Prospective Multi-Institutional Observational Study of Retreatment with Anti-PD-1/PD-L1 Antibodies in Patients with Non-Small Cell Lung Cancer Previously Treated with Anti-PD-1/PD-L1 Plus Chemotherapy: NJLCG (North Japan Lung Cancer Group) Trial 1901
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Eligibility
2.2. Treatment, Assessment, and Endpoints
2.3. Statistical Analysis
2.4. Sample Size
2.5. Ethical Considerations
3. Results
3.1. Patient Characteristics
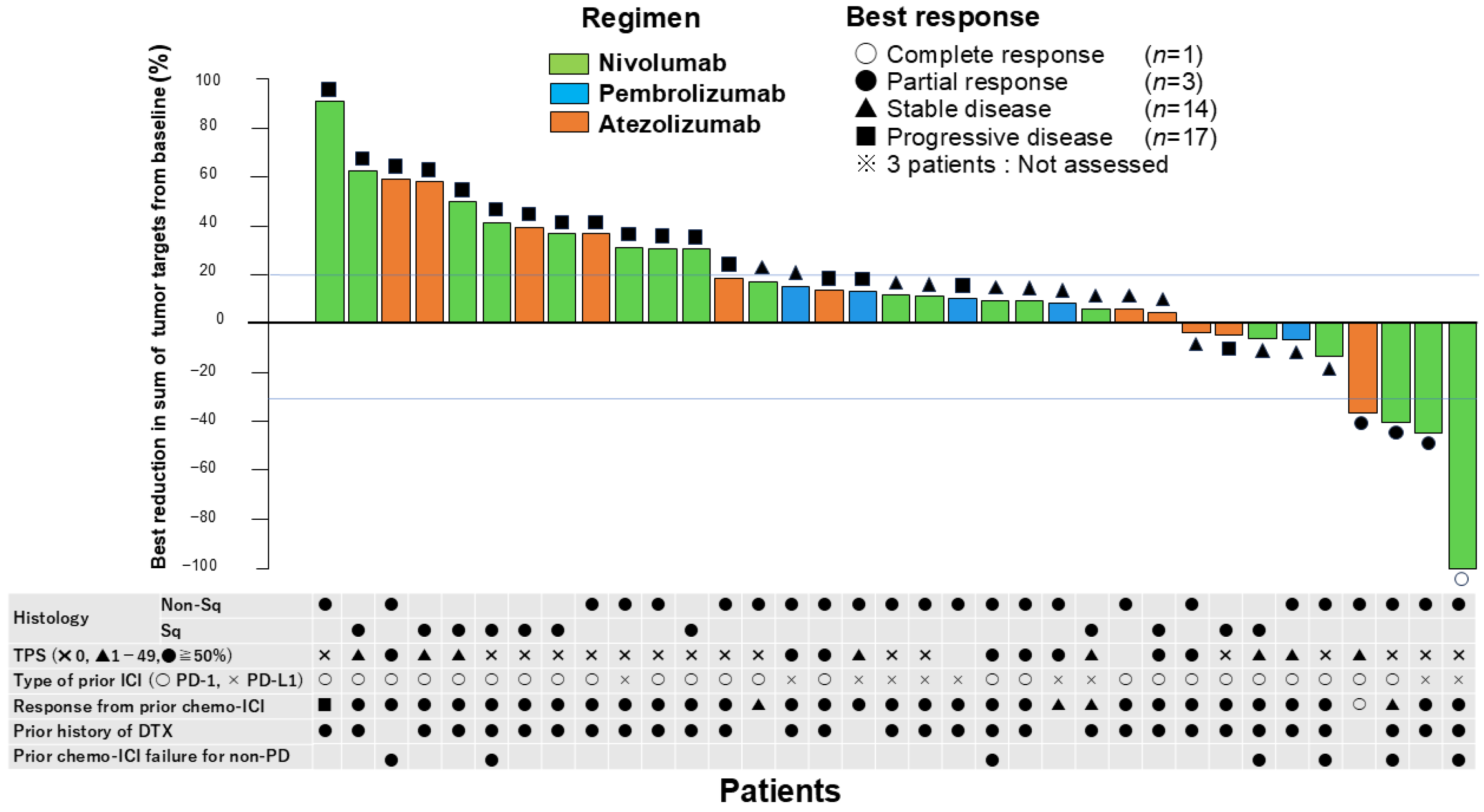
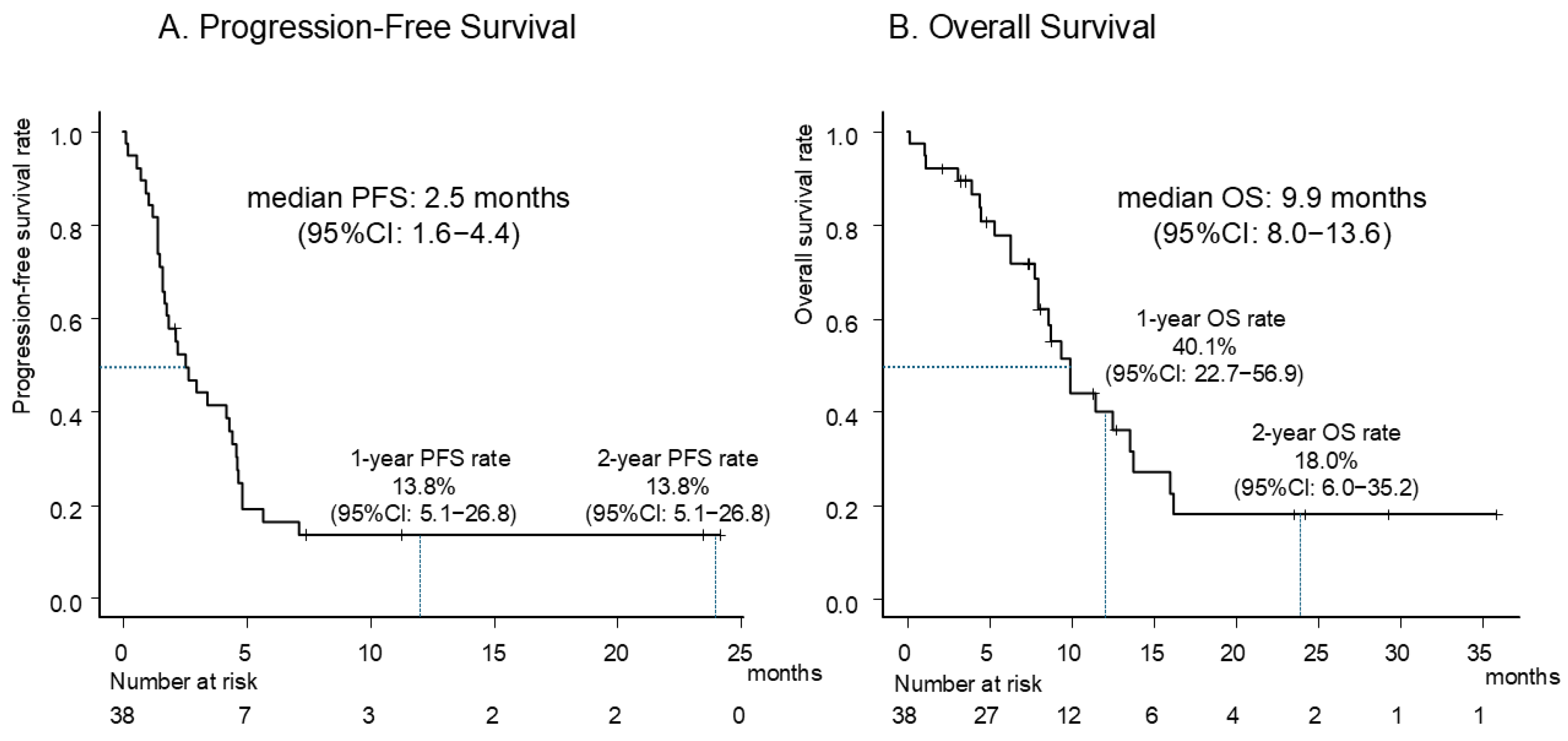
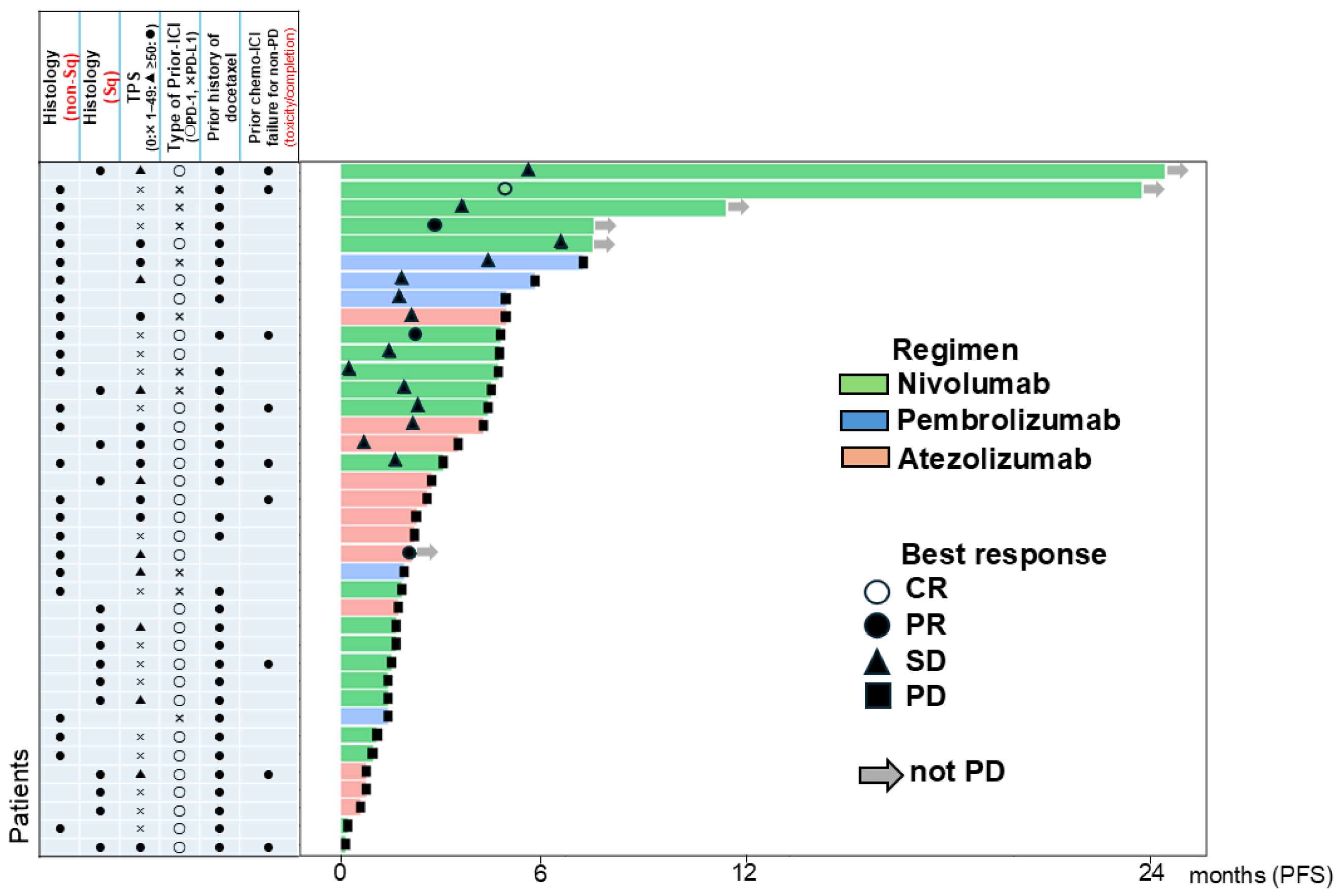
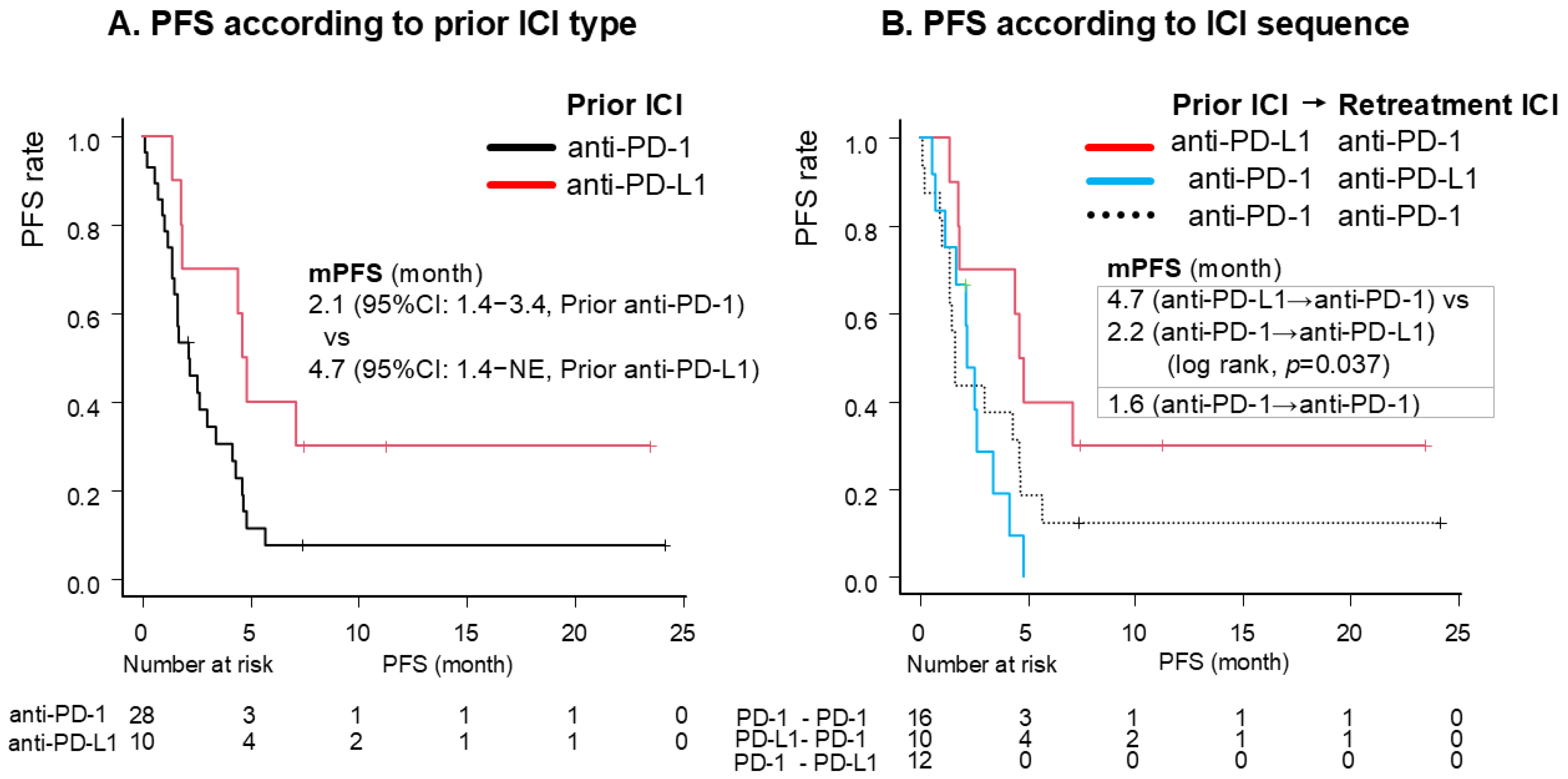
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Fossella, F.V.; DeVore, R.; Kerr, R.N.; Crawford, J.; Natale, R.R.; Dunphy, F.; Kalman, L.; Miller, V.; Lee, J.S.; Moore, M.; et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J. Clin. Oncol. 2000, 18, 2354–2362. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Dancey, J.; Ramlau, R.; Mattson, K.; Gralla, R.; O’Rourke, M.; Levitan, N.; Gressot, L.; Vincent, M.; Burkes, R.; et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 2000, 18, 2095–2103. [Google Scholar] [CrossRef]
