Exploring the Role of ADCs in Brain Metastases and Primary Brain Tumors: Insight and Future Directions
Simple Summary
Abstract
1. Introduction
2. Primary Brain Tumors
| Reference | Type of Trial | Population | Intervention | Target | Endpoints |
|---|---|---|---|---|---|
| [35] | Phase III | rGBM (first recurrence) | CB vs. gliadel wafers | IL-13 | No survival difference |
| [36] | Phase IIb | rGBM | MDNA55 (Bizaxofusp) | IL-4 | mOS: 11.64 months [80% one-sided CI 8.62, 15.02] |
3. Brain Metastases
3.1. Non-Small Cell Lung Cancer
3.1.1. Trastuzumab Deruxtecan
3.1.2. Datopotamab Deruxtecan
3.1.3. Patritumab Deruxtecan
3.1.4. Telisotuzumab–Vedotin
3.2. Breast Cancer
3.2.1. Ado-Trastuzumab Emtansine
3.2.2. Trastuzumab Deruxtecan
3.2.3. Sacituzumab Govitecan
4. Combination with Radiation Therapy
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Clinical Trial | Response Endpoint | ADC | Target | Linker Type | Payload Action | Population | CNS Metastasis Eligibility | Trial Type | Status |
|---|---|---|---|---|---|---|---|---|---|
| HER3-DXd in Breast Cancer and NSCLC Brain Metastases and Solid Tumor Leptomeningeal Disease (TUXEDO-3) (NCT05865990) | iORR as defined by RANO -BM criteria | Patritumab deruxtecan (HER3-DXd) | HER3 | Cleavable | Topoisomerase I inhibitor (Deruxtecan) | Patients with previously treated a/m NSCLC with newly diagnosed or progressive BM | Patients are eligible if steroid use is <8 mg of Dexamethasona/day | Phase II | Active, not recruiting |
| Datopotamab Deruxtecan (Dato-DXd) for Non-Small Cell Lung Cancer (NSCLC) patients with Active Brain Metastases (TUXEDO-5) (NCT06676917) | iORR as defined by RANO-BM criteria | Datopotamab Deruxtecan (Dato-DXd) | TROP2 | Cleavable | Topoisomerase I inhibitor (Deruxtecan) | Patients with previously treated a/m non squamous NSCLC with active BM | Patients with BM and leptomeningeal disease are allowed if there is no indication for immediate local therapy | Phase II | Recruitment not yet open; the estimated study start date is April 2025 |
| Sacituzumab Tirumotecan (MK-2870) Versus Chemotherapy in Previously Treated a/m Nonsquamous NSCLC with EGFR Mutations or Other Genomic Alterations (NCT06074588) | PFS as defined by RECIST v 1.1 and OS | Sacituzumab tirumotecan (MK-2870) | TROP2 | Cleavable | Topoisomerase I Inhibitor (Tirumotecan) | Patients with a/m non-squamous NSCLC with treatment-refractory progressive disease | Previously treated BM included; active BM and leptomeningeal disease excluded | Phase III | Recruiting |
| Phase III, Open-label, First-line Study of Dato-DXd in Combination with Durvalumab and Carboplatin for Advanced NSCLC Without Actionable Genomic Alterations (AVANZAR) (NCT05687266) | PFS as defined by RECIST v1.1 and OS | Datopotamab Deruxtecan (Dato-DXd) | TROP2 | Clevable | Topoisomerase I inhibitor (Deruxtecan) | Patients with a/m NSCLC without actionable genomic alterations with approved and a viable target therapy | Active BM and leptomeningeal disease excluded | Phase III | Active, not recruiting |
| Study of Pembrolizumab (MK-3475) Monotherapy Versus Sacituzumab Govitecan in Combination with Pembrolizumab for Participants With Metastatic Non-small Cell Lung Cancer (NSCLC) With Programmed Cell Death Ligand 1 (PD-L1) Tumor Proportion Score (TPS) ≥ 50% (MK-3475-D46) (NCT05609968) | PFS as defined by RECIST v1.1 and OS | Sacituzumab govitecan (SG) | TROP2 | Cleavable | Topoisomerase I Inhibitor (govitecan) | Patients with a/m NSCLC without an indication of EGFR-ALK-1, or ROS-1-targeted therapies with PD-L1 expression > 50% | Active BM and leptomeningeal disease excluded | Phase III | Recruiting |
| Study of Dato-DXd Plus Pembrolizumab vs. Pembrolizumab Alone in the First-line Treatment of Subjects with Advanced or Metastatic NSCLC Without Actionable Genomic Alterations (TROPION-Lung08) (NCT05215340) | PFS as defined by RECIST v1.1 and OS | Datopotamab Deruxtecan (Dato-DXd) | TROP2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | Patients with a/m non-squamous NSCLC without actionable genomic alterations (mKRAS eligible) with PD-L1 expression > 50% | Active and untreated BM excluded; leptomeningeal disease excluded | Phase III | Recruiting |
| A Study of YL202 in Selected Patients with Advanced Solid Tumors (NCT06107686) | ORR as defined by RECIST v1.1 | YL202 | HER3 | Cleavable | Topoisomerase I Inhibitor (YL0010014) | Patients with a/m NSCLC | Patients with BM excluded | Phase II | Recruiting |
| A Study of Disitamab Vedotin in Previously Treated Solid Tumors That Express HER2 (NCT06003231) | ORR as defined by RECIST v1.1 | Disitamab Vedotin | HER2 | Cleavable | Auristatin microtubule inhibitor (monomethyl auristatin E) | Patients with a/m solid tumors, including NSCLC, with HER2 overexpression | Active and untreated BM and leptomeningeal disease excluded | Phase II | Recruiting |
| A Phase 2 Study of BA3011 Alone and in Combination with PD-1 Inhibitor in Adult Patients with Metastatic Non-small Cell Lung Cancer (NSCLC) Who Had Prior Disease Progression on a PD-1/L-1 Inhibitor, EGFR, or ALK Inhibitor. (NCT04681131) | ORR as defined by RECIST v1.1 | CAB-AXL-ADC | AXL | Cleavable | Auristatin microtubule inhibitor (monomethyl auristatin E) | Patients with a/m NSCLC | Patients with active BM excluded | Phase II | Active, non recruiting |
| Datopotamab Deruxtecan (Dato-DXd, DS-1062a) in Advanced and/or Unresectable Non-Small Cell Lung Cancer (ICARUS-LUNG01) (NCT04940325) | ORR as defined by RECIST v1.1 | Datopotomab Deruxtecan (Dato-DXd) | TROP2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | Patients with a/m NSCLC | BM eligible only if treated and clinically stable without medical treatment | Phase II | Active, non recruiting |
| CAB-ROR2-ADC Safety and Efficacy Study in Patients with TNBC or Head & Neck Cancer (Ph1) and NSCLC or Melanoma (Ph2) (NCT03504488) | ORR | Ozuriftamab vedotin | ROR2 | Cleavable | Auristatin microtubule inhibitor (monomethyl auristatin E) | Patients with a/m solid tumors, including NSCLC, that is refractory or not amenable to the standard-of-care therapy | Uncontrolled BM excluded | Phase I/II | Active, non recruiting |
| A Clinical Trial of TQB2102 for Injection in Non-small Cell Lung Cancer with HER2 Gene Abnormality (NCT06496490) | ORR | TQB2102 | HER2 | Cleavable | Topoisomerase I Inhibitor (topotecan) | Patients with a/m NSCLC refractory to standard-of-care therapy | Stable BM included Uncontrolled BM or Leptomeningeal disease excluded | Phase II | Recruiting |
| Study to Investigate Luveltamab Tazevibulin in Adults with Advanced or Metastatic Non-small Cell Lung Cancer (NCT06555263) | ORR as defined by RECIST v1.1 | Luveltamab Tazevibulin | FOLR1 | Cleavable | Auristatin microtubule inhibitor (hemiasterina) | Patients who are a/m NSCLC-positive per FOLR1 expression per central testing | Untreated BM are excluded | Phase II | Recruiting |
| A First-in-human Study Using BDC-1001 as a Single Agent and in Combination with Nivolumab in Advanced HER2-Expressing Solid Tumors (NCT04278144) | ORR | BDC-1001 | HER2 | Non cleavable | ISAC (Trastuzumab conjugated with TLR7/8 agonist) | Patients with an a/m solid tumor with documented HER2-protein expression or gene amplification for which approved therapies have been exhausted or not clinically indicated | Untreated BM or active BM are excluded | Phase I/II | Active, non recruiting |
| Phase I–II, FIH, TROP2 ADC, Advanced Unresectable/Metastatic Solid Tumors, Refractory to Standard Therapies (A264) (NCT04152499) | ORR (phase II) | SKB264 | TROP2 | Cleavable | Microtubule inhibitor (Belotecan-based payload) | Patients with a/m solid tumors, including NSCLC, that is refractory or not amenable to the standard-of care-therapy | Symptomatic BM or any radiation or surgery for BMs within 1 month of first infusion of study drug are the exclusion criteria (Phase I). Patients with active BM or leptomeningeal disease are excluded (Phase II) | Phase I/II | Recruiting |
| PRO1184 for Advanced Solid Tumors (PRO1184-001) (NCT05579366) | ORR as defined by Recist v1.1 (phase II) | PRO1184 | FOLR1 | Cleavable | Topoisomerase I Inhibitor (exatecan) | Patients with a/m solid tumors, including NSCLC, that is refractory or not amenable to the standard-of-care therapy | Active BMs are excluded | Phase I/II | Recruiting |
| Study of DF1001 in Patients with Advanced Solid Tumors (NCT04143711) | ORR as defined by RECIST v1.1 | DF1001 | HER2 | Cleavable | Induce cell death by targeting NK cells and T-cell activation signals | A/m NSCLC patients with HER2 overexpression/amplification/mutation who progressed after a platinum-based CT and have received and progressed on or after anti PD-(L)1 therapy. Those for whom a/m NSCLC progressed during or after platinum doublet-based chemotherapy (expansion NSCLC cohort) | Active BMs or a history of BM are the exclusion criteria | Phase I/II | Recruiting |
