Predictive Risk of Radiation Induced Cerebral Necrosis in Pediatric Brain Cancer Patients after VMAT Versus Proton Therapy
Abstract
:1. Introduction
2. Methods and Materials
| Patient Index | Age at Treatment | Sex | Assigned Diagnosis * | PTV Volume (cm3) |
|---|---|---|---|---|
| 1 | 2 | F | Glioma | 89.1 |
| 2 | 4 | M | Glioma | 86.31 |
| 3 | 6 | F | Glioma | 38.34 |
| 4 | 8 | F | Glioma | 180.2 |
| 5 | 10 | F | Glioma | 139.87 |
| 6 | 4 | M | Glioma | 71.4 |
| 7 | 6 | M | Glioma | 76.74 |
| 8 | 8 | M | Glioma | 215.69 |
| 9 | 10 | M | Ependymoma | 64.38 |
| 10 | 12 | F | Ependymoma | 97.41 |
| 11 | 13 | F | Ependymoma | 105.75 |
| 12 | 16 | F | Ependymoma | 72.57 |
| 13 | 16 | F | Ependymoma | 95.49 |
3. Results and Discussion
3.1. Planning Comparison
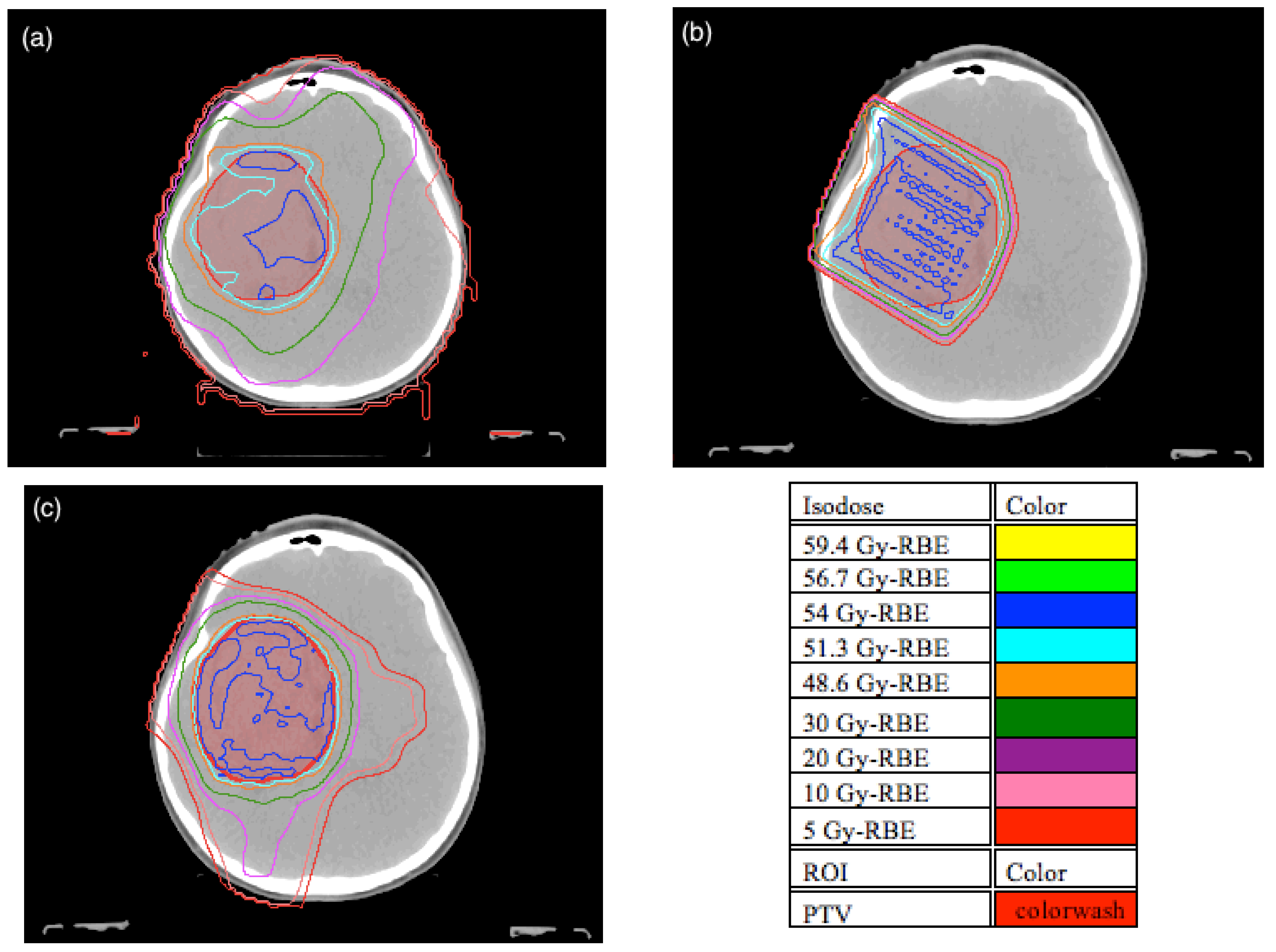
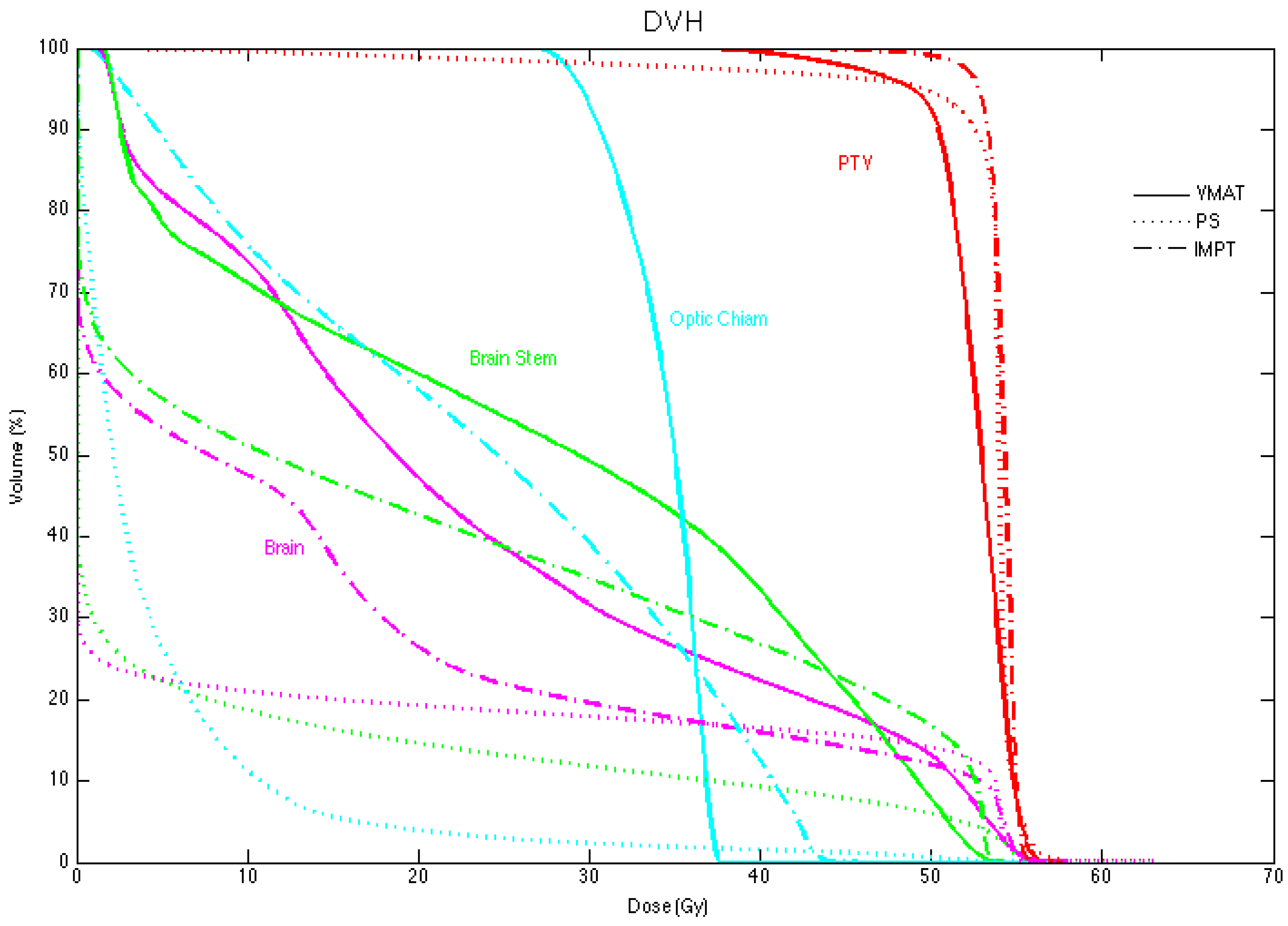
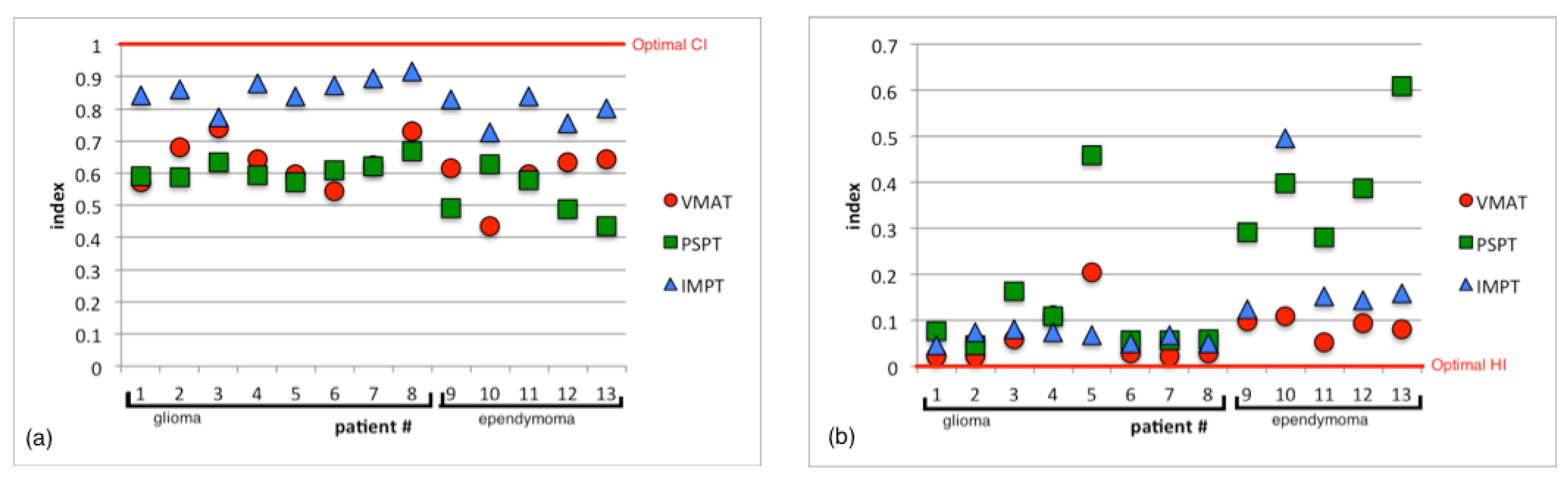
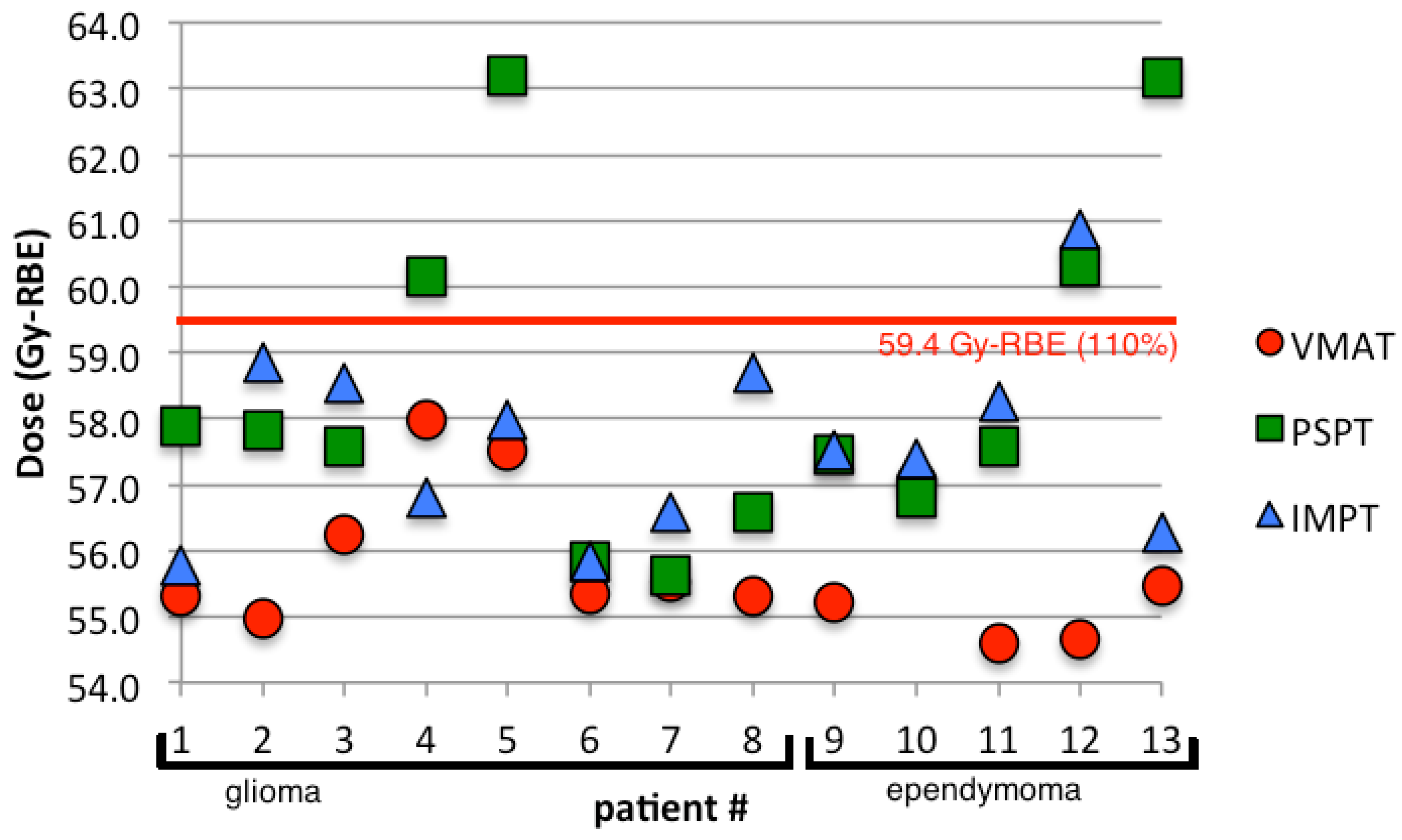
3.2. Normal Tissue Complication Probability

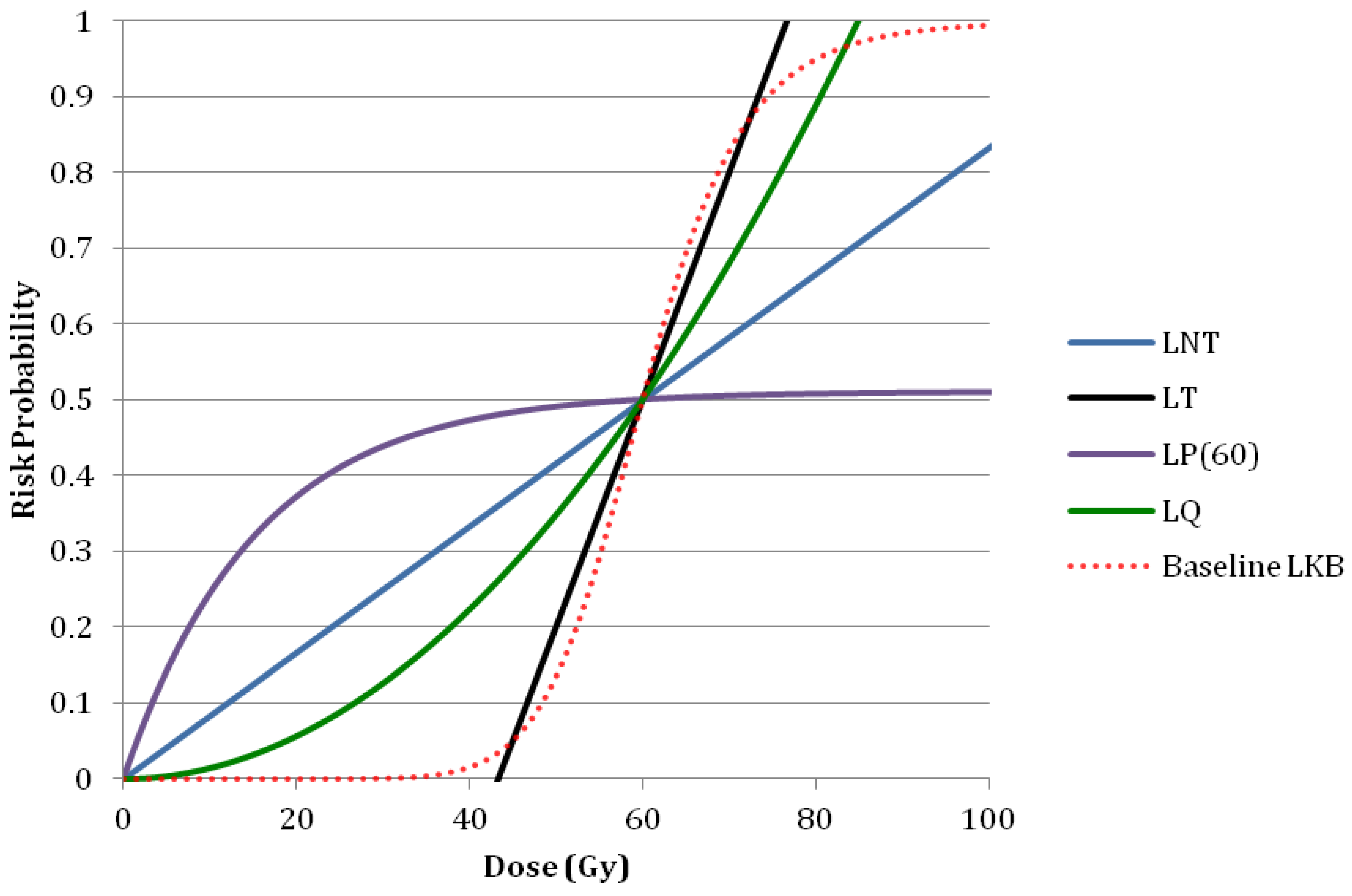
3.3. Sensitivity Analysis
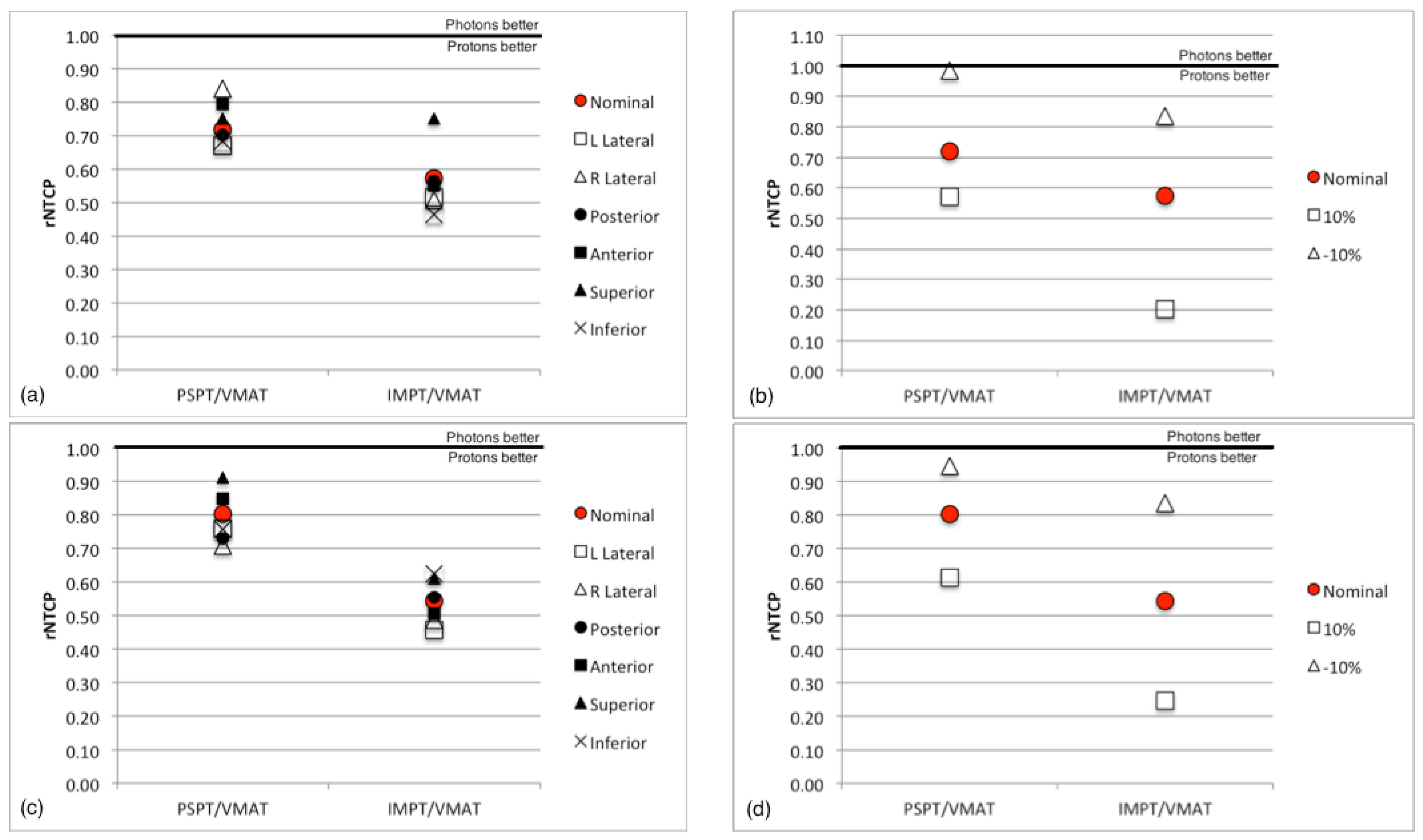
