Localization of VEGF to Vascular ECM Is an Important Aspect of Tumor Angiogenesis
Abstract
1. Introduction
2. Direct Effects on the Vascular Basement Membrane Alter VEGF-A Localization and Tumor Vascularization
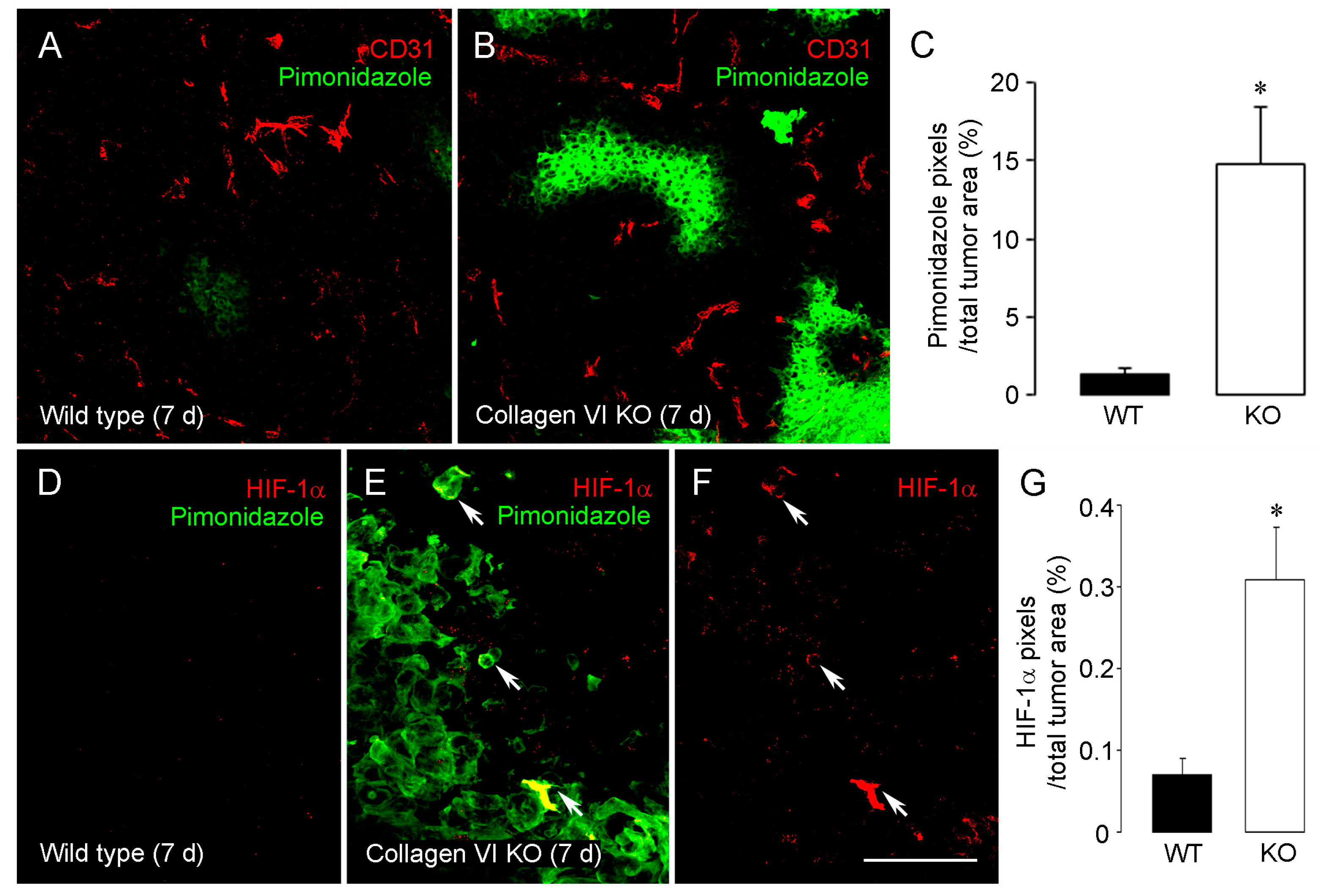
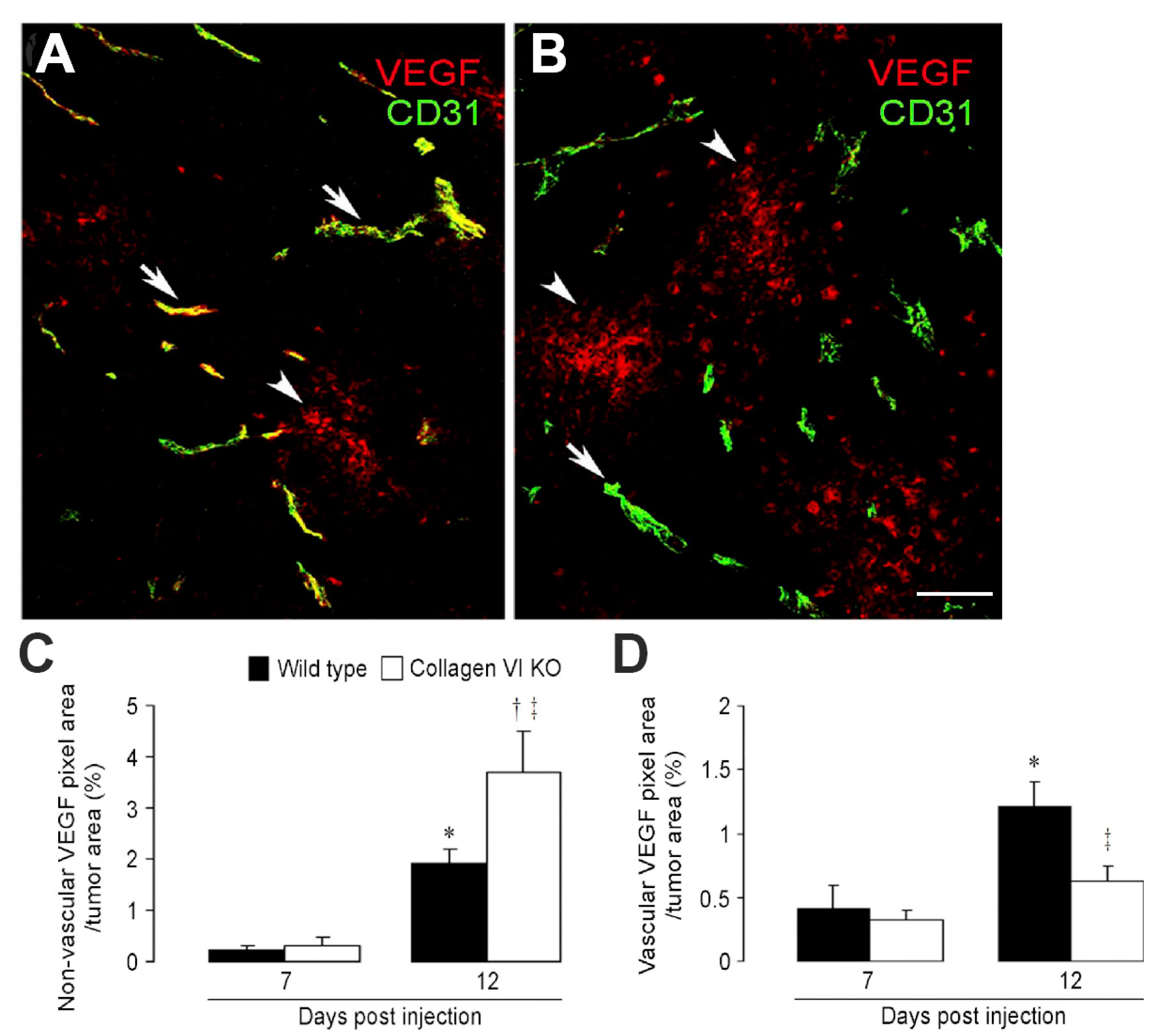
2.1. Collagen VI Ablation in Host Stroma of Intracranial Melanomas
2.2. Tks5 Knockdown in Mammary Tumor Cells
3. Indirect Effects on the Vascular Basement Membrane Alter VEGF-A Localization and Tumor Vascularization
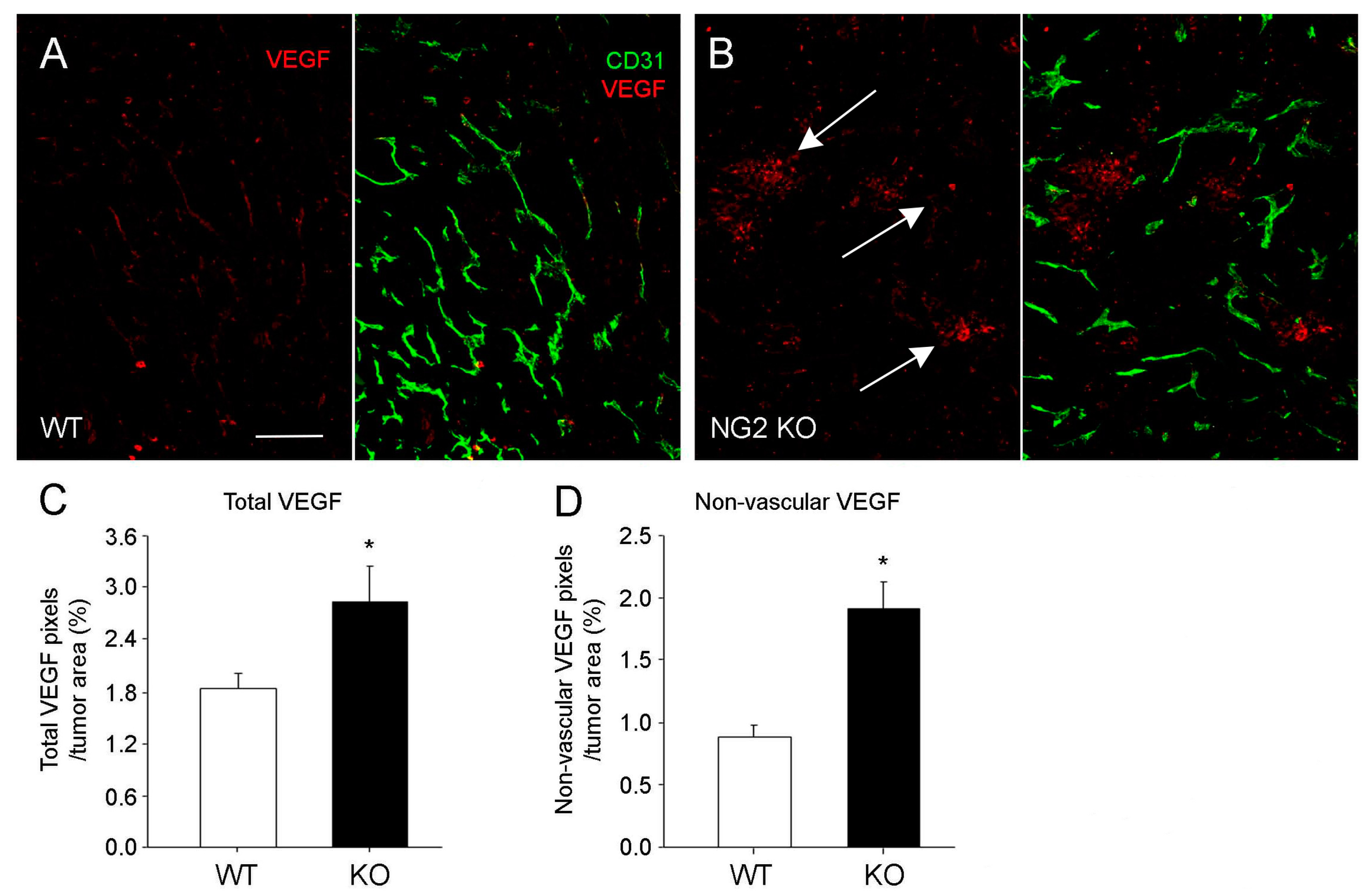
3.1. Germline NG2 Ablation MMTV-PyMT Mammary Tumor Stroma
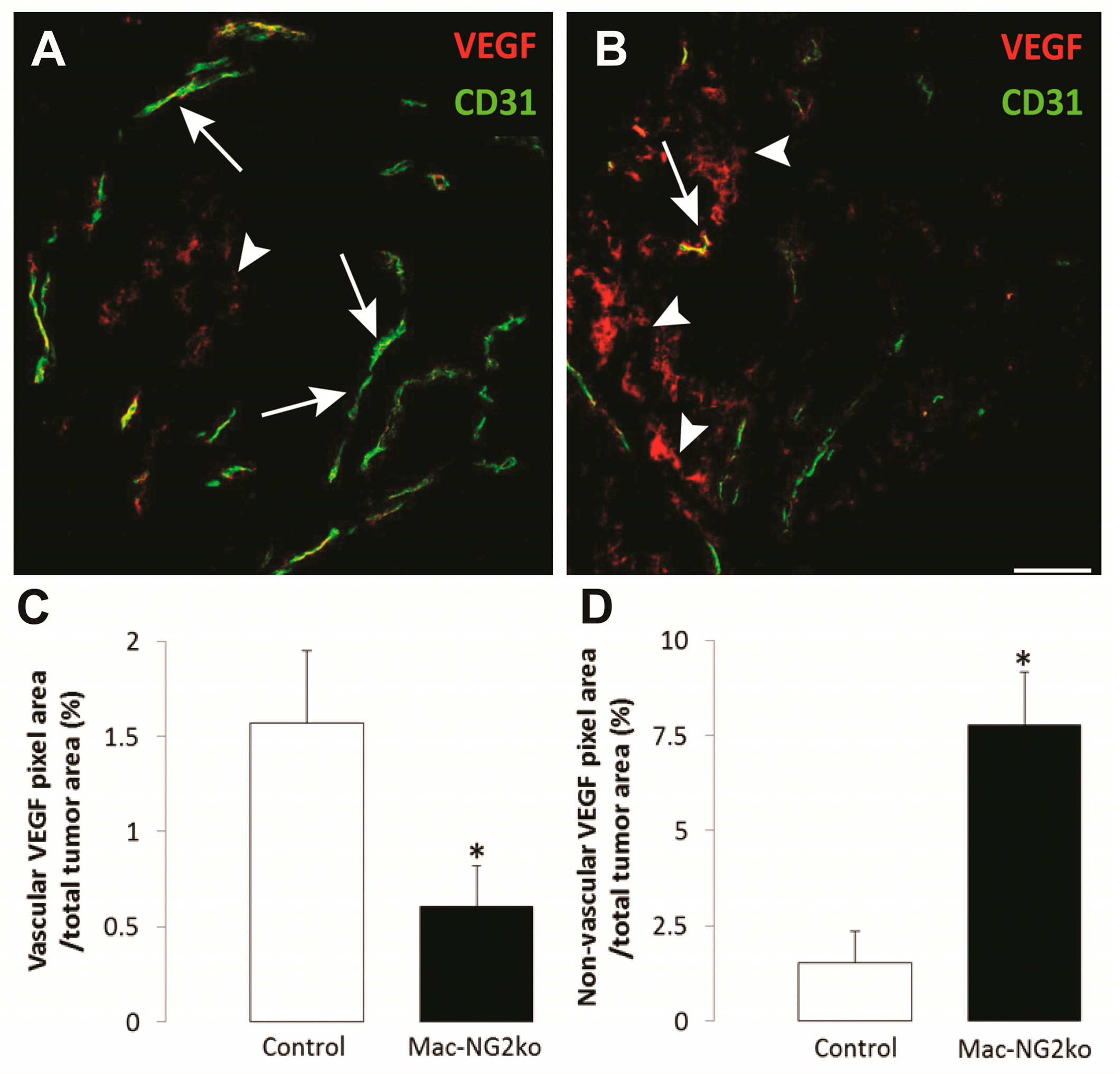
3.2. Myeloid-Specific NG2 Ablation in Host Stroma of Intracranial Melanomas
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Gaengel, K.; Genove, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Stallcup, W.B. Brain Metastases from Primary Tumors, 1st ed.; Hayat, M.A., Ed.; Academic Press: New York, NY, USA, 2015; pp. 133–143. [Google Scholar]
- Eichmann, A.; Simons, M. VEGF signaling inside vascular endothelial cells and beyond. Curr. Opin. Cell Biol. 2012, 24, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Carmeliet, P. The Link Between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F.Q.; Dermime, S.; Mohammad, R.M.; Uddin, S. Vascular endothelial growth factor (VEGF) Signaling in tumour vascularization: Potential and challenges. Curr. Vasc. Pharmacol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Fernandes, P.A.; Ramos, M.J. Vascular endothelial growth factor (VEGF) inhibition—A critical review. Anticancer Agents Med. Chem. 2007, 7, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Binding to the extracellular matrix and proteolytic processing: Two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 2010, 21, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, H.; Ortega, N.; Plouet, J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003, 17, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Keller, G.A.; Ferrara, N. Vascular endothelial growth-factor (VEGF) isoforms—Differential deposition into the subepithelial extracellular-matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 1993, 4, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, J.J.; Whitelock, J.M.; Iozzo, R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009, 28, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, M.; Aumailley, M.; Specks, U.; Knolle, J.; Zerwes, H.; Timpl, R. Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type, VI. Exp. Cell Res. 1993, 206, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Tillet, E.; Ruggiero, F.; Nishiyama, A.; Stallcup, W.B. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J. Biol. Chem. 1997, 272, 10769–10776. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R.; Chu, M.L. Extracellurar Matrix Assemby and Structure; Yurchenko, P., Bird, D., Mechan, R., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 207–241. [Google Scholar]
- Huang, F.J.; You, W.K.; Bonaldo, P.; Seyfried, T.N.; Pasquale, E.B.; Stallcup, W.B. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev. Biol. 2010, 344, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Braghetta, P.; Zanetti, M.; Piccolo, S.; Volpin, D.; Bressan, G.M. Collagen VI deficiency induces early onset myopathy in the mouse: An animal model for Bethlem myopathy. Hum. Mol. Genet. 1998, 7, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat. New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Bonaldo, P.; Stallcup, W.B. Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina. Am. J. Pathol. 2012, 180, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Blouw, B.; Seals, D.F.; Pass, I.; Diaz, B.; Courtneidge, S.A. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur. J. Cell Biol. 2008, 87, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.F.; Azucena, E.F.; Pass, I., Jr.; Tesfay, L.; Gordon, R.; Woodrow, M.; Resau, J.H.; Courtneidge, S.A. The adaptor protein Tks5/FISH is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 2005, 7, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Abram, C.L.; Seals, D.F.; Pass, I.; Salinsky, D.; Maurer, L.; Roth, T.M.; Courtneidge, S.A. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 2003, 278, 16844–16851. [Google Scholar] [CrossRef] [PubMed]
- Stylli, S.S.; Stacey, T.T.; Verhagen, A.M.; Xu, S.S.; Pass, I.; Courtneidge, S.A.; Lock, P. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J. Cell Sci. 2009, 122, 2727–2740. [Google Scholar] [CrossRef] [PubMed]
- Blouw, B.; Patel, M.; Iizuka, S.; Abdullah, C.; You, W.K.; Huang, X.; Li, J.L.; Diaz, B.; Stallcup, W.B.; Courtneidge, S.A. The invadopodia scaffold protein Tks5 is required for the growth of human breast cancer cells in vitro and in vivo. PLoS ONE 2015, 10, e0121003. [Google Scholar] [CrossRef] [PubMed]
- Stratman, A.N.; Malotte, K.M.; Mahan, R.D.; Davis, M.J.; Davis, G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114, 5091–5101. [Google Scholar] [CrossRef] [PubMed]
- Gibby, K.; You, W.K.; Kadoya, K.; Helgadottir, H.; Young, L.J.; Ellies, L.G.; Chang, Y.; Cardiff, R.D.; Stallcup, W.B. Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the neuron-glial antigen 2 proteoglycan. Breast Cancer Res. 2012, 14, R67. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, F.; You, W.K.; Cejudo-Martin, P.; Kucharova, K.; Sakimura, K.; Stallcup, W.B. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology 2015, 4, e1001204. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Jones, J.G.; Li, P.; Zhu, L.; Whitney, K.D.; Muller, W.J.; Pollard, J.W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003, 163, 2113–2126. [Google Scholar] [CrossRef]
