An Updated Meta-Analysis on Long-Term Outcomes Following Hyperthermic Intraperitoneal Chemotherapy in Advanced Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
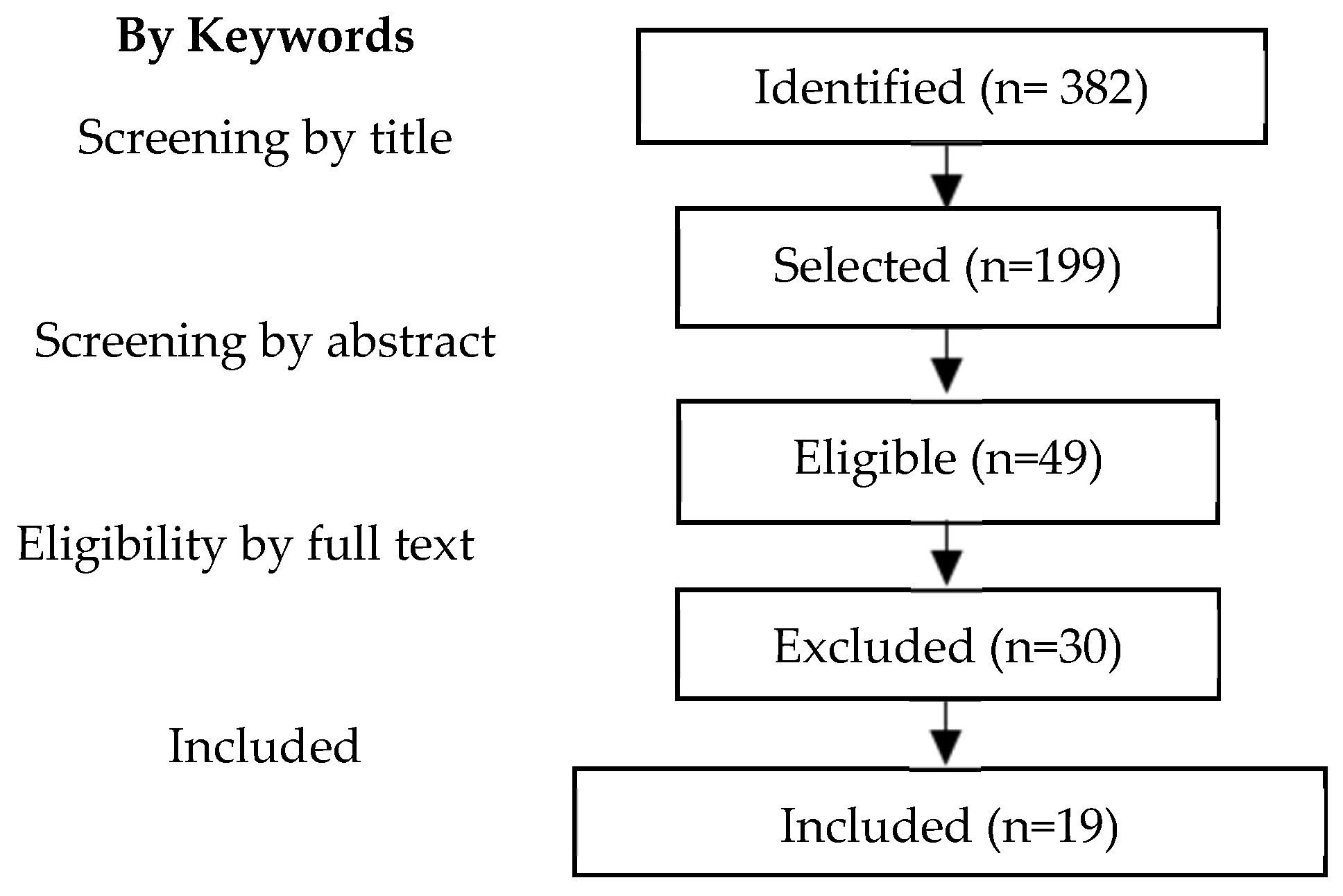
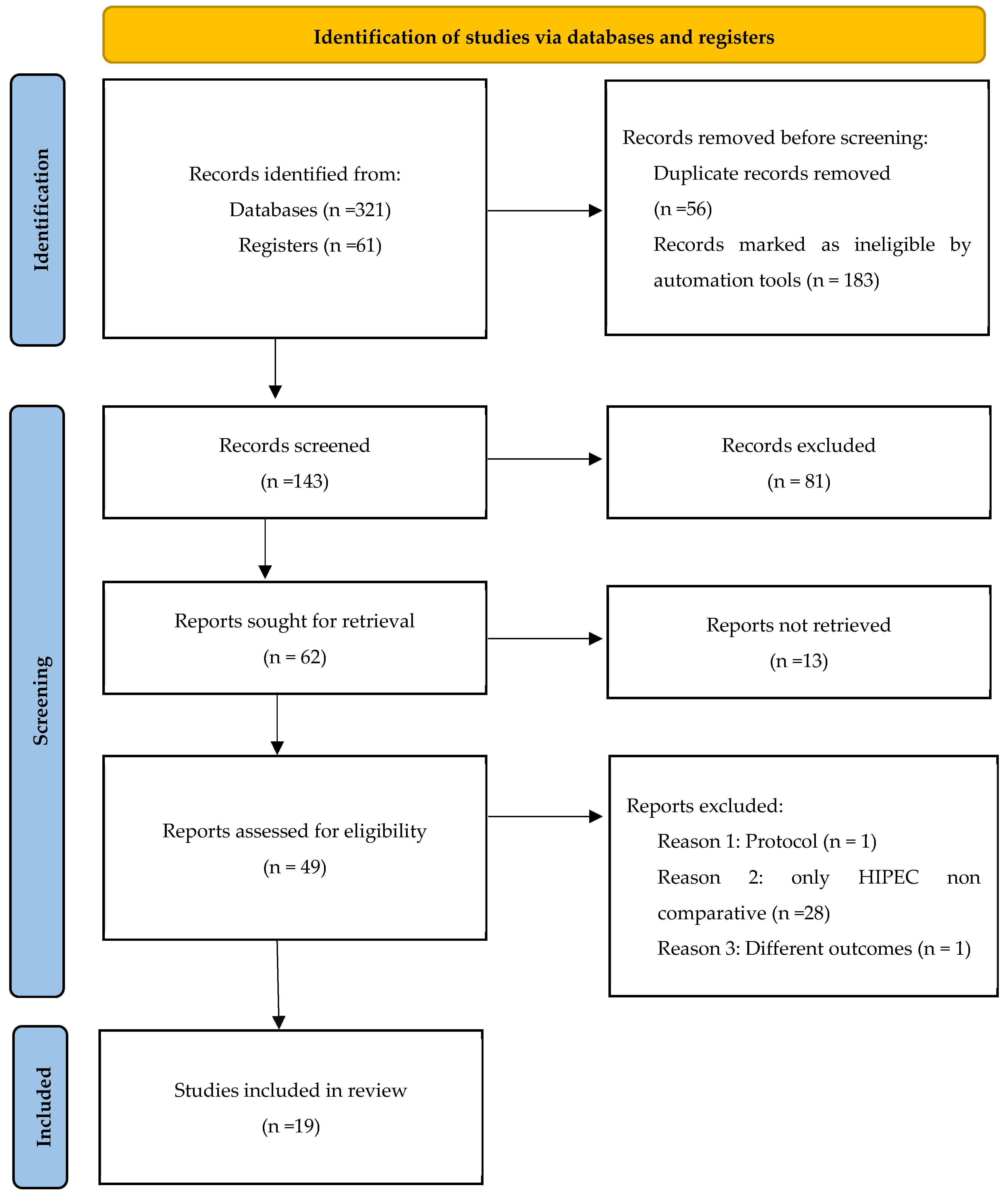
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Quality Assessment and Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
| Author (Study Type/Interval/Country) | Surgery Type | Number of Patients (Control/HIPEC arm) | Treatment | HIPEC Scheme | HIPEC Technique | HIPEC T(°C), Duration (min) | Survival Analysis |
|---|---|---|---|---|---|---|---|
| Aronson et al. [16] (RCT/2007–2016/Netherlands) | IDS | 123/122 | NACT + CRS ± HIPEC | CDDP 100 mg/m2 | Open | 40–42, 90’ | OS/PFS |
| Baiocci G. et al. [17] (Cohort/2000–2014/Brazil) | SCR | 50/29 | NACT + CRS ± HIPEC | MMC 10 mg/m2 + CDDP 50 mg/m2 or CDDP 50 mg/m2 + DXR or CDDP 50 mg/m2 or OX | Closed | 41–42, <90’ | OS/DFS |
| Campos et al. [18] (RCT/2012–2019/Spain) | PDS, IDS and SCR | 23/32 | NACT ± CRS ± HIPEC | PTX 175 mg/m2 | Closed | 42–43, 60’ | OS/RFS/AE |
| Ceresoli et al. [19] (CC/2010–2016/Italy) | IDS | 28/28 | NACT + CRS ± HIPEC | CDDP 100 mg/m2 + PTX 175 mg/m2 | Open | 41.5, 90’ | OS/DFS/AE |
| Classe et al. [20] (RCT/2011–2021/France) | SCR | 208/207 | NACT + CRS ± HIPEC | CDDP 75 mg/m2 | Open or closed | 41 ± 1, 60’ | OS/PFS/AE |
| Fagotti et al. [21] (CC/2005–2009/Italy) | SCR | 13/30 | CRS ± HIPEC | OX 460 mg/m2 | Closed | 41.5, 30’ | OS/PFS |
| Kim et al. [22] (CC/1991–2004/South Korea) | PDS | 24/19 | CRS ± HIPEC | PTX 175 mg/m2 | Open | 43–44, 90’ | PFS/OS |
| Le Brun et al. [23] (CC/1997–2011/France) | SCR | 19/23 | NACT+ CRS ± HIPEC | CDDP or OX or MMC | NA | 42, Cis 60’, OX 30’, MMC 30’ | OS |
| Lee et al., 2022 [24] (Cohort/2015–2019/South Korea) | IDS | 80/43 | NACT + CRS ± HIPEC | CDDP 100 mg/m2 or PTX 175 mg/m2 | Closed | 42, 90’ | PFS/OS/AE |
| Lee et al., 2023 [25] (Cohort/2017–2022/South Korea) | IDS | 87/109 | CRS ± HIPEC | CDDP or PTX | Open and closed | 42, 90’ | OS/PFS/AE |