| Study of REGN5093-M114 (METxMET Antibody–Drug Conjugate) in Adult Patients with Mesenchymal–Epithelial Transition Factor (MET) Overexpressing Advanced Cancer (NCT04982224) | ORR (Phase II) | REGN5093-M114 | METxMET | Cleavable | Auristatin microtubule inhibitor (M24) | Patients with a/m NSCLC with MET Overexpression | Untreated/active BM and leptomeningeal disease excluded | Phase I/II | Recruiting |
| Clinical Study of Antibody–Drug Conjugate MYTX-011 in Subjects with Non-Small Cell Lung Cancer (NCT05652868) | ORR, OS and PFS are secondary endpoints | MYTX-011 | MET | Clevable | Auristatin microtubule inhibitor (Monomethyl auristatin E) | Patients with a/m NSCLC with MET alterations that is refractory to the standard-of-care therapy | Untreated/active BM and leptomeningeal disease excluded | Phase I | Recruiting |
| An Efficacy and Safety Study of Cofetuzumab Pelidotin in Participants with PTK7-Expressing, Recurrent Non-Small Cell Lung Cancer (NCT04189614) | OS, PFS and DOR are secondary endpoints | Cofetuzumab pelidotin | PTK7 | Cleavable | Auristatin microtubule inhibitor (Auristatin-0101) | Patients with recurrent and treatment-refractory NSCLC with PTK7 expression | Asymptomatic and treated BM included, active BM excluded | Phase I | Active, non recruiting |
| A Study to Evaluate TROP2 ADC LCB84 Single Agent and in Combination with an Anti-PD-1 Ab in Advanced Solid Tumors (NCT05941507) | PFS, ORR, DOR, TTP as defined by RECIST v1.1, iRECIST, and RANO-BM OS | LCB84 | TROP2 | Cleavable | Microtubule inhibitor (Monomethyl auristatin E) | Patients with advanced solid tumors, including NSCLC that is refractory or not amenable to the standard-of-care therapy | Stable and treated BM included | Phase I/II | Recruiting |
| DS8201a and Pembrolizumab in Participants with Locally Advanced/Metastatic Breast or Non-Small Cell Lung Cancer (NCT04042701) | ORR | Trastuzumab deruxtecan | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | Patients with a/m breast cancer or NSCLC with HER2 overexpression or HER2-mutant disease | Active BM excluded | Phase I | Recruiting |
| Phase Ib Study of the Safety of T-DXd and Immunotherapy Agents with and Without Chemotherapy in Advanced or Metastatic HER2-positive, non-squamous NSCLC (DL03) (NCT04686305) | PFS, ORR, DOR, as defined by RECIST v1.1, OS | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | Patients with a/m non-squamous NSCLC that are refractory to systemic therapy or treatment-naive with HER2 overexpression | Untreated and symptomatic BM excluded | Phase I | Recruiting |
| A Study of LY4052031 in Participants with Advanced or Metastatic Urothelial Cancer or Other Solid Tumors (NEXUS-01) (NCT06465069) | ORR defined by RECIST v1.1 | LY4052031 | NECTIN4 | Cleavable | Topoisomerase I Inhibitor (Camptothecin-98) | Patients with a/m solid tumors, including NSCLC that is refractory or not amenable to the standard-of-care therapy | Known or suspected uncontrolled BM excluded | Phase I | Recruiting |
| A Study of LY4170156 in Participants with Selected Advanced Solid Tumors (NCT06400472) | ORR defined by RECIST v1.1 | LY4170156 | FOLR1 | Cleavable | Topoisomerase I Inhibitor (Camptothecin-98) | Patients with advanced solid tumors, including NSCLC that is refractory or not amenable to the standard-of-care therapy | Active and untreated BM, and history of leptomeningeal disease excluded | Phase I | Recruiting |
| A Study of LY4101174 in Participants with Recurrent, Advanced or Metastatic Solid Tumors (NCT06238479) | ORR defined by RECIST v1.1 (primary endpoint) DOR, PFS, TTR, DCF as defined by RECIST v1.1 OS | LY4101174 | NECTIN4 | Cleavable | Topoisomerase I Inhibitor (Camptothecin-98) | Patients with a/m solid tumors, including NSCLC that is refractory or not amenable to the standard-of-care therapy | Known or suspected uncontrolled BM excluded | Phase I | Recruiting |
| Safety of GQ1001 in Adult Patients with HER2-Positive Advanced Solid Tumors (NCT04450732) | ORR, DCR, PFS and DoR as defined by RECIST v1.1 | GQ1001 | HER2 | Cleavable | Microtubule inhibitor (Maytansinoide DM1) | Patients with a/m HER2-expressing solid tumors, including NSCLC that is refractory or not amenable to the standard-of-care therapy | Treated and asymptomatic BM included untreated and symptomatic BM excluded | Phase I | Recruiting |
| Phase I–II, FIH, TROP2 ADC, Advanced Unresectable/Metastatic Solid Tumors, Refractory to Standard Therapies (A264) (NCT04152499) | ORR, DoR, PFS as defined by RECIST v1.1 OS | Sacituzumab tirumotecan | TROP2 | Cleavable | Topoisomerase I inhibitor (belotecan derivative) | Patients with a/m solid tumors, including metastatic NSCLC, that is refractory or not amenable to the standard of-care-therapy | Symptomatic and active BM, history of LMD, brainstem metastasis, and spinal cord metastasis excluded | Phase I/II | Recruiting |
| Phase 1 Study of CPO301 in Adult Patients with Advanced or Metastatic Solid Tumors (NCT05948865) | Efficacy assesment not specified | CPO301 | EGFR | Cleavable | Non-specified | Patients with a/m solid tumors, including metastatic NSCLC, that is refractory or not amenable to the standard-of-care therapy | Known, active, or uncontrolled BM or leptomeningeal disease excluded | Phase I | Recruiting |
| A Study of PF-08046050 (SGN-CEACAM5C) in Adults with Advanced Solid Tumors (NCT06131840) | ORR, DoR, PFS as defined by RECIST v1.1 Best response OS | PF-08046050 | CEACAM5 | Cleavable | Topoisomerase I inhibitor | Patients with a/m solid tumors, including metastatic NSCLC, that is refractory or not amenable to the standard-of-care therapy | Stable and treated BM included | Phase I | Recruiting |
| PRO1184 for Advanced Solid Tumors (PRO1184-001) (NCT05579366) | ORR, DoR, PFS as defined by RECIST v1.1 OS | PRO1184 | FOLR1 | Cleavable | Topoisomerase I inhibitor | Patients with a/m solid tumors, including NSCLC that is refractory or not amenable to the standard-of-care therapy | Previously treated and stable BM included active BM or leptomeningeal diseaseexcluded | Phase I/II | Recruiting |
References
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, J.; Zou, Z.; Liu, H.; Liu, C.; Gong, S.; Gao, X.; Liang, G. Clinical Characteristics and Prognosis of Patients with Glioblastoma: A Review of Survival Analysis of 1674 Patients Based on SEER Database. Medicine 2022, 101, e32042. [Google Scholar] [CrossRef]
- Bondy, M.L.; Scheurer, M.E.; Malmer, B.; Barnholtz-Sloan, J.S.; Davis, F.G.; Il’yasova, D.; Kruchko, C.; McCarthy, B.J.; Rajaraman, P.; Schwartzbaum, J.A.; et al. Brain Tumor Epidemiology: Consensus from the Brain Tumor Epidemiology Consortium (BTEC). Cancer 2008, 113, 1953–1968. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain Metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, A.; Barigazzi, C.; Losurdo, A.; Persico, P.; Di Muzio, A.; Navarria, P.; Pessina, F.; van den Bent, M.; Santoro, A.; Simonelli, M. Brain Metastases and Next-Generation Anticancer Therapies: A Survival Guide for Clinicians. Crit. Rev. Oncol. Hematol. 2024, 194, 104239. [Google Scholar] [CrossRef]
- Alemany, M.; Velasco, R.; Simó, M.; Bruna, J. Late Effects of Cancer Treatment: Consequences for Long-Term Brain Cancer Survivors. Neuro-Oncol. Pract. 2020, 8, 18–30. [Google Scholar] [CrossRef]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of Radiotherapy-Associated Cognitive Disability in Patients with Brain Tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Brenner, A.W.; Patel, A.J. Review of Current Principles of the Diagnosis and Management of Brain Metastases. Front. Oncol. 2022, 12, 857622. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood-Brain Barrier and Blood-Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Freskgård, P.-O.; Urich, E. Antibody Therapies in CNS Diseases. Neuropharmacology 2017, 120, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Corti, A.; Ferreri, A.J.M. Breaching the Blood–Brain Tumor Barrier for Tumor Therapy. Cancers 2021, 13, 2391. [Google Scholar] [CrossRef]
- Chhichholiya, Y.; Ruthuparna, M.; Velagaleti, H.; Munshi, A. Brain Metastasis in Breast Cancer: Focus on Genes and Signaling Pathways Involved, Blood-Brain Barrier and Treatment Strategies. Clin. Transl. Oncol. 2023, 25, 1218–1241. [Google Scholar] [CrossRef] [PubMed]
- ESMO. Trastuzumab Deruxtecan Shows High Intracranial Response Rate in Patients with Active Brain Metastases from HER2-Positive Breast Cancer. Available online: https://www.esmo.org/oncology-news/trastuzumab-deruxtecan-shows-high-intracranial-response-rate-in-patients-with-active-brain-metastases-from-her2-positive-breast-cancer (accessed on 20 March 2024).
