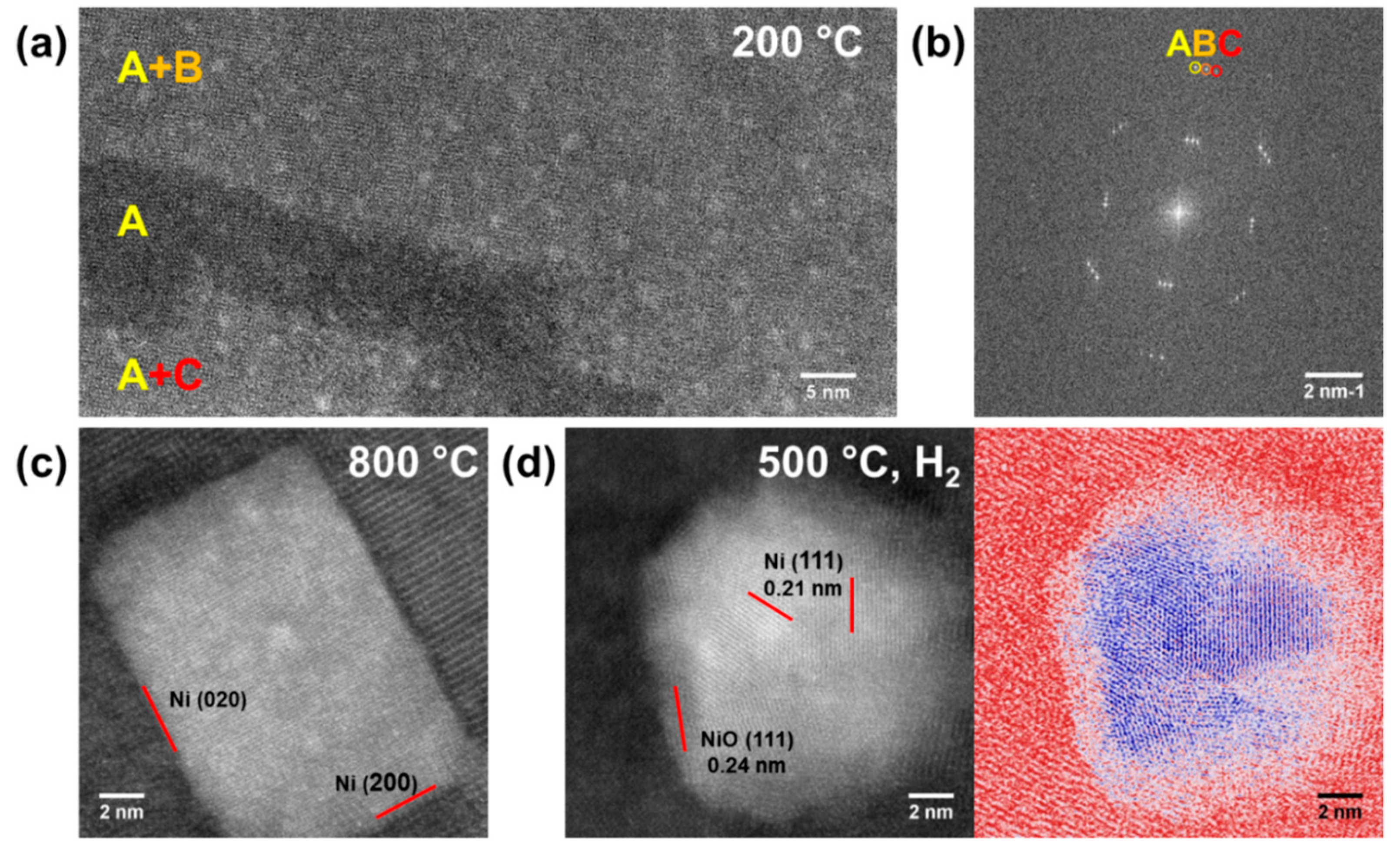
2.3. Structural Evolution of the Nanocomposite during in Situ Heating
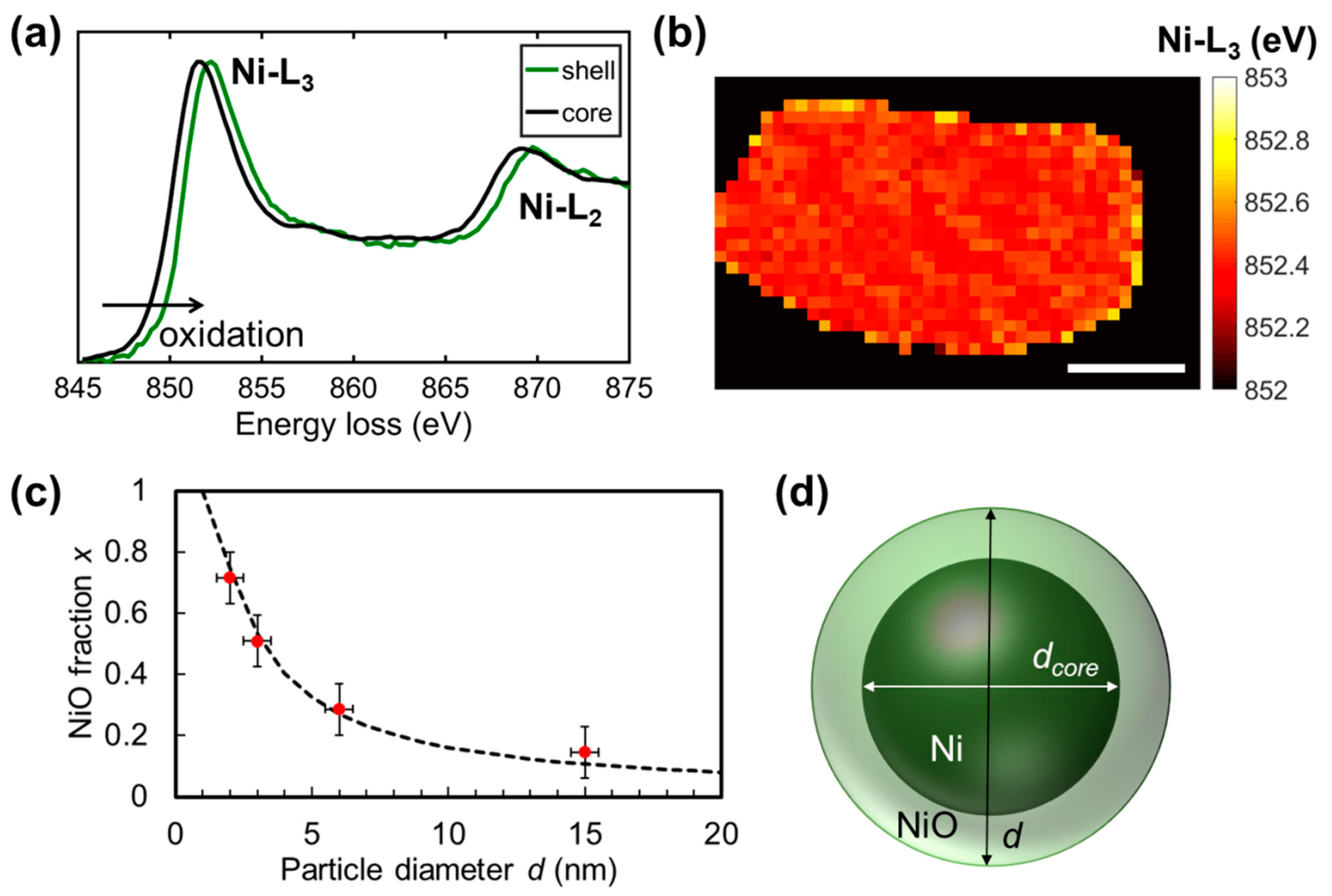
2.4. Average Oxidation States of Ni Core/NiO Shell Nanoparticles
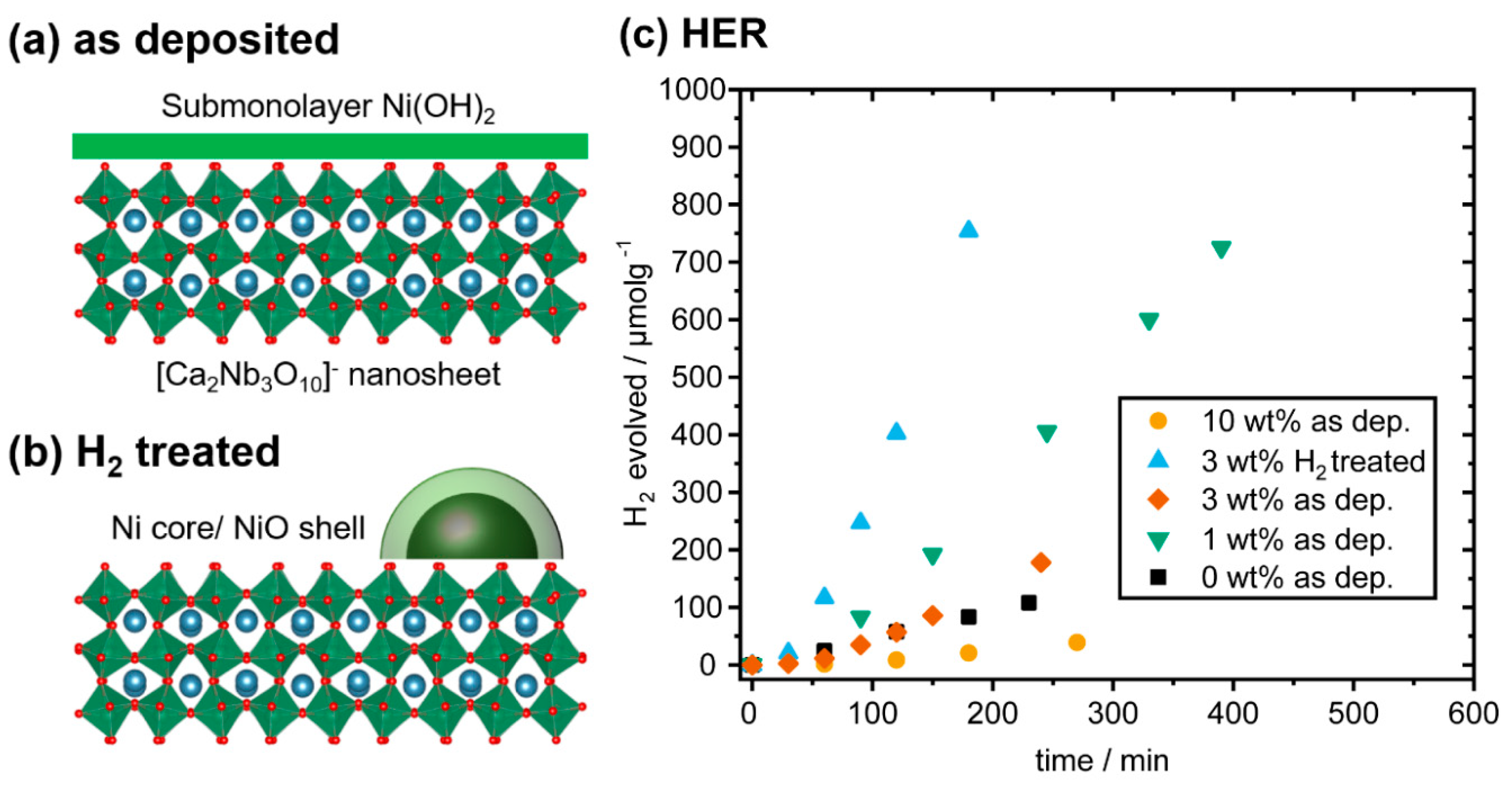
2.5. Photocatalytic Activity of the Nanocomposites
3. Materials and Methods
3.1. Synthesis of [Ca2Nb3O10]− Nanosheets
3.2. Loading of Ni Co-Catalysts
3.3. Structural Characterization
3.4. Photocatalytic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Nanoscale Effects in Water Splitting Photocatalysis. In Solar Energy for Fuels; Tüysüz, H., Chan, C.K., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 371, pp. 105–142. [Google Scholar] [CrossRef] [Green Version]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A 2014, 471, 0792. [Google Scholar] [CrossRef]
- Cronan, C.L.; Micale, F.J.; Topic, M.; Leidheiser, H.; Zettlemoyer, A.C.; Popovic, S. Surface properties of Ni(OH)2 and NiO II. Mechanism for the thermal decomposition of Ni(OH)2 and other metal hydroxides. J. Colloid Interface Sci. 1976, 55, 546–557. [Google Scholar] [CrossRef]
- Liang, Z.-H.; Zhu, Y.-J.; Hu, X.-L. β-nickel hydroxide nanosheets and their thermal decomposition to nickel oxide nanosheets. J. Phys. Chem. B 2004, 108, 3488–3491. [Google Scholar] [CrossRef]