| Lei et al. [26] (Cohort/2010–2017/China) | PDS | 159/425 | CRS ± HIPEC | CDDP 50 mg/m2 | Closed | 43, 60’ | OS/AE |
| Lim et al. [27] (RCT/2010–2016/South Korea) | PDS or IDS | 92/92 | NACT+ CRS ± HIPEC | CDDP 75 mg/m2 | Closed | 41.5, 90’ | OS/PFS/AE |
| Marocco et al. [28] (Cohort/1995–2012/Italy) | SCR | 11/19 | NACT + CRS ± HIPEC | CDDP 100 mg/m2 + DXR 15.2 mg | Semi-closed | 41.5, 60’ | PFS/OS |
| Mendivil et al. [29] (Cohort/2008–2014/USA) | PDS | 69/69 | CRS ± HIPEC | CB AUC10 | Closed | 41.5, 90’ | PFS/OS |
| Muñoz-Casares et al. [30] (Cohort/1997–2004/Spain) | SCR | 12/14 | CRS ± HIPEC | PTX 60 mg/m2 | Open | 41–43, 60’ | OS/AE |
| Spiliotis et al. (PR) [31] (RCT/2006–2013/Greece) | SCR | 24/22 | CRS ± HIPEC | DXR 35 mg/m2 + PTX 175 mg/m2 | Open and closed | 42.5, 60’ | RFS/OS |
| Spiliotis et al. (PS) [31] (RCT/2006–2013/Greece) | SCR | 36/38 | CRS ± HIPEC | CDDP 100 mg/m2 + PTX 175 mg/m2 | Open and closed | 42.5, 60’ | RFS/OS |
| Van Driel et al. [32] (RCT/2007–2016/Netherlands) | IDS | 123/122 | NACT + CRS ± HIPEC | CDDP 100 mg/m2 | Open | 40, 120’ | OS/PFS/AE |
| Warschkow et al. (PDS) [33] (Cohort/1991–2006/Switzerland) | PDS | 43/10 | CRS ± HIPEC | CDDP 50 mg/m2 | NA | 42, 90’ | OS/AE |
| Warschkow et al. (SCR) [33] (Cohort/1991–2006/Switzerland) | SCR | 47/11 | CRS ± HIPEC | CDDP 50 mg/m2 | NA | 42, 90’ | OS/AE |
| Zhang et al. [34] (Cohort/2004–2019/China) | PDS | 53/80 | CRS ± HIPEC | CDDP 120 mg + MMC 30 mg DTX/PTX 120 mg + CDDP 30 mg | Open | 43, 60’ | OS/PFS |
| Zivanovic et al. [35] (RCT/2014–2016/USA) | SCR | 49/49 | CRS ± HIPEC | CB 800 mg/m2 | Closed | 41–43, 90’ | OS/PFS |
| Population | Women with primary epithelial ovarian carcinoma in FIGO Stages III or IVa |
| Women with recurrent epithelial ovarian carcinoma | |
| Intervention | With or without neoadjuvant chemotherapy Cytoreductive surgery ± HIPEC ± adjuvant chemotherapy |
| Comparison | CRS ± chemotherapy (neoadjuvant ± adjuvant) vs. CRS + HIPEC ± chemotherapy (neoadjuvant ± adjuvant) |
