Upfront Oxaliplatin–Fluoropyrimidine Chemotherapy and Somatostatin Analogues in Advanced Well-Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
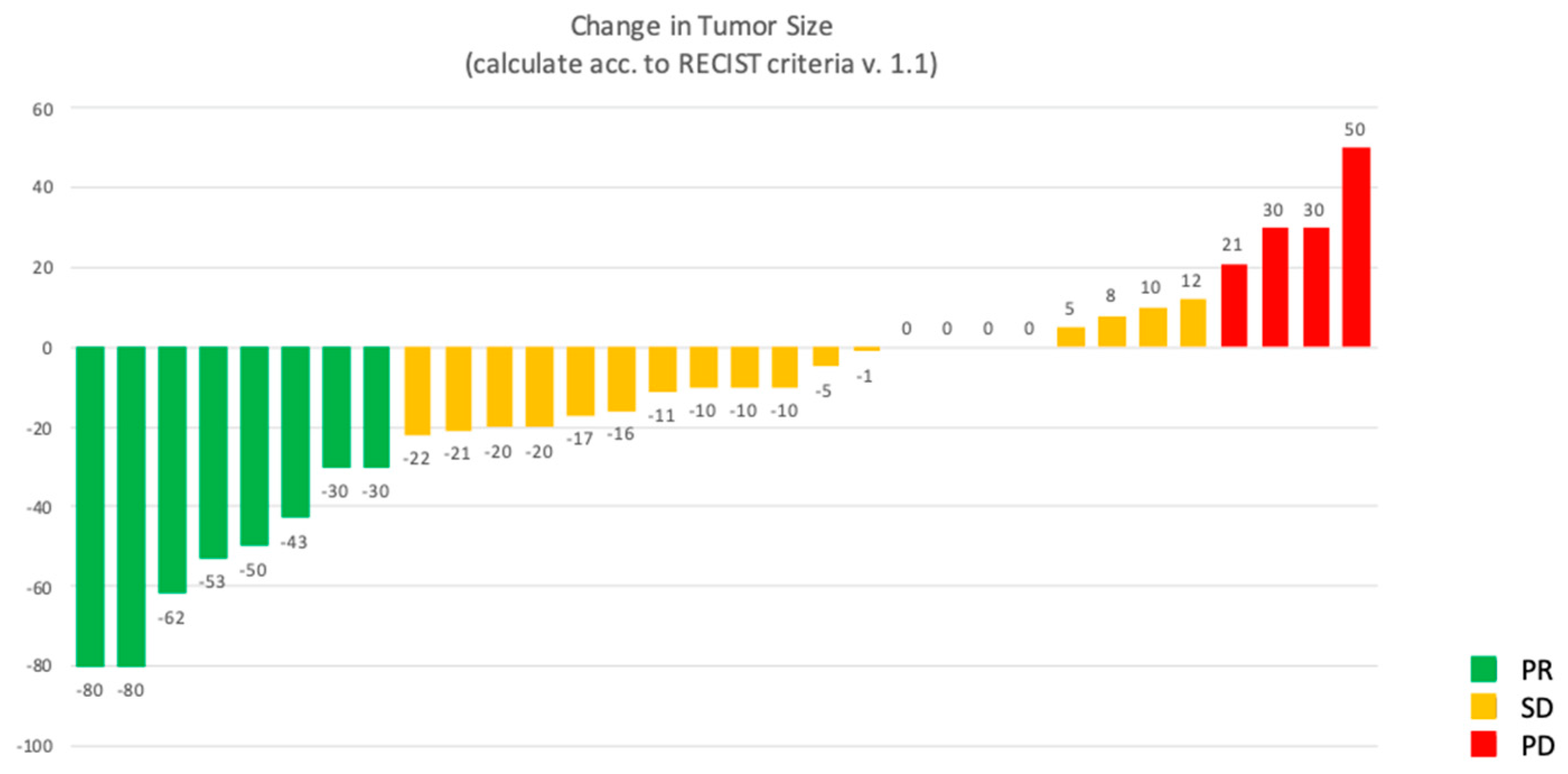
3. Results
3.1. Demographic and Baseline Characteristics of Patients
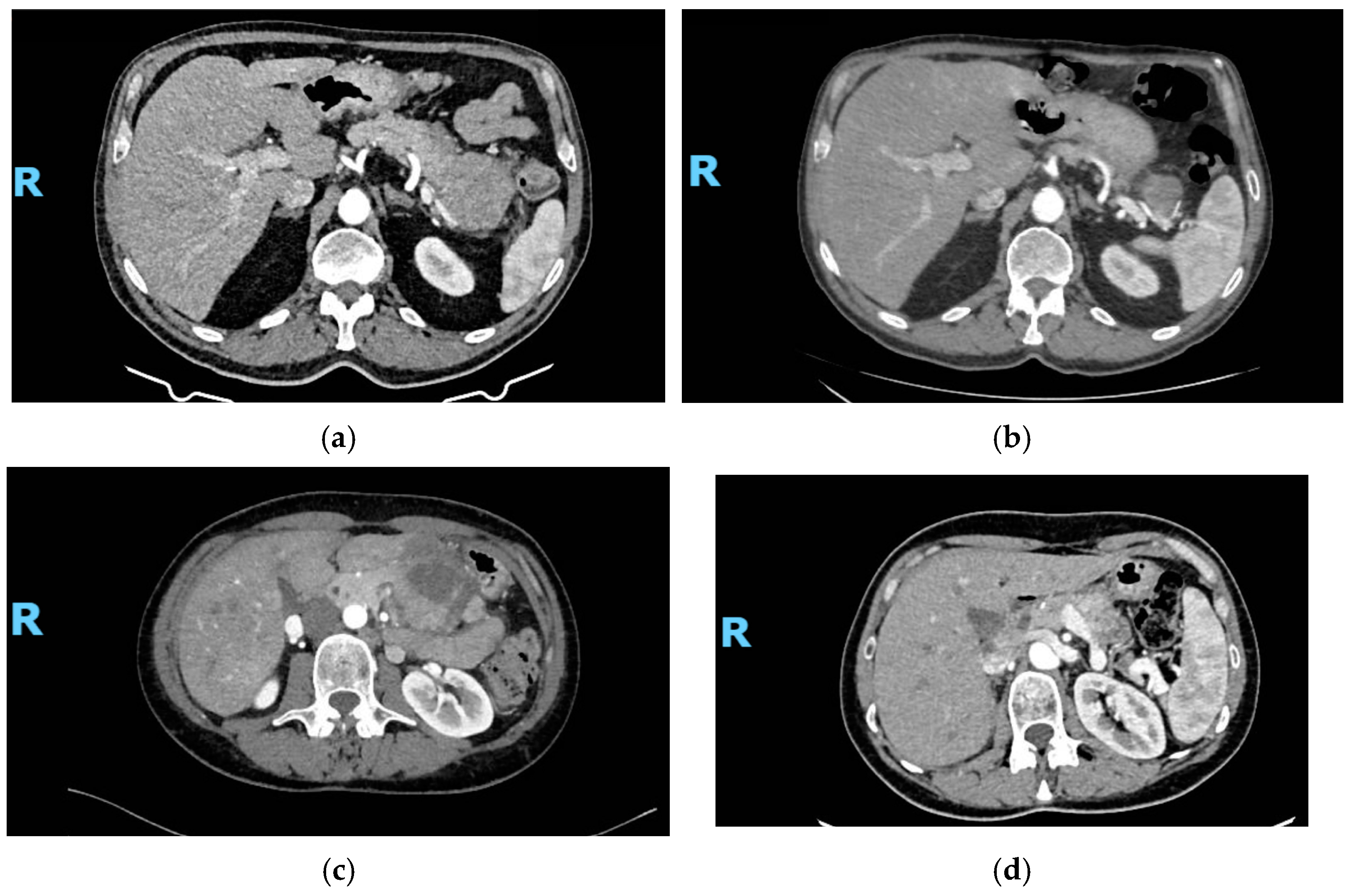
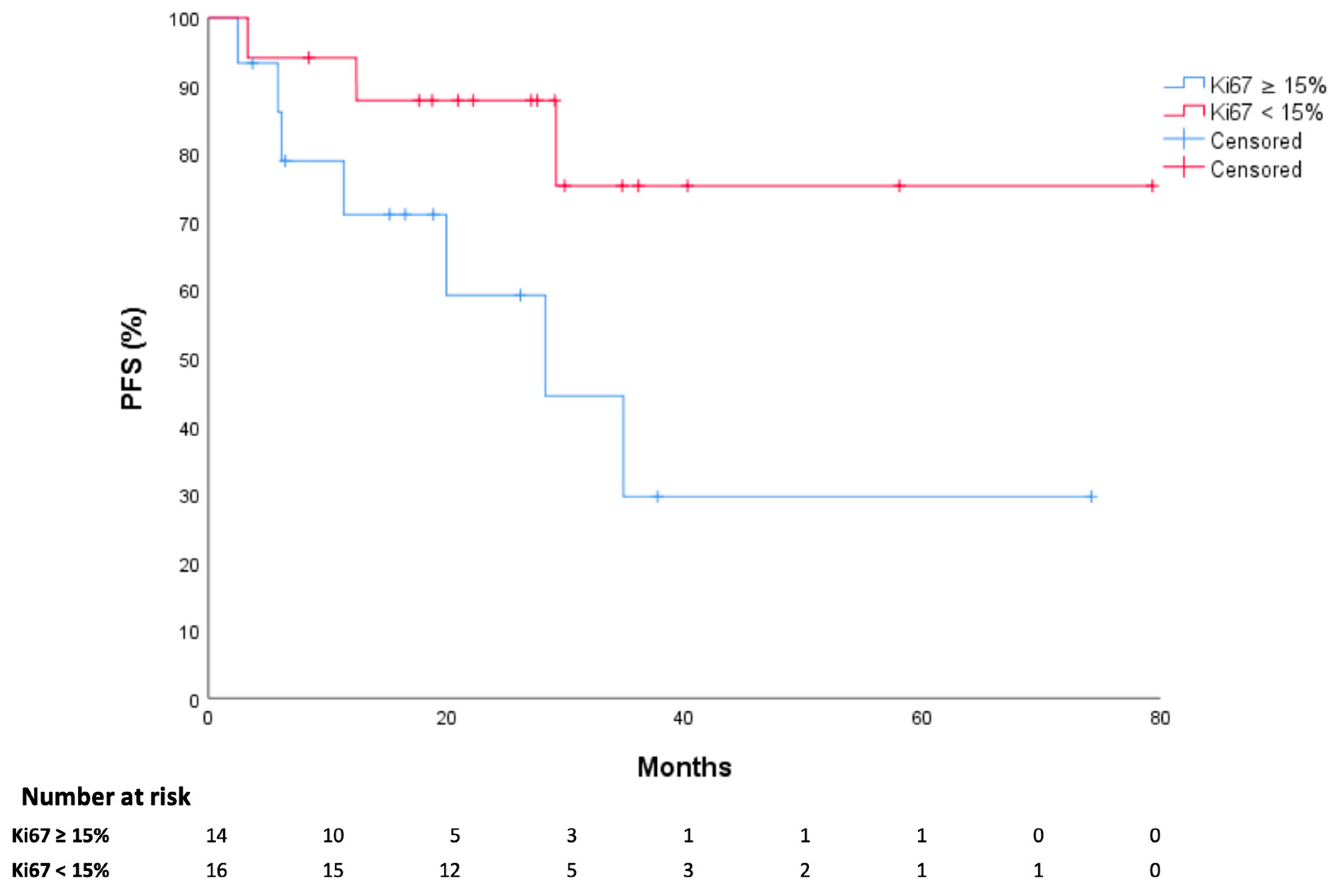
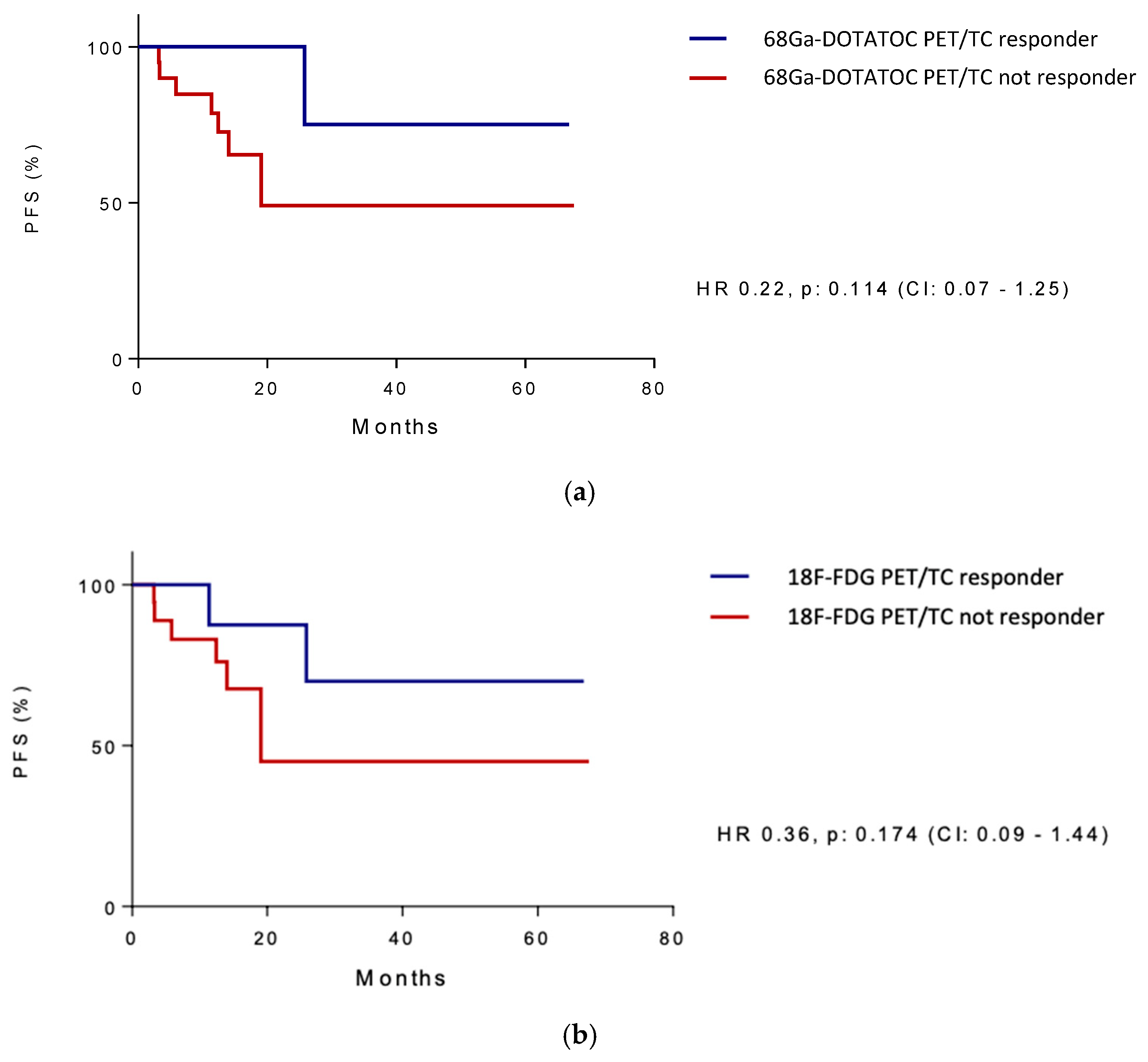
3.2. Study Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fonseca, P.; Krug, S.; Tamagno, G.; Fierro Maya, F.; Monléon Getino, A.; Rodriguez Casado, C.I.; Costa, F.; de Herder, W.W.; Jann, H. Identifying Prognostic Factors for Well-Differentiated Metastatic Pancreatic Neuroendocrine Tumours: A Retrospective International Multicentre Cohort Study. Neuroendocrinology 2018, 107, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.T.; Wolin, E.M.; Ruszniewski, P.; CLARINET Investigators. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study on behalf of the CLARINET Investigators. Endocrine. 2020, 71, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R.; PROMID Study Group. E-Mail Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival Long-Term Survival in the PROMID Cohort. