Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review
Abstract
:1. Introduction
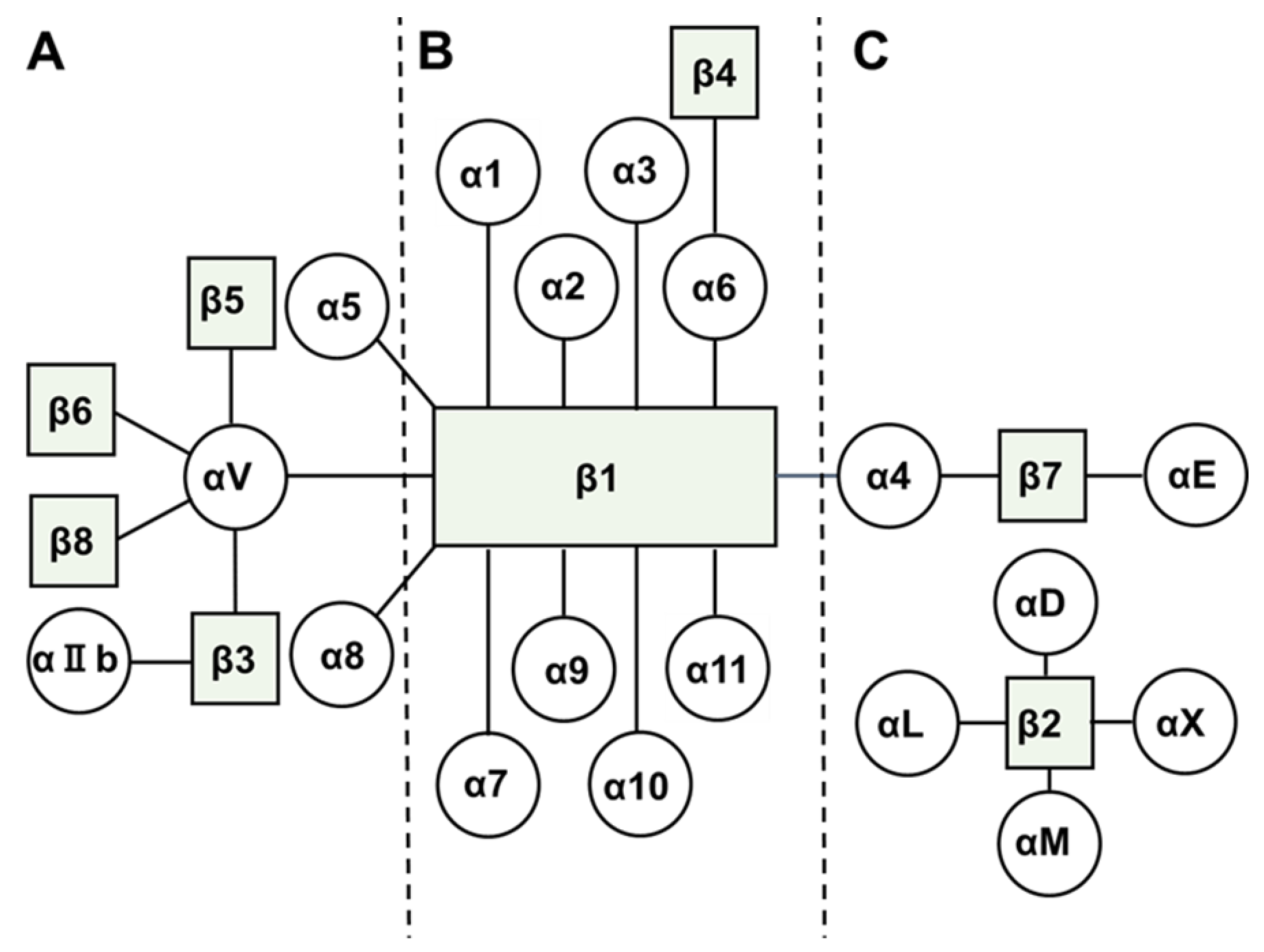
2. Biology of Integrins
3. Current Treatment of Ovarian Cancer
4. Biology of Integrins in Ovarian Cancer
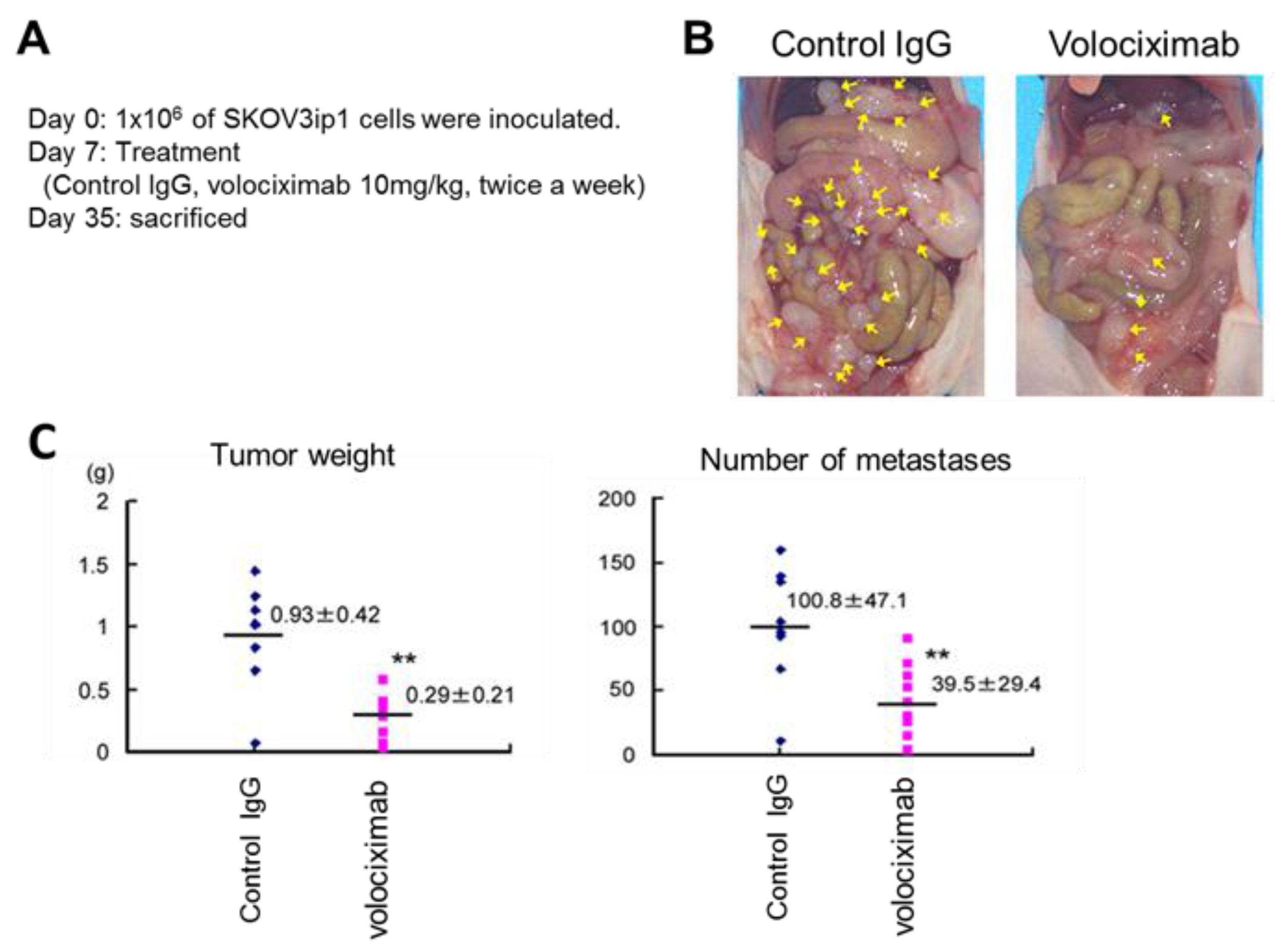
5. Clinical Trials of Integrin Inhibitors for Treating Ovarian Cancer
6. Future Directions and Conclusions
Acknowledgments
Conflicts of Interests
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Petignat, P. Purified autoantibodies against glucose-regulated protein 78 (GRP78) promote apoptosis and decrease invasiveness of ovarian cancer cells. Cancer Lett. 2011, 309, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Shen, Y.; Chen, X.; Ren, M. Molecular targeted drug therapy of ovarian cancer. ARC J. Gynecol. Obstet. 2016, 1, 1–7. [Google Scholar]
- Medeiros, F.; Muto, M.G.; Lee, Y.; Elvin, J.A.; Callahan, M.J.; Feltmate, C.; Garber, J.E.; Cramer, D.W.; Crum, C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 2006, 30, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Chianq, C.Y.; White, E.A.; Elizabeth, M.S.; Mohammed, H.; Iris, L.R.; Andras, L.; Carla, V.P.; Joshy, G.; Karl, M.; et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J. Clin. Investig. 2014, 124, 4614–4628. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Stephen, A.C. β1-Integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol. Oncol. 1999, 73, 362–367. [Google Scholar]
- Zhang, K.; Chen, F. The regulation of integrin function by divalent cations. Cell Adh. Migr. 2012, 6, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Jay, S.D.; David, A.C. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ray, C.M.; Spira, A.I.; Trepel, J.B.; Carter, C.A.; Cottrill, H.M. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med. Oncol. 2017, 34, 103. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Shih, K.K.; Chi, D.S. Maximal cytoreductive effort in epithelial ovarian cancer surgery. J. Gynecol. Oncol. 2010, 21, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org (accessed on 19 June 2017).
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Jazaeri, A.A. Recurrent epithelial ovarian cancer: Pharmacotherapy and novel therapeutics. Expert Opin. Pharmacother. 2007, 8, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swat, A.M.; Pfisterer, J.; Ledermann, J.A.; Lauraine, E.P.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, 1358–1366. [Google Scholar]