| Outcome | Overall survival (OS) |
| Progression-free survival (PFS) | |
| Complications | |
| Study design | Randomized controlled trial (RCT) |
| Case-control (CC) | |
| Cohort |
3.2. Quality Assessment
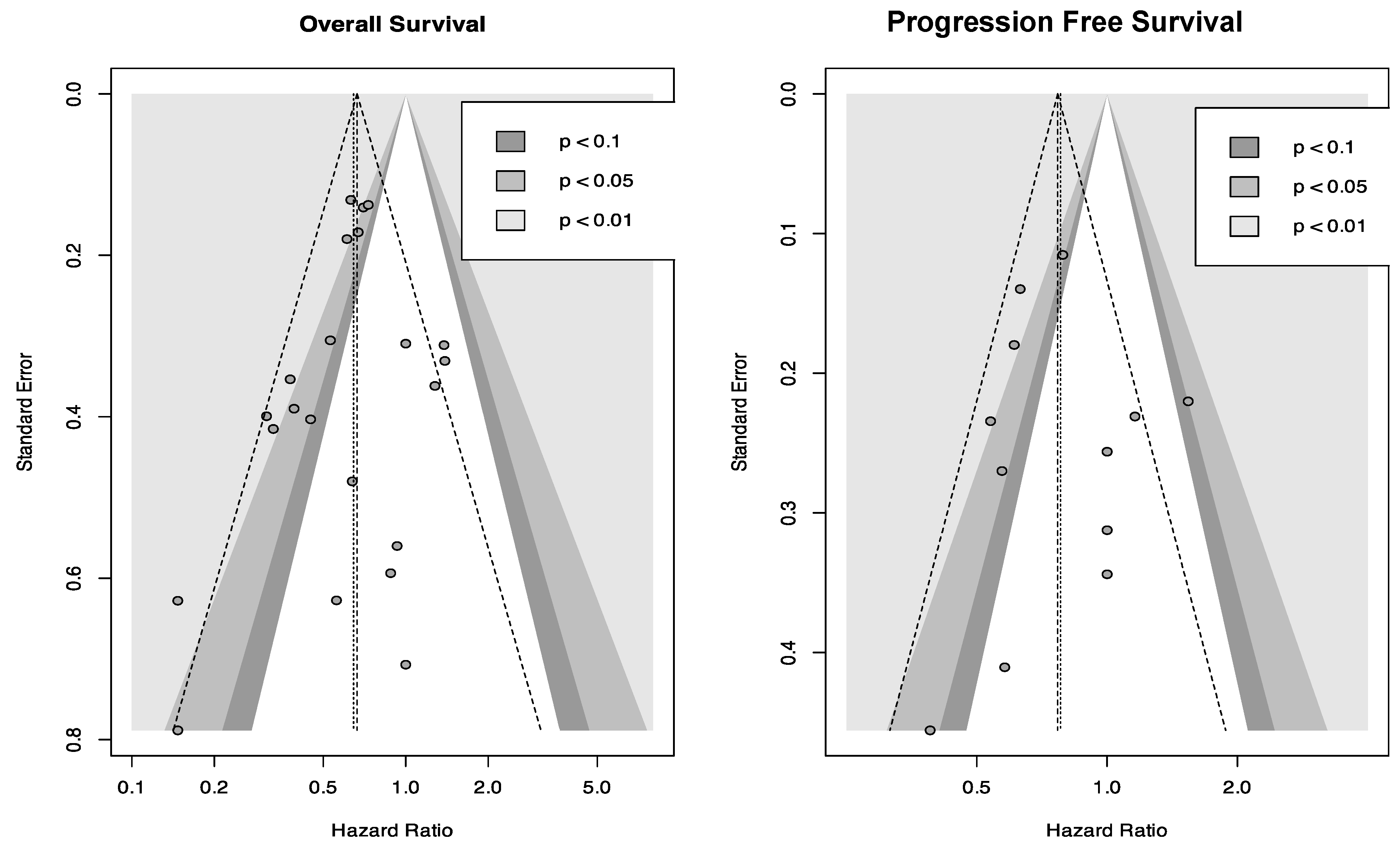
3.3. Risk of Publication Bias
3.4. Statistical Outcomes According to Study Design
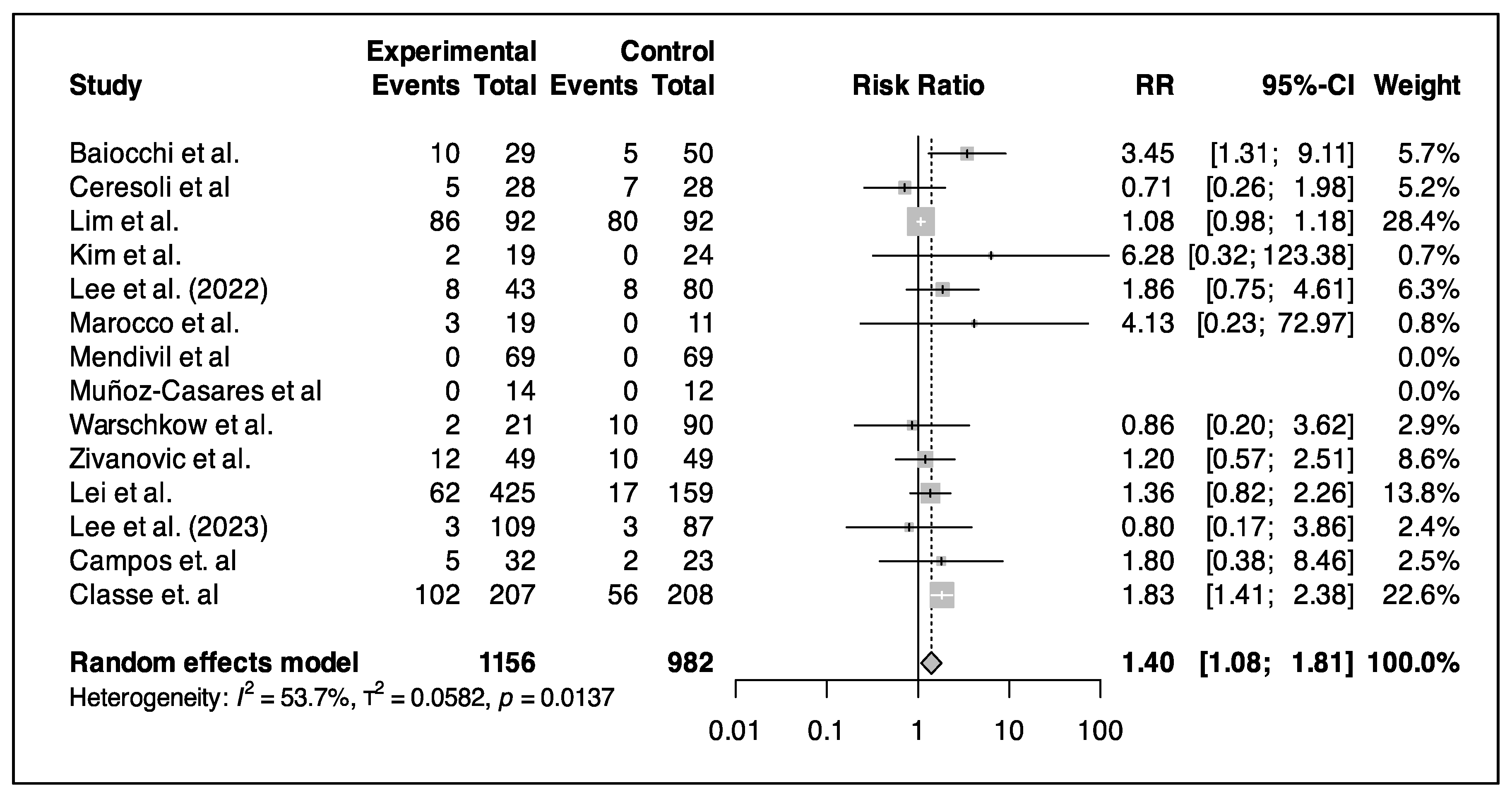
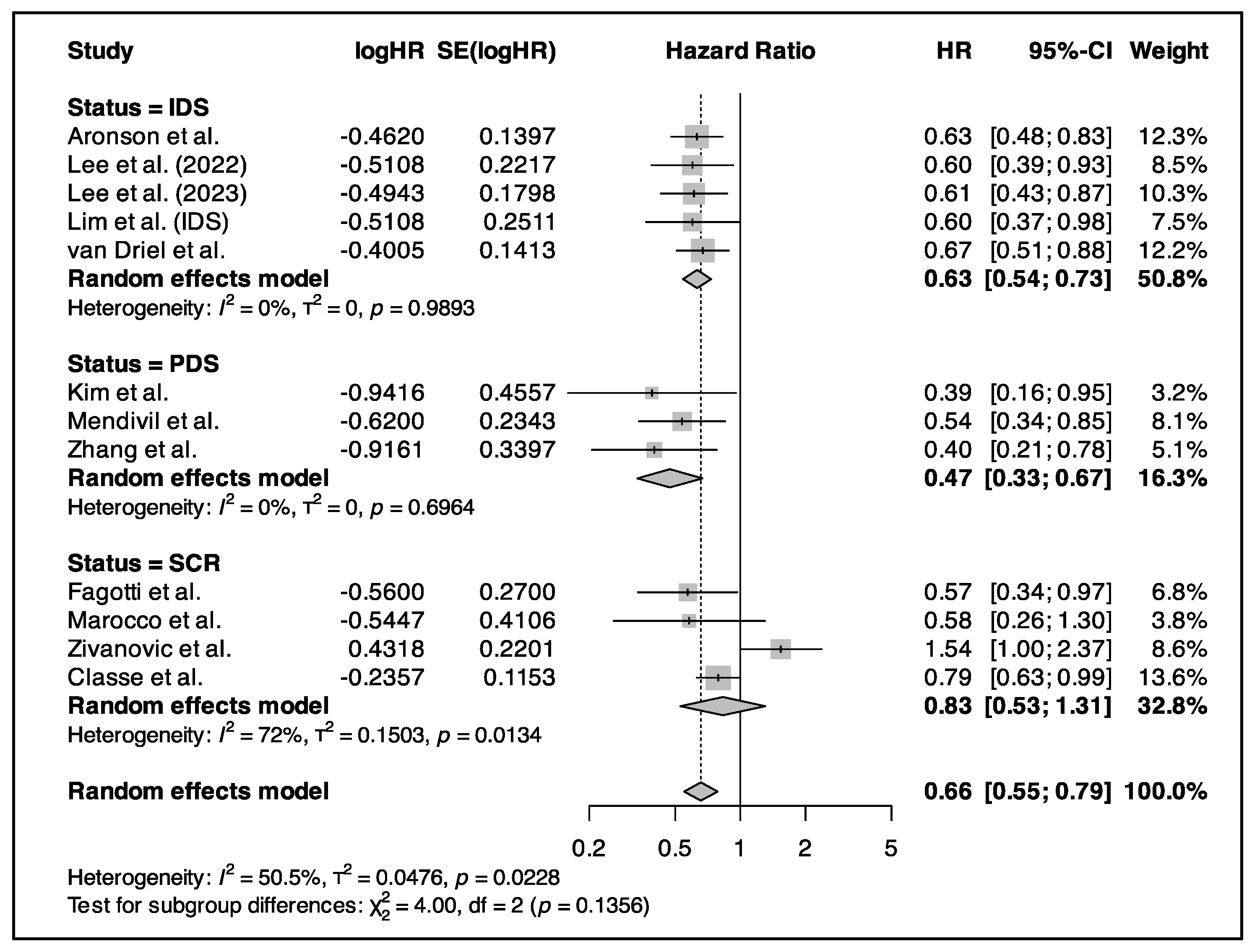
3.5. Primary Outcome (Survival Analysis)
3.5.1. HIPEC in Primary CRS
3.5.2. HIPEC in IDS
3.5.3. HIPEC in Relapse Surgery
3.5.4. HIPEC Regimen and Technique
3.5.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOC | Epithelial ovarian cancer |
| OS | Overall survival |
| PFS | Progression-free survival |
| DFS | Disease-free survival |
| RFS | Recurrence-free survival |
| CRS | Cytoreductive surgery |
| PDS | Primary debulking surgery |
| SCR | Secondary cytoreduction |
| IDS | Interval debulking surgery |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Singh, N.; Ghatage, P. Past, Present, and Future of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer. Cureus 2021, 13, e15563. [Google Scholar] [CrossRef] [PubMed]
- PRISMA Statement. Available online: https://www.prisma-statement.org (accessed on 1 May 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Risk of Bias Tools—RoB 2 Tool. Available online: https://www.riskofbias.info/welcome/rob-2-0-tool (accessed on 1 May 2024).
- Mickenautsch, S.; Yengopal, V. Selection Bias Risk in Randomized Controlled Trials Rated as Low Bias Using Risk of Bias, Version 2 (RoB2) Tool. Cureus 2024, 16, e63581. [Google Scholar] [CrossRef]
- nos_manual.pdf [Internet]. Available online: https://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf (accessed on 5 April 2025).
- automeris.io. Available online: https://automeris.io/WebPlotDigitizer.html (accessed on 1 May 2024).
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 May 2024).