- Kuang, Y.; Feng, G.; Li, P.; Bi, Y.; Li, Y.; Sun, X. Single-crystalline ultrathin nickel nanosheets array from in situ topotactic reduction for active and stable electrocatalysis. Angew. Chem. Int. Ed. 2016, 55, 693–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strayer, M.E.; Senftle, T.P.; Winterstein, J.P.; Vargas-Barbosa, N.M.; Sharma, R.; Rioux, R.M.; Janik, M.J.; Mallouk, T.E. Charge transfer stabilization of late transition metal oxide nanoparticles on a layered niobate support. J. Am. Chem. Soc. 2015, 137, 16216–16224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhukovskyi, M.; Tongying, P.; Yashan, H.; Wang, Y.; Kuno, M. Efficient photocatalytic hydrogen generation from Ni nanoparticle decorated CdS nanosheets. ACS Catal. 2015, 5, 6615–6623. [Google Scholar] [CrossRef]
- Ran, J.; Yu, J.; Jaroniec, M. Ni(OH)2 modified CdS nanorods for highly efficient visible-light-driven photocatalytic H2 generation. Green Chem. 2011, 13, 2708–2713. [Google Scholar] [CrossRef]
- Lo, I.-H.; Wang, J.-Y.; Huang, K.-Y.; Huang, J.-H.; Kang, W.P. Synthesis of Ni(OH)2 nanoflakes on ZnO nanowires by pulse electrodeposition for high-performance supercapacitors. J. Power Sources 2016, 308, 29–36. [Google Scholar] [CrossRef]
- Domen, K.; Naito, S.; Onishi, T.; Tamaru, K.; Soma, M. Study of the photocatalytic decomposition of water vapor over a NiO-SrTiO3 catalyst. J. Phys. Chem. 1982, 86, 3657–3661. [Google Scholar] [CrossRef]
- Domen, K.; Kudo, A.; Shinozaki, A.; Tanaka, A.; Maruya, K.; Onishi, T. Photodecomposition of water and hydrogen evolution from aqueous methanol solution over novel niobate photocatalysts. J. Chem. Soc. Chem. Commun. 1986, 4, 356–357. [Google Scholar] [CrossRef]
