Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Supported Au25 Nanoclusters
2.2. The Catalytic Activities of Different Supported Gold Catalysts
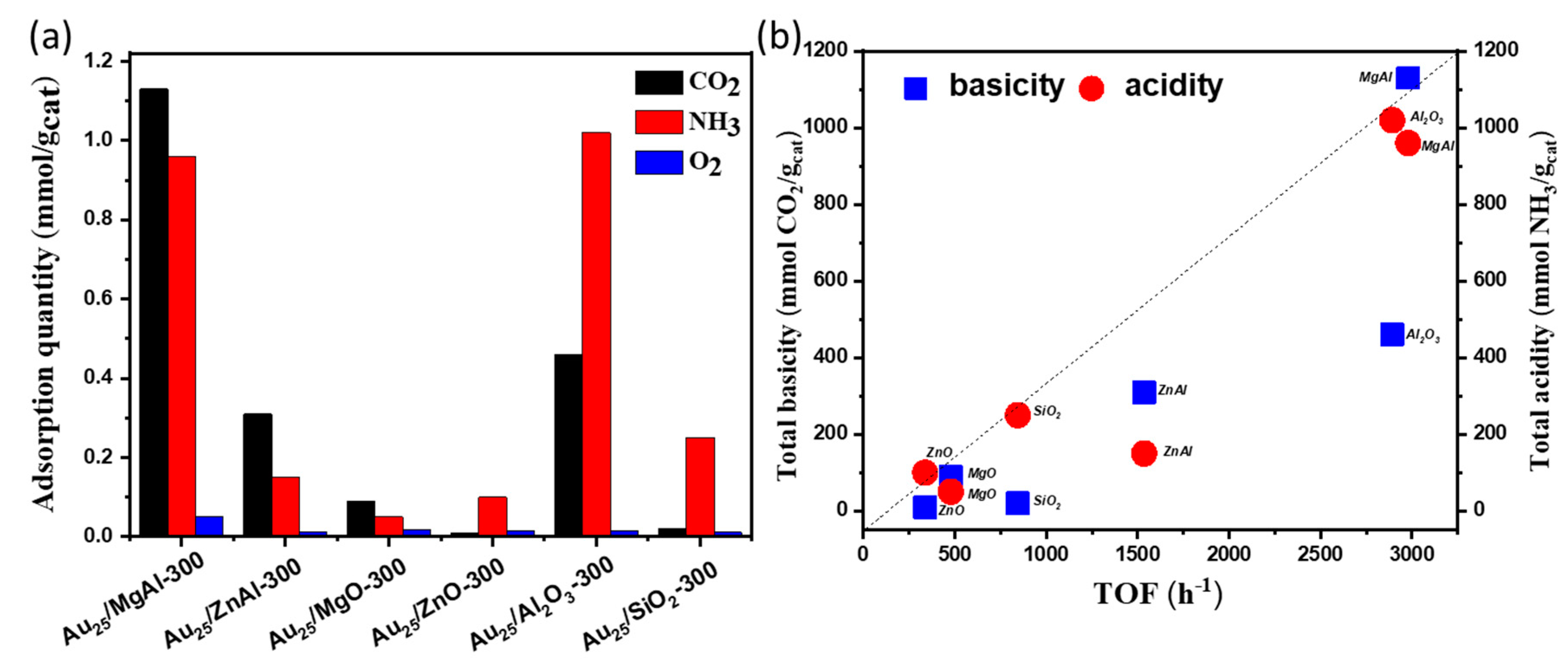
2.3. The Influence Factors for Aerobic Oxidation of Benzyl Alcohol over the Supported Gold Catalysts
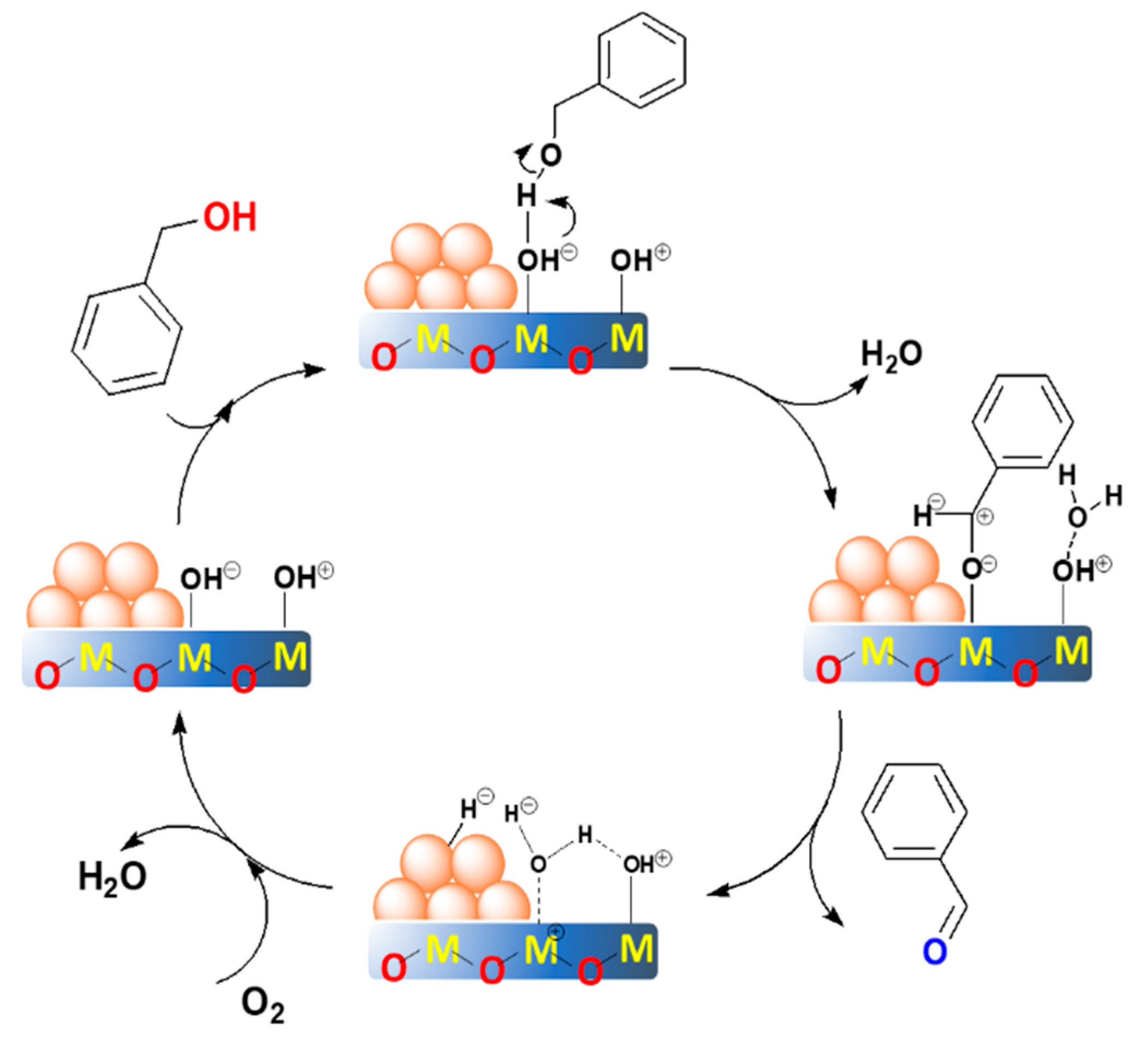
2.4. The Proposed Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Au25 NCs
3.3. Preparation of the Hydrotalcite (HT)
3.4. Preparation of the Supported Au25 Nanoclusters (NCs)
3.5. Preparation of the Supported Au Nanoparticles (NPs)
3.6. Characterization of the Catalysts
3.7. Aerobic Oxidation of Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hutchings, G.J. Vapor Phase Hydrochlorination of Acetylene: Correlation of Catalytic Acitivity of Supported Metal Chloride Catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Harauta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalyst for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Harauta, M. Size-and Support-Dependency in the Catalysis of Gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Min, B.K.; Friend, C.M. Heterogeneous Gold-Based Catalysis for Green Chemistry: Low-Temperature CO Oxidation and Propene Oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Comotti, M.; Schüth, F. Highly Reproducible Syntheses of Active Au/TiO2 Catalysts for CO Oxidation by Deposition–Precipitation or Impregnation. J. Catal. 2006, 237, 190–196. [Google Scholar] [CrossRef]
- Zhao, K.; Tang, H.; Qiao, B.; Li, L.; Wang, J. High Activity of Au/γ-Fe2O3 for CO Oxidation: Effect of Support Crystal Phase in Catalyst Design. ACS Catal. 2015, 5, 3528–3539. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Chen, H.; Cullen, D.A.; Larese, J.Z. Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over Gold Supported on Zinc Oxide Materials. J. Phys. Chem. C 2015, 119, 28885–28894. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-Gold Catalysis in Fine Chemical Synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef]
