Heart and Left Anterior Descending Coronary Artery (LAD) Exposure from Hypo-Fractionated Whole Breast Radiotherapy with a Prone Setup
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment Simulation and Verification Procedures
2.3. Definition of Planning Volumes
2.4. Treatment Planning, Dose, and Fields Definition
2.5. Data Collection and Statistics Evaluation
3. Results
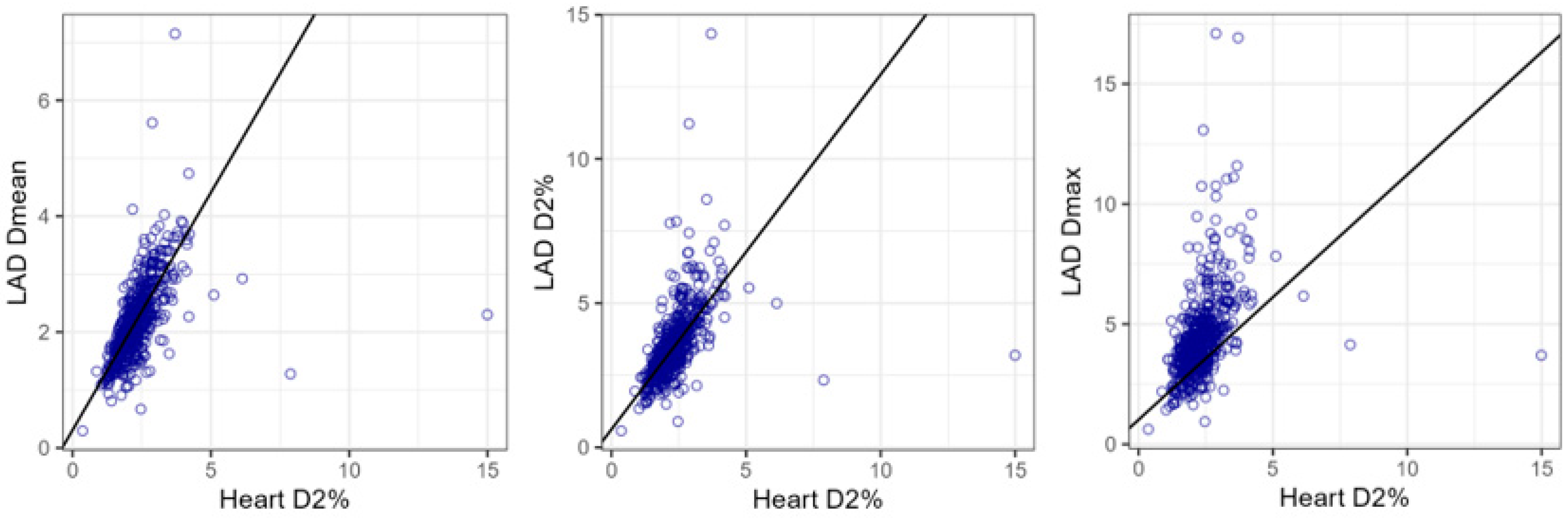
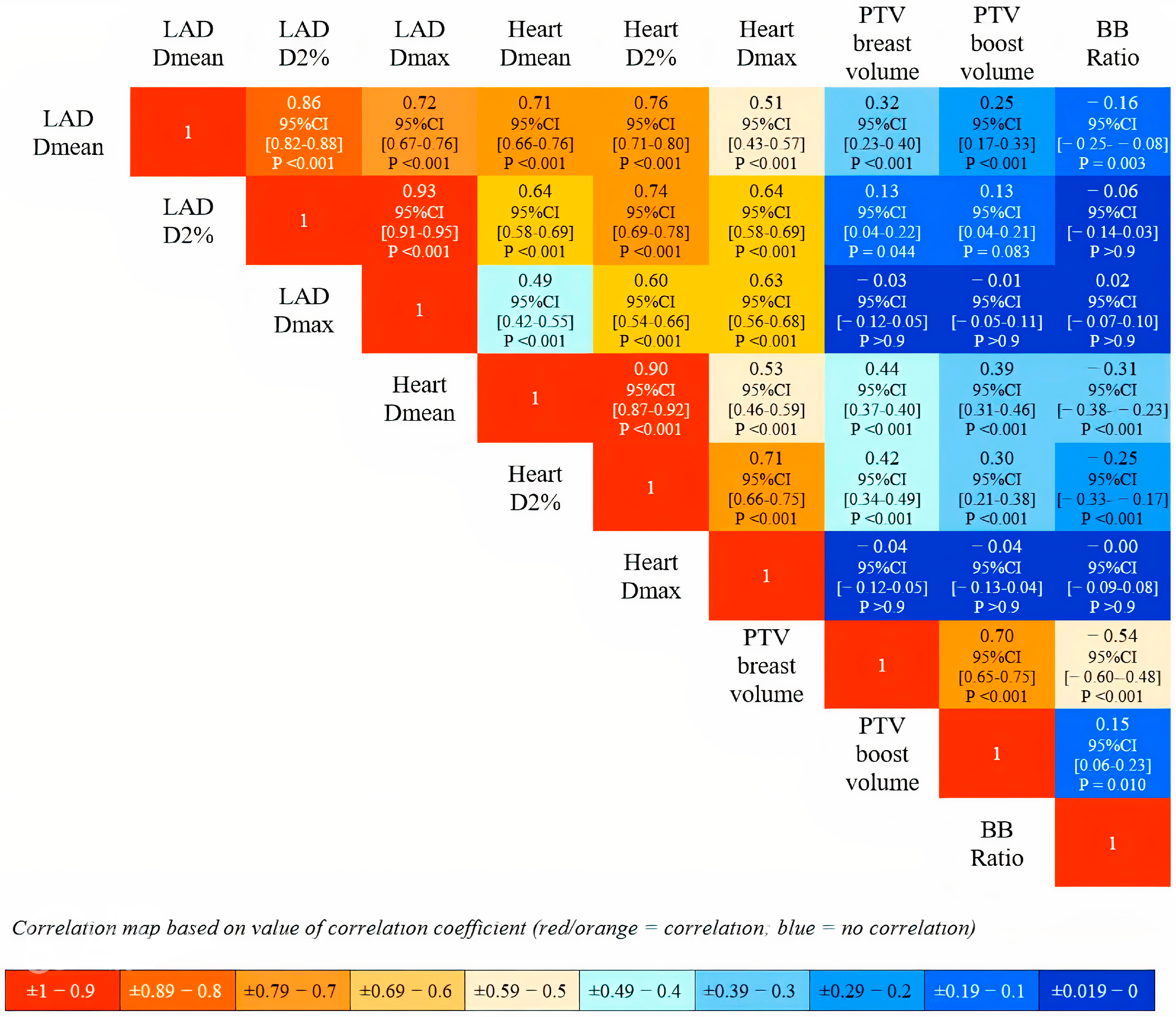
- LAD Dmean (Gy) = 0.31 + 0.82 × Heart D2% (Gy);
- LAD D2% (Gy) = 0.63 + 1.23 × Heart D2% (Gy);
- LAD Dmax (Gy) = 1.02 + 1.38 × Heart D2% (Gy).
