The Evolution of Therapies in Non-Small Cell Lung Cancer
Abstract
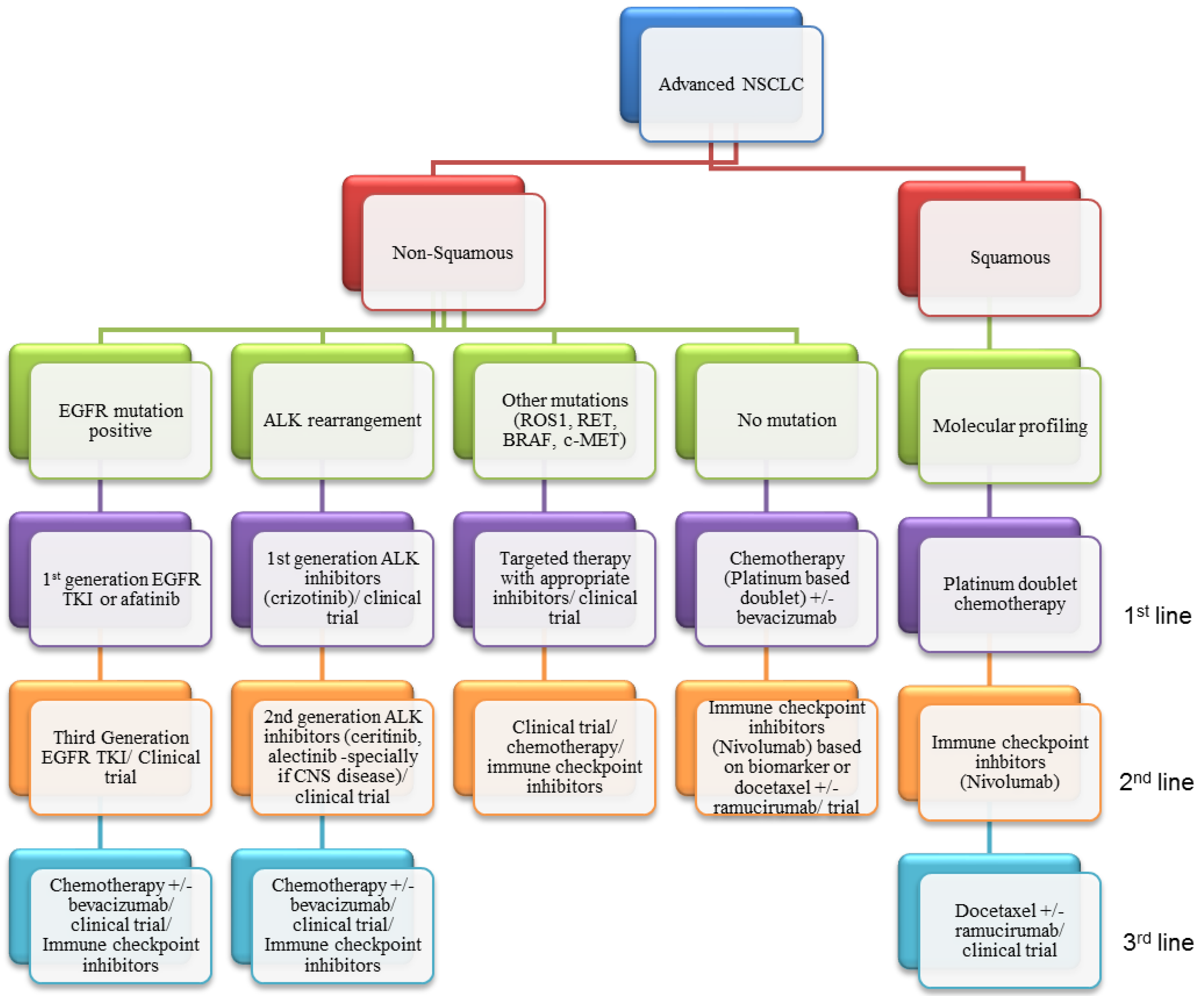
:1. Introduction
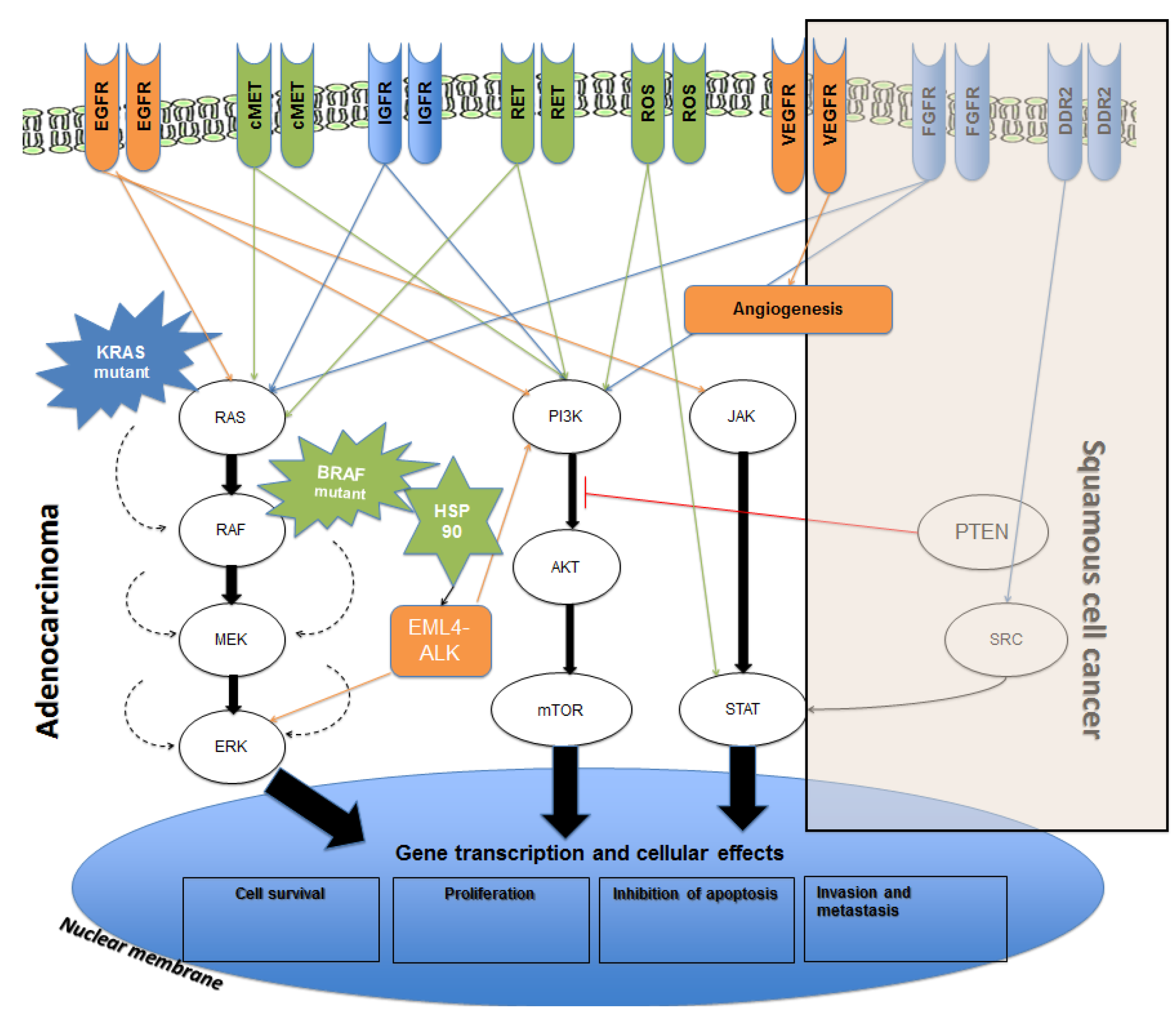
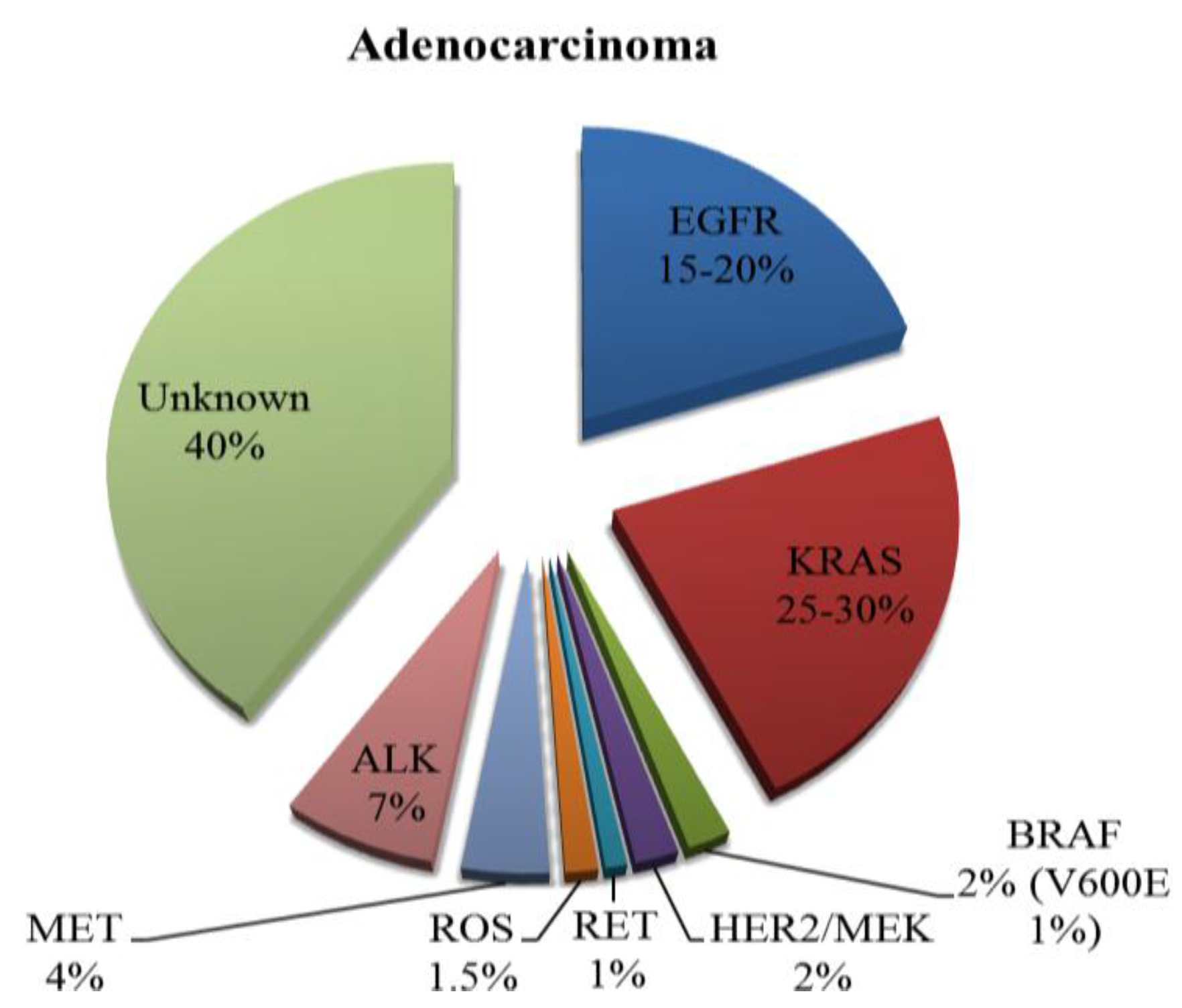
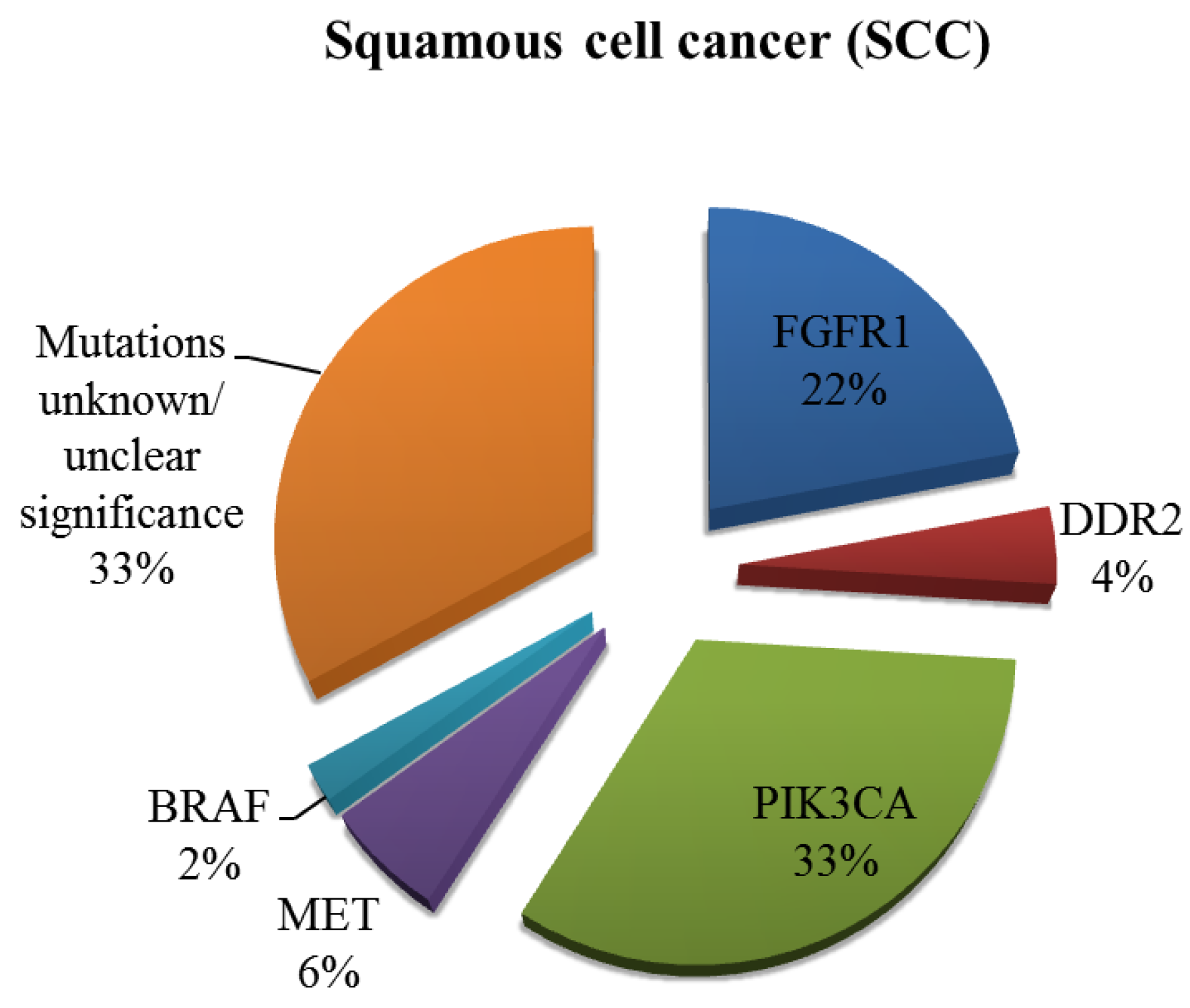
2. Current Therapies Available
2.1. Chemotherapy
2.2. Vascular Endothelial Growth Factor (VEGF) Inhibition
2.3. Proven Targeted Therapies
2.3.1. Epidermal Growth Factor Receptor (EGFR)
First-Generation EGFR Tyrosine Kinase Inhibitors (TKIs)
Second-Generation EGFR TKIs
| First Generation EGFR TKI (Erlotinib, Gefitinib)-First-Line Studies | ||||||
|---|---|---|---|---|---|---|
| Trial | Population | Number | Agent (A) | Comparator (C) | Median PFS A vs. C (Months) | HR |
| Mok et al. (2009) [40] Phase III | Adenocarcinoma, Asian, never or light smokers | 1217 | Gefitinib | Carboplatin, pacltaxel | 9.8 vs. 6.4 | 0.48 |
| Maemondo et al. (2010) [41] Phase III | EGFR mutant | 230 | Gefitinib | Carboplatin, paclitaxel | 10.8 vs. 5.4 | 0.30 |
| Mitsudomi et al. (2010) [42] Phase III | EGFR mutant | 172 | Gefitinib | Cisplatin, docetaxel | 9.2 vs. 6.3 | 0.489 |
| Han et al. (2012) [43] Phase III | Adenocarcinoma, Asian, never or light smokers | 309 | Gefitinib | Cisplatin, gemcitabine | 5.8 vs. 6.4 | 1.198 |
| 42 EGFR +ve | 8.0 vs. 6.3 | 0.544 | ||||
| Zhou et al. (2011) [44] Phase III | EGFR mutant | 154 | Erlotinib | Carboplatin, gemcitabine | 13.1 vs. 4.6 | 0.16 |