- Li, G.; Jin, R. Catalysis by Gold Nanoparticles: Carbon-Carbon Coupling Reactions. Nanotechnol. Rev. 2013, 2, 529–545. [Google Scholar]
- Zhou, Y.; Li, G.A. Critical Review on Carbon-Carbon Coupling over Ultra-Small Gold Nanoclusters. Acta Phys. Chim. Sin. 2017, 33, 1297–1309. [Google Scholar]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials 2019, 9, 838–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad, A.; Almela, C.; Corma, A.; Garía, H. Unique Gold Chemoselectivity for the Aerobic Oxidation of Allylic Alcohols. Chem. Commun. 2006, 30, 3178–3180. [Google Scholar] [CrossRef]
- Li, L.; Dou, L.; Zhang, H. Layered Double Hydroxide Supported Gold Nanoclusters by Glutathione-Capped Au Nanoclusters Precursor Method for Highly Efficient Aerobic Oxidation of Alcohols. Nanoscale 2014, 6, 3753–3763. [Google Scholar] [CrossRef]
- Pina, C.D.; Falletta, E.; Rossi, M. Update on Selective Oxidation Using Gold. Chem. Soc. Rev. 2012, 41, 350–369. [Google Scholar] [CrossRef]
- Su, F.Z.; Liu, Y.M.; Wang, L.C.; Cao, Y.; He, H.; Fan, K.N. Ga–Al Mixed-Oxide-Supported Gold Nanoparticles with Enhanced Activity for Aerobic Alcohol Oxidation. Angew. Chem. Int. Ed. 2008, 120, 340–343. [Google Scholar] [CrossRef]
- Asao, N.; Hatakeyama, N.; Menggenbateer; Minato, T.; Ito, E.; Masahiko, H.; Kim, Y.; Yamamoto, Y.; Chen, M.; Zhang, W.; et al. Aerobic Oxidation of Alcohols in the Liquid Phase with Nanoporous Gold Catalysts. Chem. Commun. 2012, 48, 4540–4542. [Google Scholar] [CrossRef]
- Gualteros, J.A.D.; Garcia, M.A.S.; da Silva, A.G.M.; Rodrigues, T.S.; Căndido, E.G.; e Silva, F.A.; Fonseca, F.C.; Quiroz, J.; de Oliveira, D.C.; Cordoba de Torresi, S.I.; et al. Synthesis of Highly Dispersed Gold Nanoparticles on Al2O3, SiO2, and TiO2 for the Solvent-Free Oxidation of Benzyl Alcohol under Low Metal Loadings. J. Mater. Sci. 2018, 54, 238–251. [Google Scholar] [CrossRef]