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Kulke, M.H.; Ruszniewski, P.; Van Cutsem, E.; Lombard-Bohas, C.; Valle, J.W.; De Herder, W.W.; Pavel, M.; Degtyarev, E.; Brase, J.C.; Bubuteishvili-Pacaud, L.; et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann. Oncol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.J.; Lee, S.H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Lefkopoulo, M.; Lipsitz, S.; Hahn, R.G.K.D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1992, 326, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, S.; Fonck, M.; Bécouarn, Y.; Brunet, R. Dacarbazine, fluorouracil, and leucovorin in patients with advanced neuroendocrine tumors: A phase II trial. Am. J. Clin. Oncol. Cancer. Clin. Trials. 1998, 21, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kouvaraki, M.A.; Ajani, J.A.; Hoff, P.; Wolff, R.; Evans, D.B.; Lozano, R.; Yao, J.C. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J. Clin. Oncol. 2004, 22, 4710–4719. [Google Scholar] [CrossRef]
- Sun, W.; Lipsitz, S.; Catalano, P.; Mailliard, J.A.; Haller, D.G. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J. Clin. Oncol. 2005, 23, 4897–4904. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Fine, R.L.; Choi, J.; Nasir, A.; Coppola, D.; Chen, D.T.; Helm, J.; Kvols, L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef]