| Rosell et al. (2012) [45] Phase III | EGFR mutant | 173 | Erlotinib | Platinum doublet | 9.7 vs. 5.2 | 0.37 |
| Second Generation EGFR TKI (Afatinib, Dacomitinib, Neratinib) | ||||||
| Sequist et al. (2013) [52] Phase III | EGFR mutant- 1st line setting | 345 | Afatinib | Cisplatin, pemetrexed | 11.1 vs. 6.9 | 0.58 |
| Wu et al. (2014) [60] Phase III | EGFR mutant- 1st line setting | 364 | Afatinib | Cisplatin, gemcitabine | 11.0 vs. 5.6 | 0.28 |
| Yang et al. (2012) [53] Phase II | EGFR mutant- 2nd line with no prior EGFR TKI | 129 | Afatinib | Single arm | 14.0 | NA |
| Miller et al. (2012) [54] Phase IIb/III | EGFR mutant- after treatment with EGFR TKI | 585 | Afatinib | Placebo | 3.3 vs. 1.1 | 0.38 |
| Katakami et al. (2013) [55] Phase II | Clinical post 1st line EGFR TKI, 72.6% mutant | 62 | Afatinib | Single arm | 4.4 | NA |
| Ramalingam et al. (2014) [57] Phase III | All NSCLC post progression with chemotherapy | 878 | Dacomitinib | Erlotinib | 2.6 vs. 2.6 | 1.022 |
| Ellist et al. (2014) [58] Phase III | All NSCLC post progression with chemotherapy and EGFR TKI | 720 | Dacomitinib | Placebo | 2.7 vs. 1.4 | 0.66 |
| Sequist et al. (2010) [59] Phase II | All NSCLC | 167 | Neratinib | Single arm | 3.7 (Severe diarrhoea) | NA |
| Third Generation EGFR TKI (Rociletinib, AZD9291) | ||||||
| Sequist et al. (2015) [61] Phase I/II | EGFR mutant post progression on EGFR TKI | 130 | Rociletinib | Single arm | 13.1 | NA |
