Mapping the Chemical Space of Antiviral Peptides with Half-Space Proximal and Metadata Networks Through Interactive Data Mining
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic Concepts
2.2. Half-Space Proximal Network
2.3. Metadata Complex Network
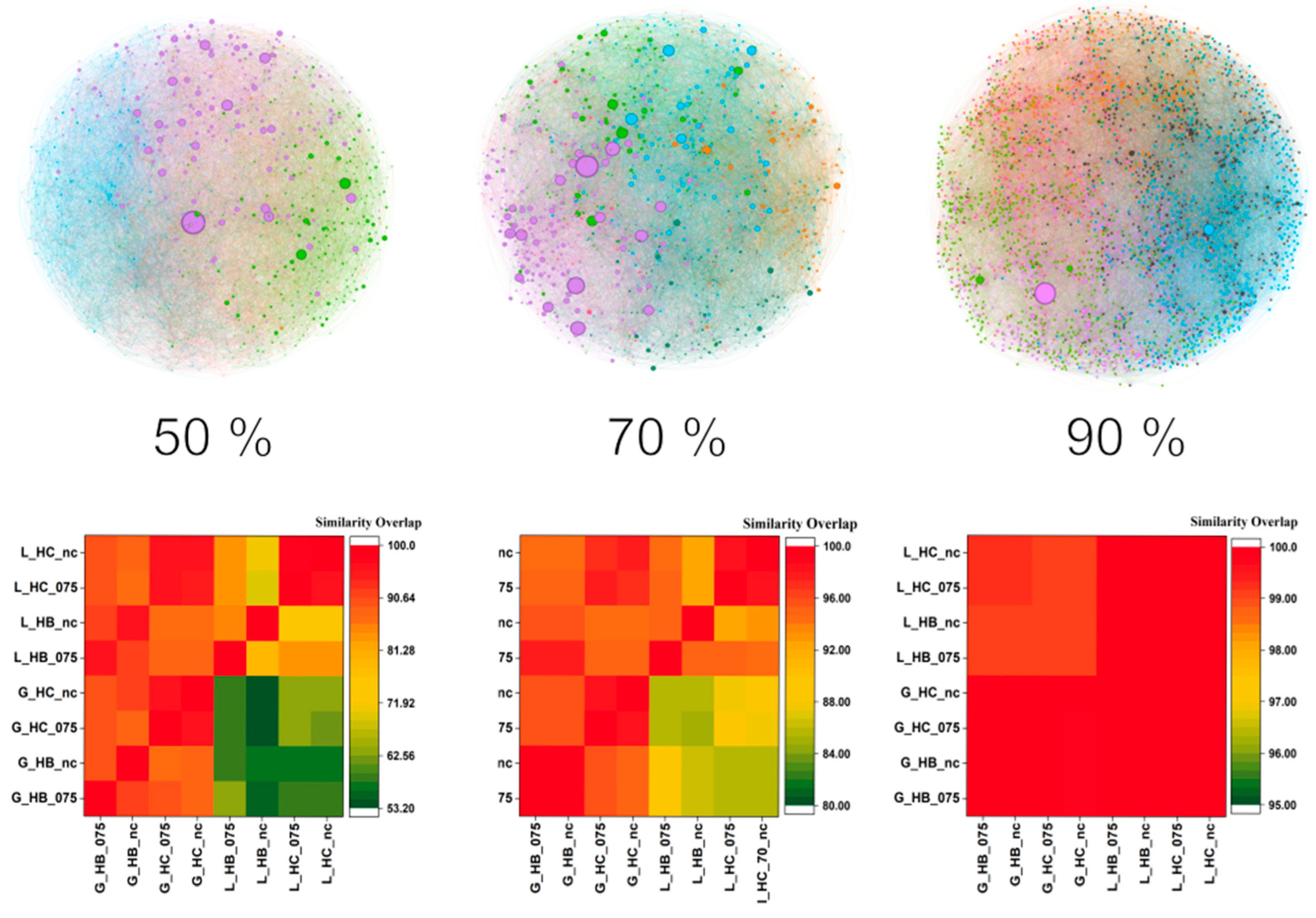
2.4. Network Visualization and Characterization
2.5. Exploration of Scaffold and Selection of Most Representative Subset
2.6. Motif Discovery
2.7. Alignment Free Motif Enrichment
- B-TS_StarPepAVP (272 positives + 623 negatives)
- Ex_StarPepAVP (1230 positives + 10,771 negatives)
- TR_StarPepAVP (2321 positives + 2321 negatives)
- TS_StarPepAVP (623 positives + 623 negatives)
2.8. Motif Scanning on Non-Antiviral Sequences
- Antibacterial (12,936 seqs)
- Antifungal (4882)
- Antiparasitic (530)