- Eric, P.L.; Felix, H.; Beatrice, W.; Alexander, R.; Andres, P.; Gunnar, K.; Roberto, S.; Ignace, V.; Petronella, W.; Aristotelis, B.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar]
- Jazaeri, A.A.; Slack-Davis, J.K. The promise of antiangiogenic therapy for ovarian cancer. Cancer Biol. Ther. 2009, 23, 2260–2261. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Mitra, A.K.; Radjabi, A.R.; Bhaskar, V.; Kistner, E.O.; Tretiakova, M.; Jagadeeswaran, S.; Montag, A.; Becker, A.; Kenny, H.A.; et al. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 2008, 68, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Ruff, L.E.; Skubitz, A.P.N. β1-Integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Hu, Z.; Gao, S.; Gao, J.; Hou, R.; Liu, C.; Liu, J.; Li, B.; Liu, D.; Zhang, S.; Lin, B. Elevated levels of Lewis y and integrin α5β1 correlate with chemotherapeutic drug resistance in epithelial ovarian carcinoma. Int. J. Mol. Sci. 2012, 13, 15588–15600. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.L.; O’Connor, K.L. Clinical significance of the integrin α6β4 in human malignancies. Lab. Investig. 2015, 95, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Pineda, J.C.; Garibay-Cerdenares, O.L.; Hernandez-Ramires, V.I.; Gallardo-Rincón, D.; David, C.L.; Pérez-Montiel-Gómez, M.D.; Talamás-Rohana, P. Integrins and haptoglobin: Molecules overexpressed in ovarian cancer. Pathol. Res. Pract. 2015, 211, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Charo, I.F.; Nannizzi, L.; Smith, J.W.; Cheresh, D.A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 1990, 111, 2795–2800. [Google Scholar] [CrossRef] [PubMed]
- Petitclerc, E.; Stromblad, S.; Schalscha, T.L.; Mitjans, F.; Piulats, J.; Montgomery, A.M.P.; Cheresh, D.A.; Brooks, P.C. Integrin αvβ3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999, 59, 2724–2730. [Google Scholar] [PubMed]