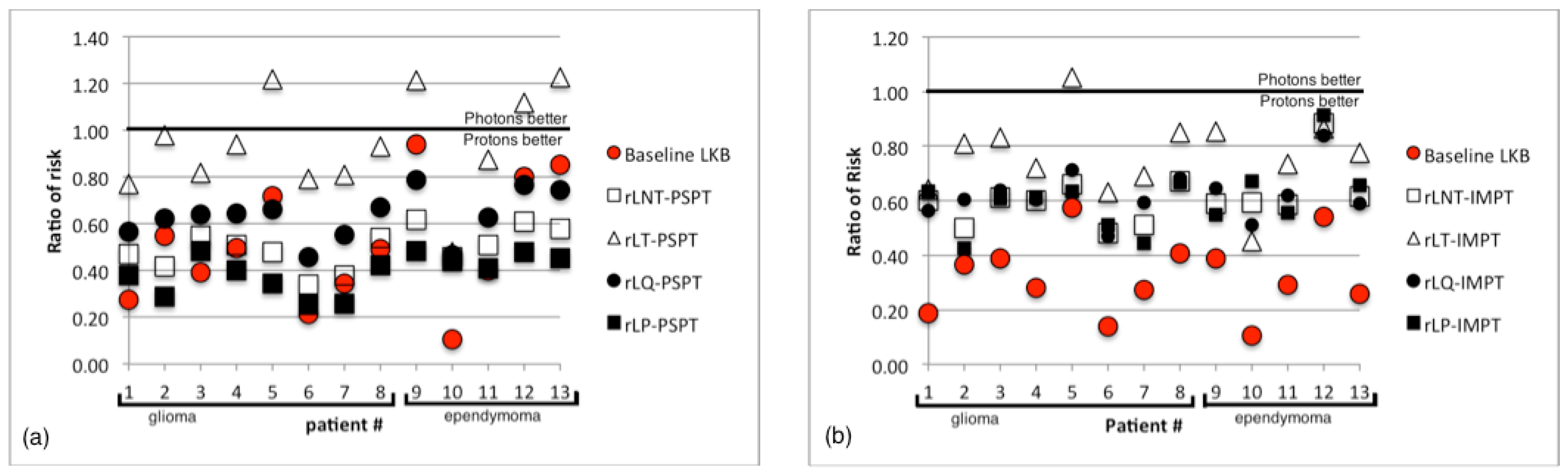
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro. Oncol. 2012, 14, v1–v49. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.; Smith, M.; Gurney, J.; Linet, M.; Tamra, T.; Young, J.; Bunin, G.E. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; National Cancer Institute: Bethesda, MD, USA, 1999; pp. 35–49. [Google Scholar]
- Pizzo, P.A.; Poplack, D.G. Principles and Practice of Pediatric Oncology, 3rd ed.; Lippincott-Raven: Philadelphia, PA, USA, 1997; p. 1522. [Google Scholar]
- Halperin, E.C.; Constine, L.S.; Tarbell, N.J. Pediatric Radiation Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- De Laney, T.F.; Kooy, H.M. Proton and Charged Particle Radiotherapy; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; p. 274. [Google Scholar]
- Haas-Kogan, D.A.; Barani, I.J.; Hayden, M.G.; Edwards, M.S.B.; Fisher, P.G. Pediatric central nervous system tumors. In Textbook of Radiation Oncology, 3rd ed.; Phillips, T.L., Hoppe, R., Roach, M., Eds.; Elsevier Publishers: Philadelphia, PA, USA, 2010; pp. 1111–1129. [Google Scholar]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neurooncology 2013, 15, ii1–ii56. [Google Scholar]