| Response Variable = LAD Dmean (Gy) | ||||||
| Explanatory variables | Estimate | 95%CI | SE | T value | p value for t-test | R2 |
| Intercept (Gy) | 0.31 | 0.19∓0.44 | 0.06 | 4.92 | 1 × 10−6 | 0.64 |
| Heart Dmean (Gy) | ∓ | – | – | – | – | |
| Heart D2% (Gy) | 0.82 | 0.76–0.87 | 0.03 | 30.08 | 5 × 10−116 | |
| Heart Dmax (Gy) | – | – | – | – | – | |
| Response variable = LAD D2% (Gy) | ||||||
| Explanatory variables | Estimate | 95%CI | SE | T value | p value for t-test | R2 |
| Intercept (Gy) | 0.63 | 0.43–0.83 | 0.10 | 6.13 | 2 × 10−9 | 0.60 |
| Heart Dmean (Gy) | – | – | – | – | – | |
| Heart D2% (Gy) | 1.23 | 1.14–1.31 | 0.04 | 28.21 | 5 × 10−107 | |
| Heart Dmax (Gy) | – | – | – | – | – | |
| Response variable = LAD Dmax (Gy) | ||||||
| Explanatory variables | Estimate | 95%CI | SE | T value | p value for t-test | R2 |
| Intercept (Gy) | 1.02 | 0.69–1.36 | 0.17 | 5.96 | 5 × 10−9 | 0.41 |
| Heart Dmean (Gy) | – | – | – | – | – | |
| Heart D2% (Gy) | 1.38 | 1.24–1.53 | 0.07 | 19.09 | 5 × 10−62 | |
| Heart Dmax (Gy) | – | – | – | – | – | |
Comparison with LAD and Heart Dosimetry from Supine Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, E.E.; Correa, C.; Hwang, W.T.; Liao, J.; Litt, H.I.; Ferrari, V.A.; Solin, L.J. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J. Clin. Oncol. 2006, 24, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Moran, J.M.; Koelling, T.; Chughtai, A.; Chan, J.L.; Freedman, L.; Hayman, J.A.; Jagsi, R.; Jolly, S.; Larouere, J.; et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 10–18. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, V.A.; Ta, B.D.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.; Bantema-Joppe, E.J.; van Dijk, L.V.; van Dijk-Peters, F.B.; Marteijn, L.A.; de Bock, G.H.; et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients with Breast Cancer Treated with Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef]
- van den Bogaard, V.A.B.; Spoor, D.S.; van der Schaaf, A.; van Dijk, L.V.; Schuit, E.; Sijtsema, N.M.; Langendijk, J.A.; Maduro, J.H.; Crijns, A.P.G. The Importance of Radiation Dose to the Atherosclerotic Plaque in the Left Anterior Descending Coronary Artery for Radiation-Induced Cardiac Toxicity of Breast Cancer Patients? Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1350–1359. [Google Scholar] [CrossRef]
- Tagami, T.; Almahariq, M.F.; Balanescu, D.V.; Quinn, T.J.; Dilworth, J.T.; Franklin, B.A.; Bilolikar, A. Usefulness of Coronary Computed Tomographic Angiography to Evaluate Coronary Artery Disease in Radiotherapy-Treated Breast Cancer Survivors. Am J Cardiol. 2021, 143, 14–20. [Google Scholar] [CrossRef]
- Zureick, A.H.; Grzywacz, V.P.; Almahariq, M.F.; Silverman, B.R.; Vayntraub, A.; Chen, P.Y.; Gustafson, G.S.; Jawad, M.S.; Dilworth, J.T. Dose to the Left Anterior Descending Artery Correlates with Cardiac Events After Irradiation for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 130–139. [Google Scholar] [CrossRef]
- DeWyngaert, J.K.; Jozsef, G.; Mitchell, J.; Rosenstein, B.; Formenti, S.C. Accelerated intensity-modulated radiotherapy to breast in prone position: Dosimetric results. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1251–1259. [Google Scholar] [CrossRef]