- Dilz, L.M.; Denecke, T.; Steffen, I.G.; Prasad, V.; von Weikersthal, L.F.; Pape, U.F.; Wiedenmann, B.; Pavel, M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur. J. Cancer 2015, 51, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Niccoli, P.; Autret, A.; Cavaglione, G.; Mineur, L.; Raoul, J.-L. Systemic chemotherapy with FOLFOX in metastatic grade 1/2 neuroendocrine cancer. Mol. Clin. Oncol. 2017, 6, 44–48. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Elliott, E.; Barriuso, J.; Backen, A.; McNamara, M.G.; Hubner, R.; Valle, J.W. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: A lost cause? Cancer Treat Rev. 2016, 44, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Brizzi, M.P.; Faggiano, A.; La Rosa, S.; Rapa, I.; Ferrero, A.; Mansueto, G.; Righi, L.; Garancini, S.; Capella, C.; et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. 2007, 20, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Honma, Y.; Machida, N.; Mizuno, N.; Hamaguchi, T.; Boku, N.; Sadachi, R.; Hiraoka, N.; Okusaka, T.; Hirano, H.; et al. A phase III study of combination therapy with everolimus plus lanreotide versus everolimus monotherapy for unresectable or recurrent gastroenteropancreatic neuroendocrine tumor (JCOG1901, STARTER-NET). JCO 2025, 43 (Suppl. 