- Kudo, A.; Tanaka, A.; Domen, K.; Maruya, K.; Aika, K.; Onishi, T. Photocatalytic decomposition of water over NiO-K4Nb6O17 catalyst. J. Catal. 1988, 111, 67–76. [Google Scholar] [CrossRef]
- Townsend, T.K.; Browning, N.D.; Osterloh, F.E. Overall photocatalytic water splitting with NiOx–SrTiO3—A revised mechanism. Energy Environ. Sci. 2012, 5, 9543. [Google Scholar] [CrossRef]
- Teichner, S.J.; Morrison, J.A. The adsorption of gases on nickel oxides. Trans. Faraday Soc. 1955, 51, 961–966. [Google Scholar] [CrossRef]
- Carney, C.S.; Chinn, R.E.; Dogan, Ö.N.; Gao, M.C. Isothermal decomposition kinetics of nickel (II) hydroxide powder. J. Alloy Compd. 2015, 644, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Parravano, G. The reduction of nickel oxide by hydrogen. J. Am. Chem. Soc. 1952, 74, 1194–1198. [Google Scholar] [CrossRef]
- Larkins, F.P.; Fensham, P.J. Decomposition of nickel oxide. Nature 1967, 215, 1268–1269. [Google Scholar] [CrossRef]
- Jeangros, Q.; Hansen, T.W.; Wagner, J.B.; Damsgaard, C.D.; Dunin-Borkowski, R.E.; Hébert, C.; Van herle, J.; Hessler-Wyser, A. Reduction of nickel oxide particles by hydrogen studied in an environmental TEM. J. Mater. Sci. 2013, 48, 2893–2907. [Google Scholar] [CrossRef] [Green Version]
- Manukyan, K.V.; Avetisyan, A.G.; Shuck, C.E.; Chatilyan, H.A.; Rouvimov, S.; Kharatyan, S.L.; Mukasyan, A.S. Nickel oxide reduction by hydrogen: Kinetics and structural transformations. J. Phys. Chem. C 2015, 119, 16131–16138. [Google Scholar] [CrossRef]