| Janne et al. (2015) [62] Phase I/II | EGFR mutant post progression on EGFR TKI | 253 | AZD9291 | Single arm | 8.2 | NA |
Third-Generation EGFR TKIs
Dual Inhibition
Treatment Paradigm Following First-Line EGFR TKI
EGFR Inhibition in SCC
2.3.2. Anaplastic Lymphoma Kinase (ALK)
Second-Generation ALK Inhibitors
Other Targets
| First Generation ALK TKI-Crizotinib | ||||||
|---|---|---|---|---|---|---|
| Trial | Population | Number | Agent (A) | Comparator (C) | Median PFS A vs. C (Months) | HR |
| Kim et al. (2012) [77] Phase II | ALK positive post 1st line chemotherapy | 439 | Crizotinib | Single arm | 8.5 | NA |
| Shaw et al. (2013) [78] Phase III | ALK positive post 1st line chemotherapy | 347 | Crizotinib | Pemetrexed or docetaxel | 7.7 vs. 3.0 | 0.49 |
| Solomon et al. (2014) [79] Phase III | ALK positive- 1st line | 343 | Crizotinib | Platinum, pemetrexed | 10.9 vs. 7.0 | 0.45 |
| Second Generation ALK TKI (Ceritinib, Alectinib) | ||||||
| Shaw et al. (2014) [83] Phase I | ALK positive (68% progressed on Crizotinib) | 130 | Ceritinib | Single arm | 7.0 overall, 10.4 for ALK inhibitor naïve, 6.9 in prev. treated | NA |
| Seto et al. (2013) [85,86] Phase I/II | ALK positive- 1st line setting | 58 | Alectinib | Single arm | Not yet reached >10.3 | NA |
2.4. Potential Targets under Study
2.4.1. ROS1
2.4.2. Mesenchymal-Epithelial Transition (MET)
