Time–Frequency Feature Fusion Approach for Hemiplegic Gait Recognition
Abstract
1. Introduction
- (1)
- CDUT Plantar Pressure Dataset—Middle-Aged and Elderly Group (CDUT-PP-MAE): A dataset of plantar pressure data was collected from 48 middle-aged and elderly individuals, including both hemiplegic and healthy participants. Based on these data, a center of pressure trajectory dataset was constructed to validate the effectiveness of the proposed hemiplegic gait recognition method.
- (2)
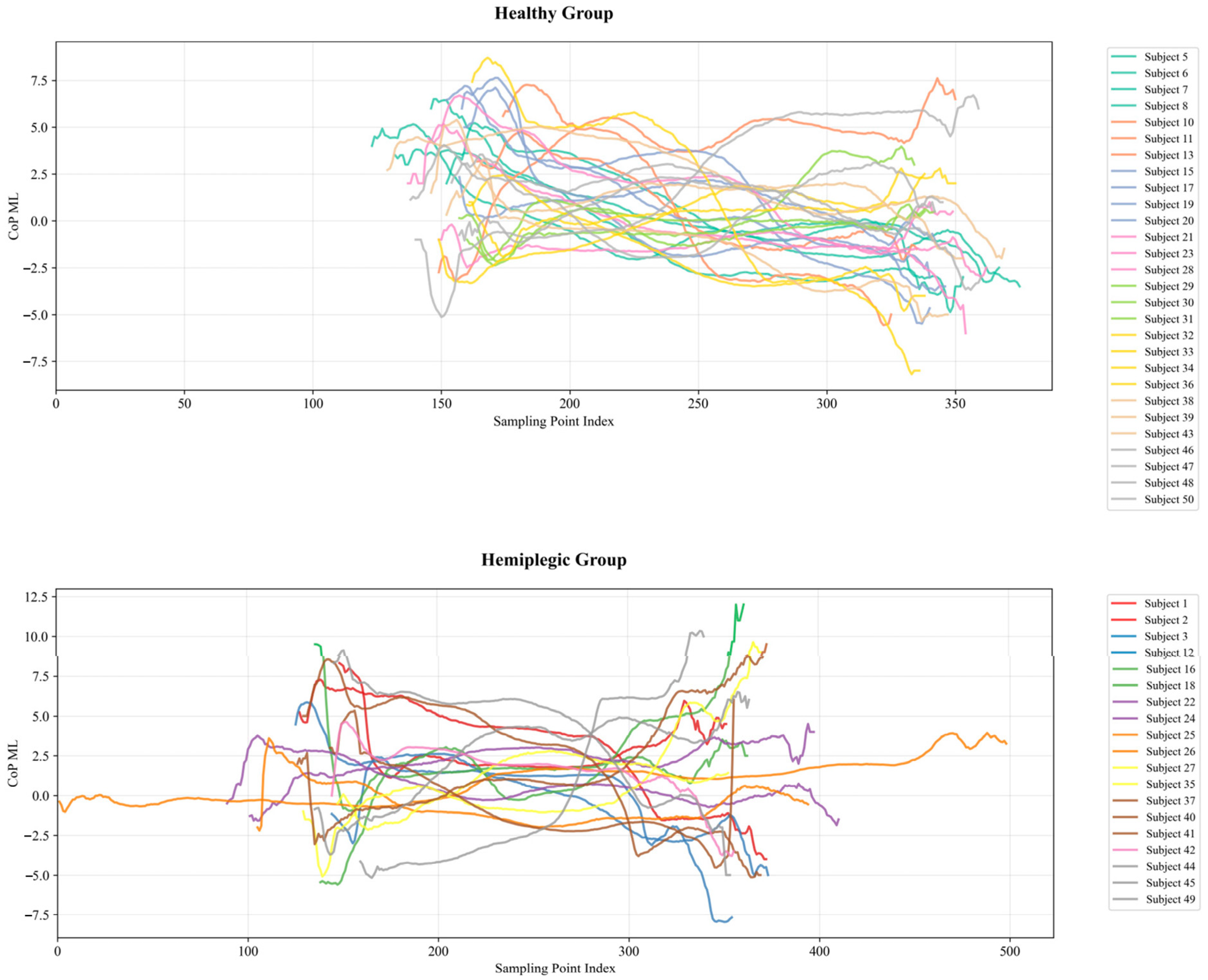
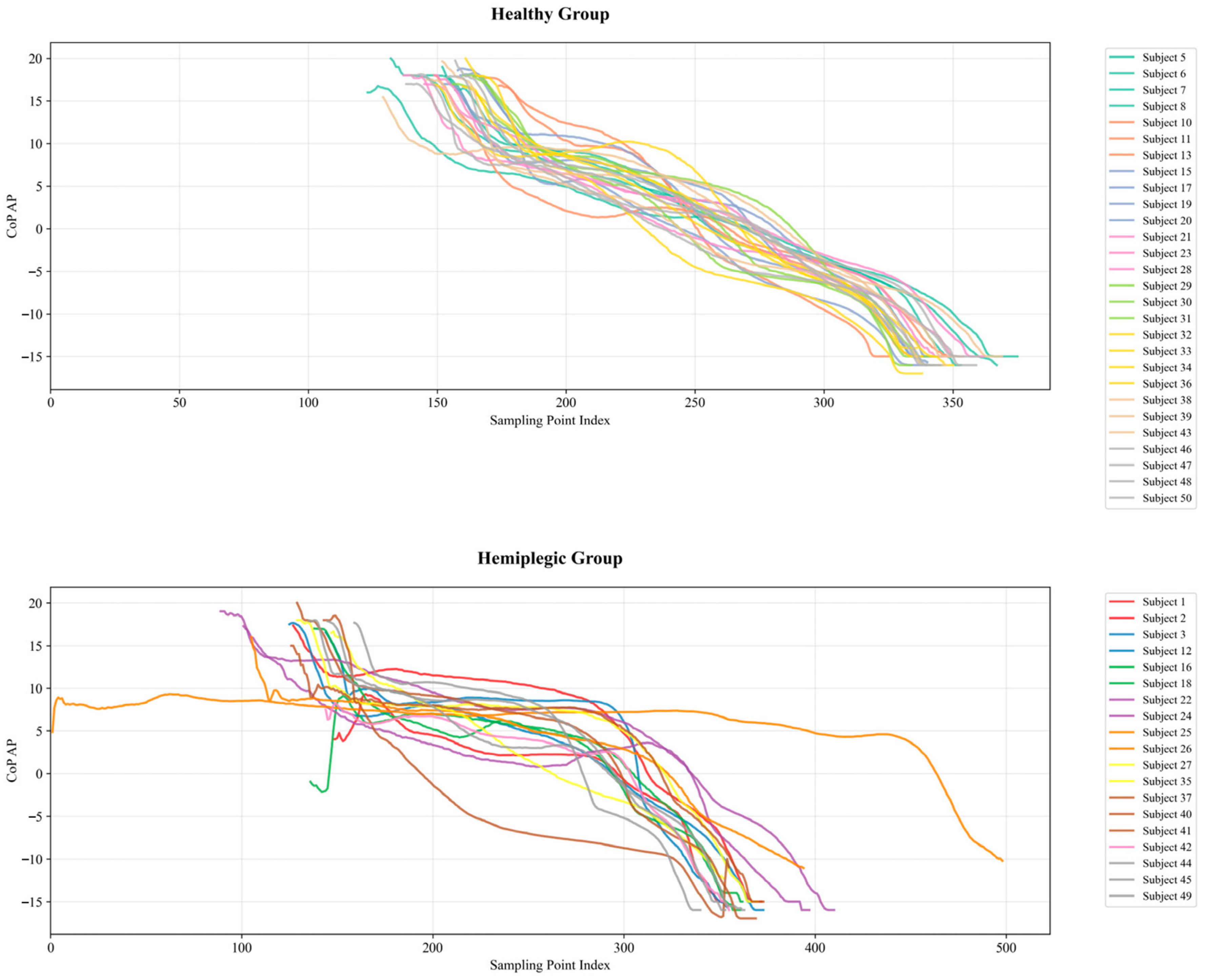
- The study compares the performance of hemiplegic gait recognition using plantar pressure center trajectories in different directional orientations.
- (3)
- A novel temporal–frequency domain interaction network (TFDI-Net) is introduced. Its superiority is demonstrated through comparisons with traditional machine learning methods, as well as state-of-the-art deep learning techniques.