- AMP dataset (13,107): antibacterial, antifungal, and antiparasitic peptides, excluding any overlap among these categories.
- Other dataset (8440): peptides classified as anticancer, antidiabetic, antihypertensive, enzymatic inhibitors, insecticidal, neuropeptides, and spermicidal.
- Toxic dataset (4653): peptides annotated as venom/toxic or toxic to mammals.
- CPP dataset (1171): cell-penetrating peptides from CPPsite 2.0.
3. Results and Discussion
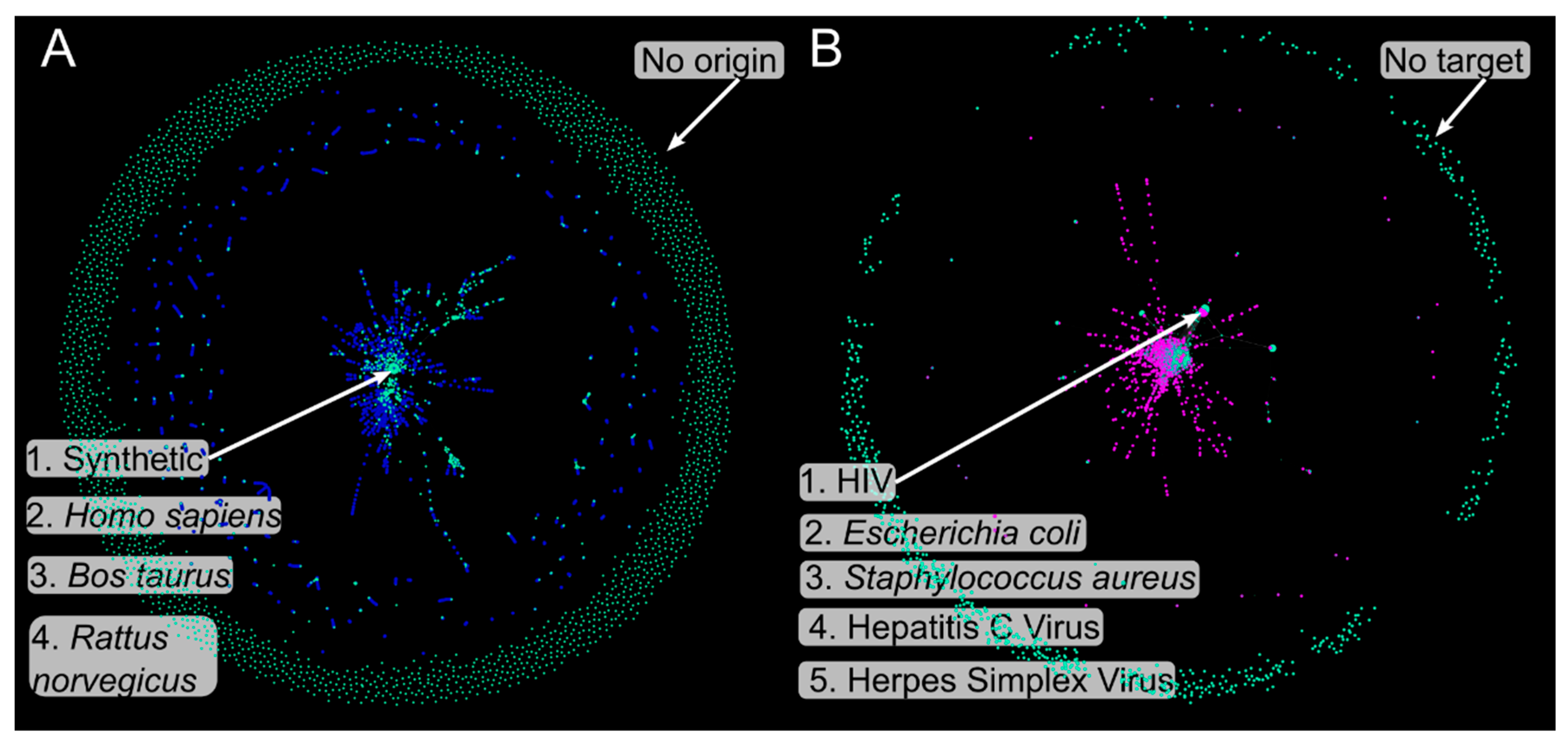
3.1. Metadata Complex Networks

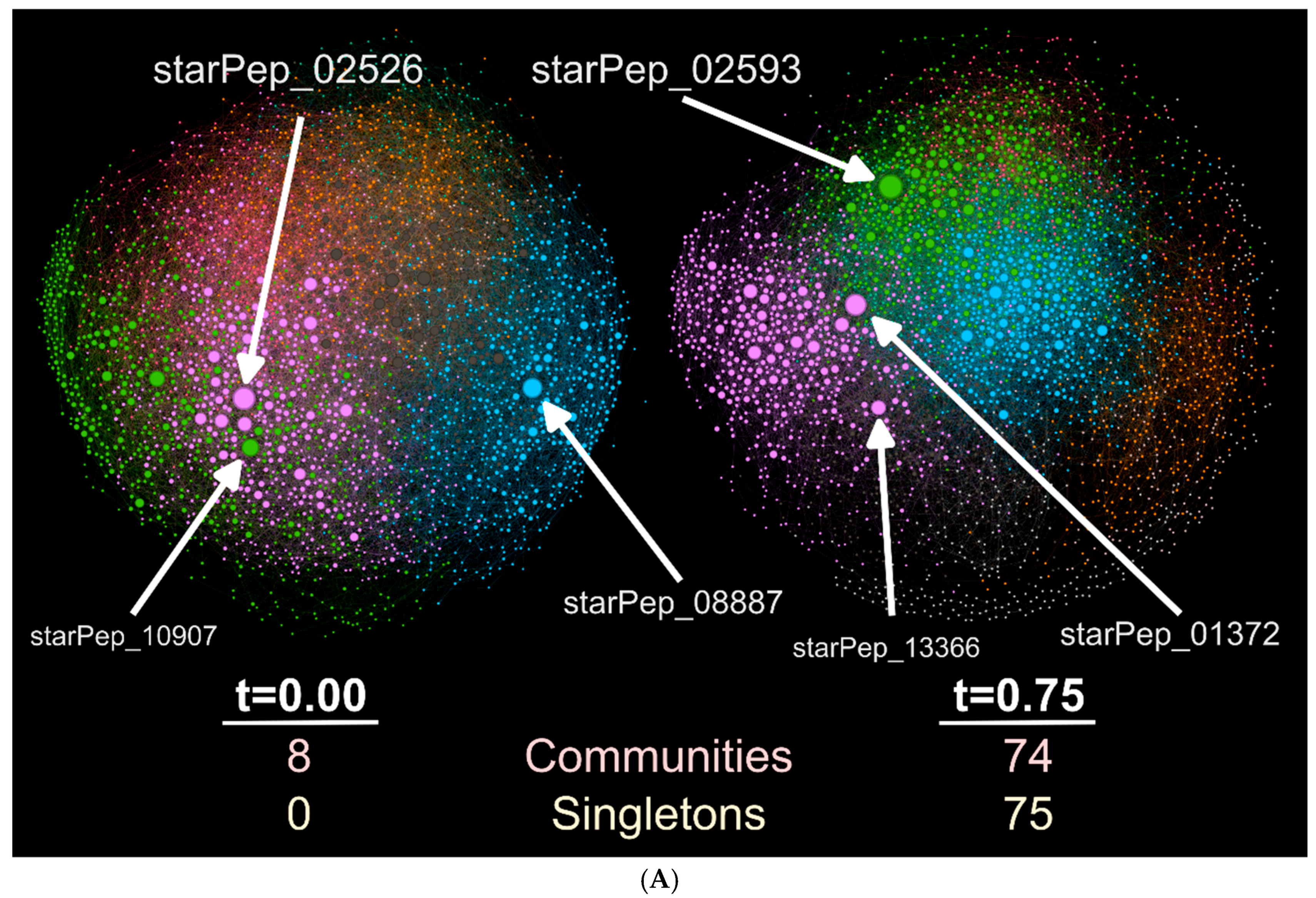
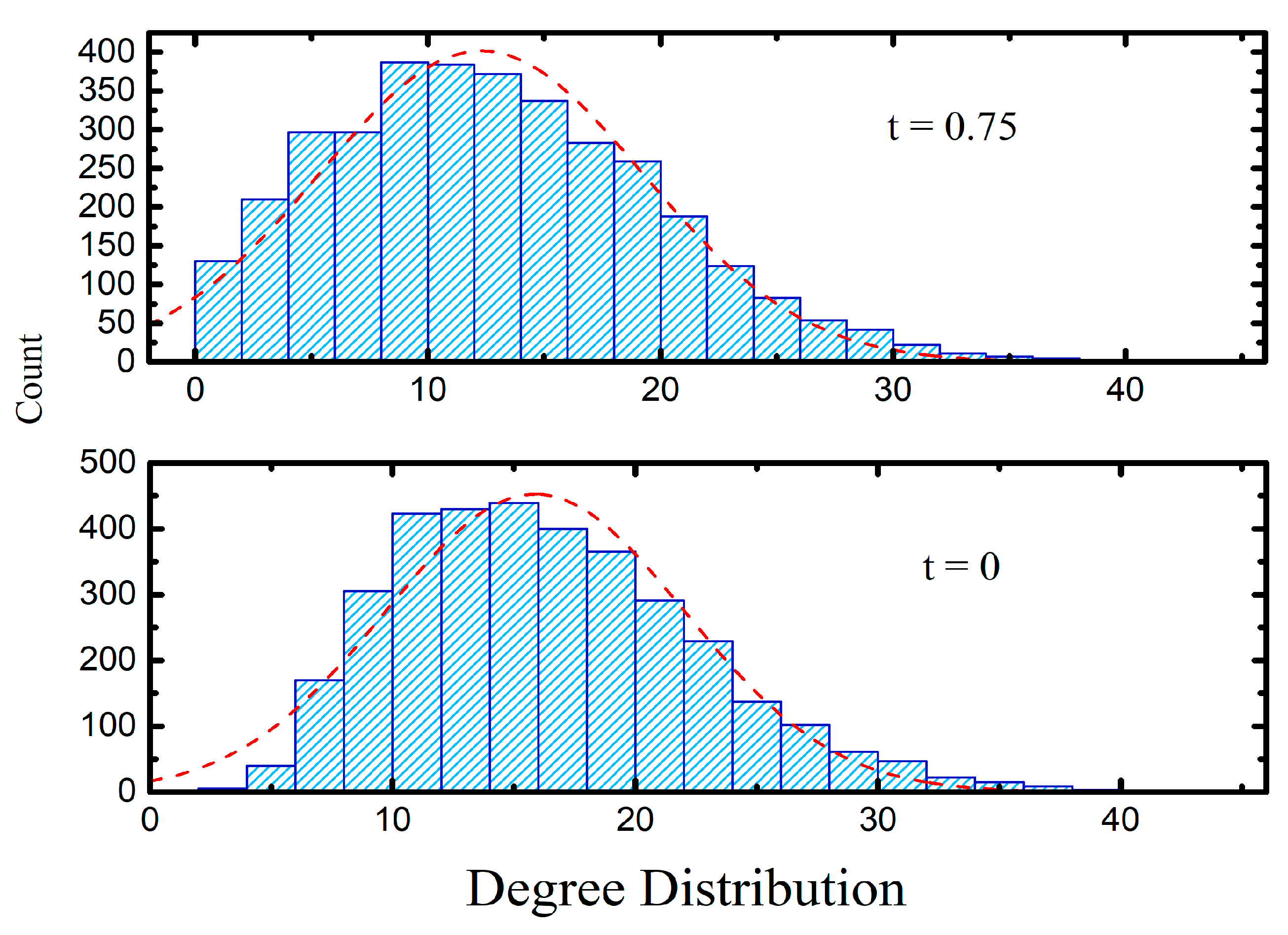
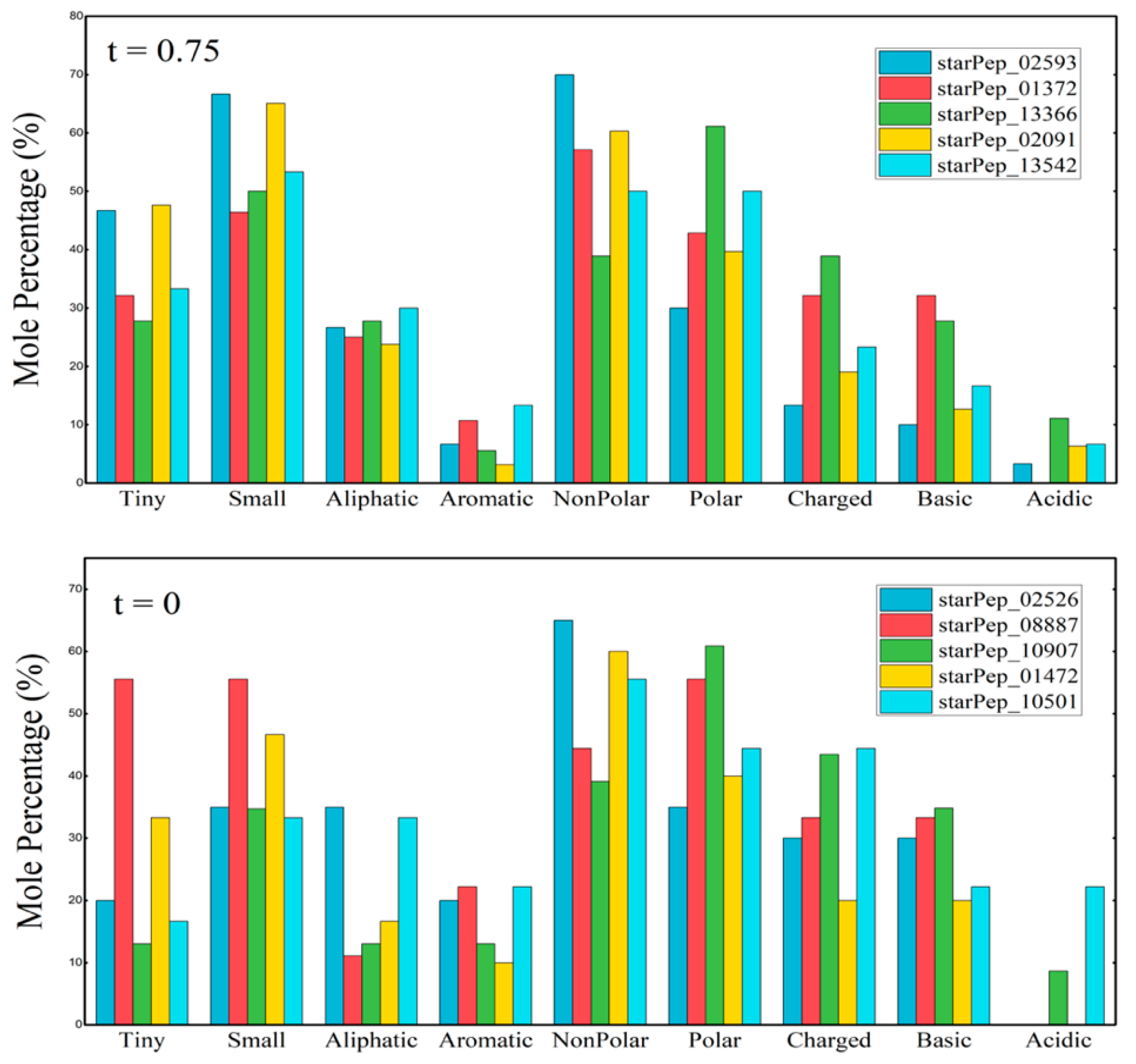
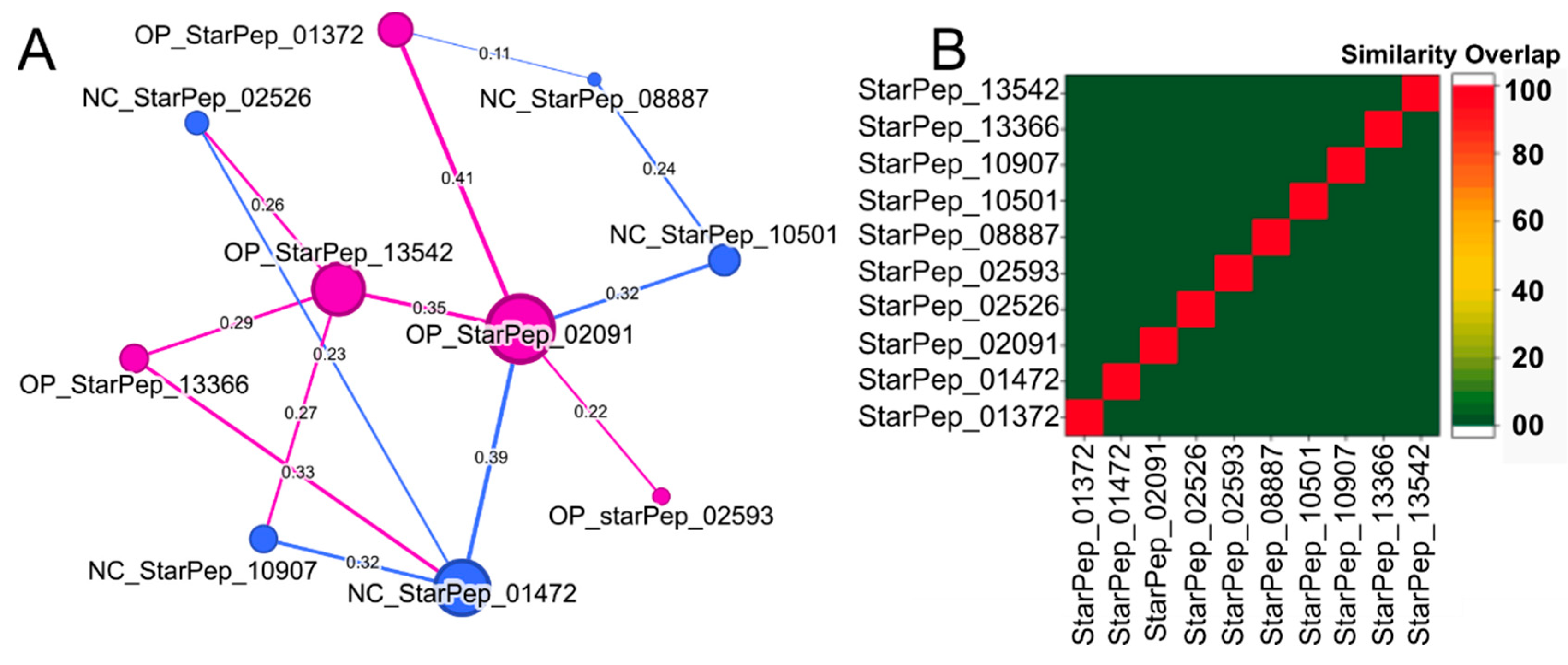
3.2. Half Space Proximal Networks
- StarPep_02593—Cycloviolacin-O17 from Viola odorata; anti-HIV, antibacterial, hemolytic. [77]. Seq: “GIPCGESCVWIPGISAAIGCSCKNKVCYRN”.