- Maglione, J.E.; McGoldrick, E.T.; Young, L.J.; Namba, R.; Gregg, J.P.; Liu, L.; Moghanaki, D.; Ellies, L.G.; Borowsky, A.D.; Cardiff, R.D.; et al. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: Single origin, divergent evolution, and multiple outcomes. Mol. Cancer Ther. 2004, 3, 941–953. [Google Scholar] [PubMed]
- Chang, Y.; She, Z.G.; Sakimura, K.; Roberts, A.; Kucharova, K.; Rowitch, D.H.; Stallcup, W.B. Ablation of NG2 proteoglycan leads to deficits in brown fat function and to adult onset obesity. PLoS ONE 2012, 7, e30637. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgen. Res. 1999, 8, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, C.; Doedens, A.; Weidemann, A.; Zhang, N.; Takeda, N.; Greenberg, J.I.; Cheresh, D.A.; Johnson, R.S. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 2008, 456, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Kucharova, K.; Stallcup, W.B. NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair. J. Neuroinflamm. 2015, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B.; You, W.K.; Kucharova, K.; Cejudo-Martin, P.; Yotsumoto, F. NG2 proteoglycan-dependent contributions of pericytes and macrophages to brain tumor vascularization and progression. Microcirculation 2016, 23, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Hughes, R.; Lewis, C.E. Tumor-associated macrophages: Effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta 2009, 1796, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Lewis, C.E.; Naldini, L.; Brown, J.M.; Ferrara, N.; de Palma, M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am. J. Pathol. 2010, 176, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Venneri, M.A.; Galli, R.; Sergi Sergi, L.; Politi, L.S.; Sampaolesi, M.; Naldini, L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005, 8, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Li, J.F.; Gnatovskiy, L.; Deng, Y.; Zhu, L.; Grzesik, D.A.; Qian, H.; Xue, X.N.; Pollard, J.W. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006, 66, 11238–11246. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Wolburg, H.; Redies, C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. 2000, 218, 472–479. [Google Scholar] [CrossRef]