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.H.; Brown, M.P. Antibody Drug Conjugates and Bystander Killing: Is Antigen-Dependent Internalisation Required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Mckertish, C.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; Im, S.-A.; Iwata, H.; Curigliano, G.; Kim, S.-B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer: Long-Term Survival Analysis of the DESTINY-Breast03 Trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef]
- Marin, B.-M.; Porath, K.A.; Jain, S.; Kim, M.; Conage-Pough, J.E.; Oh, J.-H.; Miller, C.L.; Talele, S.; Kitange, G.J.; Tian, S.; et al. Heterogeneous Delivery across the Blood-Brain Barrier Limits the Efficacy of an EGFR-Targeting Antibody Drug Conjugate in Glioblastoma. Neuro-Oncol. 2021, 23, 2042–2053. [Google Scholar] [CrossRef]
- Siegall, C.B. Targeted Toxins as Anticancer Agents. Cancer 1994, 74, 1006–1012. [Google Scholar] [CrossRef]
- Li, Y.M.; Hall, W.A. Targeted Toxins in Brain Tumor Therapy. Toxins 2010, 2, 2645–2662. [Google Scholar] [CrossRef]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Berger, M.S.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Greer, K.; Herndon, J.E.; Kunwar, S.; et al. Intracerebral Infusion of an EGFR-Targeted Toxin in Recurrent Malignant Brain Tumors. Neuro-Oncol. 2008, 10, 320–329. [Google Scholar] [CrossRef]
- Zhu, H.; Acquaviva, J.; Ramachandran, P.; Boskovitz, A.; Woolfenden, S.; Pfannl, R.; Bronson, R.T.; Chen, J.W.; Weissleder, R.; Housman, D.E.; et al. Oncogenic EGFR Signaling Cooperates with Loss of Tumor Suppressor Gene Functions in Gliomagenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; Aldape, K.D.; Ansell, P.J.; Bain, E.; Curran, W.J.; Eoli, M.; French, P.J.; Kinoshita, M.; Looman, J.; Mehta, M.; et al. Epidermal Growth Factor Receptor (EGFR) Amplification Rates Observed in Screening Patients for Randomized Trials in Glioblastoma. J. Neuro-Oncol. 2019, 144, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Muragaki, Y.; Kagawa, N.; Asai, K.; Nagane, M.; Matsuda, M.; Ueki, K.; Kuroda, J.; Date, I.; Kobayashi, H.; et al. Safety and Efficacy of Depatuxizumab Mafodotin in Japanese Patients with Malignant Glioma: A Nonrandomized, Phase 1/2 Trial. Cancer Sci. 2021, 112, 5020–5033. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III Randomized Trial of CED of IL13-PE38QQR vs. Gliadel Wafers for Recurrent Glioblastoma. Neuro-Oncol. 2010, 12, 871–881. Available online: https://academic.oup.com/neuro-oncology/article/12/8/871/1073747 (accessed on 29 August 2024). [CrossRef]
- Sampson, J.H.; Singh Achrol, A.; Aghi, M.K.; Bankiewicz, K.; Bexon, M.; Brem, S.; Brenner, A.; Chandhasin, C.; Chowdhary, S.; Coello, M.; et al. Targeting the IL4 Receptor with MDNA55 in Patients with Recurrent Glioblastoma: Results of a Phase IIb Trial. Neuro-Oncol. 2023, 25, 1085–1097. [Google Scholar] [CrossRef]
- Medicenna Therapeutics Corp. Medicenna Announces Compelling Survival Benefit from Phase 2b Study of Bizaxofusp in Recurrent Glioblastoma at the 28th Annual Meeting of the Society for NeuroOncology; Medicenna Therapeutics Corp: Toronto, ON, Canada, 2023; Available online: https://www.globenewswire.com/news-release/2023/11/17/2782496/0/en/Medicenna-Announces-Compelling-Survival-Benefit-from-Phase-2b-Study-of-Bizaxofusp-in-Recurrent-Glioblastoma-at-the-28th-Annual-Meeting-of-the-Society-for-NeuroOncology.html (accessed on 1 February 2025).
- Tortorella, S.; Karagiannis, T.C. Transferrin Receptor-Mediated Endocytosis: A Useful Target for Cancer Therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef]
- Gan, H.K.; Burgess, A.W.; Clayton, A.H.A.; Scott, A.M. Targeting of a Conformationally Exposed, Tumor-Specific Epitope of EGFR as a Strategy for Cancer Therapy. Cancer Res. 2012, 72, 2924–2930. [Google Scholar] [CrossRef]
- van den Bent, M.; Gan, H.K.; Lassman, A.B.; Kumthekar, P.; Merrell, R.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Efficacy of Depatuxizumab Mafodotin (ABT-414) Monotherapy in Patients with EGFR-Amplified, Recurrent Glioblastoma: Results from a Multi-Center, International Study. Cancer Chemother. Pharmacol. 2017, 80, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Lassman, A.B.; Van Den Bent, M.; Kumthekar, P.; Merrell, R.; Scott, A.M.; Fichtel, L.; Sulman, E.P.; Gomez, E.; Fischer, J.; et al. Efficacy and Safety Results of ABT-414 in Combination with Radiation and Temozolomide in Newly Diagnosed Glioblastoma. Neuro-Oncol. 2017, 19, 965–975. Available online: https://academic.oup.com/neuro-oncology/article/19/7/965/2760198 (accessed on 29 August 2024). [CrossRef] [PubMed]
- Gan, H.K.; Reardon, D.A.; Lassman, A.B.; Merrell, R.; van den Bent, M.; Butowski, N.; Lwin, Z.; Wheeler, H.; Fichtel, L.; Scott, A.M.; et al. Safety, Pharmacokinetics, and Antitumor Response of Depatuxizumab Mafodotin as Monotherapy or in Combination with Temozolomide in Patients with Glioblastoma. Neuro-Oncol. 2018, 20, 838–847. [Google Scholar] [CrossRef]
- Lassman, A.B.; van den Bent, M.J.; Gan, H.K.; Reardon, D.A.; Kumthekar, P.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Safety and Efficacy of Depatuxizumab Mafodotin + Temozolomide in Patients with EGFR-Amplified, Recurrent Glioblastoma: Results from an International Phase I Multicenter Trial. Neuro-Oncol. 2019, 21, 106–114. [Google Scholar] [CrossRef]
- Van Den Bent, M.; Eoli, M.; Sepulveda, J.M.; Smits, M.; Walenkamp, A.; Frenel, J.-S.; Franceschi, E.; Clement, P.M.; Chinot, O.; De Vos, F.; et al. INTELLANCE 2/EORTC 1410 Randomized Phase II Study of Depatux-M Alone and with Temozolomide vs. Temozolomide or Lomustine in Recurrent EGFR Amplified Glioblastoma. Neuro-Oncol. 2020, 22, 684–693. [Google Scholar] [CrossRef]
- Hoogstrate, Y.; Ghisai, S.A.; de Wit, M.; de Heer, I.; Draaisma, K.; van Riet, J.; van de Werken, H.J.G.; Bours, V.; Buter, J.; Vanden Bempt, I.; et al. The EGFRvIII Transcriptome in Glioblastoma: A Meta-Omics Analysis. Neuro-Oncol. 2022, 24, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; Pugh, S.L.; Wang, T.J.C.; Aldape, K.; Gan, H.K.; Preusser, M.; Vogelbaum, M.A.; Sulman, E.P.; Won, M.; Zhang, P.; et al. Depatuxizumab Mafodotin in EGFR-Amplified Newly Diagnosed Glioblastoma: A Phase III Randomized Clinical Trial. Neuro-Oncol. 2023, 25, 339–350. [Google Scholar] [CrossRef]
- Padovan, M.; Eoli, M.; Pellerino, A.; Rizzato, S.; Caserta, C.; Simonelli, M.; Michiara, M.; Caccese, M.; Anghileri, E.; Cerretti, G.; et al. Depatuxizumab Mafodotin (Depatux-M) Plus Temozolomide in Recurrent Glioblastoma Patients: Real-World Experience from a Multicenter Study of Italian Association of Neuro-Oncology (AINO). Cancers 2021, 13, 2773. [Google Scholar] [CrossRef]