- Fink, J.; Born, D.; Chamberlain, M.C. Radiation necrosis: Relevance with respect to treatment of primary and secondary brain tumors. Curr. Neurol. Neurosci. Rep. 2012, 12, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.T.; Ahluwalia, M.S.; Barnett, G.H.; Stevens, G.H.; Murphy, E.S.; Stockham, A.L.; Shiue, K.; Suh, J.H. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ruben, J.D.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.A.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Yu, M.; Jang, H.-S.; Kim, Y.-S.; Choi, B.-O.; Kang, Y.-N.; Lee, Y.-S.; Kim, D.-C.; Hong, Y.-K.; Jeun, S.-S.; et al. Radiation-induced brain injury: Retrospective analysis of twelve pathologically proven cases. Radiat. Oncol. J. 2011, 29, 147–155. [Google Scholar]
- Lawrence, Y.R.; Li, X.A.; el Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation dose-volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Foo, W.; Chappell, R.; Fowler, J.F.; Sze, W.M.; Poon, Y.F.; Law, S.C.; Ng, S.H.; O, S.K.; Tung, S.Y.; et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 35–42. [Google Scholar]
- Murphy, E.S.; Merchant, T.E.; Wu, S.; Xiong, X.; Lukose, R.; Wright, K.D.; Qaddoumi, I.; Armstrong, G.T.; Broniscer, A.; Gajjar, A. Necrosis after craniospinal irradiation: Results from a prospective series of children with central nervous system embryonal tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e655–e660. [Google Scholar] [CrossRef] [PubMed]
- Boehling, N.S.; Grosshans, D.R.; Bluett, J.B.; Palmer, M.T.; Song, X.; Amos, R.A.; Sahoo, N.; Meyer, J.J.; Mahajan, A.; Woo, S.Y. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Howell, R.; Giebeler, A.; Koontz-Raisig, W.; Mahajan, A.; Etzel, C.; D’Amelio, A.; Homann, K.; Newhauser, W. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat. Oncol. 2012, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Toward robust proton therapy planning and delivery. Transl. Cancer Res. 2012, 1, 217–226. [Google Scholar]