2.4.3. Rearranged during Transfection (RET)
2.4.4. BRAF Mutation
2.5. Targets with No Currently Effective Therapy
2.5.1. Kirsten Rat Sarcoma 2 Viral Oncogene Homolog (K-RAS) Mutation
2.5.2. Insulin-Like Growth Factor 1 Receptor (IGFR1)
2.5.3. Fibroblast Growth Factor Receptor 1 (FGFR1)
2.5.4. Discoidin Domain Receptors (DDR)
2.6. Immunotherapy in NSCLC
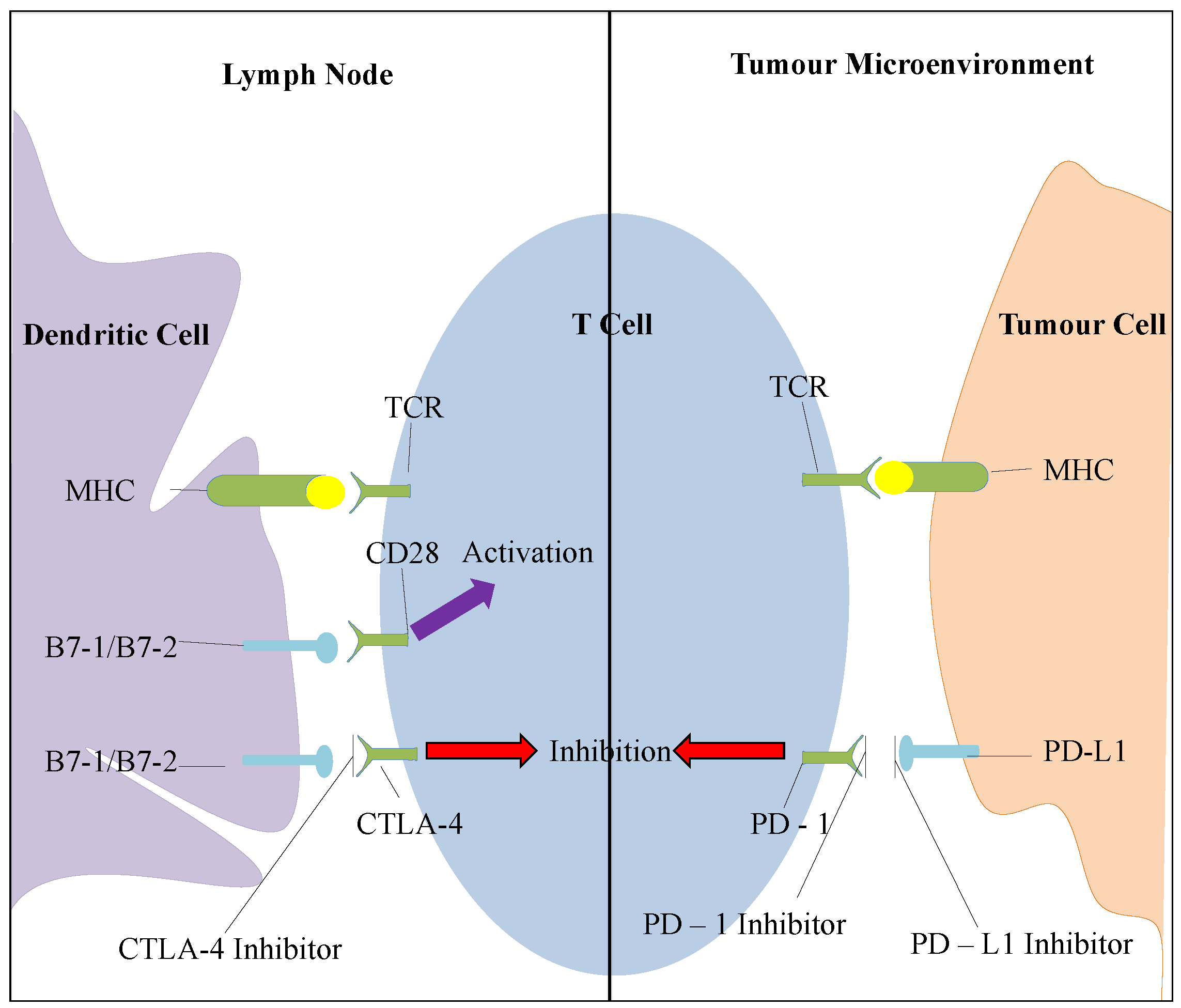
2.6.1. CTLA-4 Inhibition
2.6.2. PD-1 Pathway Inhibition
2.7. Stem Cell Inhibitors
2.8. Other Potential Biomarker Based Clinical Trials
2.9. Special Considerations for Elderly Patients
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. 2013. Available online: http://globocan.iarc.fr (accessed on 23 June 2015).
- Ettinger, D.S.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.; Govindan, R.; et al. Non-small cell lung cancer. J. Natl. Compr. Cancer Netw. 2012, 10, 1236–1271. [Google Scholar]
- Janssen-Heijnen, M.L.; Coebergh, J.W. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003, 41, 245–258. [Google Scholar] [CrossRef]
- Spiro, S.G.; Gould, M.K.; Colice, G.L. Initial evaluation of the patient with lung cancer: Symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP Evidenced-based Clinical Practice Guidelines (2nd edition). Chest 2007, 132, 149S–160S. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2012; National Cancer Institute: Bethesda, MD, USA, 2015.