- StarPep_01372—Kenojeinin I from fermented skate skin; cationic residues aid bacterial membrane binding; hydrophobic residues disrupt membranes [78]. Seq: “GKQYFPKVGGRLSGKAPLAAKTHRRLKP”.
- StarPep_13366—Hepatitis C virus genome polyprotein fragment (E1/E2 envelope region), implicated in viral entry [79]. Seq: “VATRDGKLPTTQLRRHID”.
- StarPep_02091—Ascaris suum antibacterial factor abf-2; active against Gram-positive/negative bacteria and yeast [80]. Seq: “DIDFSTCARMDVPILKKAAQGLCITSCSMQNCGTGSCKKRSGRPTCVCYRCANGGGDIPLGAL”.
- StarPep_13542—Classical swine fever virus genome polyprotein fragment, envelope glycoprotein-associated [81]. Seq: “VSRRYLASLHKKALPTSVTFELLFDGTNPS”.
- StarPep_02526—D51 synthetic AMP designed via linguistic model, amphipathic, active against Gram-positive/negative bacteria [82]. Seq: “FLFRVASKVFPALIGKFKKK”.
- StarPep_08887—Andes virus inhibitor, identified via cysteine-constrained phage display [83]. Seq: “CSLHSHKGC”.
- StarPep_10907—Feline immunodeficiency virus gp150-derived peptide, likely interfering with viral entry [84]. Seq: “KQRNRWEWRPDFKSKKVKISLPC”.
- StarPep_01472—Deer (Cervus elaphus) blood-derived AMP, especially active against Gram-negative bacteria [85]. Seq: “IRNSLTCRFNFGICLPKRCPGRMRQIGTCF”
- StarPep_10501—Amphipathic helix peptide targeting HIV envelope glycoprotein to inhibit membrane fusion [86]. Seq: “KAFEEVLAKKFYDKALWD”.
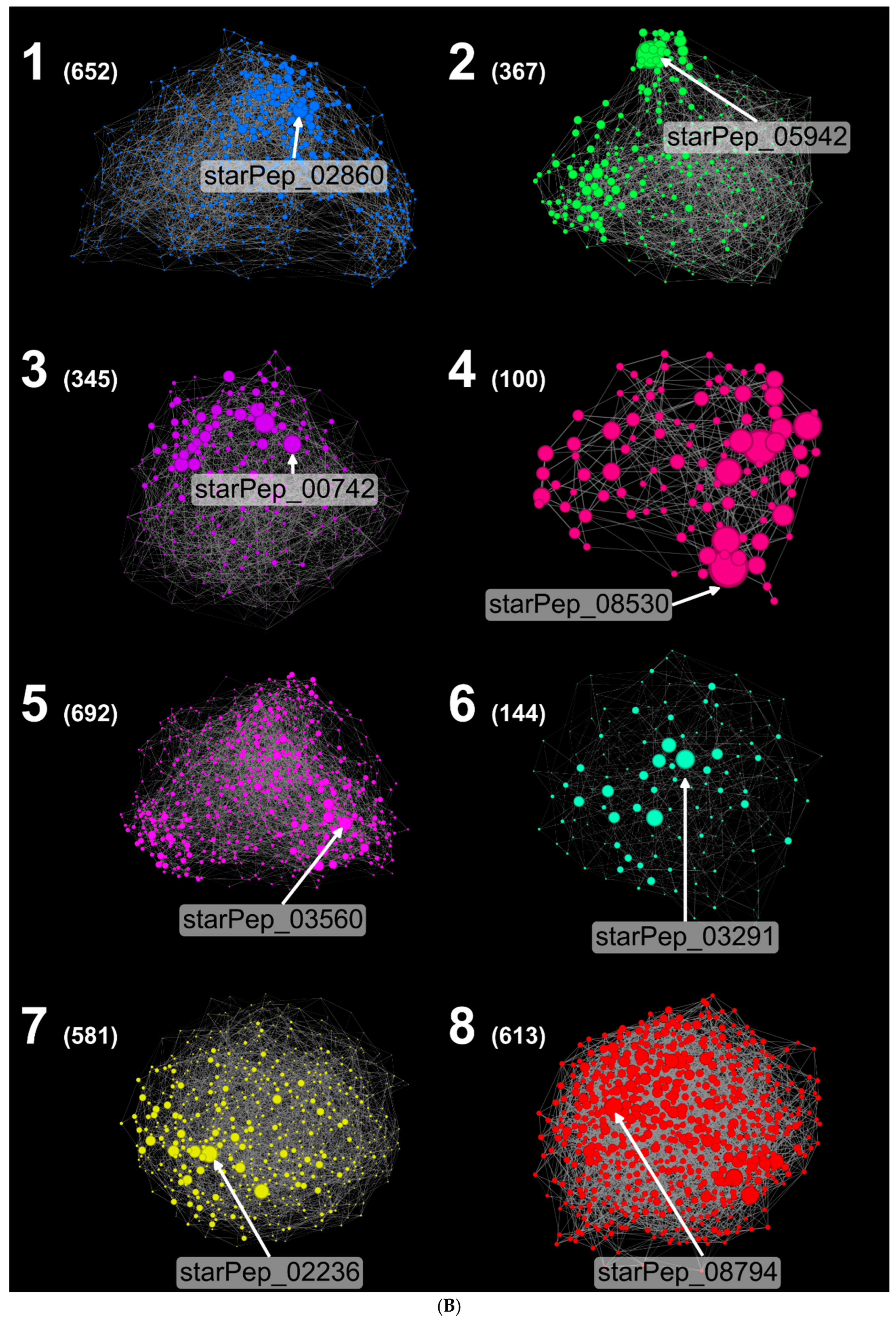
3.3. Scaffold Extraction
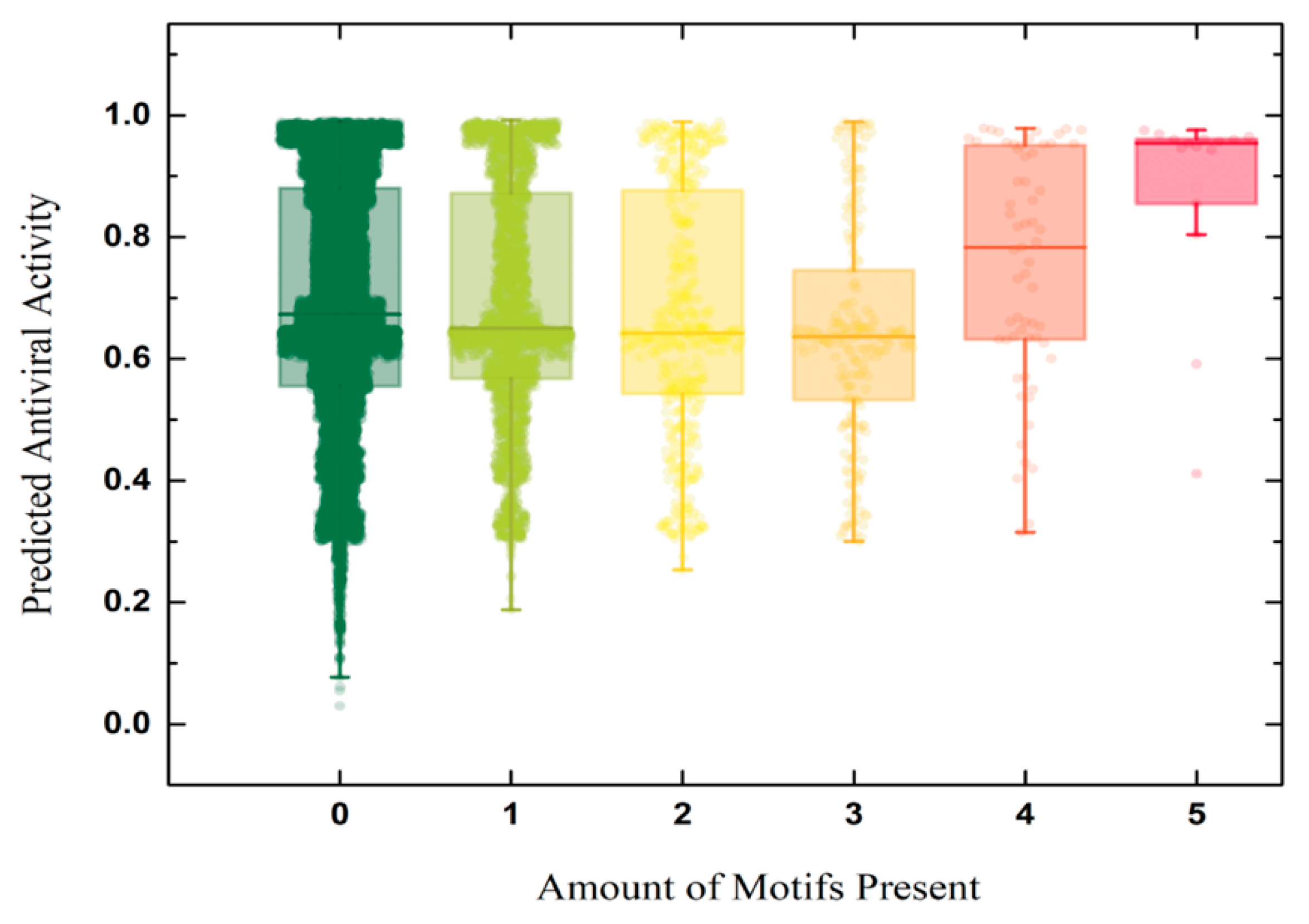
3.4. Motif Discovery
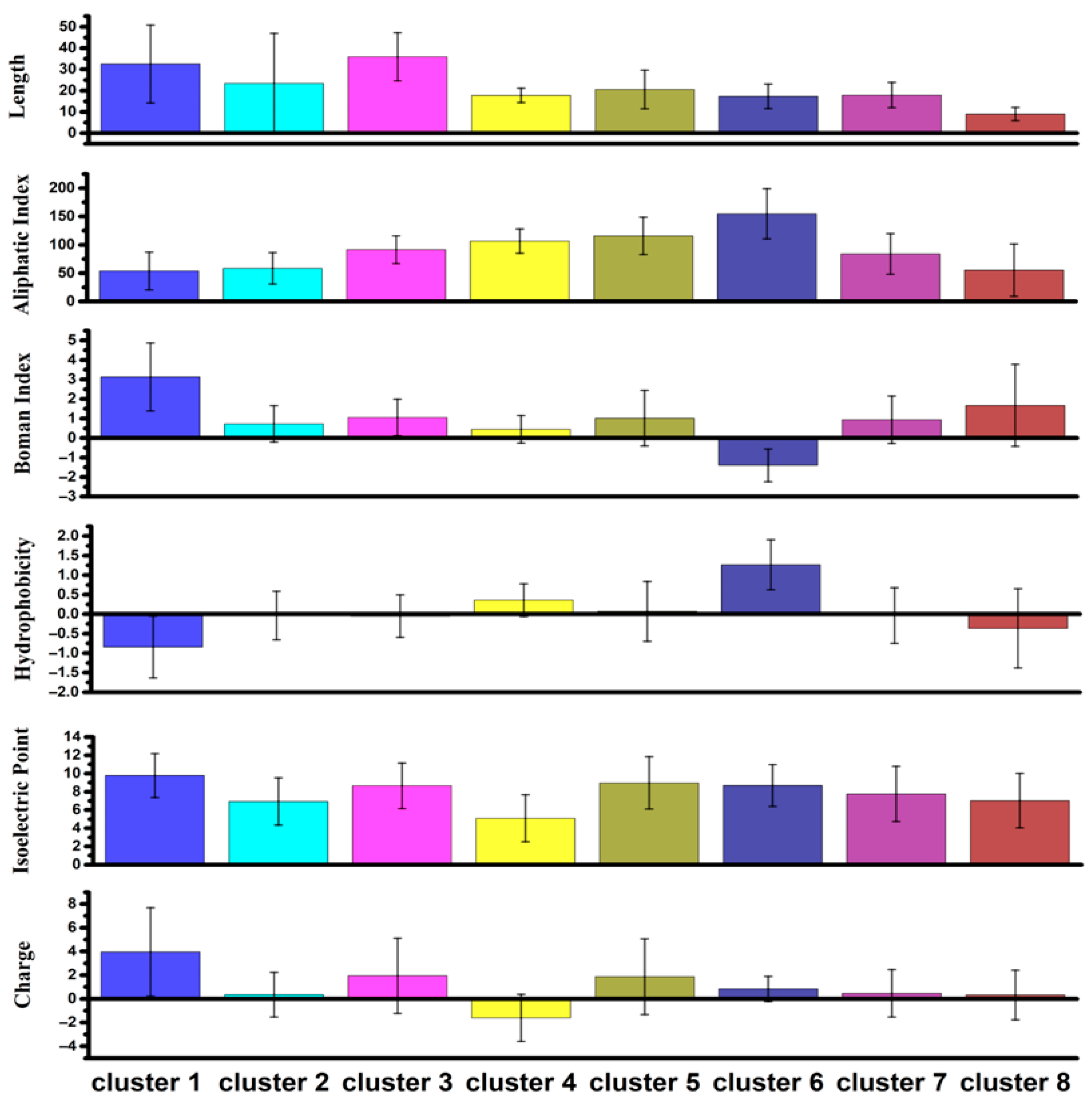
- Cluster 4: more acidic AAs, average negative charge, lowest isoelectric point.