- Formenti, S.C.; Gidea-Addeo, D.; Goldberg, J.D.; Roses, D.F.; Guth, A.; Rosenstein, B.S.; DeWyngaert, K.J. Phase I-II trial of prone accelerated intensity modulated radiation therapy to the breast to optimally spare normal tissue. J. Clin. Oncol. 2007, 25, 2236–2242. [Google Scholar] [CrossRef]
- Osa, E.O.; DeWyngaert, K.; Roses, D.; Speyer, J.; Guth, A.; Axelrod, D.; Fenton Kerimian, M.; Goldberg, J.D.; Formenti, S.C. Prone breast intensity modulated radiation therapy: 5-year results. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.T.; Li, X.; Shin, S.M.; Modrek, A.S.; Hsu, H.C.; DeWyngaert, J.K.; Jozsef, G.; Lymberis, S.C.; Goldberg, J.D.; Formenti, S.C. Preplanning prediction of the left anterior descending artery maximum dose based on patient, dosimetric, and treatment planning parameters. Adv. Radiat. Oncol. 2016, 1, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Huppert, N.; Jozsef, G.; Dewyngaert, K.; Formenti, S.C. The role of a prone setup in breast radiation therapy. Front Oncol. 2011, 1, 31. [Google Scholar] [CrossRef]
- Mitchell, J.; Formenti, S.C.; DeWyngaert, J.K. Interfraction and intrafraction setup variability for prone breast radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Wang, Z.; Macaulay, E.; Jagsi, R.; Duane, F.; Darby, S.C. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 845–853. [Google Scholar] [CrossRef]
- Drost, L.; Yee, C.; Lam, H.; Zhang, L.; Wronski, M.; McCann, C.; Lee, J.; Vesprini, D.; Leung, E.; Chow, E. A Systematic Review of Heart Dose in Breast Radiotherapy. Clin. Breast Cancer 2018, 18, e819–e824. [Google Scholar] [CrossRef]
- Lai, J.; Hu, S.; Luo, Y.; Zheng, R.; Zhu, Q.; Chen, P.; Chi, B.; Zhang, Y.; Zhong, F.; Long, X. Meta-analysis of deep inspiration breath hold (DIBH) versus free breathing (FB) in postoperative radiotherapy for left-side breast cancer. Breast Cancer 2020, 27, 299–307. [Google Scholar] [CrossRef]
- Lai, J.; Zhong, F.; Deng, J.; Hu, S.; Shen, R.; Luo, H.; Luo, Y. Prone position versus supine position in postoperative radiotherapy for breast cancer: A meta-analysis. Medicine 2021, 100, e26000. [Google Scholar] [CrossRef]
- Smyth, L.M.; Knight, K.A.; Aarons, Y.K.; Wasiak, J. The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: A systematic review. J. Med. Radiat. Sci. 2015, 62, 66–73. [Google Scholar] [CrossRef]
- Jacob, S.; Camilleri, J.; Derreumaux, S.; Walker, V.; Lairez, O.; Lapeyre, M.; Bruguière, E.; Pathak, A.; Bernier, M.O.; Laurier, D.; et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: A dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat. Oncol. 2019, 14, 29. [Google Scholar] [CrossRef]
- Bartlett, F.R.; Colgan, R.M.; Donovan, E.M.; McNair, H.A.; Carr, K.; Evans, P.M.; Griffin, C.; Locke, I.; Haviland, J.S.; Yarnold, J.R.; et al. The UK HeartSpare Study (Stage IB): Randomised comparison of a voluntary breath-hold technique and prone radiotherapy after breast conserving surgery. Radiother. Oncol. 2015, 114, 66–72. [Google Scholar] [CrossRef]