- Furuya, N.; Nishino, M.; Wakuda, K.; Ikeda, S.; Sato, T.; Ushio, R.; Tanzawa, S.; Sata, M.; Ito, K. Real-world efficacy of atezolizumab in non-small cell lung cancer: A multicenter cohort study focused on performance status and retreatment after failure of anti-PD-1 antibody. Thorac. Cancer 2021, 12, 613–618. [Google Scholar] [CrossRef]
- Gobbini, E.; Toffart, A.C.; Pérol, M.; Assié, J.B.; Duruisseaux, M.; Coupez, D.; Dubos, C.; Westeel, V.; Delaunay, M.; Guisier, F.; et al. Immune Checkpoint Inhibitors Rechallenge Efficacy in Non-Small-Cell Lung Cancer Patients. Clin. Lung Cancer 2020, 21, e497–e510. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hao, X.; Yang, K.; Wang, Q.; Wang, J.; Lin, L.; Teng, F.; Li, J.; Xing, P. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: A retrospective cohort study. J. Cancer Res. Clin. Oncol. 2022, 148, 3081–3089. [Google Scholar] [CrossRef]
- Fujita, K.; Yamamoto, Y.; Kanai, O.; Okamura, M.; Hashimoto, M.; Nakatani, K.; Sawai, S.; Mio, T. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac. Cancer 2020, 11, 15–18. [Google Scholar] [CrossRef]
- Mouri, A.; Kaira, K.; Yamaguchi, O.; Shiono, A.; Miura, Y.; Hashimoto, K.; Nishihara, F.; Murayama, Y.; Kobayashi, K.; Kagamu, H. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother. Pharmacol. 