- Alamgeer, M.; Ganju, V.; Watkins, D.N. Novel therapeutics in non-small cell lung cancer. Curr. Opin. Pharmacol. 2013, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- West, L.; Vidwans, S.J.; Campbell, N.P.; Shrager, J.; Simon, G.R.; Bueno, R.; Dennis, P.A.; Otterson, G.A.; Salgia, R. A novel classification of lung cancer into molecular subtypes. PLoS ONE 2012, 7, e31906. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, P.; Brambilla, E.; Thomas, R.; Soria, J.C. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin. Cancer Res. 2012, 18, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Tartour, E.; Zitvogel, L. Lung cancer: Potential targets for immunotherapy. Lancet Respir. Med. 2013, 1, 551–563. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Azzoli, C.G.; Baker, S.; Temin, S.; Pao, W.; Aliff, T.; Brahmer, J.; Johnson, D.H.; Laskin, J.L.; Masters, G.; Milton, D.; et al. American society of clinical oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 6251–6266. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Popat, S.; Reinmuth, N.; de Ruysscher, D.; Kerr, K.M.; Peters, S. Metastatic non-small cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann. Oncol. 2014, 25, iii27–iii39. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Parikh, P.; von Pawel, J.; Biesma, B.; Vansteenkiste, J.; Manegold, C.; Serwatowski, P.; Gatzemeier, U.; Digumarti, R.; Zukin, M.; et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Brodowicz, T.; Shepherd, F.A.; Zielinski, C.; Vansteenkiste, J.; Manegold, C.; Simms, L.; Fossella, F.; Sugarman, K.; Belani, C.P. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, P.; Volante, M.; Saviozzi, S.; Rapa, I.; Novello, S.; Cambieri, A.; Lo Iacono, M.; Cappia, S.; Papotti, M.; Scagliotti, G.V. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006, 107, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Brodowicz, T.; Krzakowski, M.; Zwitter, M.; Tzekova, V.; Ramlau, R.; Ghilezan, N.; Ciulianu, T.; Cucevic, B.; Gyurkovits, K.; Ulsperger, E.; et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: A phase III trial. Lung Cancer 2006, 52, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fidas, P.M.; Dakhil, S.R.; Lyss, A.P.; Loesch, D.M.; Waterhouse, D.M.; Bromund, J.L.; Chen, R.; Hristova-Kazmierski, M.; Treat, J.; Obasaju, C.K.; et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Dancey, J.; Ramlau, R.; Mattson, K.; Gralla, R.; O’Rourke, M.; Levitan, N.; Gressot, L.; Vincent, M.; Burkes, R.; et al. Prospective randomized trial of docetaxel vs. best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 2000, 18, 2095–2103. [Google Scholar] [PubMed]
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly nab-paclitaxel in combination with carboplatin vs. solvent based paclitaxel plus carboplatin as first-line therapy in patients with advanced non small-cell lung cancer: Final results of a phase III trial. J. Clin. Oncol. 2012, 30, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Fehrenbacher, L.; Novotny, W.F.; Herbst, R.S.; Nemunaitis, J.J.; Jablons, D.M.; Langer, C.J.; de Vore, R.F., 3rd; Gaudreault, J.; Damico, L.A.; et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small cell lung cancer: AVAiL. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Scherpereel, A.; Gorbunova, V.; Gervais, R.; Vikstrom, A.; Chouaid, C.; Chella, A.; Kim, J.H.; Ahn, M.J.; Reck, M.; et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL (MO22089) randomised phase III trial. Ann. Oncol. 2014, 25, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Socinski, M.A.; Garon, E.B.; Reynolds, C.H.; Spigel, D.R.; Olsen, M.R.; Hermann, R.C.; Jotte, R.M.; Beck, T.; Richards, D.A.; et al. PointBreak: A randomised phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab vs. paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 4349–4357. [Google Scholar] [PubMed]
- Ramlau, R.; Gorbunova, V.; Ciuleanu, T.E.; Novello, S.; Ozgurologlu, M.; Goksel, T.; Baldotto, C.; Bennouna, J.; Shepherd, F.A.; Le-Guennec, S.; et al. Aflibercept and docetaxel vs. docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: A randomised, controlled phase III trial. J. Clin. Oncol. 2012, 30, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib vs. docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel vs. placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–673. [Google Scholar] [CrossRef]
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Yatabe, Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007, 98, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, A.; Shepherd, F.A.; Tsao, M.S. Epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer: Impact of primary or secondary mutations. Clin. Lung. Cancer 2006, 7, S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Greulich, H.; Chen, T.H.; Feng, W.; Janne, P.A.; Alvarez, J.V.; Zappaterra, M.; Bulmer, S.E.; Frank, D.A.; Hahn, W.C.; Sellers, W.R.; et al. Oncogenic transformation by inhibitor-sensitive and-resistant EGFR mutants. PLoS Med. 2005, 2, e313. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Boggon, T.J.; Dayaram, T.; Janne, P.A.; Kocher, O.; Meyerson, M.; Johnson, B.E.; Eck, M.J.; Tenen, D.G.; Halmos, B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005, 352, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, Y.; Shimizu, K.; Hirato, J.; Araki, T.; Tanaka, K.; Ogawa, H.; Kakegawa, S.; Sugano, M.; Nakano, T.; Mitani, Y.; et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol. Rep. 2011, 25, 921–928. [Google Scholar] [PubMed]
- Rekhtman, N.; Paik, P.K.; Arcila, M.E.; Tafe, L.J.; Oxnard, G.R.; Moreira, A.L.; Travis, W.D.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: Lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin. Cancer Res. 2012, 18, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Ando, M.; Asami, K.; Okano, Y.; Fukuda, M.; Nakagawa, H.; Ibata, H.; Kozuki, T.; Endo, T.; Tamura, A.; et al. Randomized phase III trial of erlotinib vs. docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J. Clin. Oncol. 2014, 32, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Martelli, O.; Broggini, M.; Farina, G.; Veronese, S.; Rulli, E.; Bianchi, F.; Bettini, A.; Longo, F.; Moscetti, L.; et al. Erlotinib vs. docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): A randomised controlled trial. Lancet Oncol. 2013, 14, 981–988. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib vs. cisplatin plus docetaxel in patients with non- small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Han, J.Y.; Park, K.; Kim, S.W.; Lee, D.H.; Kim, H.Y.; Kim, H.T.; Ahn, M.J.; Yun, T.; Ahn, J.S.; Suh, C.; et al. First-SIGNAL: First-line single-agent iressa vs. gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J. Clin. Oncol. 2012, 30, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib vs. chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib vs. standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Gatzemeier, U.; Pluzanska, A.; Szczesna, A.; Kaukel, E.; Roubec, J.; de Rosa, F.; Milanowski, J.; Karnicka-Mlodkowski, H.; Pesek, M.; Serwatowski, P.; et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J. Clin. Oncol. 2007, 25, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Prager, D.; Hermann, R.; Fehrenbacher, L.; Johnson, B.E.; Sandler, A.; Kris, M.G.; Tran, H.T.; Klein, P.; Li, X.; et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non- small-cell lung cancer. J. Clin. Oncol. 2005, 23, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Orashi, K.; Maruvka, Y.E.; Michor, F.; Pao, W. Epidermal growth factor receptor tyrosine kinase inhibitor-Resistant disease. J. Clin. Oncol. 2013, 31, 1070–1080. [Google Scholar]