- Wu, M.-F.; Liang, J.-X.; Li, H.; Ye, Y.-F.; Liang, W.-F.; Wang, L.-J.; Zhang, B.-Z.; Chen, Q.; Lin, Z.-Q.; Li, J. Effects of neoadjuvant hyperthermic intraperitoneal chemotherapy on chemotherapy response score and recurrence in high-grade serous ovarian cancer patients with advanced disease: A multicentre retrospective cohort study. BJOG 2022, 129 (Suppl. 2), 5–13. [Google Scholar] [CrossRef]
- Marrelli, D.; Petrioli, R.; Cassetti, D.; D’Ignazio, A.; Marsili, S.; Mazzei, M.A.; Lazzi, S.; Roviello, F. A novel treatment protocol with 6 cycles of neoadjuvant chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in stage III primary ovarian cancer. Surg. Oncol. 2021, 37, 101523. [Google Scholar] [CrossRef]
- Batista, T.P.; Carneiro, V.C.G.; Tancredi, R.; Badiglian Filho, L.; Rangel, R.L.C.; Lopes, A.; Sarmento, B.J.Q.; Leão, C.S. A phase 2 trial of short-course Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) at interval cytoreductive surgery (iCRS) for advanced ovarian cancer. Rev. Col. Bras. Cir. 2022, 49, e20223135. [Google Scholar] [CrossRef]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef]
- Aronson, S.L.; Lopez-Yurda, M.; Koole, S.N.; van Leeuwen, J.H.S.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van Gent, M.D.J.M.; Arts, H.J.G.; Ham, M.A.P.C.v.; et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): Final survival analysis of a randomised, controlled, phase 3 trial. Lancet Oncol. 2023, 24, 1109–1118. [Google Scholar] [CrossRef]
- Baiocchi, G.; Ferreira, F.O.; Mantoan, H.; da Costa, A.A.B.A.; Faloppa, C.C.; Kumagai, L.Y.; de Mello, C.A.L.; Takahashi, R.M.; Nakagawa, W.T.; Aguiar, S.; et al. Hyperthermic Intraperitoneal Chemotherapy after Secondary Cytoreduction in Epithelial Ovarian Cancer: A Single-center Comparative Analysis. Ann. Surg. Oncol. 2016, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Villarejo Campos, P.; Sánchez García, S.; Amo-Salas, M.; García Santos, E.; López de la Manzanara, C.; Alberca, A.; Padilla-Valverde, D.; Redondo Calvo, F.J.; Martín, J. Paclitaxel as HIPEC-Drug after Surgical Cytoreduction for Ovarian Peritoneal Metastases: A Randomized Phase III Clinical Trial (HIPECOVA). Curr. Oncol. 