2019, 84, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shukuya, T.; Tamura, J.; Shimamura, S.; Kurokawa, K.; Miura, K.; Miyawaki, T.; Hayakawa, D.; Asao, T.; Yamamoto, K.; et al. Heterogeneous Outcomes of Immune Checkpoint Inhibitor Rechallenge in Patients with NSCLC: A Systematic Review and Meta-Analysis. JTO Clin. Res. Rep. 2022, 3, 100309. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, H.; Teraoka, S.; Takamori, S.; Miura, S.; Hayashi, H.; Hata, A.; Toi, Y.; Shiraishi, Y.; Mamesaya, N.; Sato, Y.; et al. Nivolumab Retreatment in Non-Small Cell Lung Cancer Patients Who Responded to Prior Immune Checkpoint Inhibitors and Had ICI-Free Intervals (WJOG9616L). Clin. Cancer Res. 2022, 28, OF1–OF7. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Mukohara, T.; Takeda, K.; Miyazaki, M.; Takifuji, N.; Terakawa, K.; Negoro, S. Japanese experience with second-line chemotherapy with low-dose (60 mg/M2) docetaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2001, 48, 356–360. [Google Scholar] [CrossRef]
- Baitsch, L.; Baumgaertner, P.; Devêvre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011, 121, 2350–2360. [Google Scholar] [CrossRef]