- Fukuoka, H.; Isami, T.; Yamanaka, S. Crystal structure of a layered perovskite niobate KCa2Nb3O10. J. Solid State Chem. 2000, 151, 40–45. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Self-assembly of tiled perovskite monolayer and multilayer thin films. Chem. Mater. 2000, 12, 2513–2516. [Google Scholar] [CrossRef]
- Ebina, Y.; Sasaki, T.; Harada, M.; Watanabe, M. Restacked perovskite nanosheets and their Pt-loaded materials as photocatalysts. Chem. Mater. 2002, 14, 4390–4395. [Google Scholar] [CrossRef]
- Compton, O.C.; Carroll, E.C.; Kim, J.Y.; Larsen, D.S.; Osterloh, F.E. Calcium niobate semiconductor nanosheets as catalysts for photochemical hydrogen evolution from water. J. Phys. Chem. C 2007, 111, 14589–14592. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhao, X.; Ma, H.; Ma, S.; Huang, G.; Makita, Y.; Bai, X.; Yang, X. Structure and dehydration of layered perovskite niobate with bilayer hydrates prepared by exfoliation/self-assembly process. J. Solid State Chem. 2008, 181, 1684–1694. [Google Scholar] [CrossRef]
- Hata, H.; Kobayashi, Y.; Bojan, V.; Youngblood, W.J.; Mallouk, T.E. Direct deposition of trivalent rhodium hydroxide nanoparticles onto a semiconducting layered calcium niobate for photocatalytic hydrogen evolution. Nano Lett. 2008, 8, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Eguchi, M.; Youngblood, W.J.; Mallouk, T.E. Calcium niobate nanosheets prepared by the polymerized complex method as catalytic materials for photochemical hydrogen evolution. Chem. Mater. 2009, 21, 3611–3617. [Google Scholar] [CrossRef]