- Brooks, P.C.; Stromblad, S.; Klemke, R.; Visscher, D.; Sarkar, F.H.; Cheresh, D.A. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J. Clin. Investig. 1995, 96, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N.; Kim, T.J.; Lin, Y.G.; Merritt, W.M.; Kamat, A.A.; Han, L.Y.; Spannuth, W.A.; Nick, A.M.; Jennnings, N.B.; Kinch, M.S.; et al. Tumor-selective response to antibody-mediated targeting of αvβ3 integrin in ovarian cancer. Neoplasia 2008, 10, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Goldberg, I.; Reich, R.; Tell, L.; Dong, H.P.; Tropé, C.G.; Risberg, B.; Kopolovic, J. αV- and β1-integrin subunits are commonly expressed in malignant effusions from ovarian carcinoma patients. Gynecol. Oncol. 2003, 90, 248–257. [Google Scholar] [CrossRef]
- Gao, J.; Hu, Z.; Liu, D.; Liu, J.; Liu, C.; Hou, R.; Gao, S.; Zhang, D.; Zhang, S.; Lin, B. Expression of Lewis y antigen and integrin αv, β3 in ovarian cancer and their relationship with chemotherapeutic drug resistance. J. Exp. Clin. Cancer Res. 2013, 32, 36. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kenny, H.A.; Jagadeeswaran, S.; Zillhardt, M.R.; Montag, A.G.; Kistner, E.; Yamada, S.D.; Mitra, A.K.; Lengyel, E. β3-Integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer. Am. J. Pathol. 2009, 175, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Li, H.; Lu, Z.; Shan, W.; Mercado-Uribe, I.; Liu, J. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. Am. J. Transl. Res. 2013, 5, 336–346. [Google Scholar] [PubMed]
- Scalici, J.M.; Harrer, C.; Allen, A.; Jazaeri, A.; Atkins, K.A.; McLachlan, K.R.; Slack-Davis, J.K. Inhibition of α4β1 integrin increases ovarian cancer response to carboplatin. Gynecol. Oncol. 2014, 132, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Slack-Davis, J.K.; Atkins, K.A.; Harrer, C.; Hershey, E.D.; Conaway, M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009, 69, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Bhaskar, V.; Law, D.A.; Wong, M.H.; DuBridge, R.B.; Breinberg, D.; O'Hara, C.; Powers, D.B.; Liu, G.; et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J. Exp. Ther. Oncol. 2006, 5, 273–286. [Google Scholar] [PubMed]
- Almokadem, S.; Belani, C.P. Volociximab in cancer. Expert Opin. Biol. Ther. 2012, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Sawada, K.; Tiwari, P.; Mui, K.; Gwin, K.; Lengyel, E. Ligand independent activation of c-Met by fibronectin and α5β1-integrin regulates ovarian cancer invasion and metastasis. Oncogene 2011, 30, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Ricart, A.D.; Tolcher, A.W.; Liu, G.; Holen, K.; Schwartz, G.; Albertini, M.; Weiss, G.; Yazji, S.; Ng, C.; Wilding, G. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: A phase I, pharmacokinetic, and biological correlative study. Clin. Cancer Res. 2008, 14, 7924–7929. [Google Scholar] [CrossRef] [PubMed]