- Mognol, G.P.; Spreafico, R.; Wong, V.; Scott-Browne, J.P.; Togher, S.; Hoffmann, A.; Hogan, P.G.; Rao, A.; Trifari, S. Exhaustion-associated regulatory regions in CD8(+) tumor-infiltrating T cells. Proc. Natl. Acad. Sci. USA 2017, 114, e2776–e2785. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Takeda, M.; Sakai, K.; Nakamura, Y.; Ito, A.; Hayashi, H.; Tanaka, K.; Nishio, K.; Nakagawa, K. Impact of cytotoxic chemotherapy on PD-L1 expression in patients with non-small cell lung cancer negative for EGFR mutation and ALK fusion. Lung Cancer 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Song, P.; Xue, X.; Guo, C.; Han, L.; Fang, Q.; Ying, J.; Gao, S.; Li, W. Variation of Programmed Death Ligand 1 Expression After Platinum-based Neoadjuvant Chemotherapy in Lung Cancer. J. Immunother. 2019, 42, 215–220. [Google Scholar] [CrossRef]
- Lin, X.; Lin, K.; Lin, C.; Liu, T.; Ba, M.; Tang, Y.; Wang, J.; Zhou, L.; Wang, J.; Xiao, C. Prognostic and clinicopathological value of PD-L2 in lung cancer: A meta-analysis. Int. Immunopharmacol. 2021, 91, 107280. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Hakozaki, T.; Kitadai, R.; Hosomi, Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review. Thorac. Cancer 2020, 11, 1927–1933. [Google Scholar] [CrossRef]
- Takahara, Y.; Tanaka, T.; Ishige, Y.; Shionoya, I.; Yamamura, K.; Sakuma, T.; Nishiki, K.; Nakase, K.; Nojiri, M.; Kato, R.; et al. Efficacy and predictors of rechallenge with immune checkpoint inhibitors in non-small cell lung cancer. Thorac. Cancer 2022, 13, 624–630. [Google Scholar] [CrossRef]