- Hou, J.; Lv, A.; Deng, Q.; Zhang, G.; Hu, X.; Cui, H. TROP2 Promotes the Proliferation and Metastasis of Glioblastoma Cells by Activating the JAK2/STAT3 Signaling Pathway. Oncol. Rep. 2019, 41, 753–764. [Google Scholar] [CrossRef]
- Alberti, S.; Trerotola, M.; Moschella, A.; Guerra, E. TROP2 Gene Copy Number Variations Are a Hallmark of Glioblastoma and MEN-2A Cancers. Cancer Res. 2023, 83 (Suppl. S7), 2589. Available online: https://www.researchgate.net/publication/369810215_Abstract_2589_TROP2_gene_copy_number_variations_are_a_hallmark_of_glioblastoma_and_MEN-2A_cancers (accessed on 1 September 2024). [CrossRef]
- Guerra, E.; Di Pietro, R.; Stati, G.; Alberti, S. A Non-Mutated TROP2 Fingerprint in Cancer Genetics. Front. Oncol. 2023, 13, 1151090. Available online: https://pubmed.ncbi.nlm.nih.gov/37456256/ (accessed on 1 September 2024). [CrossRef] [PubMed]
- Stepan, L.P.; Trueblood, E.S.; Hale, K.; Babcook, J.; Borges, L.; Sutherland, C.L. Expression of Trop2 Cell Surface Glycoprotein in Normal and Tumor Tissues: Potential Implications as a Cancer Therapeutic Target. J. Histochem. Cytochem. 2011, 59, 701–710. [Google Scholar] [CrossRef]
- Page, S.; Milner-Watts, C.; Perna, M.; Janzic, U.; Vidal, N.; Kaudeer, N.; Ahmed, M.; McDonald, F.; Locke, I.; Minchom, A.; et al. Systemic Treatment of Brain Metastases in Non-Small Cell Lung Cancer. Eur. J. Cancer 2020, 132, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.S.; Mustafa, M.A.; Richardson, G.E.; Alam, A.M.; Lee, K.S.; Hughes, D.M.; Escriu, C.; Zakaria, R. Genomic Alterations and the Incidence of Brain Metastases in Advanced and Metastatic NSCLC: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2023, 18, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, E.; Bertoli, E.; Del Conte, A.; Stanzione, B.; Berto, E.; Revelant, A.; Spina, M.; Bearz, A. Brain Metastases Management in Oncogene-Addicted Non-Small Cell Lung Cancer in the Targeted Therapies Era. Int. J. Mol. Sci. 2022, 23, 6477. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Jänne, P.A.; Planchard, D.; Kobayashi, K.; Cheng, Y.; Lee, C.K.; Valdiviezo, N.; Laktionov, K.; Yang, T.Y.; Yu, Y.; Kato, T.; et al. CNS Efficacy of Osimertinib with or Without Chemotherapy in Epidermal Growth Factor Receptor–Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 808–820. [Google Scholar] [CrossRef]
- Solomon, B.J.; Liu, G.; Felip, E.; Mok, T.S.K.; Soo, R.A.; Mazieres, J.; Shaw, A.T.; de Marinis, F.; Goto, Y.; Wu, Y.-L.; et al. Lorlatinib Versus Crizotinib in Patients with Advanced ALK-Positive Non–Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J. Clin. Oncol. 2024, 42, 3400–3409. [Google Scholar] [CrossRef]
- Powell, S.F.; Rodríguez-Abreu, D.; Langer, C.J.; Tafreshi, A.; Paz-Ares, L.; Kopp, H.-G.; Rodríguez-Cid, J.; Kowalski, D.M.; Cheng, Y.; Kurata, T.; et al. Outcomes with Pembrolizumab Plus Platinum-Based Chemotherapy for Patients with NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J. Thorac. Oncol. 2021, 16, 1883–1892. [Google Scholar] [CrossRef]
- Nadal, E.; Rodríguez-Abreu, D.; Simó, M.; Massutí, B.; Juan, O.; Huidobro, G.; López, R.; De Castro, J.; Estival, A.; Mosquera, J.; et al. Phase II Trial of Atezolizumab Combined with Carboplatin and Pemetrexed for Patients with Advanced Nonsquamous Non-Small-Cell Lung Cancer with Untreated Brain Metastases (Atezo-Brain, GECP17/05). J. Clin. Oncol. 2023, 41, 4478–4485. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, C.; Wu, G.; Lin, W.; Xie, Z.; Zhang, H.; Yi, J.; Peng, Z.; Yin, L.; Ma, C.; et al. Efficacy, Safety, and Health-Related Quality of Life with Camrelizumab Plus Pemetrexed and Carboplatin as First-Line Treatment for Advanced Nonsquamous NSCLC with Brain Metastases (CAP-BRAIN): A Multicenter, Open-Label, Single-Arm, Phase 2 Study. J. Thorac. Oncol. 2023, 18, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-Drug Conjugates in Lung Cancer: Dawn of a New Era? npj Precis. Oncol. 2023, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Choi, S.-J.; Cho, J.H.; Choi, H.J.; Lee, J.; Jung, K.; Irwin, D.; Liu, X.; Lira, M.E.; Mao, M.; et al. Lung Cancer in Never-Smoker Asian Females Is Driven by Oncogenic Mutations, Most Often Involving EGFR. Oncotarget 2015, 6, 5465–5474. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Planchard, D.; Goto, K.; Smit, E.F.; Langen, J.D.; Goto, Y.; Ninomiya, K.; Kubo, T.; Pérol, M.; Felip, E.; et al. 1321MO Trastuzumab Deruxtecan (T-DXd) in Patients (Pts) with HER2 (ERBB2)-Mutant (HER2m) Metastatic Non–Small Cell Lung Cancer (NSCLC) with and without Brain Metastases (BMs): Pooled Analyses from DESTINY-Lung01 and DESTINY-Lung02. Ann. Oncol. 2023, 34, S762–S763. [Google Scholar] [CrossRef]
- Lenárt, S.; Lenárt, P.; Šmarda, J.; Remšík, J.; Souček, K.; Beneš, P. Trop2: Jack of All Trades, Master of None. Cancers 2020, 12, 3328. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7696911/ (accessed on 23 June 2024). [CrossRef]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Ninomiya, H.; Sakakibara, R.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Association of Tumor TROP2 Expression with Prognosis Varies among Lung Cancer Subtypes. Oncotarget 2017, 8, 28725. [Google Scholar] [CrossRef]
- Bessede, A.; Peyraud, F.; Besse, B.; Cousin, S.; Cabart, M.; Chomy, F.; Rey, C.; Lara, O.; Odin, O.; Nafia, I.; et al. TROP2 Is Associated with Primary Resistance to Immune Checkpoint Inhibition in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 779–785. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, G.; Zhang, X.; Ma, W. Targeting HER3 to Overcome EGFR TKI Resistance in NSCLC. Front. Immunol. 2024, 14, 1332057. [Google Scholar] [CrossRef]
- Gandullo-Sánchez, L.; Ocaña, A.; Pandiella, A. HER3 in Cancer: From the Bench to the Bedside. J. Exp. Clin. Cancer Res. 2022, 41, 310. [Google Scholar] [CrossRef]
- Gherardi, E.; Birchmeier, W.; Birchmeier, C.; Vande Woude, G. Targeting MET in Cancer: Rationale and Progress. Nat. Rev. Cancer 2012, 12, 89–103. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.W.; Moiseenko, F.V.; Filippova, E.; Cicin, I.; Ciuleanu, T.E.; Daaboul, N.; Liu, C.; et al. Telisotuzumab Vedotin Monotherapy in Patients with Previously Treated C-Met–Overexpressing Non-Squamous EGFR Wildtype Advanced NSCLC: Primary Analysis of the LUMINOSITY Trial. J. Clin. Oncol. 2024, 42 (Suppl. S16), 103. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The Incidence of Brain Metastases among Patients with Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Neuro-Oncol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Murthy, R.K.; Abramson, V.; Anders, C.; Bachelot, T.; Bedard, P.L.; Borges, V.; Cameron, D.; Carey, L.A.; Chien, A.J.; et al. Tucatinib vs. Placebo, Both in Combination with Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients with Brain Metastases: Updated Exploratory Analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol. 2023, 9, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chehade, R.; Nofech-Mozes, S.; Plotkin, A.; Fan, K.Y.; Das, S.; Sahgal, A.; Moravan, V.; Jerzak, K.J. Human Epidermal Growth Factor Receptor 2-Low Breast Cancer Brain Metastases: An Opportunity for Targeted Systemic Therapies in a High-Need Patient Population. JCO Precis. Oncol. 2024, 8, e2300487. [Google Scholar] [CrossRef]