- Bell-McGuinn, K.M.; Matthews, C.M.; Ho, S.N.; Barve, M.; Gilbert, L.; Penson, R.T.; Lengyel, M.; Palaparthy, R.; Gilder, K.; Vassos, A.; et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol. Oncol. 2011, 121, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, W. Inhibition of integrin β1 decreases the malignancy of ovarian cancer cells and potentiates anticancer therapy via the FAK/STAT1 signaling pathway. Mol. Med. Rep. 2015, 12, 7869–7876. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Rayburn, H.; Hynes, R.O. Embryonic mesodermal defects in α5 integrin-deficient mice. Development 1993, 119, 1093–1105. [Google Scholar] [PubMed]
- Xie, X.; Long, L.; Wang, H.; Zheng, Y.; Liu, S. The specifical inhibition of the expression of integrin alpha5/beta1 probably enhances the treatment effects and improves the prognosis of epithelial ovarian cancer. Med. Hypotheses 2015, 84, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nagasoto, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef] [PubMed]
- O’Day, S.; Pavlick, A.; Loquai, C.; Lawson, D.; Gutzmer, R.; Richards, J.; Schadendorf, D.; Thompson, J.A.; Gonzalez, R.; Trefzer, U.; et al. A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br. J. Cancer 2011, 105, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Mullamitha, S.A.; Ton, N.C.; Parker, G.J.; Jackson, A.; Julyan, P.J.; Roberts, C.; Buonaccorsi, G.B.; Watson, Y.; Davies, K.; Cheung, S.; et al. Phase I evaluation of a fully human anti-alpha v integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin. Cancer Res. 2007, 13, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Delbaldo, C.; Raymond, E.; Vera, K.; Hammershaimb, L.; Kaucic, K.; Lozahic, S.; Marty, M.; Faivre, S. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest. New Drugs 2008, 26, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Sosman, J.; O’Day, S.; Richards, J.; Bedikian, A.; Gonzalez, R.; Sharfman, W.; Weber, R.; Logan, T.; Buzoianu, M.; et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrinαvβ3, ±dacarbazine in patients with stage IV metastatic melanoma. Cancer 2010, 116, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Kuhn, J.; Lamborn, K.R.; Lieberman, F.; Wen, P.Y.; Mehta, M.; Cloughesy, T.; Lassman, A.B.; DeAngelis, L.M.; Chang, S.; et al. Cilengitide in patients with recurrent glioblastoma: The results of NABTC 03-02, a phase II trial with measures of treatment delivery. J. Neurooncol. 2012, 106, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Mikkelsen, T.; Hegi, M.E.; Ye, X.; Batchelor, T.; Lesser, G.; Peereboom, D.; Rosenfeld, M.R.; Olsen, J.; Brem, S.; et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma. Cancer 2012, 118, 5601–5607. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Sara, C.E.; James, P.; Yong-Kil, H.; Kenneth, D.A.; Benoit, L.; Torsten, P.; Danica, G.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Miller, L.M.; Pritchard, J.M.; Macdonald, S.J.; Jamieson, C.; Watson, A.J. Emergence of small-molecule non-RGD-mimetic inhibitors for RGD integrins. J. Med. Chem. 2017, 60, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Breton, A.L.; Preat, V. RGD-based strategies to target αvβ3 integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Vroman, B.; Lecouturier, N.; Crokart, N.; Pourcelle, V.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J. Control. Release 2009, 140, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Su, B.; Li, W.; Ding, Y.; Tang, L.; Zhou, W.; Song, Y.; Caicun, Z. Integrin-targeted paclitaxel nanoliposomes for tumor therapy. Med. Oncol. 2011, 28, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Sawada, K.; Kimura, T. Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers 2017, 9, 83. https://doi.org/10.3390/cancers9070083
Kobayashi M, Sawada K, Kimura T. Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers. 2017; 9(7):83. https://doi.org/10.3390/cancers9070083
Chicago/Turabian StyleKobayashi, Masaki, Kenjiro Sawada, and Tadashi Kimura. 2017. "Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review" Cancers 9, no. 7: 83. https://doi.org/10.3390/cancers9070083
APA StyleKobayashi, M., Sawada, K., & Kimura, T. (2017). Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers, 9(7), 83. https://doi.org/10.3390/cancers9070083