- Cluster 1: highest positive charge and Boman index, lowest hydrophobicity.
- Clusters 2, 7, 8: near-neutral charges.
- Cluster 6: highest aliphatic index, high hydrophobicity, negative Boman index.
- Clusters 1 and 3: longest sequences; Cluster 8: shortest sequences.
3.4.1. Motif Enrichment
Stage 1—Positive Dataset Validation
Stage 2—Inverse Validation
- Anti-coronavirus peptide motifs: A second study reporting anti-coronavirus peptides provided functional AVP motifs in its Supplementary Information [109]. We observe higher motif-level similarity to our set (see Table 6), including enrichment of arginine, leucine, and valine, consistent with other studies [109].
- GKK motif and residue composition (ENNAVIA-D): The GKK motif appears among positive samples in the ENNAVIA-D model [110]. Consistently with [110], our motifs frequently include lysine, leucine, asparagine, glutamic acid, and valine; specifically, lysine, leucine, and valine occur in 36%, 30%, and 27% of validated motifs, respectively.
- Specificity caveat for lysine: While lysine is prevalent among AVPs, its occurrence is even higher in non-AVPs, an important consideration when interpreting motif specificity and when using lysine-rich motifs for design [110].
| MOTIF | Cluster | Reference Sequence | Reference |
|---|---|---|---|
| CYCR | 1 | CYCRTGRCATRERRSGTCIIQGRL | [111] |
| RRRRRH | RRRRRRRRHPAEPGSTVTTQNTASQTMS | [109] | |
| CGES | 2 | IPCGESCVWIPCITA | [109] |
| VWIPCI | IPCGESCVWIPCITA | [109] | |
| QAVG | VYSRCGFAQTLYYDYGVTDMNTLANWVCLVQYESSFNDQAVGAINYNGTQDFGLFQINNKYWCQGAVSSSDSCGIACTSLLGNLSASWSCAQLVYQQQGFSAWYGWLNNCNGTAPSVADCF | [112] | |
| FNK | 3 | GVTQNVLYENQKQIANQFNKAISQIQESLTTTSTALGKLQ | [13] |
| NGIGVTQNVLYENQKQIANQFNKAISQIQESLTTTSTA | |||
| AASFNKAMTNIVDAFTGVNDAITQTSQALQTVATALNKIQDVVNQQGNSLNHLTSQ | |||
| QIANQFNKAISQIQE | [109] | ||
| KQFNKCSLATELSRLGVPKSELPDWVCLVQHESNFKTNWINKKNSNGSWDFGLFQINDKWWCEGHIRSHNTCNVKCEELVTEDIEKALECAKVIKRERGYKAWYGWLNNCQNKKPSVDECF | [112] | ||
| KKKKVV | 5 | KKKKVVAATYV | [109] |
| WLRDI | SWLRDIWDWICEVLS | [109] | |
| WDWIC | SWLRDIWDWICEVLS | [109] | |
| SGSWLRDVWDWICTVLTDFKTWLQSKL | [19] | ||
| GKK | 6 | ILPLLKKFGKKFGKKVWKAL | [113] |
| AFAFDVTRKINPETSAVERPEVSEYPEIPKGTKLQEFVMMDIEIEEEGADNRAETIQRIKCVPSQCNQICRVLGKKCGYCKNASTCVCLG | [112] |
3.4.2. Mapping Antiviral Motifs Against Non-AVPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranda, M.N.S.; Pingarilho, M.; Pimentel, V.; Torneri, A.; Seabra, S.G.; Libin, P.J.K.; Abecasis, A.B. A Tale of Three Recent Pandemics: Influenza, HIV and SARS-CoV-2. Front. Microbiol. 2022, 13, 889643. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Meganck, R.M.; Baric, R.S. Developing Therapeutic Approaches for Twenty-First-Century Emerging Infectious Viral Diseases. Nat. Med. 2021, 27, 401–410. [Google Scholar] [CrossRef]
- Von Delft, A.; Hall, M.D.; Kwong, A.D.; Purcell, L.A.; Saikatendu, K.S.; Schmitz, U.; Tallarico, J.A.; Lee, A.A. Accelerating Antiviral Drug Discovery: Lessons from COVID-19. Nat. Rev. Drug Discov. 2023, 22, 585–603. [Google Scholar] [CrossRef]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-Directed Immunotherapy of Viral and Bacterial Infections: Past, Present and Future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Lamers, C. Overcoming the Shortcomings of Peptide-Based Therapeutics. Future Drug Discov. 2022, 4, FDD75. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Maleki, M.S.; Sardari, S.; Ghandehari Alavijeh, A.; Madanchi, H. Recent Patents and FDA-Approved Drugs Based on Antiviral Peptides and Other Peptide-Related Antivirals. Int. J. Pept. Res. Ther. 2022, 29, 5. [Google Scholar] [CrossRef]
- Usmani, S.S.; Bedi, G.; Samuel, J.S.; Singh, S.; Kalra, S.; Kumar, P.; Ahuja, A.A.; Sharma, M.; Gautam, A.; Raghava, G.P.S. THPdb: Database of FDA-Approved Peptide and Protein Therapeutics. PLoS ONE 2017, 12, e0181748. [Google Scholar] [CrossRef]
- Carter, E.P.; Ang, C.G.; Chaiken, I.M. Peptide Triazole Inhibitors of HIV-1: Hijackers of Env Metastability. Curr. Protein Pept. Sci. 2023, 24, 59–77. [Google Scholar] [CrossRef]
- Heydari, H.; Golmohammadi, R.; Mirnejad, R.; Tebyanian, H.; Fasihi-Ramandi, M.; Moosazadeh Moghaddam, M. Antiviral Peptides against Coronaviridae Family: A Review. Peptides 2021, 139, 170526. [Google Scholar] [CrossRef]
- Agamennone, M.; Fantacuzzi, M.; Vivenzio, G.; Scala, M.C.; Campiglia, P.; Superti, F.; Sala, M. Antiviral Peptides as Anti-Influenza Agents. Int. J. Mol. Sci. 2022, 23, 11433. [Google Scholar] [CrossRef]
- Jenssen, H. Anti Herpes Simplex Virus Activity of Lactoferrin/Lactoferricin—An Example of Antiviral Activity of Antimicrobial Protein/Peptide. Cell. Mol. Life Sci. CMLS 2005, 62, 3002–3013. [Google Scholar] [CrossRef]
- Becker, Y. Dengue Fever Virus and Japanese Encephalitis Virus Synthetic Peptides, with Motifs to Fit HLA Class I Haplotypes Prevalent in Human Populations in Endemic Regions, Can Be Used for Application to Skin Langerhans Cells to Prime Antiviral CD8+ Cytotoxic T Cells (CTLs)—A Novel Approach to the Protection of Humans. Virus Genes 1994, 9, 33–45. [Google Scholar] [CrossRef]
- Park, J.Y.; Yang, S.Y.; Kim, Y.C.; Kim, J.-C.; Dang, Q.L.; Kim, J.J.; Kim, I.S. Antiviral Peptide from Pseudomonas Chlororaphis O6 against Tobacco Mosaic Virus (TMV). J. Korean Soc. Appl. Biol. Chem. 2012, 55, 89–94. [Google Scholar] [CrossRef]
- Jenssen, H.; Gutteberg, T.J.; Rekdal, O.; Lejon, T. Prediction of Activity, Synthesis and Biological Testing of Anti-HSV Active Peptides. Chem. Biol. Drug Des. 2006, 68, 58–66. [Google Scholar] [CrossRef]
- Jackman, J.A.; Costa, V.V.; Park, S.; Real, A.L.C.V.; Park, J.H.; Cardozo, P.L.; Ferhan, A.R.; Olmo, I.G.; Moreira, T.P.; Bambirra, J.L.; et al. Therapeutic Treatment of Zika Virus Infection Using a Brain-Penetrating Antiviral Peptide. Nat. Mater. 2018, 17, 971–977. [Google Scholar] [CrossRef]
- Agarwal, G.; Gabrani, R. Antiviral Peptides: Identification and Validation. Int. J. Pept. Res. Ther. 2021, 27, 149–168. [Google Scholar] [CrossRef]
- Jhong, J.-H.; Yao, L.; Pang, Y.; Li, Z.; Chung, C.-R.; Wang, R.; Li, S.; Li, W.; Luo, M.; Ma, R.; et al. dbAMP 2.0: Updated Resource for Antimicrobial Peptides with an Enhanced Scanning Method for Genomic and Proteomic Data. Nucleic Acids Res. 2022, 50, D460–D470. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Lu, H.; Li, G.; Huang, Q. LAMP: A Database Linking Antimicrobial Peptides. PLoS ONE 2013, 8, e66557. [Google Scholar] [CrossRef]
- Gawde, U.; Chakraborty, S.; Waghu, F.H.; Barai, R.S.; Khanderkar, A.; Indraguru, R.; Shirsat, T.; Idicula-Thomas, S. CAMPR4: A Database of Natural and Synthetic Antimicrobial Peptides. Nucleic Acids Res. 2023, 51, D377–D383. [Google Scholar] [CrossRef]
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A Review on Antimicrobial Peptides Databases and the Computational Tools. Database 2022, 2022, baac011. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Thakur, N.; Tandon, H.; Kumar, M. AVPdb: A Database of Experimentally Validated Antiviral Peptides Targeting Medically Important Viruses. Nucleic Acids Res. 2014, 42, D1147–D1153. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Holder, L.B. Graph-Based Data Mining. IEEE Intell. Syst. 2000, 15, 32–41. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Sánchez-Cruz, N.; López-López, E.; Díaz-Eufracio, B.I. Progress on Open Chemoinformatic Tools for Expanding and Exploring the Chemical Space. J. Comput. Aided Mol. Des. 2022, 36, 341–354. [Google Scholar] [CrossRef]
- Maggiora, G.M.; Bajorath, J. Chemical Space Networks: A Powerful New Paradigm for the Description of Chemical Space. J. Comput. Aided Mol. Des. 2014, 28, 795–802. [Google Scholar] [CrossRef]
- Holzinger, A.; Dehmer, M.; Jurisica, I. Knowledge Discovery and Interactive Data Mining in Bioinformatics—State-of-the-Art, Future Challenges and Research Directions. BMC Bioinform. 2014, 15, I1. [Google Scholar] [CrossRef]