- Balinda, H.U.; Kelly, W.J.; Kaklamani, V.G.; Lathrop, K.I.; Canola, M.M.; Ghamasaee, P.; Sareddy, G.R.; Michalek, J.; Gilbert, A.R.; Surapaneni, P.; et al. Sacituzumab Govitecan in Patients with Breast Cancer Brain Metastases and Recurrent Glioblastoma: A Phase 0 Window-of-Opportunity Trial. Nat. Commun. 2024, 15, 6707. [Google Scholar] [CrossRef]
- Pestalozzi, B.C.; Holmes, E.; de Azambuja, E.; Metzger-Filho, O.; Hogge, L.; Scullion, M.; Láng, I.; Wardley, A.; Lichinitser, M.; Sanchez, R.I.L.; et al. CNS Relapses in Patients with HER2-Positive Early Breast Cancer Who Have and Have Not Received Adjuvant Trastuzumab: A Retrospective Substudy of the HERA Trial (BIG 1-01). Lancet Oncol. 2013, 14, 244–248. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab Emtansine versus Capecitabine plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA): A Descriptive Analysis of Final Overall Survival Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab Emtansine (T-DM1) versus Lapatinib plus Capecitabine in Patients with HER2-Positive Metastatic Breast Cancer and Central Nervous System Metastases: A Retrospective, Exploratory Analysis in EMILIA. Ann. Oncol. 2015, 26, 113–119. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.-B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.-M.; Smitt, M.; Yu, R.; Leung, A.C.F.; Wildiers, H. TH3RESA study collaborators Trastuzumab Emtansine versus Treatment of Physician’s Choice for Pretreated HER2-Positive Advanced Breast Cancer (TH3RESA): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Montemurro, F.; Delaloge, S.; Barrios, C.H.; Wuerstlein, R.; Anton, A.; Brain, E.; Hatschek, T.; Kelly, C.M.; Peña-Murillo, C.; Yilmaz, M.; et al. Trastuzumab Emtansine (T-DM1) in Patients with HER2-Positive Metastatic Breast Cancer and Brain Metastases: Exploratory Final Analysis of Cohort 1 from KAMILLA, a Single-Arm Phase IIIb Clinical Trial. Ann. Oncol. 2020, 31, 1350–1358. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzàlez Farré, X.; You, B.; Saura, C.; et al. Trastuzumab Deruxtecan in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A DESTINY-Breast01 Subgroup Analysis. Cancer Discov. 2022, 12, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab Deruxtecan in HER2-Positive Breast Cancer with Brain Metastases: A Single-Arm, Phase 2 Trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Ciruelos, E.; Jerusalem, G.; Müller, V.; Niikura, N.; Viale, G.; Bartsch, R.; Kurzeder, C.; Higgins, M.J.; Connolly, R.M.; et al. Trastuzumab Deruxtecan in HER2-Positive Advanced Breast Cancer with or without Brain Metastases: A Phase 3b/4 Trial. Nat. Med. 2024, 30, 3717–3727. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, J.M.; Vaz Batista, M.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab Deruxtecan in Patients with Central Nervous System Involvement from HER2-Positive Breast Cancer: The DEBBRAH Trial. Neuro-Oncol. 2023, 25, 157–166. [Google Scholar] [CrossRef]
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 Low, Ultra-Low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer. Front. Mol. Biosci. 2022, 9, 834651. [Google Scholar] [CrossRef]
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab Deruxtecan in Metastatic Breast Cancer with Variable HER2 Expression: The Phase 2 DAISY Trial. Nat. Med. 2023, 29, 2110–2120. Available online: https://www.nature.com/articles/s41591-023-02478-2 (accessed on 1 September 2024). [CrossRef]
- Epaillard, N.; Lusque, A.; Pistilli, B.; André, F.; Bachelot, T.; Pierga, J.-Y.; Ducoulombier, A.; Jouannaud, C.; Viret, F.; Salabert, L.; et al. 260P Antitumor Activity of Trastuzumab Deruxtecan (T-DXd) in Patients with Metastatic Breast Cancer (mBC) and Brain Metastases (BMs) from DAISY Trial. Ann. Oncol. 2022, 33, S656. [Google Scholar] [CrossRef]
- Tsurutani, J.; Jacot, W.; Yamashita, T.; Riaz, F.; Yerushalmi, R.; Im, S.-A.; Niikura, N.; Halser-Strub, U.; Cortés, J.; Wennstig, A.-K.; et al. 388P Subgroup Analysis of Patients (Pts) with HER2-Low Metastatic Breast Cancer (mBC) with Brain Metastases (BMs) at Baseline from DESTINY-Breast04, a Randomized Phase III Study of Trastuzumab Deruxtecan (T-DXd) vs. Treatment of Physician’s Choice (TPC). Ann. Oncol. 2023, 34, S342–S343. [Google Scholar] [CrossRef]
- Batista, M.V.; Pérez-García, J.M.; Cortez, P.; Garrigós, L.; Fernández-Abad, M.; Gion, M.; Martínez-Bueno, A.; Saavedra, C.; Teruel, I.; Fernandez-Ortega, A.; et al. Trastuzumab Deruxtecan in Patients with Previously Treated HER2-Low Advanced Breast Cancer and Active Brain Metastases: The DEBBRAH Trial. ESMO Open 2024, 9, 103699. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Bardia, A.; Punie, K.; Kalinsky, K.; Carey, L.A.; Rugo, H.S.; Diéras, V.; Phan, S.; Delaney, R.; Zhu, Y.; et al. Subgroup Analyses from the Phase 3 ASCENT Study of Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. npj Breast Cancer 2024, 10, 33. [Google Scholar] [CrossRef]
- Terasima, T.; Tolmach, L.J. Variations in Several Responses of HeLa Cells to X-Irradiation during the Division Cycle. Biophys. J. 1963, 3, 11–33. [Google Scholar] [CrossRef]
- Borges, G.S.; Rovere, R.K.; Dias, S.M.K.; Chong, F.H.; Morais, M.D.S. Safety and Efficacy of the Combination of T-DM1 with Radiotherapy of the Central Nervous System in a Patient with HER2-Positive Metastatic Breast Cancer: Case Study and Review of the Literature. Ecancermedicalscience 2015, 9, 586. [Google Scholar] [CrossRef]
- Ricciardi, G.R.R.; Russo, A.; Franchina, T.; Schifano, S.; Mastroeni, G.; Santacaterina, A.; Adamo, V. Efficacy of T-DM1 for Leptomeningeal and Brain Metastases in a HER2 Positive Metastatic Breast Cancer Patient: New Directions for Systemic Therapy—A Case Report and Literature Review. BMC Cancer 2018, 18, 97. [Google Scholar] [CrossRef]
- Orth, M.; Unger, K.; Schoetz, U.; Belka, C.; Lauber, K. Taxane-Mediated Radiosensitization Derives from Chromosomal Missegregation on Tripolar Mitotic Spindles Orchestrated by AURKA and TPX2. Oncogene 2018, 37, 52–62. [Google Scholar] [CrossRef]
- Mignot, F.; Kirova, Y.; Verrelle, P.; Teulade-Fichou, M.-P.; Megnin-Chanet, F. In Vitro Effects of Trastuzumab Emtansine (T-DM1) and Concurrent Irradiation on HER2-Positive Breast Cancer Cells. Cancer Radiother. 2021, 25, 126–134. [Google Scholar] [CrossRef]