- Li, D.; Ambrogio, L.; Shimamura, T.; Kubo, S.; Takahashi, M.; Chirieac, L.R.; Padera, R.F.; Shapiro, G.I.; Baum, A.; Himmelsbach, F.; et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008, 27, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Gale, C.M.; Lifshits, E.; Gonzales, A.J.; Shimamura, T.; Zhao, F.; Vincent, P.W.; Naumov, G.N.; Bradner, J.E.; Althaus, I.W.; et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007, 67, 11924–11932. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P. The role of irreversible EGFR inhibitors in the treatment of non-small cell lung cancer: Overcoming resistance to reversible EGFR inhibitors. Cancer Invest. 2010, 28, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Shih, J.Y.; Su, W.C.; Hsia, T.C.; Tsai, C.M.; Ou, S.H.; Yu, C.J.; Chang, J.C.; Ho, C.L.; Sequist, L.V.; et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol. 2012, 13, 539–548. [Google Scholar] [CrossRef]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.M.; Park, K.; Kim, S.W.; Zhou, C.; Su, W.C.; Wang, M.; Sun, Y.; et al. Afatinib vs. placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Katakami, N.; Atagi, S.; Goto, K.; Hida, T.; Horai, T.; Inoue, A.; Ichinose, Y.; Koboyashi, K.; Takeda, K.; Kiura, K.; et al. LUX-Lung 4: A phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J. Clin. Oncol. 2013, 31, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Sequist, L.V.; Geater, S.L.; Tsai, C.M.; Mok, T.S.; Schuler, M.; Yamamoto, N.; Yu, C.J.; Ou, S.H.; Zhou, C.; et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Janne, P.A.; Mok, T.; O’Byrne, K.; Boyer, M.J.; Von Pawel, J.; Pluzanski, A.; Shtivelband, M.; Docampo, L.I.; Bennouna, J.; et al. Dacomitinib vs. erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 1369–1378. [Google Scholar] [CrossRef]
- Ellis, P.M.; Liu, G.; Millward, M.; Perrone, F.; Shepherd, F.A.; Sun, S.; Cho, B.C.; Morabito, A.; Stockler, M.R.; et al. NCIC CTG BR.26: A phase III randomized, double blind, placebo controlled trial of dacomitinib vs. placebo in patients with advanced/metastatic non-small cell lung cancer (NSCLC) who received prior chemotherapy and an EGFR TKI. J. Clin. Oncol. 2014, 32. Abstract No. 8036. [Google Scholar]
- Sequist, L.V.; Besse, B.; Lynch, T.J.; Miller, V.A.; Wong, K.K.; Gitlitz, B.; Eaton, K.; Zacharchuk, C.; Freyman, A.; Powell, C.; et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: Results of a phase II trial in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib vs. cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Sequist, L.V.; Soria, J.C.; Goldman, J.W.; Wakelee, H.A.; Gadgeel, S.M.; Varga, A.; Papadimitrakopolou, V.; Solomon, B.J.; Oxnard, J.R.; et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Janne, P.A.; Yang, J.C.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. AZD9291 in EGFR Inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Regales, L.; Gong, Y.; Shen, R.; de Stanchina, E.; Vivanco, I.; Goel, A.; Koutcher, J.A.; Spassova, M.; Ouerfelli, O.; et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J. Clin. Invest. 2009, 119, 3000–3010. [Google Scholar] [PubMed]
- Janjigian, Y.Y.; Smit, E.F.; Groen, H.J.M.; Horn, L.; Gettinger, S.; Camidge, D.R.; Riely, G.J.; Wang, B.; Fu, Y.; Chand, V.K.; et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014, 4, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Oxnard, G.R.; Sima, C.S.; Kris, M.G.; Miller, V.A.; Riely, G.J. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin. Cancer Res. 2011, 17, 6298–6303. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ahn, M.; Yu, C.; Kim, S.; Lin, M.; Sriuranpong, V.; Tsai, C.; Lee, J.; Kang, J.; Perez-Moreno, P.; et al. ASPIRATION: First-line erlotinib (E) until and beyond RECIST progression (PD) in asian patients (pts) with EGFR mutation-positive (mut+) NSCLC. Ann. Oncol. 2014, 25, iv426–iv470. [Google Scholar]
- Mok, T.S.K.; Wu, Y.; Nakagawa, K.; Kim, S.; Yang, J.; Ahn, M.; Wang, J.; Yang, J.C.; Lu, Y.; Atagi, S.; et al. Gefitinib/chemotherapy vs. chemotherapy in epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC) after progression on first-line gefitinib: The pahse III randomised IMPRESS study. Ann. Oncol. 2014, 25, S1–S41. [Google Scholar]
- Pirker, R.; Pereira, J.R.; Szczesna, A.; von Pawel, J.; Krzakowski, M.; Ramlau, R.; Vynnychenko, I.; Park, K.; Yu, C.T.; Ganul, V.; et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet 2009, 373, 1525–1531. [Google Scholar] [CrossRef]
- Thatcher, N.; Hirsch, F.R.; Luft, A.V.; Szczesna, A.; Ciuleanu, T.E.; Dediu, M.; Ramlau, R.; Galiulin, R.K.; Bálint, B.; Losonczy, G.; et al. Necitumumab plus gemcitabine and cisplatin vs. gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): An open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015, 16, 763–774. [Google Scholar] [CrossRef]
- Soria, J.C.; Felip, E.; Cobo, M.; Lu, S.; Syrigos, K.; Lee, K.H.; Goker, S.; Georgoulias, V.; Li, W.; Isla, D.; et al. Afatinib vs. erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2015, 16, 897–907. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; Zou, H.Y.; Arango, M.E.; Li, Q.; Lee, J.H.; McDonnell, S.R.; Yamazaki, S.; Alton, G.R.; Mroczkowski, B.; Los, G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007, 6, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.L.; Bang, Y.J.; Camidge, D.R.; Shaw, A.T.; Solomon, B.; Maki, R.G.; Ou, S.H.; Dezube, B.J.; Janne, P.A.; Costa, D.B.; et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Bang, Y.J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.J.; Ou, S.H.; Kim, D.W.; et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef]
- Shaw, A.T.; Yeap, B.Y.; Solomon, B.J.; Riely, G.J.; Gainor, J.; Engelman, J.A.; Shapiro, G.I.; Costa, D.B.; Ou, S.H.; Butaney, M. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: A retrospective analysis. Lancet Oncol. 2011, 12, 1004–1012. [Google Scholar] [CrossRef]