- Wang, F.; Jin, R.; Agrawal, G.; Piontkivska, H. Graph and Topological Structure Mining on Scientific Articles. In Proceedings of the 2007 IEEE 7th International Symposium on BioInformatics and BioEngineering, Boston, MA, USA, 14–17 October 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 1318–1322. [Google Scholar]
- Romero, M.; Marrero-Ponce, Y.; Rodríguez, H.; Agüero-Chapin, G.; Antunes, A.; Aguilera-Mendoza, L.; Martinez-Rios, F. A Novel Network Science and Similarity-Searching-Based Approach for Discovering Potential Tumor-Homing Peptides from Antimicrobials. Antibiotics 2022, 11, 401. [Google Scholar] [CrossRef]
- Agüero-Chapin, G.; Antunes, A.; Mora, J.R.; Pérez, N.; Contreras-Torres, E.; Valdes-Martini, J.R.; Martinez-Rios, F.; Zambrano, C.H.; Marrero-Ponce, Y. Complex Networks Analyses of Antibiofilm Peptides: An Emerging Tool for Next-Generation Antimicrobials’ Discovery. Antibiotics 2023, 12, 747. [Google Scholar] [CrossRef]
- Castillo-Mendieta, K.; Agüero-Chapin, G.; Marquez, E.A.; Perez-Castillo, Y.; Barigye, S.J.; Vispo, N.S.; García-Jacas, C.R.; Marrero-Ponce, Y. Peptide Hemolytic Activity Analysis Using Visual Data Mining of Similarity-Based Complex Networks. Npj Syst. Biol. Appl. 2024, 10, 115. [Google Scholar] [CrossRef]
- Ayala-Ruano, S.; Marrero-Ponce, Y.; Aguilera-Mendoza, L.; Pérez, N.; Agüero-Chapin, G.; Antunes, A.; Aguilar, A.C. Network Science and Group Fusion Similarity-Based Searching to Explore the Chemical Space of Antiparasitic Peptides. ACS Omega 2022, 7, 46012–46036. [Google Scholar] [CrossRef]
- Agüero-Chapin, G.; Galpert-Cañizares, D.; Domínguez-Pérez, D.; Marrero-Ponce, Y.; Pérez-Machado, G.; Teijeira, M.; Antunes, A. Emerging Computational Approaches for Antimicrobial Peptide Discovery. Antibiotics 2022, 11, 936. [Google Scholar] [CrossRef]
- Aguilera-Mendoza, L.; Ayala-Ruano, S.; Martinez-Rios, F.; Chavez, E.; García-Jacas, C.R.; Brizuela, C.A.; Marrero-Ponce, Y. StarPep Toolbox: An Open-Source Software to Assist Chemical Space Analysis of Bioactive Peptides and Their Functions Using Complex Networks. Bioinformatics 2023, 39, btad506. [Google Scholar] [CrossRef]
- Aguilera-Mendoza, L.; Marrero-Ponce, Y.; Beltran, J.A.; Tellez Ibarra, R.; Guillen-Ramirez, H.A.; Brizuela, C.A. Graph-Based Data Integration from Bioactive Peptide Databases of Pharmaceutical Interest: Toward an Organized Collection Enabling Visual Network Analysis. Bioinformatics 2019, 35, 4739–4747. [Google Scholar] [CrossRef]
- Aguilera-Mendoza, L.; Marrero-Ponce, Y.; García-Jacas, C.R.; Chavez, E.; Beltran, J.A.; Guillen-Ramirez, H.A.; Brizuela, C.A. Automatic Construction of Molecular Similarity Networks for Visual Graph Mining in Chemical Space of Bioactive Peptides: An Unsupervised Learning Approach. Sci. Rep. 2020, 10, 18074. [Google Scholar] [CrossRef]
- Chavez, E.; Dobrev, S.; Kranakis, E.; Opatrny, J.; Stacho, L.; Tejeda, H.; Urrutia, J. Half-Space Proximal: A New Local Test for Extracting a Bounded Dilation Spanner of a Unit Disk Graph. In Principles of Distributed Systems; Anderson, J.H., Prencipe, G., Wattenhofer, R., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3974, pp. 235–245. ISBN 978-3-540-36321-7. [Google Scholar]
- Aguilera-Mendoza, L.; Marrero-Ponce, Y.; Tellez-Ibarra, R.; Llorente-Quesada, M.T.; Salgado, J.; Barigye, S.J.; Liu, J. Overlap and Diversity in Antimicrobial Peptide Databases: Compiling a Non-Redundant Set of Sequences. Bioinformatics 2015, 31, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L. STREME: Accurate and Versatile Sequence Motif Discovery. Bioinformatics 2021, 37, 2834–2840. [Google Scholar] [CrossRef]
- Bailey, T.L.; Grant, C.E. SEA: Simple Enrichment Analysis of Motifs. bioRxiv 2021. [Google Scholar] [CrossRef]
- Shirkhorshidi, A.S.; Aghabozorgi, S.; Wah, T.Y. A Comparison Study on Similarity and Dissimilarity Measures in Clustering Continuous Data. PLoS ONE 2015, 10, e0144059. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.J. Modularity and Community Structure in Networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef]
- Traag, V.A.; Waltman, L.; Van Eck, N.J. From Louvain to Leiden: Guaranteeing Well-Connected Communities. Sci. Rep. 2019, 9, 5233. [Google Scholar] [CrossRef]
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast Unfolding of Communities in Large Networks. J. Stat. Mech. Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]
- Zahoránszky, L.A.; Katona, G.Y.; Hári, P.; Málnási-Csizmadia, A.; Zweig, K.A.; Zahoránszky-Köhalmi, G. Breaking the Hierarchy—A New Cluster Selection Mechanism for Hierarchical Clustering Methods. Algorithms Mol. Biol. 2009, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective Dynamics of ‘Small-World’ Networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Mendoza, M.L.; Resendis-Antonio, O. Bipartite Graph. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 147–148. ISBN 978-1-4419-9862-0. [Google Scholar]
- Ghalmane, Z.; Hassouni, M.E.; Cherifi, H. Immunization of Networks with Non-Overlapping Community Structure. Soc. Netw. Anal. Min. 2019, 9, 45. [Google Scholar] [CrossRef]
- Rajeh, S.; Savonnet, M.; Leclercq, E.; Cherifi, H. Investigating Centrality Measures in Social Networks with Community Structure. In Complex Networks & Their Applications IX.; Benito, R.M., Cherifi, C., Cherifi, H., Moro, E., Rocha, L.M., Sales-Pardo, M., Eds.; Studies in Computational Intelligence; Springer International Publishing: Cham, Switzerland, 2021; Volume 943, pp. 211–222. ISBN 978-3-030-65346-0. [Google Scholar]
- Marchiori, M.; Latora, V. Harmony in the Small-World. Phys. Stat. Mech. Its Appl. 2000, 285, 539–546. [Google Scholar] [CrossRef]
- Ruan, Y.; Tang, J.; Hu, Y.; Wang, H.; Bai, L. Efficient Algorithm for the Identification of Node Significance in Complex Network. IEEE Access 2020, 8, 28947–28955. [Google Scholar] [CrossRef]
- Brandes, U.; Pich, C. CENTRALITY ESTIMATION IN LARGE NETWORKS. Int. J. Bifurc. Chaos 2007, 17, 2303–2318. [Google Scholar] [CrossRef]
- Xia, Z.; Cui, Y.; Zhang, A.; Tang, T.; Peng, L.; Huang, C.; Yang, C.; Liao, X. A Review of Parallel Implementations for the Smith–Waterman Algorithm. Interdiscip. Sci. Comput. Life Sci. 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the Third International Conference on Weblogs and Social Media, ICWSM 2009, San Jose, CA, USA, 17–20 May 2009. [Google Scholar] [CrossRef]
- Fruchterman, T.M.J.; Reingold, E.M. Graph Drawing by Force-Directed Placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Osorio, D.; Rondón-Villarreal, P.; Torres, R. Peptides: A Package for Data Mining of Antimicrobial Peptides. R J. 2015, 7, 4. [Google Scholar] [CrossRef]
- Needleman, S.B.; Wunsch, C.D. A General Method Applicable to the Search for Similarities in the Amino Acid Sequence of Two Proteins. J. Mol. Biol. 1970, 48, 443–453. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Pinacho-Castellanos, S.A.; García-Jacas, C.R.; Gilson, M.K.; Brizuela, C.A. Alignment-Free Antimicrobial Peptide Predictors: Improving Performance by a Thorough Analysis of the Largest Available Data Set. J. Chem. Inf. Model. 2021, 61, 3141–3157. [Google Scholar] [CrossRef] [PubMed]
- Gabere, M.N.; Noble, W.S. Empirical Comparison of Web-Based Antimicrobial Peptide Prediction Tools. Bioinformatics 2017, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.S.; Gautam, A. CPPsite 2.0: A Repository of Experimentally Validated Cell-Penetrating Peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. Meta-iAVP: A Sequence-Based Meta-Predictor for Improving the Prediction of Antiviral Peptides Using Effective Feature Representation. Int. J. Mol. Sci. 2019, 20, 5743. [Google Scholar] [CrossRef]
- Lin, T.-T.; Sun, Y.-Y.; Wang, C.-T.; Cheng, W.-C.; Lu, I.-H.; Lin, C.-Y.; Chen, S.-H. AI4AVP: An Antiviral Peptides Predictor in Deep Learning Approach with Generative Adversarial Network Data Augmentation. Bioinform. Adv. 2022, 2, vbac080. [Google Scholar] [CrossRef]