- Mitsuya, K.; Watanabe, J.; Nakasu, Y.; Hayashi, N.; Harada, H.; Ito, I. Expansive Hematoma in Delayed Cerebral Radiation Necrosis in Patients Treated with T-DM1: A Report of Two Cases. BMC Cancer 2016, 16, 391. [Google Scholar] [CrossRef]
- Adams, S.R.; Yang, H.C.; Savariar, E.N.; Aguilera, J.; Crisp, J.L.; Jones, K.A.; Whitney, M.A.; Lippman, S.M.; Cohen, E.E.W.; Tsien, R.Y.; et al. Anti-Tubulin Drugs Conjugated to Anti-ErbB Antibodies Selectively Radiosensitize. Nat. Commun. 2016, 7, 13019. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.D.; Longstreth, W.T.; Pedrosa, H.A.S.; Gil, G.O.B.; Duarte, J.M.; Filho, M.A.D. Progressively Enlarging Cerebellar Hematoma Concurrent with T-DM1 Treatment. World Neurosurg. 2018, 111, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Nooruddin, Z.; Rusthoven, C.; Elias, A.; Borges, V.F.; Diamond, J.R.; Kavanagh, B.; Kabos, P. Trastuzumab Emtansine and Stereotactic Radiosurgery: An Unexpected Increase in Clinically Significant Brain Edema. Neuro-Oncol. 2014, 16, 1006–1009. [Google Scholar] [CrossRef]
- Crone, S.A.; Zhao, Y.-Y.; Fan, L.; Gu, Y.; Minamisawa, S.; Liu, Y.; Peterson, K.L.; Chen, J.; Kahn, R.; Condorelli, G.; et al. ErbB2 Is Essential in the Prevention of Dilated Cardiomyopathy. Nat. Med. 2002, 8, 459–465. [Google Scholar] [CrossRef]
- Vartanian, T.; Goodearl, A.; Viehöver, A.; Fischbach, G. Axonal Neuregulin Signals Cells of the Oligodendrocyte Lineage through Activation of HER4 and Schwann Cells through HER2 and HER3. J. Cell Biol. 1997, 137, 211–220. [Google Scholar] [CrossRef]
- Lebow, E.S.; Pike, L.R.G.; Seidman, A.D.; Moss, N.; Beal, K.; Yu, Y. Symptomatic Necrosis with Antibody-Drug Conjugates and Concurrent Stereotactic Radiotherapy for Brain Metastases. JAMA Oncol. 2023, 9, 1729–1733. [Google Scholar] [CrossRef]
- Lee, E.Q.; Camidge, D.R.; Mehta, G. Extending Our Reach: Expanding Enrollment in Brain Metastases and Primary Brain Tumor Clinical Trials. Am. Soc. Clin. Oncol. Educ. Book. 2022, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Polley, M.-Y.C.; Alder, L.; Brastianos, P.K.; Anders, C.K.; Tawbi, H.A.; Mehta, M.; Wen, P.Y.; Geyer, S.; de Groot, J.; et al. Current Drug Development and Trial Designs in Neuro-Oncology: Report from the First American Society of Clinical Oncology and Society for Neuro-Oncology Clinical Trials Conference. Lancet Oncol. 2023, 24, e161–e171. [Google Scholar] [CrossRef]
- Sivakumar, S.; Jin, D.X.; Tukachinsky, H.; Murugesan, K.; McGregor, K.; Danziger, N.; Pavlick, D.; Gjoerup, O.; Ross, J.S.; Harmon, R.; et al. Tissue and Liquid Biopsy Profiling Reveal Convergent Tumor Evolution and Therapy Evasion in Breast Cancer. Nat. Commun. 2022, 13, 7495. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Ji, C.; Li, F.; Yuan, Y.; Zhang, H.; Bian, L.; Zhang, S.; Wang, T.; Li, J.; Jiang, Z. Novel Anti-HER2 Antibody-Drug Conjugates Versus T-DM1 for HER2-Positive Metastatic Breast Cancer After Tyrosine Kinase Inhibitors Treatment. Oncologist 2023, 28, e859–e866. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Griffith, J.I.; Porath, K.A.; Rathi, S.; Le, J.; Pasa, T.I.; Decker, P.A.; Gupta, S.K.; Hu, Z.; Carlson, B.L.; et al. Bystander Effects, Pharmacokinetics, and Linker-Payload Stability of EGFR-Targeting Antibody-Drug Conjugates Losatuxizumab Vedotin and Depatux-M in Glioblastoma Models. Clin. Cancer Res. 2024, 30, 3287–3297. [Google Scholar] [CrossRef] [PubMed]
- Whalen, K.A.; White, B.H.; Quinn, J.M.; Kriksciukaite, K.; Alargova, R.; Au Yeung, T.P.; Bazinet, P.; Brockman, A.; DuPont, M.M.; Oller, H.; et al. Targeting the Somatostatin Receptor 2 with the Miniaturized Drug Conjugate, PEN-221: A Potent and Novel Therapeutic for the Treatment of Small Cell Lung Cancer. Mol. Cancer Ther. 2019, 18, 1926–1936. [Google Scholar] [CrossRef]
- Anami, Y.; Otani, Y.; Xiong, W.; Ha, S.Y.Y.; Yamaguchi, A.; Rivera-Caraballo, K.A.; Zhang, N.; An, Z.; Kaur, B.; Tsuchikama, K. Homogeneity of Antibody-Drug Conjugates Critically Impacts the Therapeutic Efficacy in Brain Tumors. Cell Rep. 2022, 39, 110839. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef]
- Kaempffe, A.; Dickgiesser, S.; Rasche, N.; Paoletti, A.; Bertotti, E.; De Salve, I.; Sirtori, F.R.; Kellner, R.; Könning, D.; Hecht, S.; et al. Effect of Conjugation Site and Technique on the Stability and Pharmacokinetics of Antibody-Drug Conjugates. J. Pharm. Sci. 2021, 110, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- El Alaoui, M.; Sivado, E.; Jallas, A.-C.; Mebarki, L.; Dyson, M.R.; Perrez, F.; Valsesia-Wittmann, S.; El Alaoui, S. Antibody and Antibody Fragments Site-Specific Conjugation Using New Q-Tag Substrate of Bacterial Transglutaminase. Cell Death Discov. 2024, 10, 79. [Google Scholar] [CrossRef]
- Matsuda, Y.; Shikida, N.; Hatada, N.; Yamada, K.; Seki, T.; Nakahara, Y.; Endo, Y.; Shimbo, K.; Takahashi, K.; Nakayama, A.; et al. AJICAP-M: Traceless Affinity Peptide Mediated Conjugation Technology for Site-Selective Antibody-Drug Conjugate Synthesis. Org. Lett. 2024, 26, 5597–5601. [Google Scholar] [CrossRef]
- Mair, M.J.; Bartsch, R.; Le Rhun, E.; Berghoff, A.S.; Brastianos, P.K.; Cortes, J.; Gan, H.K.; Lin, N.U.; Lassman, A.B.; Wen, P.Y.; et al. Understanding the Activity of Antibody-Drug Conjugates in Primary and Secondary Brain Tumours. Nat. Rev. Clin. Oncol. 2023, 20, 372–389. [Google Scholar] [CrossRef]
- Ballestín, P.; López de Sá, A.; Díaz-Tejeiro, C.; Paniagua-Herranz, L.; Sanvicente, A.; López-Cade, I.; Pérez-Segura, P.; Alonso-Moreno, C.; Nieto-Jiménez, C.; Ocaña, A. Understanding the Toxicity Profile of Approved ADCs. Pharmaceutics 2025, 17, 258. [Google Scholar] [CrossRef]
- Watanabe, T.; Arashida, N.; Fujii, T.; Shikida, N.; Ito, K.; Shimbo, K.; Seki, T.; Iwai, Y.; Hirama, R.; Hatada, N.; et al. Exo-Cleavable Linkers: Enhanced Stability and Therapeutic Efficacy in Antibody–Drug Conjugates. J. Med. Chem. 2024, 67, 18124–18138. [Google Scholar] [CrossRef] [PubMed]
- Chuprakov, S.; Ogunkoya, A.O.; Barfield, R.M.; Bauzon, M.; Hickle, C.; Kim, Y.C.; Yeo, D.; Zhang, F.; Rabuka, D.; Drake, P.M. Tandem-Cleavage Linkers Improve the In Vivo Stability and Tolerability of Antibody–Drug Conjugates. Bioconjugate Chem. 2021, 32, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Vaz Batista, M.; Pérez-García, J.M.; Garrigós, L.; García-Sáenz, J.Á.; Cortez, P.; Racca, F.; Blanch, S.; Ruiz-Borrego, M.; Fernández-Ortega, A.; Fernández-Abad, M.; et al. The DEBBRAH Trial: Trastuzumab Deruxtecan in HER2-Positive and HER2-Low Breast Cancer Patients with Leptomeningeal Carcinomatosis. Med 2025, 6, 100502. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
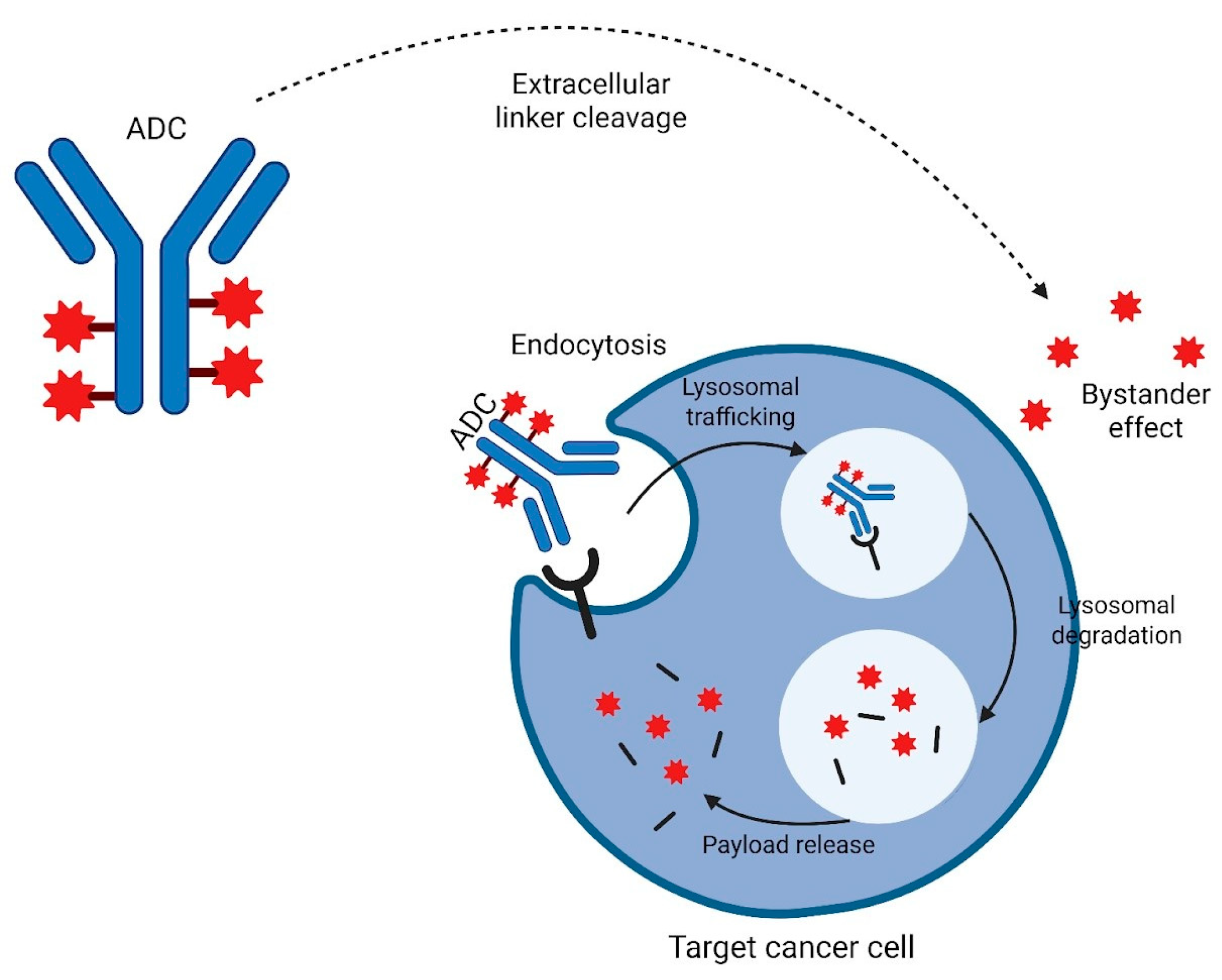
| ADC | Target | Linker Type | Payload | Indications | Registration Trial |
|---|---|---|---|---|---|