- Luo, Y.; Radice, G.L. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J. Cell Biol. 2005, 169, 29–34. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Yotsumoto, F.; Sakimura, K.; Adams, R.H.; Stallcup, W.B. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis 2014, 17, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B. NG2 proteoglycan enhances brain tumor progression by promoting beta-1 integrin activation in both cis and trans orientations. Cancers 2017, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Mpekris, F.; Baish, J.W.; Stylianopoulos, T.; Jain, R.K. Role of vascular normalization in benefit from metronomic chemotherapy. Proc. Natl. Acad Sci. USA 2017, 114, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Christov, C.; Adle-Biassette, H.; Le Guerinel, C.; Natchev, S.; Gherardi, R.K. Immunohistochemical detection of vascular endothelial growth factor (VEGF) in the vasculature of oligodendrogliomas. Neuropathol. Appl. Neurobiol. 1998, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Strickland-Marmol, L.B.; Brem, S.; Rojiani, A.M.; Rojiani, M.V. Vessel morphometric parameters-correlation with histologic grade and VEGF expression in oligodendroglioma. Am. J. Cancer Res. 2017, 7, 973–981. [Google Scholar] [PubMed]
- Pietsch, T.; Valter, M.M.; Wolf, H.K.; von Deimling, A.; Huang, H.J.; Cavenee, W.K.; Wiestler, O.D. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol. 1997, 93, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Matsumoto, K.; Irie, F.; Fukushi, J.; Stallcup, W.B.; Yamaguchi, Y. Conditional ablation of the heparan sulfate-synthesizing enzyme Ext1 leads to dysregulation of bone morphogenic protein signaling and severe skeletal defects. J. Biol. Chem. 