4), 652. [Google Scholar] [CrossRef]
- Frilling, A.; Li, J.; Malamutmann, E.; Schmid, K.W.; Bockisch, A.; Broelsch, C.E. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br. J. Surg. 2009, 96, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; deBraud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. 2017, 43, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Spinoglio, G.; Chiappa, A.; Ribero, D.; Biffi, R.; Partelli, S.; Falconi, M. Assessing the role of primary tumour resection in patients with synchronous unresectable liver metastases from pancreatic neuroendocrine tumour of the body and tail. A propensity score survival evaluation. Eur. J. Surg. Oncol. 2017, 43, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Alevroudis, E.; Spei, M.E.; Chatziioannou, S.N.; Tsoli, M.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Clinical Utility of 18F-FDG PET in Neuroendocrine Tumors Prior to Peptide Receptor Radionuclide Therapy: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristics | Patients Numbers (%) |
| Primitive Tumor Location | |
| Pancreas | 19 (59.3) |
| Small Intestine | 5 (15.6) |
| Other | 8 (25) |
| - Stomach | 2 (6.2) |
| - Liver | 1 (3.1) |
| - Unknown | 5 (15.6) |
| Grading acc. WHO | |
| G2 | 26 (81.2) |
| G3 | 6 (18.8) |
| Metastatic site distribution | |
| Liver | 23 (71.9) |
| Lymph nodes | 19 (59.3) |
| Bone | 13 (40.6) |
| Lung | 7 (21.9) |
| Peritoneum | 5 (15.6) |
| SSTR2A by IHC acc. Volante Score | |
| 0 | 2 (6.2) |
| 1 | 0 (0) |
| 2 | 3 (9.4) |
| 3 | 27 (84.4) |
| CTCAE Term | Incidence (%) | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| Paresthesia | 21.8 | 15.6 | 3.1 | 0 |
| Neutropenia | 9.3 | 0 | 3.1 | 0 |
| Febrile Neutropenia | - | - | - | 0 |
| Platelet Count Decrease | 3.1 | 9.3 | 6.2 | 0 |
| Anemia | 25 | 0 | 0 | 0 |
| Nausea/Vomiting | 18.7 | 9.3 | 0 | 0 |
| Oral Mucositis | 6.2 | 0 | 0 | 0 |
| Diarrhea | 12.5 | 12.5 | 3.1 | 0 |
| Fatigue | 21.8 | 15.6 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maratta, M.G.; Sparagna, I.; Occhipinti, D.; Roca, L.; Sgambato, M.; Raia, S.; Bianchi, A.; Chiloiro, S.; Rossi, E.; Rindi, G.; et al. Upfront Oxaliplatin–Fluoropyrimidine Chemotherapy and Somatostatin Analogues in Advanced Well-Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors. Cancers 2025, 17, 1561. https://doi.org/10.3390/cancers17091561