- Nagy, G.; Beck, A.; Sáfrán, G.; Schay, Z.; Liu, S.; Li, T.; Qiao, B.; Wang, J.; Lázár, K. Nanodisperse Gold Catalysts in Oxidation of Benzyl Alcohol: Comparison of Various Supports under Different Conditions. React. Kinet. Mech. Catal. 2019, 128, 71–95. [Google Scholar] [CrossRef] [Green Version]
- Long, N.Q.; Quan, N.A. Highly Selective Oxidation of Benzyl Alcohol to Benzaldehyde Catalyzed by Nano Au/γ-Al2O3 under Environment-Friendly Conditions. React. Kinet. Mech. Catal. 2014, 114, 147–155. [Google Scholar] [CrossRef]
- Zhan, B.Z.; Thompson, A. Recent Developments in the Aerobic Oxidation of Alcohols. Tetrahedron 2004, 60, 2917–2935. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, H.; Das, A.; Jin, R. Comparison of the Catalytic Properties of 25-Atom Gold Nanospheres and Nanorods. Chin. J. Catal. 2011, 32, 1149–1155. [Google Scholar] [CrossRef]
- Taketoshi, A.; Haruta, M. Size- and Structure-Specificity in Catalysis by Gold Clusters. Chem. Lett. 2014, 43, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Chen, J.; Zhang, Q.; Deng, W.; Wang, Y. Hydrotalcite-Supported Gold Catalyst for the Oxidant-Free Dehydrogenation of Benzyl Alcohol: Studies on Support and Gold Size Effects. Chemistry 2011, 17, 1247–1256. [Google Scholar] [CrossRef] [Green Version]
- Comotti, M.; Li, W.C.; Spliethoff, B.; Schüth, F. Support Effect in High Activity Gold Catalysts for CO Oxidation. J. Am. Chem. Soc. 2006, 128, 917–924. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.; Zhang, T.; Mou, C.Y. Catalysis by Gold: New insights into the Support Effect. Nano Today 2013, 8, 403–416. [Google Scholar] [CrossRef]
- Cárdenas Lizana, F.; Gómez Quero, S.; Perret, N.; Keane, M.A. Support Effects in the Selective Gas Phase Hydrogenation of p-chlronitrobenzene over Gold. Gold Bull. 2009, 42, 124–132. [Google Scholar]