- Otović, E.; Njirjak, M.; Kalafatovic, D.; Mauša, G. Sequential Properties Representation Scheme for Recurrent Neural Network-Based Prediction of Therapeutic Peptides. J. Chem. Inf. Model. 2022, 62, 2961–2972. [Google Scholar] [CrossRef]
- Singh, S.; Chaudhary, K.; Dhanda, S.K.; Bhalla, S.; Usmani, S.S.; Gautam, A.; Tuknait, A.; Agrawal, P.; Mathur, D.; Raghava, G.P.S. SATPdb: A Database of Structurally Annotated Therapeutic Peptides. Nucleic Acids Res. 2016, 44, D1119–D1126. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Gabrielian, A.; Cruz, P.; Griggs, H.L.; Squires, R.B.; Hurt, D.E.; Grigolava, M.; Chubinidze, M.; Gogoladze, G.; Vishnepolsky, B.; et al. DBAASP v.2: An Enhanced Database of Structure and Antimicrobial/Cytotoxic Activity of Natural and Synthetic Peptides. Nucleic Acids Res. 2016, 44, D1104–D1112. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An Enhanced Comprehensive Data Repository of Antimicrobial Peptides. Nucleic Acids Res. 2022, 50, D488–D496. [Google Scholar] [CrossRef]
- Théolier, J.; Fliss, I.; Jean, J.; Hammami, R. MilkAMP: A Comprehensive Database of Antimicrobial Peptides of Dairy Origin. Dairy Sci. Technol. 2014, 94, 181–193. [Google Scholar] [CrossRef]
- Wang, C.K.L.; Kaas, Q.; Chiche, L.; Craik, D.J. CyBase: A Database of Cyclic Protein Sequences and Structures, with Applications in Protein Discovery and Engineering. Nucleic Acids Res. 2008, 36, D206–D210. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mendieta, K.; Agüero-Chapin, G.; Mora, J.R.; Pérez, N.; Contreras-Torres, E.; Valdes-Martini, J.R.; Martinez-Rios, F.; Marrero-Ponce, Y. Unraveling the Hemolytic Toxicity Tapestry of Peptides Using Chemical Space Complex Networks. Toxicol. Sci. 2024, 202, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a Continuous Graph Layout Algorithm for Handy Network Visualization Designed for the Gephi Software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef]
- Chandrashekar, I.R.; Cowsik, S.M. Three-Dimensional Structure of the Mammalian Tachykinin Peptide Neurokinin A Bound to Lipid Micelles. Biophys. J. 2003, 85, 4002–4011. [Google Scholar] [CrossRef]
- Zahoránszky-Kőhalmi, G.; Bologa, C.G.; Oprea, T.I. Impact of Similarity Threshold on the Topology of Molecular Similarity Networks and Clustering Outcomes. J. Cheminform. 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.C.; Colgrave, M.L.; Craik, D.J. A Novel Suite of Cyclotides from Viola odorata: Sequence Variation and the Implications for Structure, Function and Stability. Biochem. J. 2006, 400, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Lee, B.-D.; An, H.; Eun, J.-B. Kenojeinin I, Antimicrobial Peptide Isolated from the Skin of the Fermented Skate, Raja Kenojei. Peptides 2005, 26, 581–587. [Google Scholar] [CrossRef]
- Op De Beeck, A.; Voisset, C.; Bartosch, B.; Ciczora, Y.; Cocquerel, L.; Keck, Z.; Foung, S.; Cosset, F.-L.; Dubuisson, J. Characterization of Functional Hepatitis C Virus Envelope Glycoproteins. J. Virol. 2004, 78, 2994–3002. [Google Scholar] [CrossRef]
- Kato, Y.; Aizawa, T.; Hoshino, H.; Kawano, K.; Nitta, K.; Zhang, H. Abf-1 and Abf-2, ASABF-Type Antimicrobial Peptide Genes in Caenorhabditis Elegans. Biochem. J. 2002, 361, 221–230. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhao, D.; Zhang, G.; Luo, J.; Deng, R.; Yang, Y. Identification of Host Cell Binding Peptide from an Overlapping Peptide Library for Inhibition of Classical Swine Fever Virus Infection. Virus Genes 2011, 43, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Loose, C.; Jensen, K.; Rigoutsos, I.; Stephanopoulos, G. A Linguistic Model for the Rational Design of Antimicrobial Peptides. Nature 2006, 443, 867–869. [Google Scholar] [CrossRef]
- Hall, P.R.; Hjelle, B.; Njus, H.; Ye, C.; Bondu-Hawkins, V.; Brown, D.C.; Kilpatrick, K.A.; Larson, R.S. Phage Display Selection of Cyclic Peptides That Inhibit Andes Virus Infection. J. Virol. 2009, 83, 8965–8969. [Google Scholar] [CrossRef]
- Lombardi, S.; Massi, C.; Indino, E.; Rosa, C.L.; Mazzetti, P.; Falcone, M.L.; Rovero, P.; Fissi, A.; Pieroni, O.; Bandecchi, P.; et al. Inhibition of Feline Immunodeficiency Virus Infectionin Vitroby Envelope Glycoprotein Synthetic Peptides. Virology 1996, 220, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Treffers, C.; Chen, L.; Anderson, R.C.; Yu, P.-L. Isolation and Characterisation of Antimicrobial Peptides from Deer Neutrophils. Int. J. Antimicrob. Agents 2005, 26, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.J.; Anantharamaiah, G.M.; Kahlon, J.B.; Srinivas, R.V.; Compans, R.W.; Segrest, J.P. Apolipoprotein A-I and Its Amphipathic Helix Peptide Analogues Inhibit Human Immunodeficiency Virus-Induced Syncytium Formation. J. Clin. Investig. 1990, 86, 1142–1150. [Google Scholar] [CrossRef]
- Shiba, K. Natural and Artificial Peptide Motifs: Their Origins and the Application of Motif-Programming. Chem. Soc. Rev. 2010, 39, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Davey, N.E.; Gibson, T.J.; Babu, M.M. A Million Peptide Motifs for the Molecular Biologist. Mol. Cell 2014, 55, 161–169. [Google Scholar] [CrossRef]
- Neduva, V.; Linding, R.; Su-Angrand, I.; Stark, A.; Masi, F.D.; Gibson, T.J.; Lewis, J.; Serrano, L.; Russell, R.B. Systematic Discovery of New Recognition Peptides Mediating Protein Interaction Networks. PLoS Biol. 2005, 3, e405. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Cheng, C.-S.; Lai, S.-M.; Hsu, M.-P.; Chen, C.-S.; Lyu, P.-C. Solution Structure of the Plant Defensin VrD1 from Mung Bean and Its Possible Role in Insecticidal Activity against Bruchids. Proteins Struct. Funct. Bioinforma. 2006, 63, 777–786. [Google Scholar] [CrossRef]
- Lay, F.T.; Schirra, H.J.; Scanlon, M.J.; Anderson, M.A.; Craik, D.J. The Three-Dimensional Solution Structure of NaD1, a New Floral Defensin from Nicotiana Alata and Its Application to a Homology Model of the Crop Defense Protein alfAFP. J. Mol. Biol. 2003, 325, 175–188. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a Novel Antimicrobial Peptide from Jellyfish Aurelia Aurita with Structural Features of Defensins and Channel-Blocking Toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef]
- Craik, D.J.; Daly, N.L.; Bond, T.; Waine, C. Plant Cyclotides: A Unique Family of Cyclic and Knotted Proteins That Defines the Cyclic Cystine Knot Structural Motif. J. Mol. Biol. 1999, 294, 1327–1336. [Google Scholar] [CrossRef]
- Göransson, U.; Luijendijk, T.; Johansson, S.; Bohlin, L.; Claeson, P. Seven Novel Macrocyclic Polypeptides from Viola arvensis. J. Nat. Prod. 1999, 62, 283–286. [Google Scholar] [CrossRef]
- Yang, X.; Lee, W.-H.; Zhang, Y. Extremely Abundant Antimicrobial Peptides Existed in the Skins of Nine Kinds of Chinese Odorous Frogs. J. Proteome Res. 2012, 11, 306–319. [Google Scholar] [CrossRef]
- Basir, Y.J.; Knoop, F.C.; Dulka, J.; Conlon, J.M. Multiple Antimicrobial Peptides and Peptides Related to Bradykinin and Neuromedin N Isolated from Skin Secretions of the Pickerel Frog, Rana Palustris. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 2000, 1543, 95–105. [Google Scholar] [CrossRef]
- Agopian, A.; Gros, E.; Aldrian-Herrada, G.; Bosquet, N.; Clayette, P.; Divita, G. A New Generation of Peptide-Based Inhibitors Targeting HIV-1 Reverse Transcriptase Conformational Flexibility. J. Biol. Chem. 2009, 284, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Ständker, L.; Adermann, K.; Schulz, A.; Schindler, M.; Chinnadurai, R.; Pöhlmann, S.; Chaipan, C.; Biet, T.; Peters, T.; et al. Discovery and Optimization of a Natural HIV-1 Entry Inhibitor Targeting the Gp41 Fusion Peptide. Cell 2007, 129, 263–275. [Google Scholar] [CrossRef]
- Umetsu, Y.; Tenno, T.; Goda, N.; Shirakawa, M.; Ikegami, T.; Hiroaki, H. Structural Difference of Vasoactive Intestinal Peptide in Two Distinct Membrane-Mimicking Environments. Biochim. Biophys. Acta BBA—Proteins Proteom. 2011, 1814, 724–730. [Google Scholar] [CrossRef]