Maratta MG, Sparagna I, Occhipinti D, Roca L, Sgambato M, Raia S, Bianchi A, Chiloiro S, Rossi E, Rindi G, et al. Upfront Oxaliplatin–Fluoropyrimidine Chemotherapy and Somatostatin Analogues in Advanced Well-Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors. Cancers. 2025; 17(9):1561. https://doi.org/10.3390/cancers17091561
Chicago/Turabian StyleMaratta, Maria Grazia, Ileana Sparagna, Denis Occhipinti, Luigi Roca, Margherita Sgambato, Salvatore Raia, Antonio Bianchi, Sabrina Chiloiro, Ernesto Rossi, Guido Rindi, and et al. 2025. "Upfront Oxaliplatin–Fluoropyrimidine Chemotherapy and Somatostatin Analogues in Advanced Well-Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors" Cancers 17, no. 9: 1561. https://doi.org/10.3390/cancers17091561
APA StyleMaratta, M. G., Sparagna, I., Occhipinti, D., Roca, L., Sgambato, M., Raia, S., Bianchi, A., Chiloiro, S., Rossi, E., Rindi, G., Tortora, G., & Schinzari, G. (2025). Upfront Oxaliplatin–Fluoropyrimidine Chemotherapy and Somatostatin Analogues in Advanced Well-Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors. Cancers, 17(9), 1561. https://doi.org/10.3390/cancers17091561