- Sabio, E.M.; Chi, M.; Browning, N.D.; Osterloh, F.E. Charge separation in a niobate nanosheet photocatalyst studied with photochemical labelling. Langmuir 2010, 26, 7254–7261. [Google Scholar] [CrossRef] [PubMed]
- Virdi, K.S.; Kauffmann, Y.; Ziegler, C.; Ganter, P.; Blaha, P.; Lotsch, B.V.; Kaplan, W.D.; Scheu, C. Band gap extraction from individual two-dimensional perovskite nanosheets using valence electron energy loss spectroscopy. J. Phys. Chem. C 2016, 120, 11170–11179. [Google Scholar] [CrossRef]
- Compton, O.C.; Osterloh, F.E. Niobate nanosheets as catalysts for photochemical water splitting into hydrogen and hydrogen peroxide. J. Phys. Chem. C 2009, 113, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.-F.; Ebina, Y.; Bando, Y.; Sasaki, T. In-situ transmission electron microscopic study of perovskite-type niobate nanosheets under electron-irradiation and heating. J. Phys. Chem. B 2003, 107, 6698–6703. [Google Scholar] [CrossRef]
- Spectral Database for Organic Compounds SDBS. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/landingpage?sdbsno=10891 (accessed on 16 December 2019).
- Zhang, S.; Scheu, C. Evaluation of EELS spectrum imaging data by spectral components and factors from multivariate analysis. Microscopy 2018, 67, i133–i141. [Google Scholar] [CrossRef]