2024, 31, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, M.; Verrengia, A.; Montori, G.; Busci, L.; Coccolini, F.; Ansaloni, L.; Frigerio, L. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: A propensity score based case-control study. J. Gynecol. Oncol. 2018, 29, e53. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.-M.; Meeus, P.; Hudry, D.; Wernert, R.; Quenet, F.; Marchal, F.; Houvenaeghel, G.; Bats, A.-S.; Lecuru, F.; Ferron, G.; et al. Hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer (CHIPOR): A randomised, open-label, phase 3 trial. Lancet Oncol. 2024, 25, 1551–1562. [Google Scholar] [CrossRef]
- Fagotti, A.; Costantini, B.; Petrillo, M.; Vizzielli, G.; Fanfani, F.; Margariti, P.A.; Turco, L.C.; Piovano, E.; Scambia, G. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: A case-control study on survival in patients with two year follow-up. Gynecol. Oncol. 2012, 127, 502–505. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.M.; Ryu, K.S.; Lee, Y.S.; Park, Y.G.; Hur, S.Y.; Lee, K.H.; Lee, S.H.; Kim, S.J. Consolidation hyperthermic intraperitoneal chemotherapy using paclitaxel in patients with epithelial ovarian cancer. J. Surg. Oncol. 2010, 101, 149–155. [Google Scholar] [CrossRef]
- Le Brun, J.-F.; Campion, L.; Berton-Rigaud, D.; Lorimier, G.; Marchal, F.; Ferron, G.; Oger, A.S.; Dravet, F.; Jaffre, I.; Classe, J.-M. Survival benefit of hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer: A multi-institutional case control study. Ann. Surg. Oncol. 2014, 21, 3621–3627. [Google Scholar] [CrossRef]
- Lee, Y.J.; Seon, K.E.; Jung, D.C.; Lee, J.-Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Interval debulking surgery with or without hyperthermic intraperitoneal chemotherapy in advanced-stage ovarian cancer: Single-institution cohort study. Front. Oncol. 2022, 12, 936099. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, Y.J.; Son, J.