2. Related Work
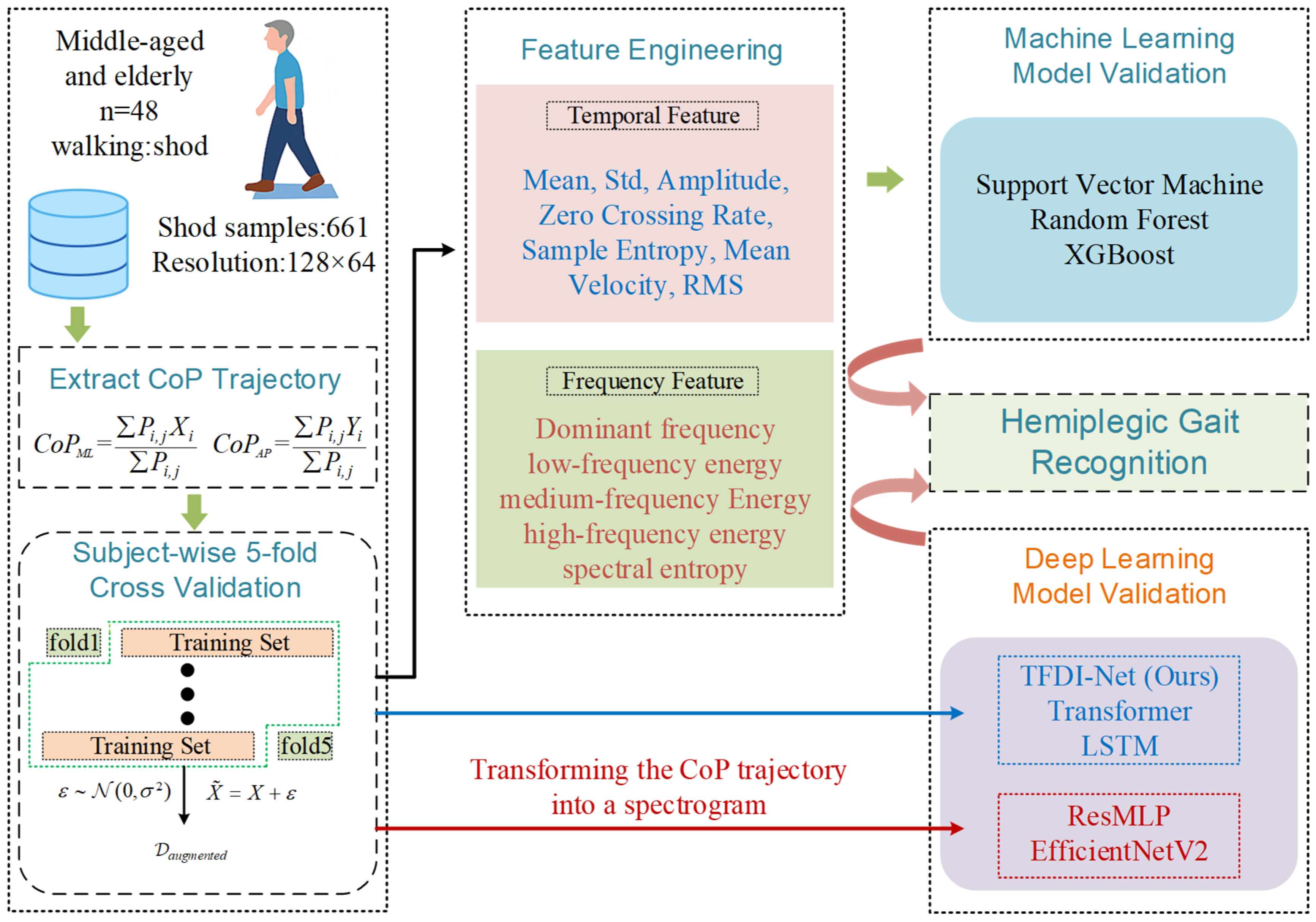
3. Materials and Methods
3.1. Data Acquisition from Student Participants
3.2. Feature Engineering
3.3. Experimental Setup
3.4. Data Augmentation
| Algorithm 1: Gaussian Noise-Based Data Augmentation |
| Input: |
| X: Original time series sample ∈ |
| N: 5//Number of augmented copies |
| , : 0.01, 0.05//Minimum and maximum noise standard deviation |
| Output: |
| Augmented dataset containing N noisy copies of X |
|
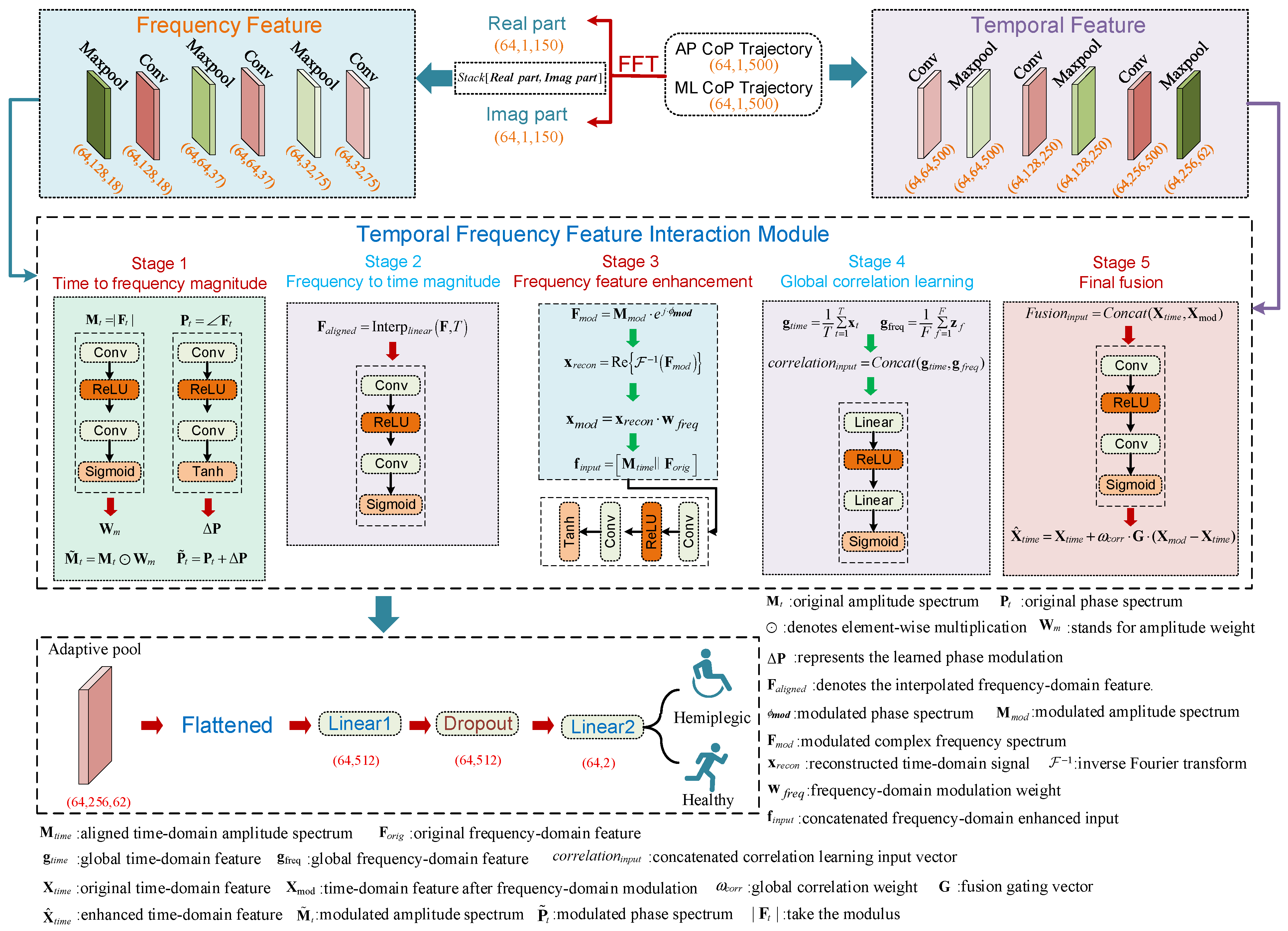
3.5. TFDI-Net
3.5.1. Temporal Domain Convolution Module
3.5.2. Frequency Domain Feature Extraction Module
3.5.3. Temporal–Frequency Modal Interaction Module
- (1)
- Time-to-Frequency Guidance
- (2)
- Frequency-to-Time Guidance
- (3)
- Frequency Feature Enhancement
- (4)
- Global Time–Frequency Correlation Learning
- (5)
- Gated Fusion
4. Results
4.1. Hemiplegic Gait Recognition Results Based on CoP Trajectories
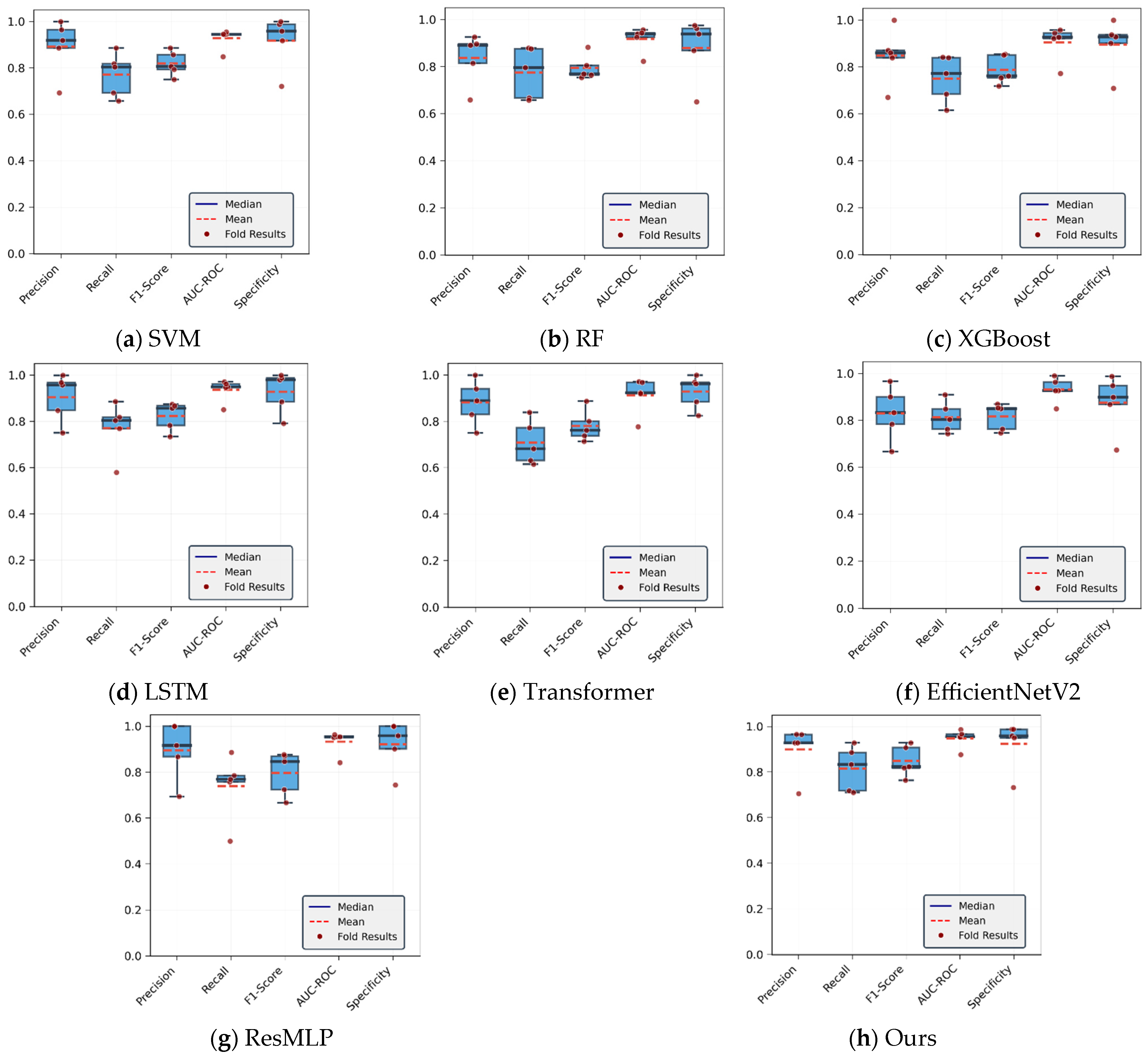
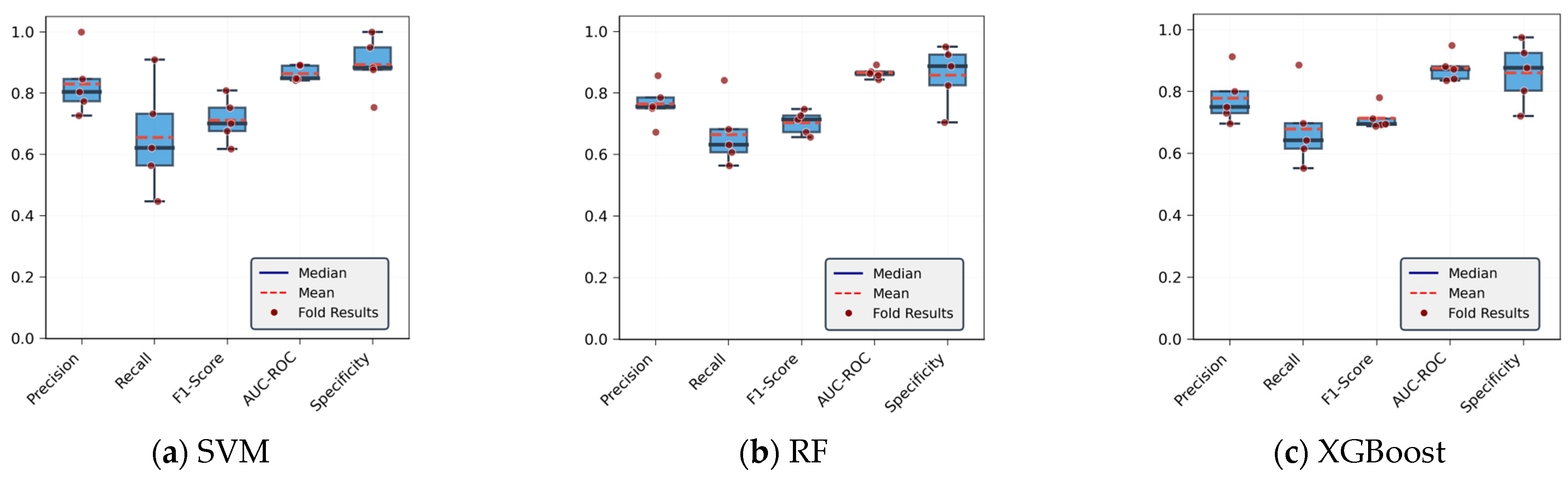
4.1.1. Analysis of CoP Trajectory Recognition Results Using Machine Learning Methods
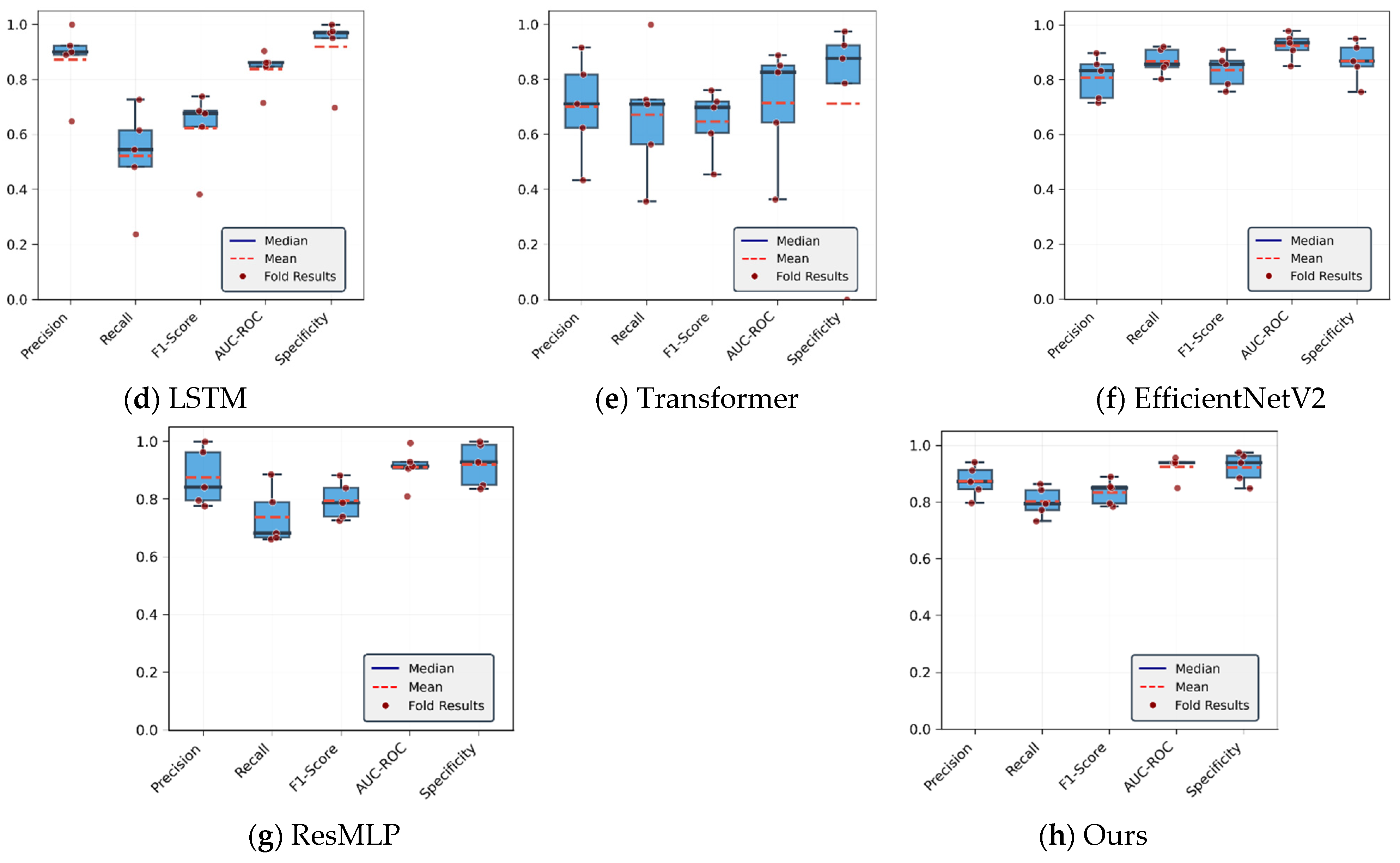
4.1.2. Analysis of CoP Trajectory Recognition Results Using Deep Learning Methods
4.2. Ablation Experiment
4.3. Computational Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, G.; Xu, J.; Zhang, C.; Jiang, J.; Chen, G.; Chen, J.; Liu, Q.; Xu, G.; Lan, Y. Identifying Biomarkers Related to Motor Function in Chronic Stroke: A fNIRS and TMS Study. CNS Neurosci. Ther. 2024, 30, e14889. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Gao, D.; Chen, X.; Ning, S.; Liu, Z.; Li, P. Analysis of Motor Functions of Hemiplegic Patients Based on Dual-Mode Signal Fusion. IEEE Sens. J. 2024, 24, 32694–32706. [Google Scholar] [CrossRef]