- Pierce, L.J.; Feng, M.; Griffith, K.A.; Jagsi, R.; Boike, T.; Dryden, D.; Gustafson, G.S.; Benedetti, L.; Matuszak, M.M.; Nurushev, T.S.; et al. Recent Time Trends and Predictors of Heart Dose from Breast Radiation Therapy in a Large Quality Consortium of Radiation Oncology Practices. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1154–1161. [Google Scholar] [CrossRef]
- Jimenez, R.B.; Hickey, S.; DePauw, N.; Yeap, B.Y.; Batin, E.; Gadd, M.A.; Specht, M.; Isakoff, S.J.; Smith, B.L.; Liao, E.C.; et al. Phase II Study of Proton Beam Radiation Therapy for Patients With Breast Cancer Requiring Regional Nodal Irradiation. J. Clin. Oncol. 2019, 37, 2778–2785. [Google Scholar] [CrossRef]
- Correa, C.R.; Litt, H.I.; Hwang, W.T.; Ferrari, V.A.; Solin, L.J.; Harris, E.E. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J. Clin. Oncol. 2007, 25, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Duvernoy, O.; Sjögren, I.; Lagerqvist, B.; Blomqvist, C. Distribution of coronary artery stenosis after radiation for breast cancer. J. Clin. Oncol. 2012, 30, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665. [Google Scholar] [CrossRef]
- López-Fernández, T.; Marco, I.; Aznar, M.C.; Barac, A.; Bergler-Klein, J.; Meattini, I.; Scott, J.M.; Cardinale, D.; Dent, S. Breast cancer and cardiovascular health. Eur. Heart J. 2024, 45, 4366–4382. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, T.; Farmakis, D.; Ameri, P.; Asteggiano, R.; de Azambuja, E.; Aznar, M.; Barac, A.; Bayes-Genis, A.; Bax, J.J.; Bergler-Klein, J.; et al. European Society of Cardiology core curriculum for cardio-oncology. Eur. J. Heart Fail. 2024, 26, 754–771. [Google Scholar] [CrossRef]
- Brenner, D.J.; Shuryak, I.; Jozsef, G.; Dewyngaert, K.J.; Formenti, S.C. Risk and risk reduction of major coronary events associated with contemporary breast radiotherapy. JAMA Intern. Med. 2014, 174, 158–160. [Google Scholar] [CrossRef]
- Piroth, M.D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther. Onkol. 2019, 195, 1–12. [Google Scholar] [CrossRef]
- Griem, K.L.; Fetherston, P.; Kuznetsova, M.; Foster, G.S.; Shott, S.; Chu, J. Three-dimensional photon dosimetry: A comparison of treatment of the intact breast in the supine and prone position. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 891–899. [Google Scholar] [CrossRef]
- Chino, J.P.; Marks, L.B. Prone positioning causes the heart to be displaced anteriorly within the thorax: Implications for breast cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Lymberis, S.C.; Formenti, S.C. Prone-breast radiotherapy: Too early for conclusions: In regard to Chino et al. (Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 916–920). Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 301–302, author reply 302. [Google Scholar] [CrossRef] [PubMed]
- Vakaet, V.; Deseyne, P.; Bultijnck, R.; Post, G.; West, C.; Azria, D.; Bourgier, C.; Farcy-Jacquet, M.P.; Rosenstein, B.; Green, S.; et al. Comparison of prone and supine positioning for breast cancer radiotherapy using REQUITE data: Dosimetry, acute and two years physician and patient reported outcomes. Acta Oncol. 2023, 62, 1036–1044. [Google Scholar] [CrossRef]