- Kim, D.W.; Ahn, M.J.; Shi, Y.; De Pas, T.M.; Yang, P.C.; Riely, G.J.; Crino, L.; Evans, T.L.; Liu, X.; Han, J.Y.; et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer. J. Clin. Oncol. 2012, 30. Abstract No. 7533. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crino, L.; Ahn, M.J.; de Pas, T.M.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib vs. chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Capuzzo, F.; Paolini, J.; Usari, T.; et al. First line crizotinib vs. chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Y.; Li, Q.; Lee, J.H.; Arango, M.E.; McDonnell, S.R.; Yamazaki, S.; Koudriakova, T.B.; Alton, G.; Cui, J.J.; Kung, P.P.; et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007, 67, 4408–4417. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; de Figueiredo-Pontes, L.L.; Kobayashi, S.; Costa, D.B. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J. Thorac. Oncol. 2012, 7, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Shaw, A.T. ALK inhibitors in non-small cell lung cancer: Crizotinib and beyond. Clin. Adv. Hematol. Oncol. 2014, 12, 429–439. [Google Scholar] [PubMed]
- Shaw, A.T.; Kim, D.W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, R.; Vansteenkiste, J.; Sharma, S.; de Pas, T.M.; et al. Ceritinib in ALK-rearranged non-small cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Tan, D.S.; de Pas, T.M.; Solomon, B.J.; Ahmad, A.; Lazzari, C.; de Marinis, F.; Spitaleri, G.; Schultz, K.; et al. Progression-free and overall survival in ALK-positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin. Cancer Res. 2015, 21, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Kiura, K.; Nishio, M.; Nakagawa, K.; Maemondo, M.; Inoue, A.; Hida, T.; Yamamoto, N.; Yoshioka, H.; Harada, M.; et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): A single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013, 14, 590–598. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiura, K.; Nishio, M.; Seto, T.; Maemondo, M.; Inoue, A.; Hida, T.; Yamamoto, N.; Yoshioka, H.; Harada, M.; et al. A phase I/II study with a highly selective ALK inhibitor CH5424802 in ALK-positive non-small cell lung cancer patients: Updated safety and efficacy results from AF-001JP. J. Clin. Oncol. 2013, 31. Abstract No. 8033. [Google Scholar]
- Gainor, J.F.; Sherman, C.A.; Willoughby, K.; Logan, J.; Kennedy, E.; Brastianos, P.K.; Chi, A.S.; Shaw, A.T. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J. Thorac. Oncol. 2015, 10, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Acquaviva, J.; Friedland, J.C.; Smith, D.L.; Sequeira, M.; Zhang, C.; Jiang, Q.; Xue, L.; Lovly, C.M.; Jimenez, J.P.; et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013, 3, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Le, A.T.; Theodoro, M.F.; Skokan, M.C.; Aisner, D.L.; Berge, E.M.; Terracciano, L.M.; Cappuzzo, F.; Incarbone, M.; Roncalli, M.; et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin. Cancer Res. 2012, 18, 4570–4579. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, R.; Solomon, B.J.; Saglia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Reddy, M.M.; Hasina, R.; Gangadhar, T.; Salgia, R. The role of the c-Met pathway in lung cancer and the potential for targeted therapy. Ther. Adv. Med. Oncol. 2011, 3, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Drilon, A.; Yu, H.; Rekhtman, N.; Ginsberg, M.S.; Borsu, L.; Schultz, N.; Berger, M.F.; Rudin, C.M.; Ladanyi, M. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015, 5, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Pan, Y.; Li, Y.; Ye, T.; Li, C.; Luo, X.; Wang, L.; Li, H.; Zhang, Y.; et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 4352–4359. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Wang, L.; Hasanovic, A.; Suehara, Y.; Lipson, D.; Stephens, P.; Ross, J.; Miller, V.; Ginsberg, M.; Zakowski, M.F.; et al. Response to cabozantinib in RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013, 3, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Mazieres, J.; Riely, G.J.; Rudin, C.M.; Barlesi, F.; Quoix, E.A.; Souquet, P.J.; Socinski, M.A.; Switzky, J.; Ma, B.; et al. Interim results of phase 2 study BRF113928 in BRAF V600E mutation-positive non-small cell lung cancer (NSCLC) patients. J. Clin. Oncol. 2013, 31. Abstract No. 8009. [Google Scholar]
- Forbes, S.; Clements, J.; Dawson, E.; Bamford, S.; Webb, T.; Dogan, A.; Flanagan, A.; Teague, J.; Wooster, R.; Futreal, P.A.; et al. Cosmic 2005. Br. J. Cancer 2006, 94, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Varella-Garcia, M.; Tang, X.; Xavier, A.C.; Ozburn, N.C.; Liu, D.D.; Bekele, B.N.; Herbst, R.S. Wistuba II: KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin. Cancer Res. 2007, 13, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Janne, P.A.; Shaw, A.T.; Pereira, J.R.; Jeannin, G.; Vansteenkiste, J.; Barrios, C.; Franke, F.A.; Grinsted, L.; Zazulina, V.; Smith, P.; et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small cell lung cancer: A randomised, multicentre, placebo controlled, phase 2 study. Lancet Oncol. 2013, 14, 38–47. [Google Scholar] [CrossRef]
- Langer, C.J.; Novello, S.; Park, K.; Krzakowski, M.; Karp, D.D.; Mok, T.; Benner, R.J.; Scranton, J.R.; Olszanski, A.J.; Jassem, J.; et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin vs. paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2014, 32, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.D.; Paz-Ares, L.G.; Novello, S.; Haluska, P.; Garland, L.; Cardenal, F.; Blakely, L.J.; Eisenberg, P.D.; Langer, C.J.; Blumenschein, G., Jr.; et al. Retraction: Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 4179. [Google Scholar]
- Kim, H.R.; Kim, D.J.; Kang, D.R.; Lee, J.G.; Lim, S.M.; Lee, C.Y.; Rha, S.Y.; Bae, M.K.; Lee, Y.J.; Kim, S.H.; et al. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J. Clin. Oncol. 2012, 31, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Shen, R.; Ferry, D.; Soria, J.; Mathewson, A.; Kilgour, E.; Landers, D.; Frewer, P.; Brooks, N.; Andre, F.; et al. A phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers: Preliminary antitumor activity and pharmacodynamics data. J. Clin. Oncol. 2014, 32. Abstract No. 8035. [Google Scholar]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bondarenko, I.; Luft, A.; Serwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Neal, J.; Lu, H.; Cuillerot, J.M.; et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012, 30, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef] [PubMed]
- Soo, A.R.; Brahmer, J.R.; Cho, B.C.; Sundar, R. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015, 7, 85–96. [Google Scholar]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab vs. docetaxel in advanced squamous-cell non-small-cell lung cancer. N.Engl.J.Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Horn, L.; Borghaei, H.; Spigel, D.R.; Steins, M.; Ready, N.; Chow, L.Q.M.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) vs. docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2015, 33. Abstract No. LBA 109. [Google Scholar]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.T.; Park, C.Y.; Ailles, L.E.; Weissman, I.L. The cancer stem cell hypothesis: A work in progress. Lab. Invest. 2006, 86, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.; Kotasek, D.; Millward, M.; Markman, B.; Hidalgo, M.; Jameson, M.; Harris, D.; Stagg, R.; Dupont, J.; Hughes, B. A phase Ib study of demcizumab plus pemetrexed and carboplatin in patients with 1st line non-squamous non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2014, 32. Abstract No. 2544. [Google Scholar]