- Hulleck, A.A.; Menoth Mohan, D.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and Future of Gait Assessment in Clinical Practice: Towards the Application of Novel Trends and Technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xie, L. Gait Feature Construction and Selection Based on Plantar Pressure Insole. In Proceedings of the 2023 5th International Academic Exchange Conference on Science and Technology Innovation (IAECST), Guangzhou, China, 8–10 December 2023; pp. 982–987. [Google Scholar]
- Potluri, S.; Ravuri, S.; Diedrich, C.; Schega, L. Deep Learning Based Gait Abnormality Detection Using Wearable Sensor System. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3613–3619. [Google Scholar]
- Hayashi, Y.; Yasuda, K.; Kitaji, Y.; Harashima, H.; Iwata, H. A Haptic-Based Perception-Empathy Biofeedback Device That Supplements Foot Pressure Pattern during Gait in Stroke Patients. In Proceedings of the 2019 IEEE/SICE International Symposium on System Integration (SII), Paris, France, 14–16 January 2019; pp. 124–128. [Google Scholar]
- LeMoyne, R.; Kerr, W.; Mastroianni, T.; Hessel, A. Implementation of Machine Learning for Classifying Hemiplegic Gait Disparity through Use of a Force Plate. In Proceedings of the 2014 13th International Conference on Machine Learning and Applications, Detroit, MI, USA, 3–6 December 2014; pp. 379–382. [Google Scholar]