2010, 285, 19227–19234. [Google Scholar] [CrossRef] [PubMed]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor. Arterioscler Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Ambesi, A.; McKeown-Longo, P.J. Conformational remodeling of the fibronectin matrix selectively regulates VEGF signaling. J. Cell Sci. 2014, 127, 3805–3816. [Google Scholar] [CrossRef] [PubMed]
- Goerges, A.L.; Nugent, M.A. pH regulates vascular endothelial growth factor binding to fibronectin: A mechanism for control of extracellular matrix storage and release. J. Biol. Chem. 2004, 279, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.D.; Teran, M.; Nugent, M.A. Extracellular matrix stiffness controls VEGF signaling and processing in Endothelial Cells. J. Cell Physiol. 2016, 231, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Wijelath, E.S.; Rahman, S.; Namekata, M.; Murray, J.; Nishimura, T.; Mostafavi-Pour, Z.; Patel, Y.; Suda, Y.; Humphries, M.J.; Sobel, M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: Enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 2006, 99, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; San Antonio, J.D. Heparan sulfate proteoglycans: Heavy hitters in the angiogenesis arena. J. Clin. Investig. 2001, 108, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ruhrberg, C.; Gerhardt, H.; Golding, M.; Watson, R.; Ioannidou, S.; Fujisawa, H.; Betsholtz, C.; Shima, D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002, 16, 2684–2698. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, M.; Hong, Z.; Costello, C.E.; Nugent, M.A. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry 2006, 45, 10319–10328. [Google Scholar] [CrossRef] [PubMed]
- Aoudjit, F.; Vuori, K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: A role for c-flip and implications for anoikis. J. Cell Biol. 2001, 152, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Short, S.M.; Talbott, G.A.; Juliano, R.L. Integrin-mediated signaling events in human endothelial cells. Mol. Biol. Cell 1998, 9, 1969–1980. [Google Scholar] [CrossRef] [PubMed]