- Hodes, G. When Small is Different: Some Recent Advances in Concepts and Applications of Nanoscale Phenomena. Adv. Mater. 2007, 19, 639–655. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Nie, X.; Qian, H.; Ge, Q.; Xu, H.; Jin, R. CO Oxidation Catalyzed by Oxide-Supported Au25(SR)18 Nanoclusters and Identification of Perimeter Sites as Active Centers. ACS Nano 2012, 6, 6014–6022. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, D.; Mann, A.K.P.; Mullins, D.R.; Qiao, Z.A.; Allard, L.F.; Zeng, C.; Jin, R.; Overbury, S.H. Thiolate Ligands as a Double-Edged Sword for CO Oxidation on CeO2 Supported Au25(SCH2CH2Ph)18 Nanoclusters. J. Am. Chem. Soc. 2014, 136, 6111–6122. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Tsukuda, T. Efficient and Selective Epoxidation of Styrene with TBHP Catalyzed by Au25 Clusters on Hydroxyapatite. Chem. Commun. 2010, 46, 550–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Li, J.; Zhang, B.; Yuan, X.; Asakura, H.; Tanaka, T.; Teramura, K.; Xie, J.; Yan, N. The Support Effect on the Size and Catalytic Activity of Thiolated Au25 Nanoclusters as Precatalysts. Nanoscale 2015, 7, 6325–6333. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, C.; Lei, Y.; Jin, R. Au25 Nanocluster-catalyzed Ullmann-Type Homocoupling Reaction of Aryl Iodides. Chem. Commun. 2012, 48, 12005–12007. [Google Scholar] [CrossRef] [PubMed]
- Shivhare, A.; Ambrose, S.J.; Zhang, H.; Purves, R.W.; Scott, R.W.J. Stable and Recyclable Au25 Clusters for the Reduction of 4-Nitrophenol. Chem. Commun. 2013, 49, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, X.Y.; Zhang, L.; Wang, A.; Li, L.; Pan, X.; Miao, S.; Haruta, M.; Wei, H.; Wang, H.; et al. ZnAl-Hydrotalcite-Supported Au25 Nanoclusters as Precatalysts for Chemoselective Hydrogenation of 3-Nitrostyrene. Angew. Chem. Int. Ed. 2017, 56, 2709–2713. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.Y.; Li, L.; Kang, L.; Wang, A.; Zhang, T. Effects of Divalent Metal Ions of Hydrotalcites on Catalytic Behavior of Supported Gold Nanocatalysts for Chemoselective Hydrogenation of 3-Nitrostyrene. J. Catal. 2018, 364, 174–182. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, H.; Drake, B.A.; Jin, R. Atomically Precise Au25(SR)18 Nanoparticles as Catalysts for the Selective Hydrogenation of α,β-Unsaturated Ketones and Aldehydes. Angew. Chem. Int. Ed. 2010, 122, 1317–1320. [Google Scholar] [CrossRef]