| Mirvetuximab soravtansine | Folate receptor alpha | Cleavable | Maytansinoid DM4 | Folate receptor-alpha (FRα)-positive, platinum-resistant high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer patients who have received 1 to 3 prior systemic treatment regimens | SORAYA (NCT04296890) |
| Tisotumab vedotin | Tissue Factor | Cleavable | Monomethyl Auristatin E (MMAE) | Recurrent or metastatic cervical cancer with disease progression to chemotherapy | InnovaTV 301 (NCT04697628) |
| Ado-trastuzumab emtansine | HER2 | Non cleavable | Maytansine DM1 | EBC: Adjuvant treatment of patients with HER2-positive EBC who have residual invasive disease after neoadjuvant taxane- and trastuzumab-based treatment. MBC: Patients with HER2-positive MBC who previously received trastuzumab and a taxane, separately or in combination | KATHERINE (NCT01772472) EMILIA (NCT00829166) |
| Enfortumab vedotin | Nectin-4 | Clevable | Monomethyl Auristatin E (MMAE) | Adult patients with locally advanced or metastatic urothelial cancer who have received an anti PD-(L)1 agent, and platinum-based chemotherapy | EV-301 (NCT03474107) |
| Trastuzumab deruxtecan | HER2 | Cleavable | Deruxtecan | Breast cancer: Unresectable or metastatic HER2-positive (IHC 3+ or ISH positive) BC patients who have received a prior anti-HER2-based regimen Patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH−) BC, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting Non-small cell lung cancer: Unresectable or metastatic NSCLC patients whose tumors have activating HER2 mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy (accelerated approval) Gastric cancer: Locally advanced or metastatic HER2-positive (IHC 3+ or IHC 2+/ISH positive) gastric or gastroesophageal junction (GEJ) adenocarcinoma patients who have received a prior trastuzumab-based regimen Solid tumors: Patients with unresectable or metastatic HER2-positive (IHC3+) solid tumors who have received prior systemic treatment (accelerated approval) | DESTINY-Breast03 (NCT03529110) DESTINY-Breast04 (NCT03734029) DESTINY-Lung02 (NCT04644237) DESTINY-Gastric01 (NCT03329690) DESTINY-PanTumor02 (NCT04482309) |
| Sacituzumab Govitecan | TROP2 | Cleavable | Camptothecin analog (SN38) | BC: Unresectable locally advanced or metastatic triple-negative BC patients who have received 2 or more prior systemic therapies Urothelial cancer: Locally advanced or metastatic urothelial cancer (mUC) patients who have previously received platinum-based chemotherapy and an anti PD-(L)1 inhibitor. (accelerated approval) | ASCENT (NCT02574455) IMMU-132-01 (NCT01631552) TROPHY (NCT03547973) |
| Datopotomab Deruxtecan | TROP2 | Cleavable | Topoisomerase I inhibitor (Deruxtecan) | Patients with unresectable or metastatic hormone receptor-positive, HER2-breast cancer that has progressed after endocrine-based therapy and chemotherapy for unresectable or metastatic disease | TROPION-Breast01 (NCT05104866) |
| Clinical Trial | Response Endpoint | Treatment | Target | Linker Type | Payload Action | Population | Trial Type | Status |
|---|---|---|---|---|---|---|---|---|
| AMG 595 First-in-Human in Recurrent Gliomas (NCT01475006) | ORR by Macdonald criteria | AMG 595 | EGFRvIII | Non-Cleavable | Microtubule inhibitor (Maytansinoid DM1) | Patients with rGBM (Part 1 and 2) or WHO Grade III anaplastic astrocytoma (Part 1 only) expressing EGFRvIII | Phase I | Completed. No results published (last update 2016) |
| Safety and Efficacy of L19TNF in Patients with Isocitrate Dehydrogenase (IDH) Wildtype WHO Grade III/IV Glioma at First Relapse (GLIOMOON) (NCT03779230) | PFS, OS, ORR by iRANO criteria | Onfekafusp alfa (L19TNF) | Fibronectin | NA | TNFalpha | Patients with WHO Grade III/IV Glioma at First Relapse | Phase I/II | Completed |
| Safety and Efficacy of L19TNF Plus Temozolomide Chemoradiotherapy in Patients with Newly Diagnosed Glioblastoma (GLIOSUN) (NCT04443010) | OS, PFS, ORR, DCR, BORR by iRANO | Onfekafusp alfa (L19TNF) + TMZ | Fibronectin | NA | TNFalpha | Patients with newly diagnosed GBM | Phase II | Recruiting |
| A Study to Evaluate Safety and Efficacy of L19TNF Plus Lomustine in Patients with Glioblastoma at First Progression (GLIOSTAR) (NCT04573192) | OS, PFS by iRANO | Onfekafusp alfa (L19TNF) + lomustine | Fibronectin | NA | TNFalpha | Patients with GBM at first progression | Phase I/II | Recruiting |
| Neuro/Sacituzumab Govitecan/Breast Brain Metastasis/Glioblastoma/Ph 0 (NCT03995706) | Ratio of SN-38 and its metabolites relative to serum concentration | Sacituzumab Govitecan | TROP2 | Cleavable | Topoisomerase I Inhibitor (govitecan) | Patients with rGBM/BM from breast | Phase 0 | Completed |
| Clinical Trial | Type of Trial | ADC | Target | Linker Type | Payload Action | Population | CNS Metastasis Eligibility | Intracranial Activity | Intracranic Response Criteria |
|---|---|---|---|---|---|---|---|---|---|
| TROPION-Lung05 (NCT04484142) | Phase II | Datopotamab Deruxtecan (Dato-DXd) | TROP-2 | Cleavable | Topoisomerase I inhibitor (Deruxtecan) | a/m NSCLC with AGAs who progressed following targeted therapy and platinum-based CT | Clinically inactive BM (not requiring medical therapy) eligible. Leptomeningeal disease excluded | iORR 22% iDCR 72% | RECIST v1.1 |
| DESTINY-Lung01 (NCT03505710) DESTINY-Lung02 (NCT04644237) | Phase II | Trastuzumab-Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I inhibitor (Deruxtecan) | HER2-overexpressing or HER2-mutant unresectable or metastatic NSCLC | No exclusion criteria for patients with brain metastases, including leptomeningeal carcinomatosis | 5.4 mg/kg Cohort (DL-02): iORR 25% iDCR 81.3% 6.4 mg/kg Cohort (DL-01/02): iORR 18.5% iDCR 76% | RECIST v1.1 |
| HERTHENA-Lung01 (NCT04619004) | Phase II | Patritumab Deruxtecan (HER3-DXd) | HER3 | Cleavable | Topoisomerase I inhibitor (Deruxtecan) | a/m NSCLC with canonical EGFR mutation who progressed on at least one EGFR TKI and one platinum-based CT | Clinically inactive BM (not requiring medical therapy) eligible. Leptomeningeal disease excluded | iORR 33.3% iDCR 76.6% | RECIST v1.1 |
| Clinical Trial | Type of Trial | ADC | Target | Linker Type | Payload Action | Population | CNS Metastasis Eligibility | CNS Endpoints | Intracranic Response Criteria |
|---|---|---|---|---|---|---|---|---|---|
| EMILIA (NCT008291) | Retrospective exploratory analysis | Trastuzumab emtansine (T-DM1) | HER2 | Non-cleavable | Microtubule Inhibitor (Maytansinoid DM1) | HER2-positive a/m BC, previously treated with trastuzumab and a taxane | Treated, asymptomatic | Patients with baseline BM mOS: 26.8 vs. 12.9 mo | RECIST v1.1 |
| TH3RESA (NCT014191) | Phase III | Trastuzumab emtansine (T-DM1) | HER2 | Non-cleavable | Microtubule Inhibitor (Maytansinoid DM1) | HER2-positive mBC pretreated with ≥2 HER2 directed regimens | Treated, asymptomatic | Patients with baseline BM mPFS: 5.8 vs. 2.9 mo | RECIST v1.1 |