-H.; Kim, S.; Choi, M.C.; Suh, D.H.; Song, J.-Y.; Hong, D.G.; Kim, M.K.; Kim, J.-H.; et al. Hyperthermic Intraperitoneal Chemotherapy After Interval Cytoreductive Surgery for Patients With Advanced-Stage Ovarian Cancer Who Had Received Neoadjuvant Chemotherapy. JAMA Surg. 2023, 158, 1133–1140. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Y.; Wang, J.; Wang, K.; Tian, J.; Zhao, Y.; Chen, L.; Wang, J.; Luo, J.; Jia, M.; et al. Evaluation of Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer. JAMA Netw. Open 2020, 3, e2013940. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Chang, S.-J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.-Y.; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Marocco, F.; Vaira, M.; Milani, A.; Genta, S.; Maggiorotto, F.; Magistris, A.; Cinquegrana, A.; Robella, M.; De Simone, M.; Aglietta, M.; et al. Secondary cytoreductive surgery, hyperthermic intraperitoneal intraoperative chemotherapy, and chemotherapy alone: A retrospective comparison of alternative approaches in relapsed platinum sensitive ovarian cancer. Eur. J. Gynaecol. Oncol. 2016, 37, 638–643. [Google Scholar] [PubMed]
- Mendivil, A.A.; Rettenmaier, M.A.; Abaid, L.N.; Brown, J.V.; Mori, K.M.; Lopez, K.L.; Goldstein, B.H. Consolidation hyperthermic intraperitoneal chemotherapy for the treatment of advanced stage ovarian carcinoma: A 3 year experience. Cancer Chemother. Pharmacol. 2017, 80, 405–410. [Google Scholar] [CrossRef]
- Muñoz-Casares, F.C.; Rufián, S.; Rubio, M.J.; Díaz, C.J.; Díaz, R.; Casado, A.; Arjona, A.; Muñoz-Villanueva, M.C.; Muntané, J. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin. Transl. Oncol. 2009, 11, 753–759. [Google Scholar] [CrossRef]
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Warschkow, R.; Tarantino, I.; Lange, J.; Müller, S.A.; Schmied, B.M.; Zünd, M.; Steffen, T. Does hyperthermic intraoperative chemotherapy lead to improved outcomes in patients with ovarian cancer? A single center cohort study in 111 consecutive patients. Patient Saf. Surg. 2012, 6, 12. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.-B.; Ma, R.; Ji, Z.-H.; Bai, W.; Li, Y. Long term survival of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy in advanced epithelial ovarian cancer. Transl. Cancer Res. TCR 2021, 10, 3705–3715. [Google Scholar] [CrossRef]
- Zivanovic, O.; Chi, D.S.; Zhou, Q.; Iasonos, A.; Konner, J.A.; Makker, V.; Grisham, R.N.; Brown, A.K.; Nerenstone, S.; Diaz, J.P.; et al. Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study. J. Clin. Oncol. 2021, 39, 2594–2604. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.H.; Lee, S.; Cho, H.; van Driel, W.J.; Sonke, G.S.; Bristow, R.E.; Park, S.-Y.; Fotopoulou, C.; Lim, M.C. Hyperthermic intraperitoneal chemotherapy for epithelial ovarian cancer: A meta-analysis. Gynecol. Oncol. 2022, 167, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Sehouli, J.; Mahner, S.; Harter, P.; Van Nieuwenhuysen, E.; Gonzalez-Martin, A.; Vergote, I.; Chiva, L.; Du Bois, A. HIPEC: HOPE or HYPE in the fight against advanced ovarian cancer? Ann. Oncol. 2018, 29, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, L.; Conte, C.; Palumbo, M.; Guerra, S.; Colacurci, D.; Riemma, G.; De Franciscis, P.; Giampaolino, P.; Fagotti, A.; Bifulco, G.; et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer-Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7012. [Google Scholar] [CrossRef] [PubMed]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef]
- Cianci, S.; Riemma, G.; Ronsini, C.; De Franciscis, P.; Torella, M.; Schiattarella, A.; La Verde, M.; Colacurci, N. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer recurrence: Systematic review and meta-analysis. Gland. Surg. 2020, 9, 1140–1148. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.-M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Canc. Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Santana, B.N.; Garcia-Torralba, E.; Viveros-Carreño, D.; Rodriguez, J.; Pareja, R.; Martin, A.; Forte, S.; Krause, K.J.; González-Martín, J.M.; Ramirez, P.T. Complications of HIPEC for ovarian cancer surgery: Evaluation over two time periods. Int. J. Gynecol. Cancer 2024, 34, 1–9. [Google Scholar] [CrossRef]