- International Comission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Proton-Beam Therapy ICRU Report 78; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Merchant, T.E. Clinical controversies: Proton therapy for pediatric tumors. Semin. Radiat. Oncol. 2013, 23, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Feuvret, L.; Noel, G.; Mazeron, J.J.; Bey, P. Conformity index: A review. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Park, S.Y.; Shin, D.; Lee, S.B.; Pyo, H.R.; Kim, D.Y.; Cho, K.H. A new homogeneity index based on statistical analysis of the dose-volume histogram. J. Appl. Clin. Med. Phys. 2007, 8, 9–17. [Google Scholar] [PubMed]
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 123–135. [Google Scholar]
- Merchant, T.E.; Hua, C.-H.; Shukla, H.; Ying, X.; Nill, S.; Oelfke, U. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr. Blood Cancer 2008, 51, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.G.; Lomax, A.J.; Miltchev, V.; Davis, J.B. A comparison of dose distributions of proton and photon beams in stereotactic conformal radiotherapy of brain lesions. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Bolsi, A.; Fogliata, A.; Cozzi, L. Radiotherapy of small intracranial tumours with different advanced techniques using photon and proton beams: A treatment planning study. Radiother. Oncol. 2003, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.R.; Adams, J.; Krejcarek, S.J.; Tarbell, N.J.; Yock, T.I. A Dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.G.; Norton, I.A.; Lomax, A.J.; Davis, J.B. Dose conformation of intensity-modulated stereotactic photon beams, proton beams, and intensity-modulated proton beams for intracranial lesions. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Urie, M.; Goitein, M.; Wagner, M. Compensating for heterogeneities in proton radiation therapy. Phys. Med. Biol. 1984, 29, 553. [Google Scholar] [CrossRef] [PubMed]
- Fuss, M.; Hug, E.B.; Schaefer, R.A.; Nevinny-Stickel, M.; Miller, D.W.; Slater, J.M.; Slater, J.D. Proton radiation therapy (PRT) for pediatric optic pathway gliomas: Comparison with 3d planned conventional photons and a standard photon technique. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Hug, E.B.; Schaefer, R.A.; Miller, D.W.; Slater, J.M.; Slater, J.D. Conformal proton radiation therapy of the posterior fossa: A study comparing protons with three-dimensional planned photons in limiting dose to auditory structures. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- St Clair, W.H.; Adams, J.A.; Bues, M.; Fullerton, B.C.; La Shell, S.; Kooy, H.M.; Loeffler, J.S.; Tarbell, N.J. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Pedroni, E.; Lomax, A. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys. Med. Biol. 1996, 41, 111. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, B.; Pedroni, E. The precision of proton range calculations in proton radiotherapy treatment planning: Experimental verification of the relation between CT-HU and proton stopping power. Phys. Med. Biol. 1998, 43, 1579. [Google Scholar] [CrossRef] [PubMed]
- Moyers, M.F.; Miller, D.W.; Bush, D.A.; Slater, J.D. Methodologies and tools for proton beam design for lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freund, D.; Zhang, R.; Sanders, M.; Newhauser, W. Predictive Risk of Radiation Induced Cerebral Necrosis in Pediatric Brain Cancer Patients after VMAT Versus Proton Therapy. Cancers 2015, 7, 617-630. https://doi.org/10.3390/cancers7020617
Freund D, Zhang R, Sanders M, Newhauser W. Predictive Risk of Radiation Induced Cerebral Necrosis in Pediatric Brain Cancer Patients after VMAT Versus Proton Therapy. Cancers. 2015; 7(2):617-630. https://doi.org/10.3390/cancers7020617
Chicago/Turabian StyleFreund, Derek, Rui Zhang, Mary Sanders, and Wayne Newhauser. 2015. "Predictive Risk of Radiation Induced Cerebral Necrosis in Pediatric Brain Cancer Patients after VMAT Versus Proton Therapy" Cancers 7, no. 2: 617-630. https://doi.org/10.3390/cancers7020617
APA StyleFreund, D., Zhang, R., Sanders, M., & Newhauser, W. (2015). Predictive Risk of Radiation Induced Cerebral Necrosis in Pediatric Brain Cancer Patients after VMAT Versus Proton Therapy. Cancers, 7(2), 617-630. https://doi.org/10.3390/cancers7020617