- Cui, C.; Bian, G.-B.; Hou, Z.-G.; Zhao, J.; Su, G.; Zhou, H.; Peng, L.; Wang, W. Simultaneous Recognition and Assessment of Post-Stroke Hemiparetic Gait by Fusing Kinematic, Kinetic, and Electrophysiological Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, H.; Wang, Z.; Dai, P. Correlation Analysis of Balance Function with Plantar Pressure Distribution and Gait Parameters in Patients with Cerebral Infarction in the Basal Ganglia Region. Front. Neurosci. 2023, 17, 1099843. [Google Scholar] [CrossRef] [PubMed]
- Sanghan, S.; Chatpun, S.; Leelasamran, W. Gait Characterizations under Dynamic Load during Walking in Hemiplegic Patients. In Proceedings of the 4th 2011 Biomedical Engineering International Conference, Chiang Mai, Thailand, 29–31 January 2012; pp. 22–25. [Google Scholar]
- Rastegarpanah, A.; Scone, T.; Saadat, M.; Rastegarpanah, M.; Taylor, S.J.; Sadeghein, N. Targeting Effect on Gait Parameters in Healthy Individuals and Post-Stroke Hemiparetic Individuals. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668318766710. [Google Scholar] [CrossRef] [PubMed]
- Kokolevich, Z.M.; Biros, E.; Tirosh, O.; Reznik, J.E. Distinct Ground Reaction Forces in Gait between the Paretic and Non-Paretic Leg of Stroke Patients: A Paradigm for Innovative Physiotherapy Intervention. Healthcare 2021, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Woo, Y.; Won, J.; Kim, S. Immediate Effects of Insoles Applied to the Sound Side Lower Extremity of Patients with Chronic Hemiplegia during Walking. Restor. Neurol. Neurosci. 2024, 42, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Chu, H.; Kang, G.-H.; Sung, K.-K.; Kang, D.G.; Lee, H.S.; Lee, S. Difference in Gait Recovery Rate of Hemiparetic Stroke Patients According to Paralyzed Side: A Cross-Sectional Study Based on a Retrospective Chart Review. Medicine 2019, 98, e18023. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Tan, M.; Le, Q. EfficientNetV2: Smaller Models and Faster Training. In Proceedings of the Proceedings of the 38th International Conference on Machine Learning, PMLR, Online, 18–24 July 2021; pp. 10096–10106. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention Is All You Need. arXiv 2017, arXiv:1706.03762v7. [Google Scholar]
- Touvron, H.; Bojanowski, P.; Caron, M.; Cord, M.; El-Nouby, A.; Grave, E.; Izacard, G.; Joulin, A.; Synnaeve, G.; Verbeek, J.; et al. ResMLP: Feedforward Networks for Image Classification With Data-Efficient Training. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 5314–5321. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, E.; Larracy, R.; Phinyomark, A.; Scheme, E. Underfoot Pressure-Based Left and Right Foot Classification Algorithms: The Impact of Footwear. IEEE Access 2023, 11, 137937–137947. [Google Scholar] [CrossRef]

| Healthy | Hemiplegic | Total | |
|---|---|---|---|
| Age | 58.9 ± 8.9 | 61.6 ± 10.9 | 60.0 ± 9.8 |
| Sex | 8/21 | 16/5 | 24/26 |
| Height (cm) | 164 ± 14 | 161 ± 11 | 164 ± 14 |
| Weight (kg) | 65 ± 22 | 65 ± 17 | 65 ± 22 |
| Shoe Size (EU) | 39 ± 3 | 39 ± 3 | 39 ± 3 |
| Hemiplegic Side | 0/0 | 11/10 | 11/10 |
| Module | Layer Name | Input Channels | Output Channels | Kernel Size | Activation | Pool |
|---|---|---|---|---|---|---|
| time feature | conv1 | 500 | 64 | 5 | ReLU | Maxpool |
| conv2 | 64 | 128 | 5 | ReLU | Maxpool | |
| conv3 | 128 | 256 | 5 | ReLU | Maxpool | |
| fft | - | - | - | - | - | |
| freq feature | conv1 | 2 | 32 | 3 | ReLU | Maxpool |