- Kannan, S. Influence of Synthesis Methodology and Post Treatments on Structural and Textural Variations in MgAlCO3 Hydrotalcite. J. Mater. Sci. 2004, 39, 6591–6596. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–784. [Google Scholar] [CrossRef]
- Stasiak-Włodarczyk, M.; Jamroz, J. Specific Surface Area and Porosity of Starch Extrudates Determined from Nitrogen Adsorption Data. J. Food Eng. 2009, 93, 379–385. [Google Scholar] [CrossRef]
- Gao, J.; Fan, G.; Yang, L.; Cao, X.; Zhang, P.; Li, F. Oxidative Esterification of Methacrolein to Methyl Methacrylate over Gold Nanoparticles on Hydroxyapatite. ChemCatChem 2017, 9, 1230–1241. [Google Scholar] [CrossRef]
- Kepinski, L. Thermal Stability of Ce0.5Gd0.5O1.75 Nanoparticles in Contact with an Amorphous SiO2. J. Non-Cryst. Solids 2015, 409, 170–177. [Google Scholar] [CrossRef]
- Feng, J.; Ma, C.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Li, D.; Du, Y.; Morgan, D.J.; Hutchings, G.J. Au-Pd Nanoalloys Supported on Mg-Al Mixed Metal Oxides as a Multifunctional Catalyst for Solvent-free Oxidation of Benzyl Alcohol. Dalton. Dalton Trans. 2013, 42, 14498–14508. [Google Scholar] [CrossRef]
- Caux, M.; Menard, H.; AlSalik, Y.M.; Irvine, J.T.S.; Idriss, H. Photo-Catalytic Hydrogen Production over Au/g-C3N4: Effect of Gold Particle Dispersion and Morphology. Phys. Chem. Chem. Phys. 2019, 21, 15974–15987. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Liu, F.; Wei, J.; Qiao, B.; Zhao, K.; Su, Y.; Jin, C.; Li, L.; Liu, J.; Wang, J.; et al. Ultrastable Hydroxyapatite/Titanium-Dioxide-Supported Gold Nanocatalyst with Strong Metal-Support Interaction for Carbon Monoxide Oxidation. Angew. Chem. Int. Ed. 2016, 55, 10606–10611. [Google Scholar] [CrossRef]