- Deseyne, P.; Speleers, B.; De Neve, W.; Boute, B.; Paelinck, L.; Van Hoof, T.; Van de Velde, J.; Van Greveling, A.; Monten, C.; Post, G.; et al. Whole breast and regional nodal irradiation in prone versus supine position in left sided breast cancer. Radiat. Oncol. 2017, 12, 89. [Google Scholar] [CrossRef]
- Speleers, B.; Schoepen, M.; Belosi, F.; Vakaet, V.; De Neve, W.; Deseyne, P.; Paelinck, L.; Vercauteren, T.; Parkes, M.J.; Lomax, T.; et al. Effects of deep inspiration breath hold on prone photon or proton irradiation of breast and regional lymph nodes. Sci. Rep. 2021, 11, 6085. [Google Scholar] [CrossRef] [PubMed]
- Deseyne, P.; Speleers, B.; Paelinck, L.; De Gersem, W.; De Neve, W.; Schoepen, M.; Van Greveling, A.; Van Hulle, H.; Vakaet, V.; Post, G.; et al. Reproducibility of repeated breathhold and impact of breathhold failure in whole breast and regional nodal irradiation in prone crawl position. Sci. Rep. 2022, 12, 1887. [Google Scholar] [CrossRef]
- Kim, D.W.; Hong, C.S.; Son, J.; Kim, S.Y.; Park, Y.I.; Chung, M.; Chung, W.K.; Han, M.C.; Kim, J.; Kim, H.; et al. Dosimetric analysis of six whole-breast irradiation techniques in supine and prone positions. Sci. Rep. 2024, 14, 14347. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, B.; Zhang, R. Cost-effectiveness analysis of radiotherapy techniques for whole breast irradiation. PLoS ONE 2021, 16, e0248220. [Google Scholar] [CrossRef]
- Jozsef, G.; DeWyngaert, J.K.; Becker, S.J.; Lymberis, S.; Formenti, S.C. Prospective study of cone-beam computed tomography image-guided radiotherapy for prone accelerated partial breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 568–574. [Google Scholar] [CrossRef]
- Macrie, B.D.; Donnelly, E.D.; Hayes, J.P.; Gopalakrishnan, M.; Philip, R.T.; Reczek, J.; Prescott, A.; Strauss, J.B. A cost-effective technique for cardiac sparing with deep inspiration-breath hold (DIBH). Phys. Med. 2015, 31, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Mutter, R.W.; Choi, J.I.; Jimenez, R.B.; Kirova, Y.M.; Fagundes, M.; Haffty, B.G.; Amos, R.A.; Bradley, J.A.; Chen, P.Y.; Ding, X.; et al. Proton Therapy for Breast Cancer: A Consensus Statement From the Particle Therapy Cooperative Group Breast Cancer Subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Bekelman, J.E.; Lu, H.; Pugh, S.; Baker, K.; Berg, C.D.; Berrington de González, A.; Braunstein, L.Z.; Bosch, W.; Chauhan, C.; Ellenberg, S.; et al. Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: The Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open 2019, 9, e025556. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. The DBCG proton trial: Photon versus proton radiation therapy for early breast cancer. The DBCG Proton Trial: Photon Versus Proton Radiation Therapy for Early Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT04291378 (accessed on 9 March 2025).
- Sethi, R.A.; No, H.S.; Jozsef, G.; Ko, J.P.; Formenti, S.C. Comparison of three-dimensional versus intensity-modulated radiotherapy techniques to treat breast and axillary level III and supraclavicular nodes in a prone versus supine position. Radiother. Oncol. 2012, 102, 74–81. [Google Scholar] [CrossRef]
- Formenti, S.C.; DeWyngaert, J.K.; Jozsef, G.; Goldberg, J.D. Prone vs. supine positioning for breast cancer radiotherapy. JAMA 2012, 308, 861–863. [Google Scholar] [CrossRef]