| conv2 | 32 | 64 | 3 | ReLU | Maxpool | |
| conv3 | 64 | 128 | 3 | ReLU | Maxpool | |
| time2freq mag | conv | 256 | 64 | 1 | ReLU | - |
| conv | 64 | 256 | 1 | Segmoid | - | |
| freq2time mag | conv | 128 | 64 | 1 | ReLU | - |
| conv | 64 | 128 | 1 | Segmoid | - | |
| freq_enhancement | conv | 384 | 64 | 1 | ReLU | - |
| conv | 64 | 384 | 1 | Tanh | - | |
| time2freq correlation | Linear1 | 384 | 64 | - | ReLU | - |
| Linear2 | 64 | 1 | - | Sigmoid | - | |
| feature fusion | conv | 512 | 62 | 1 | ReLU | - |
| conv | 62 | 512 | 1 | Sigmoid | - | |
| adaptive pool | - | 62 | - | - | - | |
| fc1 | - | - | ReLU | - | ||
| dropout | - | - | - | - | - | |
| fc2 | - | - | - | - | - |
| Model | Accuracy | Precision | F1-Score | AUC-ROC | Specificity | Recall |
|---|---|---|---|---|---|---|
| SVM | 86.99 | 89.23 | 82.76 | 92.75 | 91.7 | 77.17 |
| RF | 84.81 | 83.73 | 80.48 | 91.7 | 87.9 | 77.48 |
| XGBoost | 84.75 | 84.81 | 79.65 | 90.44 | 89.53 | 75.05 |
| LSTM | 87.66 | 90.46 | 83.26 | 93.60 | 92.85 | 77.13 |
| Transformer | 85.47 | 88.78 | 79.60 | 92.31 | 92.37 | 72.15 |
| EfficientNetV2 | 85.88 | 83.01 | 82.18 | 93.12 | 87.56 | 81.36 |
| ResMLP | 86.07 | 89.56 | 81.03 | 93.24 | 92.09 | 73.98 |
| Ours | 89.88 | 89.39 | 87.36 | 94.9 | 91.93 | 85.42 |
| Model | Accuracy | Precision | F1-Score | AUC-ROC | Specificity | Recall |
|---|---|---|---|---|---|---|
| SVM | 81.13 | 83.02 | 73.21 | 86.33 | 89.27 | 65.48 |
| RF | 79.24 | 76.42 | 71.12 | 86.56 | 85.82 | 66.51 |
| XGBoost | 79.98 | 77.79 | 72.5 | 87.59 | 85.98 | 67.88 |
| LSTM | 77.85 | 87.21 | 65.26 | 83.76 | 91.84 | 52.14 |
| Transformer | 73.29 | 72.75 | 71.81 | 74.39 | 71.87 | 70.89 |