- Hsieh, P.T.; Chen, Y.C.; Kao, K.S.; Wang, C.M. Luminescence Mechanism of ZnO Thin Film Investigated by XPS Measurement. Appl. Phys. A 2007, 90, 317–321. [Google Scholar] [CrossRef]
- Chen, Z.; Zhan, G.; Lu, Z. Solvothermal Synthesis and Conductive Properties of Nanorod-constructed Al-Doped ZnO Microflowers. J. Mater. Sci. Mater. 2014, 25, 1724–1730. [Google Scholar] [CrossRef]
- Chen, J.L.; Dong, X.Y.M.; Shi, C.L.; Li, S.H.; Wang, Y.; Zhu, J.H. Fabrication of strong solid base FeO—MgO for warm CO2 capture. Clean 2019, 47, 1800447. [Google Scholar]
- Umchoo, W.; Sriakkarin, C.; Donphai, W.; Warakulwit, C.; Poo-arporn, Y.; Jantaratana, P.; Witoon, T.; Chareonpanich, M. Green and sustainable methanol production from CO2 over magnetized FeCu/core–shell and infiltrate mesoporous silica-aluminosilicates. Energy Convers. Manag. 2018, 159, 342–352. [Google Scholar] [CrossRef]
- Campelo, J.M.; Climent, M.S.; Marinas, J.M. Michael addition of nitromethane to 3-buten-2-one catalyzed by potassium fluoride supported on Al2O3, ZnO, SnO2, sepiolite, AlPO4, AlPO4−Al2O3 and AlPO4−ZnO. React. Kinet. Catal. Lett. 1992, 47, 7–11. [Google Scholar] [CrossRef]
- Antunes, W.M.; de Oliveira Veloso, C.; Henriques, C.A. Transesterification of soybean oil with methanol catalyzed by basic solids. Catal. Today 2008, 133, 548–554. [Google Scholar] [CrossRef]
- Tanabe, K.; Yamaguchi, T. Basicity and acidity of solid surfaces. J. Res. Inst. Catal. 1964, 11, 179–184. [Google Scholar]
- Bancquart, S.; Vanhove, C.; Pouilloux, Y.; Barrault, J. Glycerol transesterification with methyl stearate over solid basic catalysts: I. Relationship between activity and basicity. Appl. Catal. A 2001, 218, 1–11. [Google Scholar] [CrossRef]