- Krebs, D.; Maroun, R.G.; Sourgen, F.; Troalen, F.; Davoust, D.; Fermandjian, S. Helical and Coiled-Coil-Forming Properties of Peptides Derived from and Inhibiting Human Immunodeficiency Virus Type 1 Integrase Assessed by 1H-NMR. Use of NH Temperature Coefficients to Probe Coiled-Coil Structures. Eur. J. Biochem. 1998, 253, 236–244. [Google Scholar] [CrossRef]
- Spriggs, M.K.; Olmsted, R.A.; Venkatesan, S.; Coligan, J.E.; Collins, P.L. Fusion Glycoprotein of Human Parainfluenza Virus Type 3: Nucleotide Sequence of the Gene, Direct Identification of the Cleavage-Activation Site, and Comparison with Other Paramyxoviruses. Virology 1986, 152, 241–251. [Google Scholar] [CrossRef]
- Conlon, J.M.; Demandt, A.; Nielsen, P.F.; Leprince, J.; Vaudry, H.; Woodhams, D.C. The Alyteserins: Two Families of Antimicrobial Peptides from the Skin Secretions of the Midwife Toad Alytes Obstetricans (Alytidae). Peptides 2009, 30, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Mechkarska, M.; Arafat, K.; Attoub, S.; Sonnevend, A. Analogues of the Frog Skin Peptide Alyteserin-2a with Enhanced Antimicrobial Activities against Gram-Negative Bacteria: ALYTESERIN-2: STRUCTURE-ACTIVITY. J. Pept. Sci. 2012, 18, 270–275. [Google Scholar] [CrossRef]
- Muñoz-Camargo, C.; Salazar, V.; Barrero-Guevara, L.; Camargo, S.; Mosquera, A.; Groot, H.; Boix, E. Unveiling the Multifaceted Mechanisms of Antibacterial Activity of Buforin II and Frenatin 2.3S Peptides from Skin Micro-Organs of the Orinoco Lime Treefrog (Sphaenorhynchus Lacteus). Int. J. Mol. Sci. 2018, 19, 2170. [Google Scholar] [CrossRef]
- Verly, R.M.; Moraes, C.M.D.; Resende, J.M.; Aisenbrey, C.; Bemquerer, M.P.; Piló-Veloso, D.; Valente, A.P.; Almeida, F.C.L.; Bechinger, B. Structure and Membrane Interactions of the Antibiotic Peptide Dermadistinctin K by Multidimensional Solution and Oriented 15N and 31P Solid-State NMR Spectroscopy. Biophys. J. 2009, 96, 2194–2203. [Google Scholar] [CrossRef]
- Lequin, O.; Ladram, A.; Chabbert, L.; Bruston, F.; Convert, O.; Vanhoye, D.; Chassaing, G.; Nicolas, P.; Amiche, M. Dermaseptin S9, an α-Helical Antimicrobial Peptide with a Hydrophobic Core and Cationic Termini. Biochemistry 2006, 45, 468–480. [Google Scholar] [CrossRef]
- Jabeen, M.; Biswas, P.; Islam, M.T.; Paul, R. Antiviral Peptides in Antimicrobial Surface Coatings—From Current Techniques to Potential Applications. Viruses 2023, 15, 640. [Google Scholar] [CrossRef]
- Manavalan, B.; Basith, S.; Lee, G. Comparative Analysis of Machine Learning-Based Approaches for Identifying Therapeutic Peptides Targeting SARS-CoV-2. Brief. Bioinform. 2022, 23, bbab412. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, Z.; Jhong, J.-H.; Lee, T.-Y. Identifying Anti-Coronavirus Peptides by Incorporating Different Negative Datasets and Imbalanced Learning Strategies. Brief. Bioinform. 2021, 22, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Timmons, P.B.; Hewage, C.M. ENNAVIA Is a Novel Method Which Employs Neural Networks for Antiviral and Anti-Coronavirus Activity Prediction for Therapeutic Peptides. Brief. Bioinform. 2021, 22, bbab258. [Google Scholar] [CrossRef]
- Singh, S.; Chauhan, P.; Sharma, V.; Rao, A.; Kumbhar, B.V.; Prajapati, V.K. Identification of Multi-Targeting Natural Antiviral Peptides to Impede SARS-CoV-2 Infection. Struct. Chem. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A Bioinformatic Study of Antimicrobial Peptides Identified in the Black Soldier Fly (BSF) Hermetia Illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Tucs, A.; Tran, D.P.; Yumoto, A.; Ito, Y.; Uzawa, T.; Tsuda, K. Generating Ampicillin-Level Antimicrobial Peptides with Activity-Aware Generative Adversarial Networks. ACS Omega 2020, 5, 22847–22851. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG Databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for Occurrences of a given Motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef]


| ID a | Cluster b | Aliphatic Index | Boman Index | Hydrophobicity | Isoelectric Point | Charge | Length |
|---|---|---|---|---|---|---|---|
| HSPN t = 0.75 (HSPN_OP) | |||||||
| StarPep_02593 | 193 | 78.00 | 0.42 | 0.34 | 7.99 | 1.69 | 30 |
| StarPep_01372 | 91 | 62.86 | 2.07 | −00.90 | 12.25 | 8.09 | 28 |
| StarPep_13366 | 91 | 86.67 | 3.56 | −00.95 | 11.28 | 2.09 | 18 |
| StarPep_02091 | 91 | 66.67 | 1.37 | −00.01 | 8.17 | 3.50 | 63 |
| StarPep_13542 | 93 | 91.00 | 1.53 | −00.14 | 10.21 | 2.09 | 30 |
| Mean (±SD) | 77.04 (±10.93) | 1.79 (±1.03) | −00.33 (±0.51) | 9.98 (±1.68) | 3.49 (±2.38) | 33.8 (±15.26) | |
| HSPN t = 0 (HSPN_NC) | |||||||
| StarPep_02526 | 518 | 97.50 | 0.34 | 0.43 | 11.90 | 6.00 | 20 |
| StarPep_08887 | 831 | 43.33 | 1.47 | −00.39 | 8.16 | 1.06 | 9 |
| StarPep_10907 | 19 | 46.52 | 3.66 | −1.56 | 11.09 | 5.94 | 23 |
| StarPep_01472 | 19 | 65.00 | 2.12 | −00.07 | 11.16 | 5.75 | 30 |
| StarPep_10501 | 518 | 76.11 | 1.43 | −00.50 | 7.02 | 0.00 | 18 |
| Mean (±SD) | 65.69 (±19.94) | 1.81 (±1.09) | −00.42 (±0.65) | 9.87 (±1.91) | 3.75 (±2.65) | 20 (±6.84) | |
| HB Centrality | HC Measure | ||||||
|---|---|---|---|---|---|---|---|
| Identity Percent | Edges | Nodes | Coverage (%) | Identity Percent | Edges | Nodes | Coverage (%) |
| Local Alignment | |||||||
| 90 | 22,343 | 2996 | 86 | 90 | 22,229 | 3003 | 86 |
| 80 | 16,396 | 2363 | 68 | 80 | 16,027 | 2369 | 68 |
| 70 | 12,820 | 2044 | 59 | 70 | 12,764 | 2028 | 58 |
| 60 | 8108 | 1536 | 44 | 60 | 8395 | 1557 | 45 |
| 50 | 3633 | 950 | 27 | 50 | 4530 | 1030 | 29 |
| Global Alignment | |||||||
| 90 | 23,768 | 3123 | 89 | 90 | 23,836 | 3124 | 89 |
| 80 | 18,585 | 2566 | 73 | 80 | 18,569 | 2560 | 73 |
| 70 | 15,612 | 2278 | 65 | 70 | 15,674 | 2273 | 65 |
| 60 | 13,004 | 2007 | 57 | 60 | 13,132 | 2005 | 57 |
| 50 | 8721 | 1587 | 45 | 50 | 8798 | 1582 | 45 |
| HB Centrality | HC Measure | ||||||
|---|---|---|---|---|---|---|---|
| Identity Percent | Edges | Nodes | Coverage (%) | Identity Percent | Edges | Nodes | Coverage (%) |
| Local Alignment | |||||||
| 90 | 16,997 | 3005 | 86 | 90 | 17,015 | 3005 | 86 |
| 80 | 12,801 | 2368 | 68 | 80 | 12,819 | 2369 | 68 |
| 70 | 10,221 | 2022 | 58 | 70 | 10,504 | 2046 | 59 |
| 60 | 6817 | 1534 | 44 | 60 | 7212 | 1559 | 45 |
| 50 | 4110 | 1034 | 30 | 50 | 4311 | 1044 | 30 |
| Global Alignment | |||||||
| 90 | 18,442 | 3119 | 89 | 90 | 18,397 | 3126 | 89 |
| 80 | 14,832 | 2562 | 73 | 80 | 14,667 | 2566 | 73 |
| 70 | 12,620 | 2277 | 65 | 70 | 12,410 | 2275 | 65 |
| 60 | 10,669 | 1991 | 57 | 60 | 10,564 | 2006 | 57 |
| 50 | 7529 | 1589 | 45 | 50 | 7287 | 1592 | 46 |
| Cluster | Name | Sequence a | Comments (Reference) |
|---|---|---|---|
| 1 | starPep_02860 | RTCMIKKEGWGKCLIDTTCAHSCKNRGYIGGDCKGMTRTCYCLVNC | Part of a plant defensin extracted from Vigna radiata [90] |
| starPep_02843 | RECKTESNTFPGICITKPPCRKACISEKFTDGHCSKLLRRCLCTKPC | Part of a floral defensin from Nicotiana tabacum [91] | |
| starPep_00566 | AACSDRAHGHICESFKSFCKDSGRNGVKLRANCKKTCGLC | Antimicrobial peptide from Aurelia aurita with defensin feature [92] | |
| 2 | StarPep_05942 | ICGETCVGGTCNTPGCSCSWPVCTRNGLP | Plant cyclotide [93] |
| StarPep_01071 | GLPICGETCVGGTCNTPGCSCSWPVCTRN | Varv peptide E from Viola arvensis [94] | |
| StarPep_40805 | TCVGGTCNTPGCSCSWPVCTRNGLPICGE | Produced by Viola arvensis (StarPep DB) | |
| 3 | StarPep_00742 | GVFTLIKGATQLIGKTLGKELGKTGLEIMACKITKQC | Antimicrobial peptide extracted from Chinese odorous frog [95] |
| StarPep_00745 | GVFTLIKGATQLIGKTLGKEVGKTGLELMACKITKQC | ||
| StarPep_01042 | GLFPKINKKKAKTGVFNIIKTVGKEAGMDLIRTGIDTIGCKIKGEC | Antimicrobial peptide obtained from pickerel frog [96] | |
| 4 | StarPep_08530 | ATKALTEVIPLTEEAEC | Inhibitors targeting HIV-1 reverse transcriptase [97] |
| StarPep_08254 | AEAIPMSIPPEVKFNKPFVF | HIV-1 entry inhibitor [98] | |
| StarPep_09666 | GAKALTEVIPLTEEAEC | Inhibitors targeting HIV-1 reverse transcriptase [97] | |