| KAMILLA (NCT017025) | Exploratory analysis | Trastuzumab emtansine (T-DM1) | HER2 | Non-cleavable | Microtubule Inhibitor (Maytansinoid DM1) | HER2-positive a/m BC progressing after CT and anti-HER2 therapy or ≤6 mo after adjuvant therapy | Controlled or untreated and asymptomatic | iORR: 21.4%, iCR: 2.4% | RECIST v1.1 |
| DESTINY-Breast01 (NCT032484) | Phase II | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-positive mBC previously treated with T-DM1 | Treated, asymptomatic | iORR: 41.2% | RECIST v1.1 |
| DESTINY-Breast03 (NCT035291) | Phase III | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-positive mBC previously treated with trastuzumab and a taxane | Treated or asymptomatic | iORR: 79 vs. 35% | RECIST v1.1 |
| TUXEDO-1 (NCT047520) | Phase II | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-positive mBC and newly diagnosed untreated BM/BM progressing after previous local therapy, previous exposure to trastuzumab and pertuzumab and no indication for immediate local therapy | Newly diagnosed untreated/progressing after previous local therapy | iORR: 73.3% iCR: 13.3% iPR: 60% iSD: 20% | RANO-BM |
| DESTINY-Breast12 (NCT047397) | Phase IIIb/IV | Trastuzumab- Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-positive mBC treated with one or more prior anti-HER2–based regimens | Stable or active (untreated/previously treated and progressing) | iORR: 71.7% (79.2% with stable BM and 62.3% with active BM) | RECIST v1.1 |
| DEBBRAH (NCT044205) | Phase II | Trastuzumab- Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-positive or HER-2 low a/m BC pretreated | Cohort 2: asymptomatic, untreated Cohort 3: progressing after local therapy | CNS ORR: 50% (cohort 2) 44.4% (cohort 3) | RANO-BM |
| ASCENT (NCT025744) | Subgroup analysis | Sacituzumab Govitecan (SG) | TROP2 | Cleavable | Topoisomerase I Inhibitor (govitecan) | relapsed or refractory mTNBC | Stable | mOS in BM population: 6.8 vs. 7.5 mo | RECIST v1.1 |
| Clinical Trial | Response Endpoint | ADC | Target | Linker Type | Payload Action | Population | CNS Metastasis Eligibility | Trial Type | Status |
|---|---|---|---|---|---|---|---|---|---|
| DATO-BASE: DATOpotamab-deruxtecan for Breast Cancer Brain MetAstaSEs (NCT06176261) | iORR as defined by RANO-BM criteria | Datopotamab Deruxtecan (Dato-DXd) | TROP2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-negative mBC | Cohorts A and B: newly diagnosed or progressing after local and/or systemic therapy Cohort C: leptomeningeal disease | Phase II | Recruiting |
| T-DXd Therapy for HER2-low Breast Cancer Patients with Brain Metastases (TUXEDO-4) (NCT06048718) | iORR as defined by RANO-BM criteria | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-low mBC with newly diagnosed or progressing Bm with or without untreated type II LMD, who received ≥1 line of systemic treatment in the advanced setting | Newly diagnosed or progressive, without indication for immediate local therapy | Phase II | Recruiting |
| Testing Sacituzumab Govitecan Therapy in Patients With HER2-Negative Breast Cancer and Brain Metastases (NCT04647916) | iORR as defined by RANO-BM criteria | Sacituzumab govitecan (SG) | TROP2 | Cleavable | Topoisomerase I Inhibitor (govitecan) | HER2-negative mBC with BM | ≥1 BM not previously treated or progressed to RT | Phase II | Recruiting |
| HER3-DXd in Breast Cancer and NSCLC Brain Metastases and Solid Tumor Leptomeningeal Disease (TUXEDO-3) (NCT05865990) | iORR as defined by RANO-BM criteria | Patritumab deruxtecan (HER3-DXd) | HER3 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | mBC or aNSCLC with active BM), who received ≥1 line of systemic treatment in the advanced setting or with active LMD after RT from an advanced solid tumor who do not need immediate local treatment, and have not received prior treatment with an anti-HER3 | Active BM from MBC (Cohort 1) and aNSCLC (Cohort 2), untreated LMD (Cohort 3) | Phase II | Active, not recruiting |
| Patritumab Deruxtecan (U3-1402) in Unresectable Locally Advanced or Metastatic Breast Cancer (ICARUS BREAST) (NCT04965766) | ORRas defined by RECIST | Patritumab deruxtecan (HER3-DXd) | HER3 | Clevable | Topoisomerase I Inhibitor (Deruxtecan) | HR+, ABC, resistant to ET and CDK4/6i, that must have received only one line of chemotherapy for ABC | Clinically inactive, treated, asymptomatic | Phase II | Active, not recruiting |
| A Phase 1b/2 Study of T-DXd Combinations in HER2-positive Metastatic Breast Cancer (DESTINY-Breast07) (NCT04538742) | ORR, PFS, PFS2, DoR as defined by RECIST, OS | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2-positive a/mBC, ≥1 line of systemic treatment in the advanced setting (Part 1), no prior lines for a/mBC (Part 2 Modules 0–5), zero or one prior lines for a/mBC allowed (Part 2 Modules 6 and 7 | Stable (Modules 0–5); untreated, not needing local therapy or previously treated that have progressed since prior local therapy (Module 6 and 7) (<2 mg dexamethasone/day) | Phase Ib/II | Active, not recruiting |
| PRE-I-SPY Phase I/Ib Oncology Platform Program (PRE-I-SPY-PI) (NCT05868226) | ORR, DoR, PFS, CBR as defined by RECIST | Trastuzumab Deruxtecan (T-DXd) | HER2 | Cleavable | Topoisomerase I Inhibitor (Deruxtecan) | HER2 expressing (>HER2 1+) mBC | Treated and clinically stable | Phase I/Ib | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzzone, F.; Barigazzi, C.; Di Muzio, A.; Tallarico, I.; Dipasquale, A.; Losurdo, A.; Persico, P.; Navarria, P.; Pessina, F.; Santoro, A.; et al. Exploring the Role of ADCs in Brain Metastases and Primary Brain Tumors: Insight and Future Directions. Cancers 2025, 17, 1591. https://doi.org/10.3390/cancers17091591
Bruzzone F, Barigazzi C, Di Muzio A, Tallarico I, Dipasquale A, Losurdo A, Persico P, Navarria P, Pessina F, Santoro A, et al. Exploring the Role of ADCs in Brain Metastases and Primary Brain Tumors: Insight and Future Directions. Cancers. 2025; 17(9):1591. https://doi.org/10.3390/cancers17091591
Chicago/Turabian StyleBruzzone, Francesco, Chiara Barigazzi, Antonio Di Muzio, Isabel Tallarico, Angelo Dipasquale, Agnese Losurdo, Pasquale Persico, Pierina Navarria, Federico Pessina, Armando Santoro, and et al. 2025. "Exploring the Role of ADCs in Brain Metastases and Primary Brain Tumors: Insight and Future Directions" Cancers 17, no. 9: 1591. https://doi.org/10.3390/cancers17091591
APA StyleBruzzone, F., Barigazzi, C., Di Muzio, A., Tallarico, I., Dipasquale, A., Losurdo, A., Persico, P., Navarria, P., Pessina, F., Santoro, A., & Simonelli, M. (2025). Exploring the Role of ADCs in Brain Metastases and Primary Brain Tumors: Insight and Future Directions. Cancers, 17(9), 1591. https://doi.org/10.3390/cancers17091591