- Drouilly, C.; Krafft, J.M.; Averseng, F.; Lauron-Pernota, H.; Bazer-Bachi, D.; Chizallet, C.; Lecocq, V.; Costentin, G. Role of oxygen vacancies in the basicity of ZnO: From the model methylbutynol conversion to the ethanol transformation application. Appl. Catal. A 2013, 453, 121–129. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.; Carley, A.; Prati, L.; Hutchings, G.J. Solvent free Liquid Phase Oxidation of Benzyl Alcohol Using Au Supported Catalysts Prepared Using a Sol Immobilization Technique. Catal. Today 2007, 122, 317–324. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.X.; Lu, J. Disentangling the Size-Dependent Geometric and Electronic Effects of Palladium Nanocatalysts Beyond Selectivity. Sci. Adv. 2019, 5, 6413–6421. [Google Scholar] [CrossRef] [Green Version]
- Skupien, E.; Berger, R.; Santos, V.P.; Gascon, J.; Makkee, M.; Kreutzer, M.T.; Kooyman, P.J.; Moulijn, J.A.; Kapteijn, F. Inhibition of a Gold-Based Catalyst in Benzyl Alcohol Oxidation: Understanding and Remediation. Catalyst 2014, 4, 89–115. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.T. Catalysis by Gold. Catal. Rev.-Sci. Eng. 1999, 41, 319–388. [Google Scholar]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple Size-Controlled Synthesis of Au Nanoparticles and their Size-Dependent Catalytic Activity. Sci. Rep. 2018, 8, 4589–4599. [Google Scholar] [CrossRef] [Green Version]
- Qiao, B.; Liang, J.X.; Wang, A.; Xu, C.Q.; Li, J.; Zhang, T.; Liu, J. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res. 2015, 8, 2913–2924. [Google Scholar] [CrossRef]
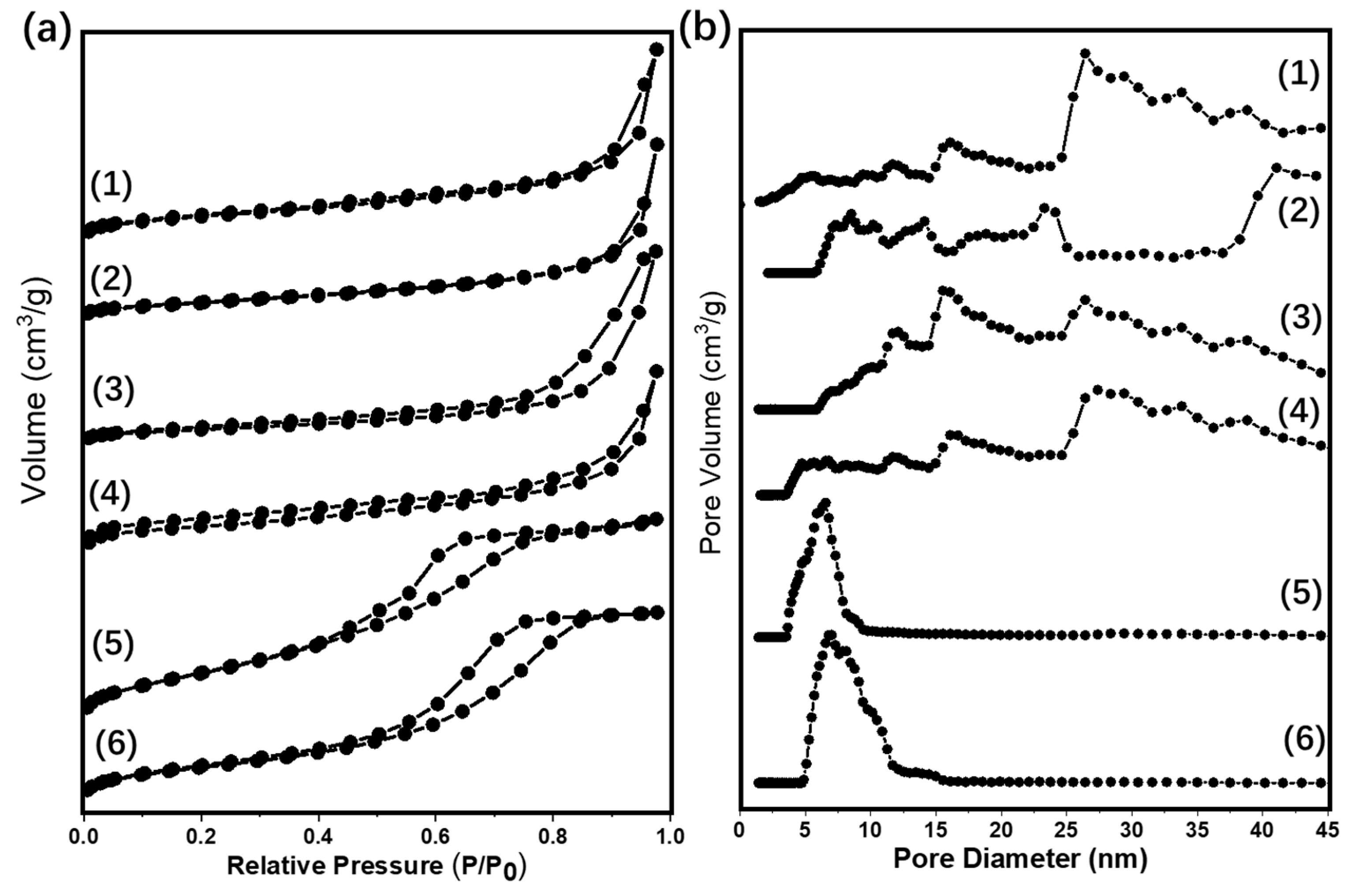
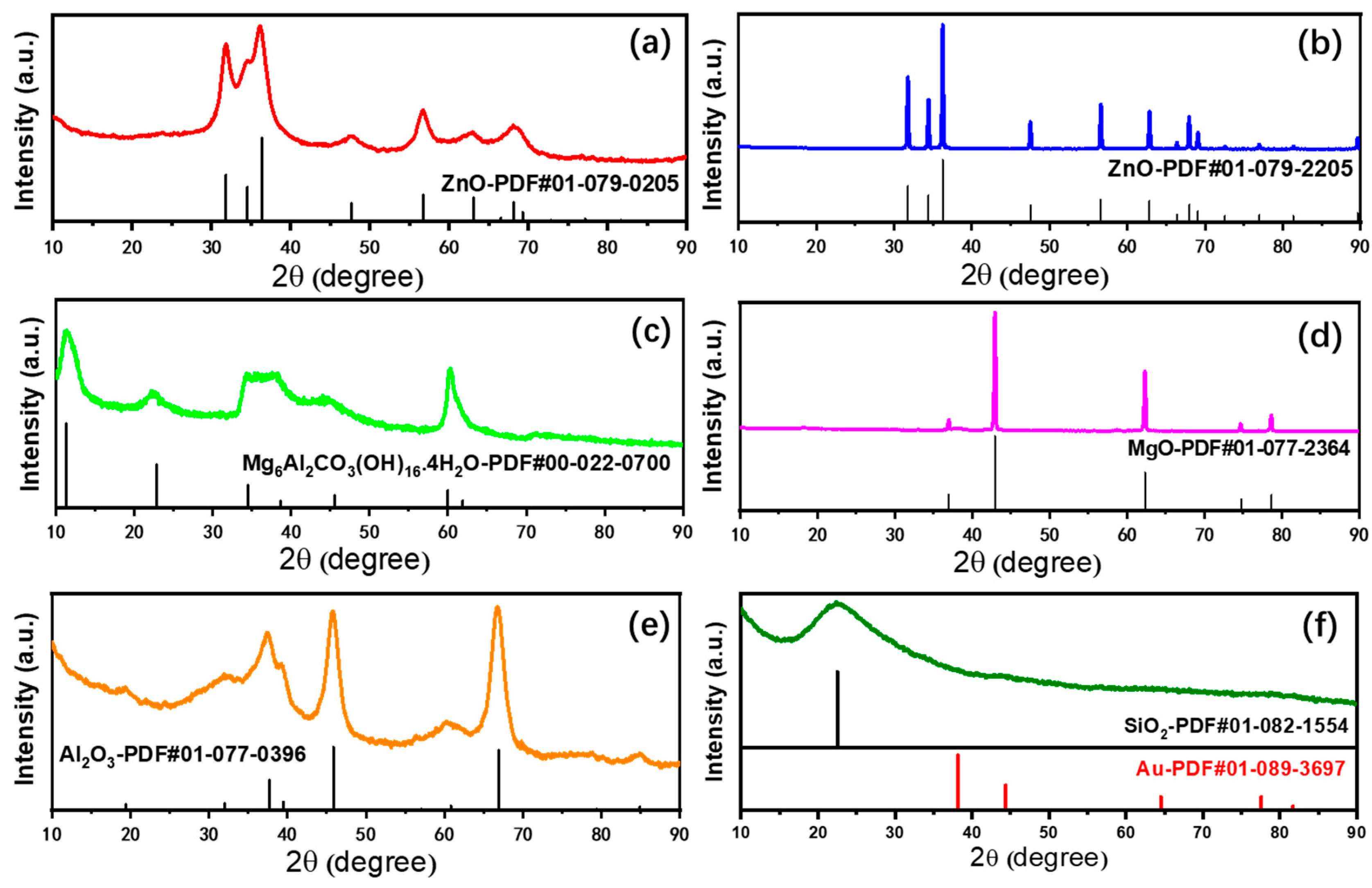
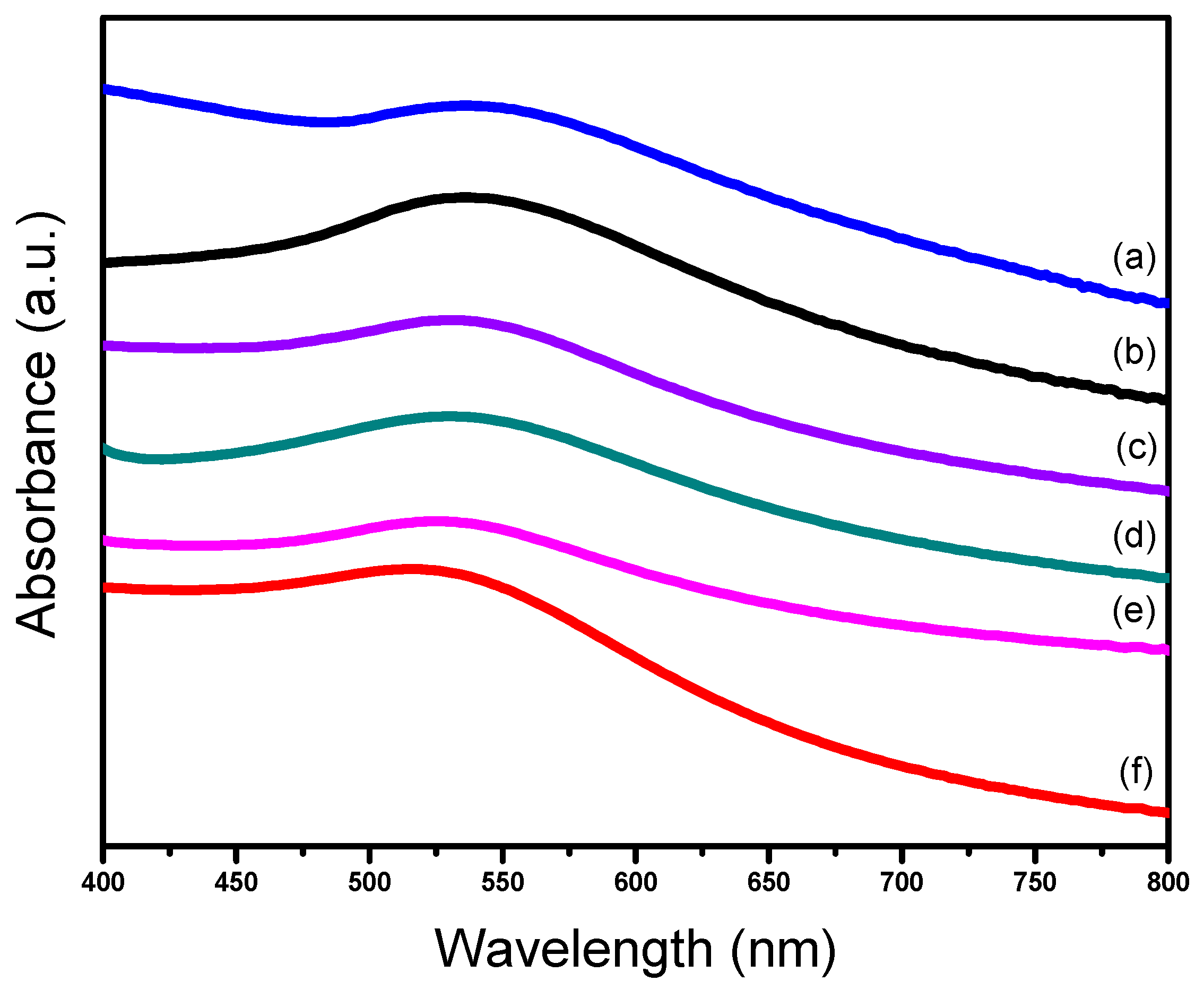

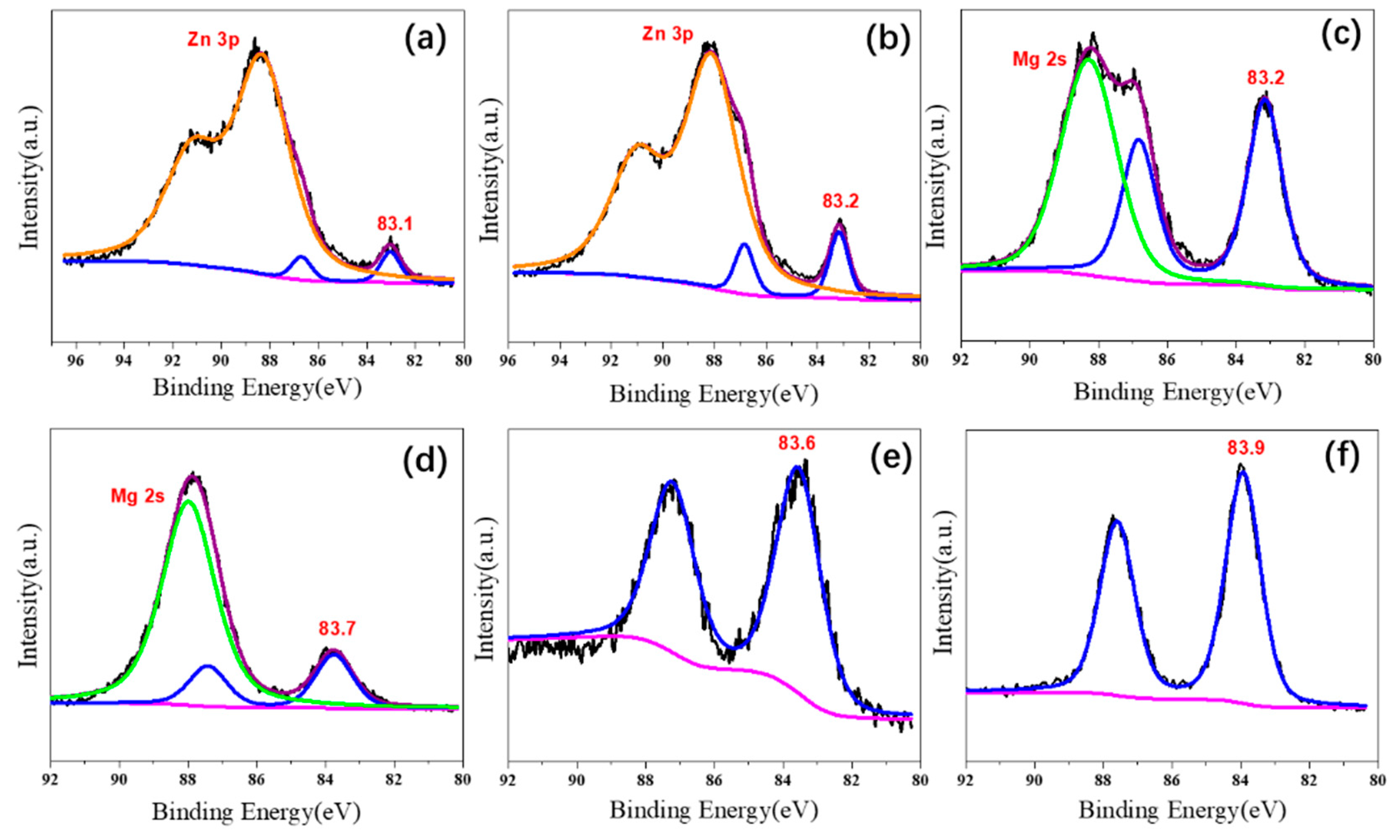
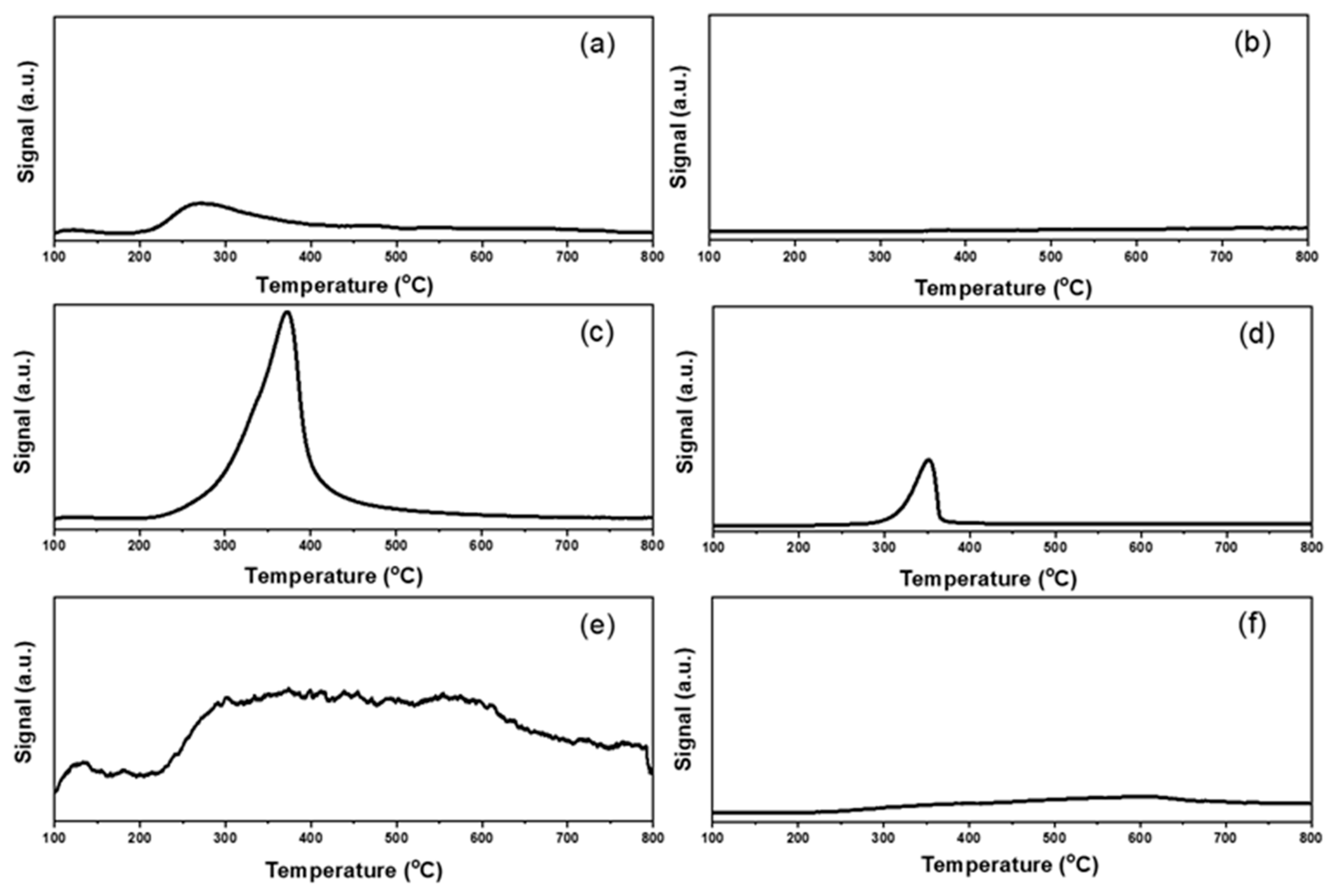
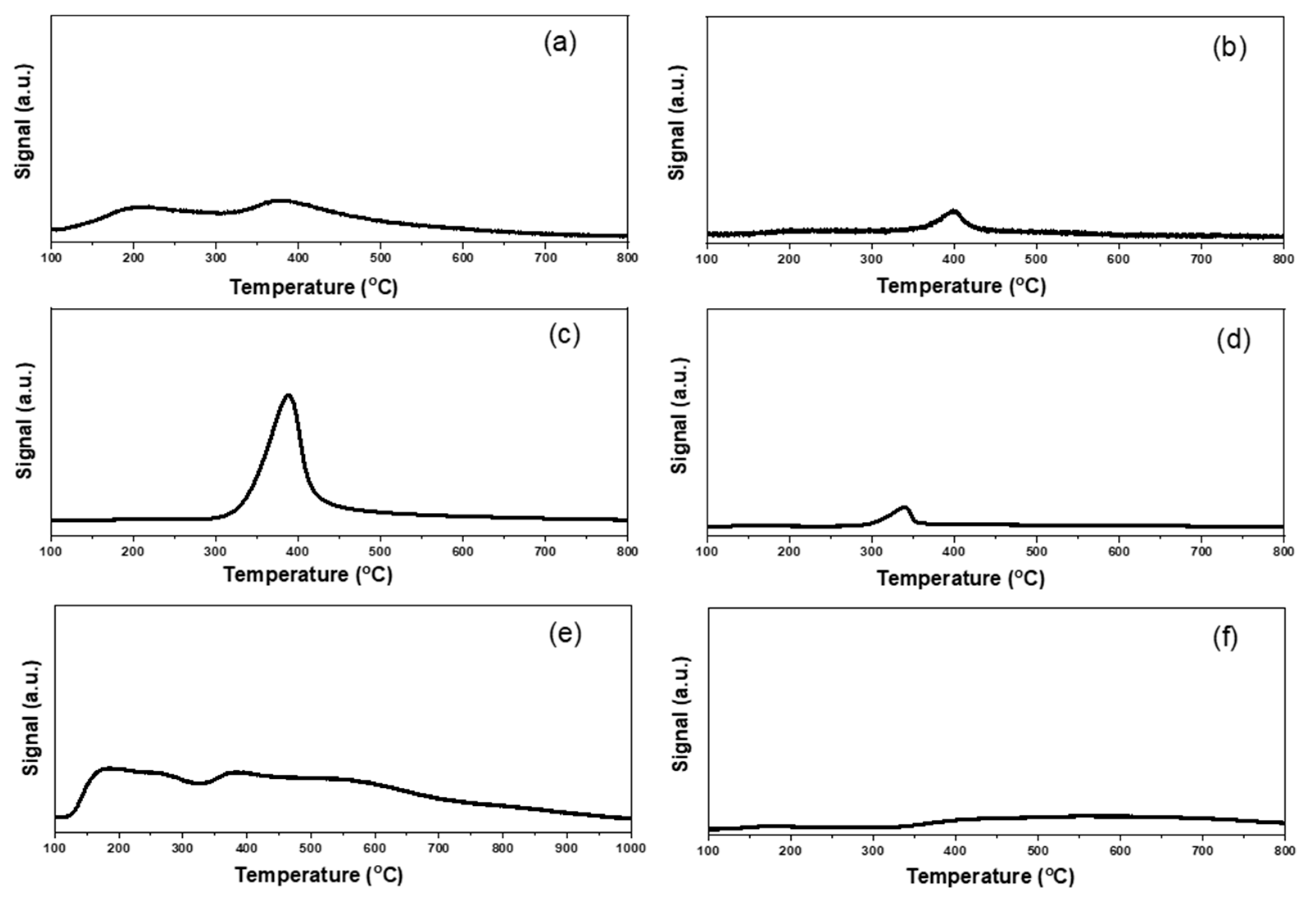
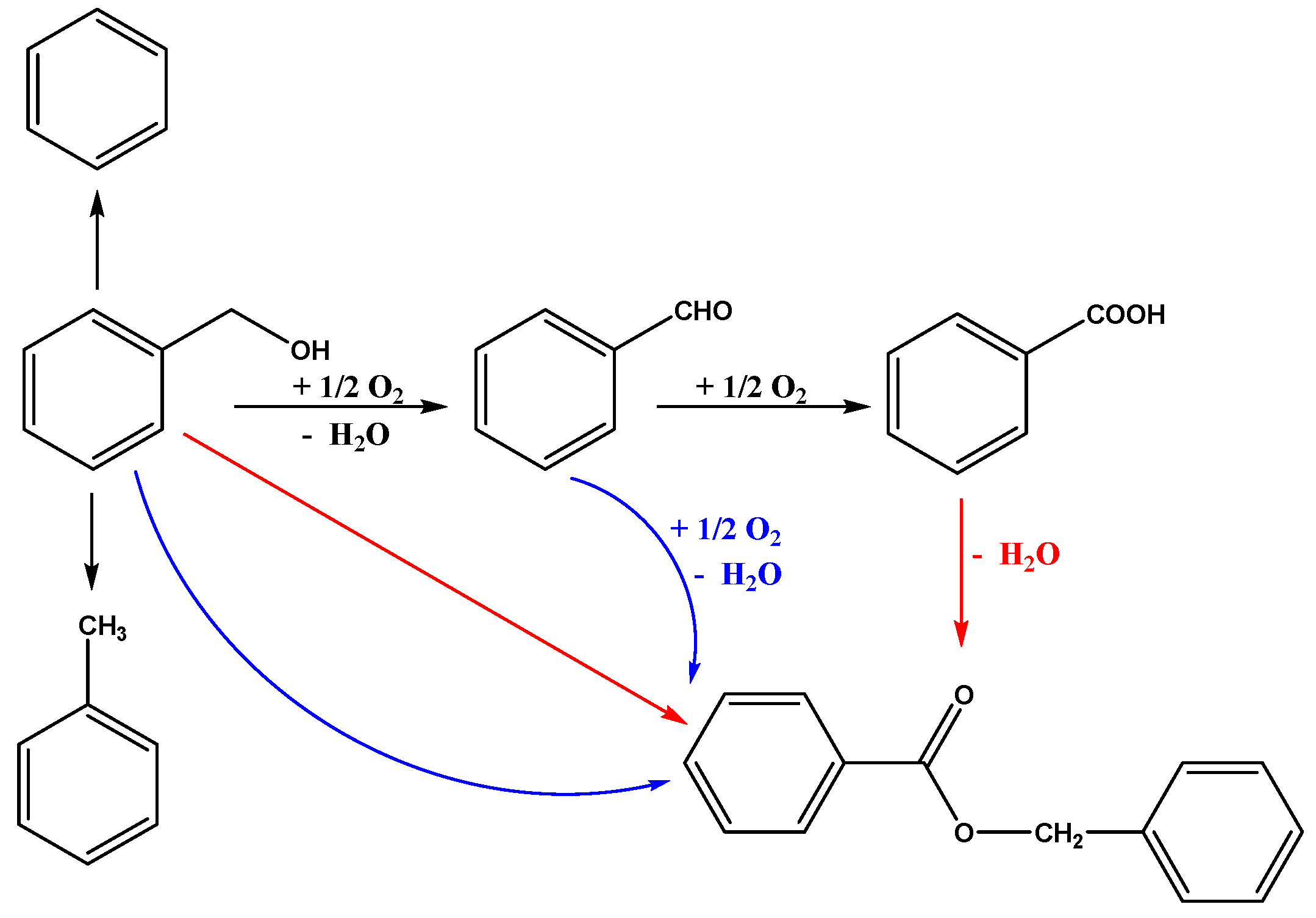
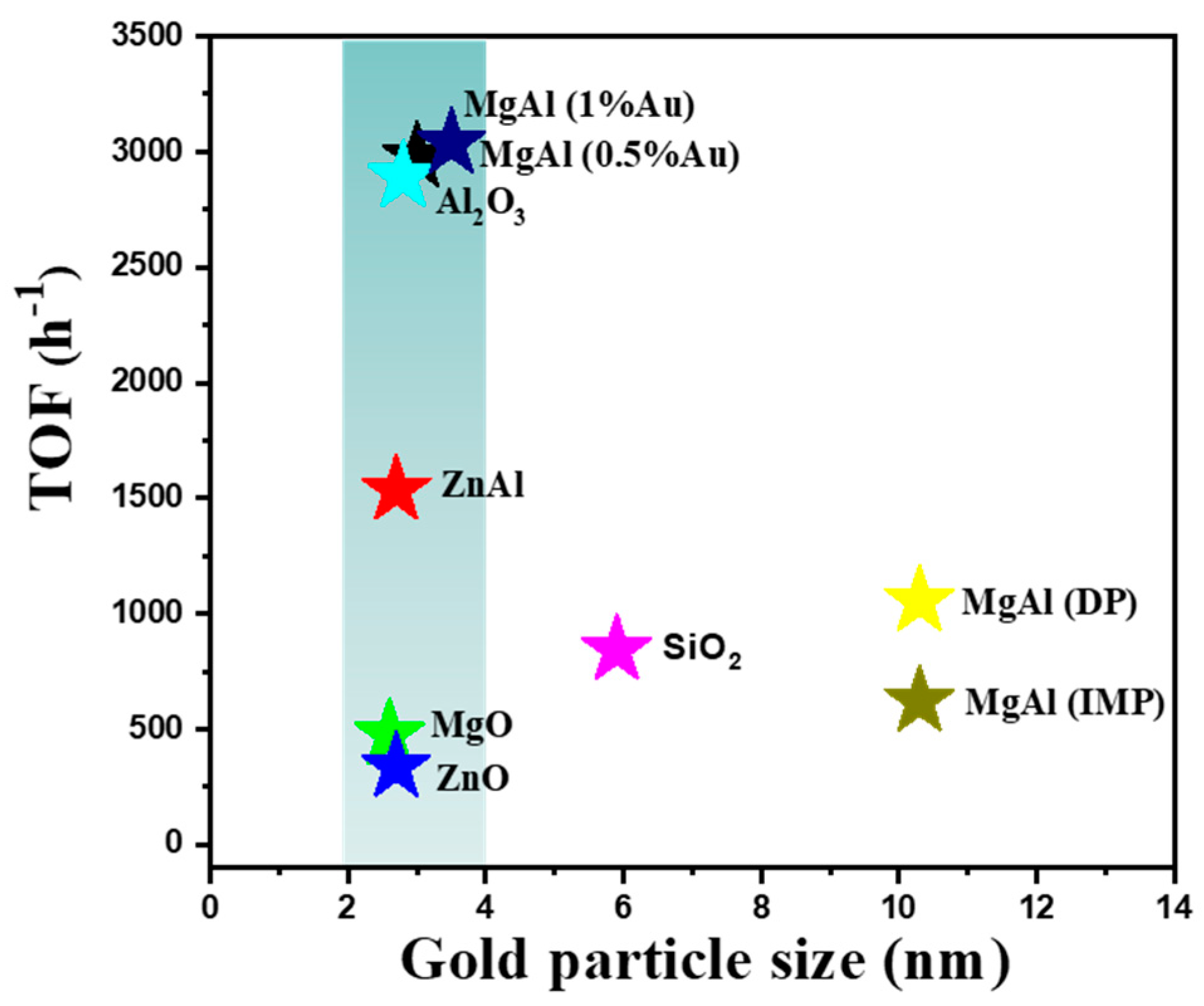
| Entry | Sample | Loadings of Au (%) a | SBET (m2/g) b | Vmesopore (cm3/g) b | Dpore (nm) b | Size (nm) c |
|---|---|---|---|---|---|---|
| 1 | Au25/MgAl-300 | 0.5 | 131.2 | 0.6 | 15.5 | 3.1 |
| 2 | Au25/ZnAl-300 | 0.7 | 112.6 | 0.3 | 26.4 | 2.7 |
| 3 | Au25/MgO-300 | 0.5 | 10.1 | 0.03 | 27.3 | 2.6 |
| 4 | Au25/ZnO-300 | 0.4 | 4.0 | 0.02 | 27.4 | 2.7 |