| 5 | StarPep_00500 | HSDAVFTDNYTRLRKQMAVKKYLNSILN | Vasoactive intestinal peptide [99] |
| StarPep_13041 | SQGVVESMNKELKKIIGQVRDQAEHLKTAY | Synthetic peptide from HIV type 1 integrase [100] | |
| StarPep_03560 | QARSDIEKLKEAIRDTNKAVQSVQSSIGNLIVAIK | Fusion glycoprotein F0 related to Human parainfluenza 3 virus [101] | |
| 6 | StarPep_03291 | ILGAILPLVSGLLSSKL | Antimicrobial peptide from the skin secretions of the midwife toad [102] |
| StarPep_02672 | ILGAILPLVSGLLSNKL | Analog of the frog skin peptide [103] | |
| StarPep_09950 | GLVGTLLGHIGKAILGG | Antibacterial peptide from Skin Micro-Organs of the Orinoco Lime Treefrog [104] | |
| 7 | StarPep_02236 | GLWSKIKEAAKTAGKMAMGFVNDMV | Antimicrobial peptide from Phyllomedusa distinta [105] |
| StarPep_02230 | GLRSKIKEAAKTAGKMALGFVNDMA | Antimicrobial peptide Dermaseptin S9 [106] | |
| StarPep_00483 | GLWSKIKEAAKAAGKAALNAVTGLVNQGDQPS | Antimicrobial peptide from Phyllomedusa distinta [105] | |
| 8 | StarPep_08794 | CNSHSPVHC | Cyclic peptide for Andes Virus inhibition [83] |
| StarPep_34731 | NXXLYSARGARGH | Antiviral/Antimicrobial (StarPepDB) | |
| StarPep_44046 | XNXLYSARGARGH |
| Motif | Cluster | p-Value | E-Value | TP a | Dataset b |
|---|---|---|---|---|---|
| CYCR | 1 | 0.00 | 0.00 | 279/1097 (25.4%) | 1 |
| 0.27 | 1.60 | 23/520 (4.4%) | 2 | ||
| 0.00 | 0.02 | 31/1935 (1.6%) | 3 | ||
| 0.50 | 3.00 | 11/217 (5.1%) | 4 | ||
| RRRRH | 0.50 | 3.00 | 4/1097 (0.4%) | 1 | |
| 0.43 | 2.55 | 15/520 (2.88%) | 2 | ||
| 0.00 | 0.00 | 26/1935 (1.34%) | 3 | ||
| 0.17 | 1.03 | 7/217 (3.22%) | 4 | ||
| RRWWC | 0.79 | 4.73 | 11/1097 (1.0%) | 1 | |
| 0.23 | 1.36 | 5/520 (0.9%) | 2 | ||
| 0.16 | 0.98 | 16/1935 (0.8%) | 3 | ||
| 0.94 | 5.63 | 2/217 (0.9%) | 4 | ||
| YDISDD | 0.99 | 5.94 | 4/1097 (0.4%) | 1 | |
| 0.00 | 0.03 | 20/520 (3.8%) | 2 | ||
| 0.00 | 0.00 | 57/1935 (2.9%) | 3 | ||
| 0.05 | 0.29 | 12/217 (5.52%) | 4 | ||
| CGES | 2 | 0.00 | 0.00 | 102/1097 (9.3%) | 1 |
| 0.14 | 5.91 | 6/217 (2.8%) | 4 | ||
| GCSCK | 0.00 | 0.00 | 96/1097 (8.7%) | 1 | |
| 0.09 | 3.58 | 10/520 (1.9%) | 2 | ||
| 0.09 | 3.67 | 7/217 (3.2%) | 4 | ||
| VCYRN | 0.00 | 0.00 | 126/1097 (11.5%) | 1 | |
| 0.01 | 0.01 | 16/520 (3.1%) | 2 | ||
| GLPV | 0.00 | 0.00 | 41/1097 (3.7%) | 1 | |
| GTCNTP | 0.00 | 0.00 | 49/1097 (4.5%) | 1 | |
| 0.22 | 9.15 | 5/520 (0.9%) | 2 | ||
| 0.15 | 6.28 | 15/217 (6.9%) | 4 | ||
| VWIPCI | 0.00 | 0.00 | 62/1097 (5.7%) | 1 | |
| 0.01 | 0.42 | 13/520 (2.5%) | 2 | ||
| 0.00 | 0.16 | 8/217 (3.7%) | 4 | ||
| SAAJ | 0.06 | 2.27 | 275/1097 (25.1%) | 1 | |
| 0.00 | 0.00 | 43/520 (8.3%) | 2 | ||
| 0.04 | 1.66 | 35/217 (16.1%) | 4 | ||
| QAVG | 0.14 | 5.87 | 170/1097 (15.5%) | 1 | |
| 0.06 | 2.52 | 4/520 (0.8%) | 2 | ||
| CKITG | 3 | 0.00 | 0.00 | 110/1097 (10.0%) | 1 |
| GJMDT | 0.00 | 0.00 | 63/1097 (5.7%) | 1 | |
| 0.11 | 4.41 | 5/520 (0.9%) | 2 | ||
| AGKSVA | 0.00 | 0.00 | 81/1097 (7.4%) | 1 | |
| JFSKI | 0.00 | 0.00 | 50/1097 (4.6%) | 1 | |
| 0.12 | 5.07 | 37/520 (7.1%) | 2 | ||
| 0.03 | 1.17 | 23/217 (10.6%) | 4 | ||
| LLDK | 0.00 | 0.00 | 29/1097 (2.6%) | 1 | |
| 0.09 | 3.67 | 10/217 (4.6%) | 4 | ||
| EAIPLT | 4 | 0.06 | 2.52 | 4/520 (0.8%) | 2 |
| 0.00 | 0.08 | 9/217 (4.14%) | 4 | ||
| FNK | 0.04 | 1.55 | 15/520 (2.9%) | 2 | |
| IPPEVK | 0.20 | 8.29 | 14/1097 (1.3%) | 1 | |
| 0.11 | 4.47 | 5/217 (2.3%) | 4 | ||
| KKKKVV | 5 | 0.00 | 0.00 | 26/520 (5.0%) | 2 |
| 0.06 | 2.56 | 6/217 (2.8%) | 4 | ||
| ATYVL | 0.00 | 0.00 | 41/520 (7.9%) | 2 | |
| TKKC | 0.00 | 0.00 | 168/1097 (15.3%) | 1 | |
| WLRDI | 0.20 | 8.10 | 38/1097 (3.5%) | 1 | |
| 0.00 | 0.01 | 23/520 (4.4%9 | 2 | ||
| 0.15 | 6.28 | 15/217 (6.9%) | 4 | ||
| LSDFK | 0.00 | 0.00 | 43/520 (8.3%) | 2 | |
| WDWIC | 0.18 | 7.18 | 18/520 (3.5%) | 2 | |
| GLSGL | 6 | 0.00 | 0.00 | 32/1097 (2.9%) | 1 |
| 0.11 | 4.47 | 5/217 (2.3%) | 4 | ||
| GKK | 0.19 | 7.72 | 153/1097 (13.9%) | 1 | |
| 0.12 | 5.06 | 17/217 (7.8%) | 4 | ||
| FLPIV | 0.00 | 0.00 | 84/1097 (7.6%) | 1 | |
| 0.17 | 6.91 | 7/520 (1.3%) | 2 | ||
| KAAGKA | 7 | 0.00 | 0.00 | 122/1097 (11.1%) | 1 |
| SLLGRM | 0.03 | 1.22 | 27/1097 (2.5%) | 1 | |
| 0.01 | 0.44 | 11/520 (2.1%) | 2 | ||
| YFL | 0.07 | 2.93 | 29/217 (13.4%) | 4 | |
| HCKFWW | 8 | 0.15 | 5.95 | 26/1097 (2.4%) | 1 |
| 0.16 | 6.63 | 11/520 (2.1%) | 2 |
| MOTIF | Antibacterial | Antifungal | Antiparasitic | AMP | Toxic | Others | CPP |
|---|---|---|---|---|---|---|---|
| CYCR | 2.10% | 23% | 0.70% | 0.90% | 2.30% | - | - |
| RRRRH | - | - | - | 0.10% | - | - | - |
| RRWWC | - | 1.40% | 1.80% | 4.90% | 3.10% | - | 6.20% |
| YDISDD | 0.20% | - | 3.60% | 2.70% | - | - | - |
| CGES | 8.10% | - | 5.40% | 2.60% | 16.50% | - | 7.40% |
| GCSCK | 0.30% | 0.10% | 0.90% | 0.20% | 1.80% | 0.80% | - |
| VCYRN | 0.60% | 1.50% | 5% | 0.80% | 1.00% | 1.10% | - |
| GLPV | 32.50% | 23.90% | 4% | 33.20% | 1.50% | - | - |
| GTCNTP | 7.4% | 9.10% | 4.90% | - | 0.90% | 2.60% | - |
| VWIPCI | 3.10% | - | 0.90% | 0.90% | 1.60% | 1.80% | - |
| SAAJ | 28.90% | 29.50% | - | 1.50% | 0.50% | 19.90% | - |
| QAVG | - | - | 31.50% | - | - | - | - |
| CKITG | 3.00% | 3.80% | - | 1.60% | 1.40% | 0.30% | - |
| GJMDT | 1.30% | 2.00% | 6% | 1.30% | 0.60% | 1.60% | - |
| AGKSVA | 1.40% | 2.30% | - | 1.00% | 7.30% | - | - |
| JFSKI | 4.6% | 16.30% | 15.80% | 2.80% | 10.10% | 3.10% | - |
| LLDK | 0.80% | 5% | 4% | 0.50% | - | 1.30% | 4.50% |
| EAIPLT | - | - | - | 1.10% | 1.10% | - | - |
| FNK | - | - | 27.90% | 18.20% | - | - | |
| IPPEVK | 10.30% | 1.80% | 6.70% | 2.20% | - | 0.20% | 2.50% |
| KKKKVV | - | - | - | - | - | - | 1.90% |
| ATYVL | 5.70% | 6% | - | 5.80% | 2.50% | 2.60% | 1.90% |
| TKKC | 5% | 1.10% | 12.10% | 24.60% | 5.00% | 25.20% | - |
| WLRDI | 1% | - | - | 2.60% | 2.20% | 0.10% | - |
| LSDFK | - | - | - | - | - | 0.20% | 3.60% |
| WDWIC | 4.90% | 0.10% | 5.80% | 3.20% | 0.60% | 0.10% | 3.60% |
| GLSGL | 2.40% | 2.90% | - | 1.90% | 6.40% | - | - |
| GKK | 56.90% | - | - | 58% | 42.30% | 24.50% | 33.70% |
| FLPIV | 11.00% | 7.70% | 4.90% | 11.40% | 5.50% | 2.60% | 1.60% |
| KAAGKA | 13.80% | 10.50% | 3.30% | 13.40% | 4.70% | 7.30% | - |
| SLLGRM | 1.10% | 13.80% | 5.80% | 0. 6% | - | - | - |
| YFL | 23.00% | 1.00% | - | 24.70% | 12.70% | - | - |
| HCKFWW | - | - | 5.80% | 2.50% | - | - | - |
| Peptide ID | Sequence |
|---|---|
| starPep_40757 | TCGECVGGTCNTPGCTCSWPVCTRNGLPV |
| starPep_23212 | GLPVCGETCVGGTCNAPGCTCSWPVCTRN |
| starPep_23254 | GLPVCGETCVGGTCNTPGCTCSWPVCARN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Llano García, D.; Marrero-Ponce, Y.; Agüero-Chapin, G.; Rodríguez, H.; Ferri, F.J.; Márquez, E.A.; Mora, J.R.; Martinez-Rios, F.; Pérez-Castillo, Y. Mapping the Chemical Space of Antiviral Peptides with Half-Space Proximal and Metadata Networks Through Interactive Data Mining. Computers 2025, 14, 423. https://doi.org/10.3390/computers14100423
de Llano García D, Marrero-Ponce Y, Agüero-Chapin G, Rodríguez H, Ferri FJ, Márquez EA, Mora JR, Martinez-Rios F, Pérez-Castillo Y. Mapping the Chemical Space of Antiviral Peptides with Half-Space Proximal and Metadata Networks Through Interactive Data Mining. Computers. 2025; 14(10):423. https://doi.org/10.3390/computers14100423
Chicago/Turabian Stylede Llano García, Daniela, Yovani Marrero-Ponce, Guillermin Agüero-Chapin, Hortensia Rodríguez, Francesc J. Ferri, Edgar A. Márquez, José R. Mora, Felix Martinez-Rios, and Yunierkis Pérez-Castillo. 2025. "Mapping the Chemical Space of Antiviral Peptides with Half-Space Proximal and Metadata Networks Through Interactive Data Mining" Computers 14, no. 10: 423. https://doi.org/10.3390/computers14100423
APA Stylede Llano García, D., Marrero-Ponce, Y., Agüero-Chapin, G., Rodríguez, H., Ferri, F. J., Márquez, E. A., Mora, J. R., Martinez-Rios, F., & Pérez-Castillo, Y. (2025). Mapping the Chemical Space of Antiviral Peptides with Half-Space Proximal and Metadata Networks Through Interactive Data Mining. Computers, 14(10), 423. https://doi.org/10.3390/computers14100423