- Taguchi, F.; Solomon, B.; Gregorc, V.; Roder, H.; Gray, R.; Kasahara, K.; Nishio, M.; Brahmer, J.; Spreafico, A.; Ludovini, V.; et al. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: A multicohort cross-institutional study. J. Natl. Cancer Inst. 2007, 99, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, V.; Novello, S.; Lazzari, C.; Barni, S.; Aieta, M.; Mencoboni, M.; Grossi, F.; de Pas, T.; De Marinis, F.; Bearz, A.; et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): A biomarker-stratified, randomised phase 3 trial. Lancet Oncol. 2014, 15, 713–721. [Google Scholar] [CrossRef]
- Lord, R.V.; Brabender, J.; Gandara, D.; Alberola, V.; Camps, C.; Domine, M.; Cardenal, F.; Sanchez, J.M.; Gumerlock, P.H.; Taron, M.; et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin. Cancer Res. 2002, 8, 2286–2289. [Google Scholar] [PubMed]
- Rosell, R.; Danenberg, K.D.; Alberola, V.; Bepler, G.; Sanchez, J.J.; Camps, C.; Provencio, M.; Isla, D.; Taron, M.; Diz, P.; et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin. Cancer Res. 2004, 10, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Isaac, S.; Souquet, P.J.; Bejui-Thivolet, F.; Pacheco, Y.; Peloux, N.; Frankfurter, A.; Luduena, R.; Perol, M. Expression of class III beta tubulin in non-small cell lung cancer is correlated with resistance to taxane chemotherapy. Bull Cancer 2005, 92, 10025–10030. [Google Scholar]
- Simon, G.; Sharma, A.; Li, X.; Hazelton, T.; Walsh, F.; Williams, C.; Chiappori, A.; Haura, E.; Tanvetyanon, T.; Antona, S.; et al. Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2007, 25, 12741–12746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, X.; Zhang, L.; Sun, S.; Huang, J.; Lin, Y. A prospective study of biomarker-guided chemotherapy in patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 2014, 74, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.H.; Socinski, M.A.; Edelman, M.J.; Belani, C.P.; Gonin, R.; Catalano, R.B.; Marinucci, D.M.; Comis, R.L.; Obasaju, C.K.; Chen, R.; et al. A retrospective analysis of outcomes by age in a three-arm phase III trial of gemcitabine in combination with carboplatin or paclitaxel vs. paclitaxel plus carboplatin for advanced non-small cell lung cancer. Crit. Rev. Oncol. Hematol. 2011, 78, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Zalcman, G.; Oster, J.P.; Weestel, V.; Pichon, E.; Lavolé, A.; Dauba, J.; Debieuvre, D.; Souquet, P.J.; Bigay-Game, L.; et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011, 378, 1079–1088. [Google Scholar] [CrossRef]
- The Elderly Lung Cancer Vinorelbine Italian Study Group. Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 1999, 91, 66–72. [Google Scholar] [CrossRef]
- Gridelli, C.; Cigolari, S.; Gallo, C.; Manzione, L.; Ianniello, G.P.; Frontini, L.; Ferrau, F.; Robbiati, S.F.; Adamo, V.; Gasparini, G.; et al. Activity and toxicity of gemcitabine and gemcitabine + vinorelbine in advanced non-small-cell lung cancer elderly patients: Phase II data from the Multicenter Italian Lung Cancer in the Elderly Study (MILES) randomised trial. Lung Cancer 2001, 31, 277–284. [Google Scholar] [CrossRef]
- Fidias, P.; Supko, J.G.; Martins, R.; Boral, A.; Carey, R.; Grossbard, M.; Shapiro, G.; Ostler, P.; Lucca, J.; Johnston, B.E.; et al. A phase II study of weekly paclitaxel in in elderly patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 3942–3949. [Google Scholar] [PubMed]
- Kudoh, S.; Takeda, K.; Nakagawa, K.; Takada, M.; Katakami, N.; Matsui, K.; Shinkai, T.; Sawa, T.; Goto, I.; Semba, H.; et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: Results of the West Japan Thoracic Oncology Group trial (WJTOG 9904). J. Clin. Oncol. 2006, 24, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Uruga, H.; Kishi, K.; Fujii, T.; Beika, Y.; Enomoto, T.; Takaya, H.; Miyamoto, A.; Morokawa, N.; Kurosaki, A.; Yoshimura, K. Efficacy of gefitinib for elderly patients with advanced non-small lung cancer harboring epidermal growth factor receptor gene mutations: A retrospective analysis. Intern. Med. 2010, 49, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Asami, K.; Koizumi, T.; Hirai, K.; Ameshima, S.; Tsukadaira, A.; Morozumi, N.; Morikawa, A.; Atagi, S.; Kawahara, M. Gefitinib as first line treatment in elderly epidermal growth factor receptor-mutated patients with advanced lung adenocarcinoma: Results of a Nagano Lung Cancer Research Group study. Clin. Lung Cancer 2011, 12, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Minegishi, Y.; Inoue, A.; Kobayashi, K.; Harada, M.; Okinaga, S.; Morikawa, N.; Oizumi, S.; Tanaka, T.; Isobe, H.; et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J. Thorac. Oncol. 2012, 7, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Muramatsu, H.; Sone, K.; Aoki, S.; Akiko, H.; Kagawa, Y.; Sato, H.; Kunieda, T. Apidermal growth factor receptor-tyrosine kinase inhibitors for non-small cell lung cancer patients aged 80 years or older: A retrospective analysis. Mol. Clin. Oncol. 2015, 3, 403–407. [Google Scholar] [PubMed]
- Yoshida, T.; Yamada, K.; Azuma, K.; Kawahara, A.; Abe, H.; Hattori, S.; Yamashita, F.; Zaiken, Y.; Kage, M.; Hoshino, T. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: A retrospective analysis. Med. Oncol. 2013, 30, 349. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Kobayashi, K.; Usui, K.; Maemondo, M.; Okinaga, S.; Mikami, I.; Ando, M.; Yamazaki, K.; Saijo, Y.; Gemma, A.; et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J. Clin. Oncol. 2009, 27, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Khan, I.; Upadhyay, S.; Lewanski, C.; Falk, S.; Skailes, G.; Marshall, E.; Woll, P.J.; Hatton, M.; Lal, R.; et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): A double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2012, 13, 1161–1170. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boolell, V.; Alamgeer, M.; Watkins, D.N.; Ganju, V. The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers 2015, 7, 1815-1846. https://doi.org/10.3390/cancers7030864
Boolell V, Alamgeer M, Watkins DN, Ganju V. The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers. 2015; 7(3):1815-1846. https://doi.org/10.3390/cancers7030864
Chicago/Turabian StyleBoolell, Vishal, Muhammad Alamgeer, David N. Watkins, and Vinod Ganju. 2015. "The Evolution of Therapies in Non-Small Cell Lung Cancer" Cancers 7, no. 3: 1815-1846. https://doi.org/10.3390/cancers7030864
APA StyleBoolell, V., Alamgeer, M., Watkins, D. N., & Ganju, V. (2015). The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers, 7(3), 1815-1846. https://doi.org/10.3390/cancers7030864