| EfficientNetV2 | 86.91 | 80.75 | 83.63 | 92.44 | 86.81 | 86.73 |
| ResMLP | 85.54 | 87.51 | 80.01 | 91.07 | 92 | 73.7 |
| Ours | 88.61 | 88.73 | 85.06 | 91.97 | 92.65 | 81.71 |
| T | F | Fusion | Accuracy | Precision | F1-Score | AUC-ROC | Specificity | Recall |
|---|---|---|---|---|---|---|---|---|
| ✓ | 88.81 | 89.77 | 85.14 | 95.16 | 92.70 | 80.97 | ||
| ✓ | 88.56 | 88.51 | 85.74 | 94.16 | 90.72 | 83.13 | ||
| ✓ | ✓ | 89.4 | 88.24 | 86.74 | 94.97 | 90.82 | 85.29 | |
| ✓ | ✓ | ✓ | 89.88 | 89.39 | 87.36 | 94.9 | 91.93 | 85.42 |
| Model | Parameters (M) | Size (MB) | Training Time (s) | Inference Time (ms) |
|---|---|---|---|---|
| SVM | - | - | 35 | - |
| RF | - | - | 59 | - |
| XGBoost | - | - | 25 | - |
| LSTM | 0.17 | 0.69 | 55.4 | 0.5 |
| Transformer | 31.9 | 62.7 | 606 | 2 |
| EfficientNetV2 | 20.3 | 78.08 | 1055 | 13.48 |
| ResMLP | 14.36 | 54.77 | 874 | 3.35 |
| Ours | 12.77 | 48.73 | 195 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Mei, Z. Time–Frequency Feature Fusion Approach for Hemiplegic Gait Recognition. Computers 2025, 14, 334. https://doi.org/10.3390/computers14080334
Mao L, Mei Z. Time–Frequency Feature Fusion Approach for Hemiplegic Gait Recognition. Computers. 2025; 14(8):334. https://doi.org/10.3390/computers14080334
Chicago/Turabian StyleMao, Linglong, and Zhanyong Mei. 2025. "Time–Frequency Feature Fusion Approach for Hemiplegic Gait Recognition" Computers 14, no. 8: 334. https://doi.org/10.3390/computers14080334
APA StyleMao, L., & Mei, Z. (2025). Time–Frequency Feature Fusion Approach for Hemiplegic Gait Recognition. Computers, 14(8), 334. https://doi.org/10.3390/computers14080334