| 1. Collagen VI Null (Brain) | 2. Tks5 Knockdown (Mammary) | 3. NG2 Null (Mammary) | 4. Mac-NG2ko (Brain) | |
|---|---|---|---|---|
| decreased tumor growth | 2-fold | 4-fold | 2-fold | 6-fold |
| decreased vessel density | no change | no change | no change | no change |
| decreased PC/EC sheathing | no change | ND | 1.6-fold | 2.5-fold |
| decreased basement membrane | 2-fold | ND | 1.5-fold | 8-fold |
| decreased PC maturation | 2-fold | ND | 2.1-fold | 5-fold |
| decreased EC sprouting | 2-fold | ND | 2.3-fold | 3-fold |
| decreased vessel diameter | 1.5-fold | 3-fold | 1.4-fold | 2.3-fold |
| increased vessel leakage | 3-fold | 2.4-fold | 3.4-fold | 5-fold |
| decreased vessel patency | 1.4-fold | ND | ND | 2-fold |
| increased tumor hypoxia | 7-fold | 3.3-fold | 2.3-fold | 12-fold |
| increased total VEGF-A | 1.3-fold | ND | 1.5-fold | 3-fold |
| decreased vessel VEGF-A | 2-fold | 3-fold | ND | 3-fold |
| increased diffuse VEGF-A | 2-fold | ND | 2-fold | 4-fold |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, W.-K.; Stallcup, W.B. Localization of VEGF to Vascular ECM Is an Important Aspect of Tumor Angiogenesis. Cancers 2017, 9, 97. https://doi.org/10.3390/cancers9080097
You W-K, Stallcup WB. Localization of VEGF to Vascular ECM Is an Important Aspect of Tumor Angiogenesis. Cancers. 2017; 9(8):97. https://doi.org/10.3390/cancers9080097
Chicago/Turabian StyleYou, Weon-Kyoo, and William B. Stallcup. 2017. "Localization of VEGF to Vascular ECM Is an Important Aspect of Tumor Angiogenesis" Cancers 9, no. 8: 97. https://doi.org/10.3390/cancers9080097
APA StyleYou, W.-K., & Stallcup, W. B. (2017). Localization of VEGF to Vascular ECM Is an Important Aspect of Tumor Angiogenesis. Cancers, 9(8), 97. https://doi.org/10.3390/cancers9080097