| Structure | Variable | Mean | SD | Median | IQR | Range |
|---|---|---|---|---|---|---|
| Heart | ||||||
| MHD (Gy) | 0.69 | 0.19 | 0.67 | 0.56–0.78 | 0.12–1.88 | |
| EQD2 (Gy) | 0.35 | 0.10 | 0.34 | 0.29–0.40 | 0.06–1.00 | |
| Dmax (Gy) | 7.92 | 6.32 | 5.02 | 3.71–8.75 | 0.57–39.82 | |
| EQD2 (Gy) | 5.01 | 3.83 | 2.93 | 2.08–5.65 | 0.29–46.34 | |
| D2% (Gy) | 2.35 | 0.88 | 2.27 | 1.86–2.65 | 0.35–7.87 | |
| EQD2 (Gy) | 1.27 | 0.45 | 1.22 | 0.99–1.44 | 0.18–4.97 | |
| LAD | ||||||
| Dmean (Gy) | 2.20 | 0.68 | 2.13 | 1.74–2.56 | 0.29–5.61 | |
| EQD2 (Gy) | 1.18 | 0.35 | 1.14 | 0.92–1.39 | 0.15–3.33 | |
| Dmax (Gy) | 4.44 | 1.82 | 4.09 | 3.37–5.07 | 0.61–16.92 | |
| EQD2 (Gy) | 2.55 | 0.97 | 2.32 | 1.87–2.96 | 0.31–13.23 | |
| D2% (Gy) | 3.57 | 1.25 | 3.32 | 2.83–4.08 | 0.57–11.22 | |
| EQD2 (Gy) | 2.00 | 0.65 | 1.84 | 1.55–2.32 | 0.29–7.71 | |
| PTV breast | ||||||
| V95% (%) | 101.4 | 1.52 | 101.3 | 100.5–101.9 | 99.61–107.58 | |
| Breast volume | ||||||
| Volume (cc) | 699.6 | 545.5 | 560.2 | 320.8–901.1 | 41.54–3747.50 | |
| PTV boost | ||||||
| V98% (%) | 100.8 | 0.95 | 100.8 | 100.5–101.1 | 99.51–104.73 | |
| Post-operative cavity volume | ||||||
| Volume (cc) | 110.8 | 75.6 | 94.88 | 61.4–141.8 | 9.53–701.04 | |
| Author/ Publication Year | Evaluation Period | Study Design | Treatment Planning, Field Type, Dose/Fractions | Sample Size (Patients/Studies) | MHD for FB Supine Position | MHD for DIBH Supine Position | MHD for Prone Position | LAD Dmean for FB Supine Position | LAD Dmean for DIBH Supine Position | LAD Dmean for Prone Position | LAD Dmax for FB Supine Position | LAD Dmax for DIBH Supine Position | LAD Dmax for Prone Position |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Darby 2013 | 1958–2001 (Sweden) 1977–2000 (Denmark) | Large retrospective study | Oblique anterior, direct anterior, tangential pair; 40–52 Gy/20–28 fx | 2168 patients | 4.9 Gy ^ (SD 4.4) | - | - | - | - | - | - | - | - |
| Taylor 2015 | 2003–2013 | Systematic Review | 3D conformal tangents; IMRT, 40–50 Gy/15–28 fx | NA (149 studies) | 3.8 Gy * (SE 0.2) | 1.3 Gy * (SE 0.1) | 2.4 Gy * (SE 0.5) | - | - | - | - | - | - |
| Smyth 2015 | 1966–2014 | Systematic Review | 3D conformal tangents; IMRT; 50 Gy/25 fx, 40–42 Gy/15–16 fx | 268 patients (10 studies) | 4.29 Gy * | 2.16 Gy * | - | 20.5 Gy * | 10.36 Gy * | - | - | - | - |
| Bartlett 2015 | 2013–2014 | Randomized study | 3D conformal tangents; 40 Gy/15 fx | 34 patients | - | 0.44 Gy ^ (95% CI 0.38–0.51) | 0.66 Gy ^ (95% CI 0.61–0.71) | - | 2.9 Gy ^ (95% CI 1.8–3.9) | 7.8 Gy ^ (95% CI 6.4–9.2) | - | 21.0 Gy ^ (95% CI 15.8–26.2) | 36.8 Gy ^ (95% CI 35.2–38.4) |
| Pierce 2017 | 2012–2015 | Prospective observational large study | 3D conformal tangents; conventional + accelerated fractionation | 4688 patients | 1.32 Gy ^ (Range 1.20–1.45) | 1.08 Gy ^ (Range 1.03–1.14) | 0.89 Gy ^ (Range 0.79–1.00) | - | - | - | - | - | - |
| Drost 2018 | 2014–2017 | Systematic Review | 2D, 3D conformal tangents, IMRT; 50 Gy/25–28 fx, 40–42 Gy/15–16 fx | 11,545 patients (99 studies) | 4.7 Gy * (Range 0.1–18.7) | 1.7 Gy * (Range 0.4–4.8) | 2.3 Gy * (Range 0.7–4.2) | - | - | - | - | - | - |