- Narasimhan, V.; Tan, S.; Kong, J.; Pham, T.; Michael, M.; Ramsay, R.; Warrier, S.; Heriot, A. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: A systematic review and meta-analysis. Colorectal. Dis. 2020, 22, 1482–1495. [Google Scholar] [CrossRef]
- Koole, S.; van Stein, R.; Sikorska, K.; Barton, D.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.E.; et al. OVHIPEC-2 Steering Committee and the Dutch OVHIPEC group. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer 2020, 30, 888–892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kassis, N.; Jerbaka, M.; Abou Chakra, R.; El Hadi, C.; Arab, W.; El Hajj, H.; Brennan, D.J.; Atallah, D. An Updated Meta-Analysis on Long-Term Outcomes Following Hyperthermic Intraperitoneal Chemotherapy in Advanced Ovarian Cancer. Cancers 2025, 17, 1569. https://doi.org/10.3390/cancers17091569
El Kassis N, Jerbaka M, Abou Chakra R, El Hadi C, Arab W, El Hajj H, Brennan DJ, Atallah D. An Updated Meta-Analysis on Long-Term Outcomes Following Hyperthermic Intraperitoneal Chemotherapy in Advanced Ovarian Cancer. Cancers. 2025; 17(9):1569. https://doi.org/10.3390/cancers17091569
Chicago/Turabian StyleEl Kassis, Nadine, Myriam Jerbaka, Rime Abou Chakra, Christopher El Hadi, Wissam Arab, Houssein El Hajj, Donal J. Brennan, and David Atallah. 2025. "An Updated Meta-Analysis on Long-Term Outcomes Following Hyperthermic Intraperitoneal Chemotherapy in Advanced Ovarian Cancer" Cancers 17, no. 9: 1569. https://doi.org/10.3390/cancers17091569
APA StyleEl Kassis, N., Jerbaka, M., Abou Chakra, R., El Hadi, C., Arab, W., El Hajj, H., Brennan, D. J., & Atallah, D. (2025). An Updated Meta-Analysis on Long-Term Outcomes Following Hyperthermic Intraperitoneal Chemotherapy in Advanced Ovarian Cancer. Cancers, 17(9), 1569. https://doi.org/10.3390/cancers17091569