| 5 | Au25/Al2O3-300 | 0.4 | 235.4 | 0.3 | 6.5 | 2.8 |
| 6 | Au25/SiO2-300 | 0.4 | 367.1 | 0.7 | 7.0 | 5.9 |
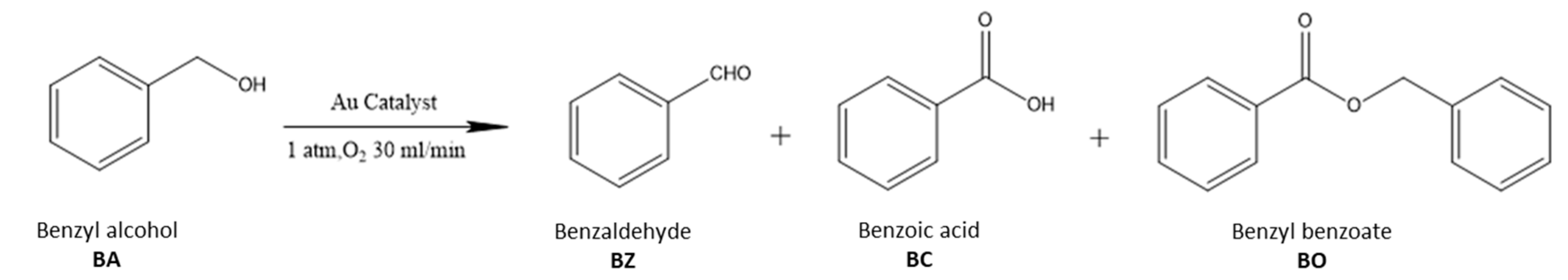
| Entry | Catalyst | Au mmol% | Conversion (%) a | Selectivity (%) a | TOF (h−1) b | TOF (h−1) c | |
|---|---|---|---|---|---|---|---|
| BZ | BO | ||||||
| 1 | Au25/MgAl-300 | 0.27 | 89.5 | 95.0 | 5.0 | 993 | 2979 |
| 2 | Au25/ZnAl-300 | 0.37 | 69.7 | 94.7 | 5.3 | 569 | 1536 |
| 3 | Au25/MgO-300 | 0.26 | 26.8 | 97.4 | 2.6 | 184 | 478 |
| 4 | Au25/ZnO-300 | 0.21 | 14.8 | 49.7 | 0.5 | 125 | 338 |
| 5 | Au25/Al2O3-300 | 0.19 | 87.2 | 94.9 | 5.1 | 1033 | 2892 |
| 6 | Au25/SiO2-300 | 0.20 | 14.2 | 0.6 | 0 | 143 | 844 |
| 7 | Au25/MgAl-300 (1%) d | 0.28 | 74.0 | 96.9 | 3.1 | 846 | 2792 |
| 8 | Au/MgAl-300-DP e | 0.20 | 10.9 | 56.3 | 0.5 | 102 | 1051 |
| 9 | Au/MgAl-300-IMP e | 0.25 | 7.4 | 38.6 | 0 | 61 | 628 |
| 10 | Au25/MgAl-HT | 0.25 | 8.1 | 4.3 | 0 | - | - |
| 11 | MgAl-300 | - | 5.4 | 1.3 | 0 | - | - |
| 12 | Blank | - | 0 | 0 | 0 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, H.; Tan, Y.; Chen, X.; Lin, R.; Yang, W.; Huang, C.; Wang, S.; Wang, X.; Liu, X.Y.; et al. Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts 2020, 10, 107. https://doi.org/10.3390/catal10010107
Liu L, Li H, Tan Y, Chen X, Lin R, Yang W, Huang C, Wang S, Wang X, Liu XY, et al. Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts. 2020; 10(1):107. https://doi.org/10.3390/catal10010107
Chicago/Turabian StyleLiu, Lu, Huayin Li, Yuan Tan, Xingkun Chen, Ronghe Lin, Wenshao Yang, Chuanqi Huang, Saisai Wang, Xuepeng Wang, Xiao Yan Liu, and et al. 2020. "Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols" Catalysts 10, no. 1: 107. https://doi.org/10.3390/catal10010107
APA StyleLiu, L., Li, H., Tan, Y., Chen, X., Lin, R., Yang, W., Huang, C., Wang, S., Wang, X., Liu, X. Y., Zhao, M., & Ding, Y. (2020). Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts, 10(1), 107. https://doi.org/10.3390/catal10010107