- Nakibli, Y.; Kalisman, P.; Amirav, L. Less is more: The case of metal cocatalysts. J. Phys. Chem. Lett. 2015, 6, 2265–2268. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.Y.; Song, H. Regulation of electron-hole recombination kinetics on uniform metal-semiconductor nanostructures for photocatalytic hydrogen evolution. APL Mater. 2019, 7, 100702. [Google Scholar] [CrossRef]
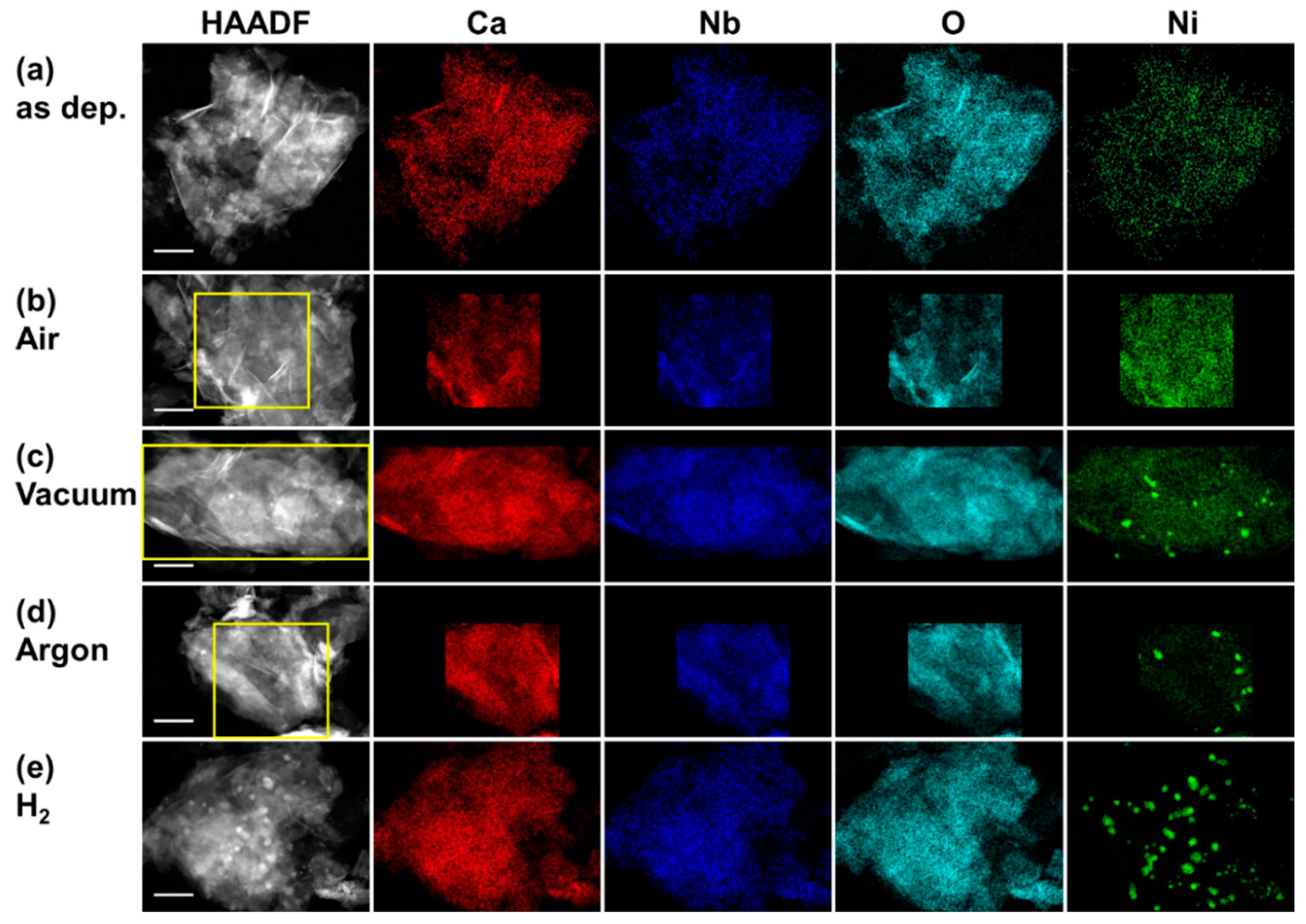
| 500 °C Heating | Nb/Ca | Ni/Ca |
|---|---|---|
| As deposited | 1.62 | 0.14 |
| Air | 1.59 | 0.14 |
| Vacuum | 1.64 | 0.16 |
| Argon | 1.64 | 0.14 |
| H2 | 1.63 | 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Diehl, L.; Wrede, S.; Lotsch, B.V.; Scheu, C. Structural Evolution of Ni-Based Co-Catalysts on [Ca2Nb3O10]− Nanosheets during Heating and Their Photocatalytic Properties. Catalysts 2020, 10, 13. https://doi.org/10.3390/catal10010013
Zhang S, Diehl L, Wrede S, Lotsch BV, Scheu C. Structural Evolution of Ni-Based Co-Catalysts on [Ca2Nb3O10]− Nanosheets during Heating and Their Photocatalytic Properties. Catalysts. 2020; 10(1):13. https://doi.org/10.3390/catal10010013
Chicago/Turabian StyleZhang, Siyuan, Leo Diehl, Sina Wrede, Bettina V. Lotsch, and Christina Scheu. 2020. "Structural Evolution of Ni-Based Co-Catalysts on [Ca2Nb3O10]− Nanosheets during Heating and Their Photocatalytic Properties" Catalysts 10, no. 1: 13. https://doi.org/10.3390/catal10010013
APA StyleZhang, S., Diehl, L., Wrede, S., Lotsch, B. V., & Scheu, C. (2020). Structural Evolution of Ni-Based Co-Catalysts on [Ca2Nb3O10]− Nanosheets during Heating and Their Photocatalytic Properties. Catalysts, 10(1), 13. https://doi.org/10.3390/catal10010013