| Jacob 2019 | 2015–2017 | Prospective study | 3D conformal tangents; 47–50 Gy/20–25 fx | 89 patients | 2.95 Gy ^ (SD 1.49) | - | - | 15.68 Gy ^ (SD 8.13) | - | - | 37.97 Gy ^ (SD 15.93) | - | - |
| Lai 2020 | 2011–2018 | Meta-analysis | NA; 50 Gy/25 fx | 1019 patients (12 studies) | 3.19 Gy * (SD 1.8) | 1.53 Gy * (SD 0.87) | - | 20.42 Gy * (SD 10.15) | 8.57 Gy * (SD 6.68) | - | 36.2 Gy * (SD 11.52) | 18.2 Gy * (SD 13.99) | - |
| Lai 2021 | 2007–2020 | Meta-analysis | NA; 50 Gy/25 fx, 40–42 Gy/15–16 fx | 751 patients (19 studies) | 4.15 Gy * (SD 1.93) | - | 3.18 Gy * (SD 1.45) | 15.49 Gy * (SD 6.8) | - | 12.29 Gy * (SD 6.8) | 35.56 Gy * (SD 11.1) | - | 26.76 Gy * (SD 12.48) |
| van den Bogaard 2021 | 2005–2008 | Large retrospective study | 3D conformal tangents; 50 Gy/28 fx | 910 patients | 2.35 Gy ^ (Range 0.51–15.25) | - | - | 2.82 Gy ^ (Range 0.41–59.33) | - | - | - | - | - |
| Zureick 2022 | 2012–2018 | Large retrospective study | 3D-conformal tangent fields; 50 Gy/25–28 fx; 42 Gy/16 fx | 375 patients | - | 0.8 Gy ^ (IQR 0.6–1.1) | - | - | 1.9 Gy ^ (IQR 1.4–3.2) | - | - | 4.0 Gy ^ (IQR 3–11) | - |
| # Jimenez 2019 | 2011–2019 | Phase II study | 3D-conformal proton therapy; 50.4 Gy (RBE) in 28 fx (chest wall) 45.0 Gy (RBE) in 25 fx (breast) | 63 patients | 0.5 Gy (RBE) (Range 0.1–1.7) | - | - | 1.16 Gy (RBE) (Range 0.09–12) | - | - | - | - | - |
| Our study 2024 | 2016–2023 | Large Retrospective study | 3D conformal tangents; IMRT; 40.5 Gy/15 fx + SIB | 524 patients | - | - | 0.69 Gy ^ (SD 0.19) EQD2 0.35 Gy ^ (SD 0.10) | - | - | 2.20 Gy ^ (SD 0.68) EQD2 1.18 Gy ^ (SD 0.35) | - | - | 4.44 Gy ^ (SD 1.82) EQD2 2.55 Gy ^ (SD 0.97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregucci, F.; Bonzano, E.; Ng, J.; Talebi, F.; Patel, M.; Trick, D.; Chandrasekhar, S.; Zhou, X.K.; Fenton-Kerimian, M.; Pennell, R.; et al. Heart and Left Anterior Descending Coronary Artery (LAD) Exposure from Hypo-Fractionated Whole Breast Radiotherapy with a Prone Setup. Cancers 2025, 17, 1562. https://doi.org/10.3390/cancers17091562
Gregucci F, Bonzano E, Ng J, Talebi F, Patel M, Trick D, Chandrasekhar S, Zhou XK, Fenton-Kerimian M, Pennell R, et al. Heart and Left Anterior Descending Coronary Artery (LAD) Exposure from Hypo-Fractionated Whole Breast Radiotherapy with a Prone Setup. Cancers. 2025; 17(9):1562. https://doi.org/10.3390/cancers17091562
Chicago/Turabian StyleGregucci, Fabiana, Elisabetta Bonzano, John Ng, Fereshteh Talebi, Maahi Patel, Dakota Trick, Sharanya Chandrasekhar, Xi Kathy Zhou, Maria Fenton-Kerimian, Ryan Pennell, and et al. 2025. "Heart and Left Anterior Descending Coronary Artery (LAD) Exposure from Hypo-Fractionated Whole Breast Radiotherapy with a Prone Setup" Cancers 17, no. 9: 1562. https://doi.org/10.3390/cancers17091562
APA StyleGregucci, F., Bonzano, E., Ng, J., Talebi, F., Patel, M., Trick, D., Chandrasekhar, S., Zhou, X. K., Fenton-Kerimian, M., Pennell, R., & Formenti, S. C. (2025). Heart and Left Anterior Descending Coronary Artery (LAD) Exposure from Hypo-Fractionated Whole Breast Radiotherapy with a Prone Setup. Cancers, 17(9), 1562. https://doi.org/10.3390/cancers17091562