- Mouri, A.; Watanabe, S.; Tokito, T.; Nagai, Y.; Saida, Y.; Imai, H.; Yamaguchi, O.; Kobayashi, K.; Kaira, K.; Kagamu, H. Clinical Outcome of Nivolumab Plus Ipilimumab in Patients with Locally Advanced Non-Small-Cell Lung Cancer with Relapse after Concurrent Chemoradiotherapy followed by Durvalumab. Cancers 2024, 16, 1409. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.-W.; Costa, E.C.; Park, K.; Alexandru, A.; Lupinacci, L.; Jimenez, E.d.l.M.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab with or without Tremelimumab in Combination with Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J. Clin. Oncol. 2023, 41, 1213–1227. [Google Scholar] [CrossRef]
- Fujisaki, T.; Watanabe, S.; Ota, T.; Kushiro, K.; Sato, Y.; Takahashi, M.; Ohtsubo, A.; Shoji, S.; Nozaki, K.; Ichikawa, K.; et al. The Prognostic Significance of the Continuous Administration of Anti-PD-1 Antibody via Continuation or Rechallenge After the Occurrence of Immune-Related Adverse Events. Front. Oncol. 2021, 11, 704475. [Google Scholar] [CrossRef] [PubMed]
| All Patients, n = 38 | ||
|---|---|---|
| Age | Median (range), years | 69.3 (37–83) |
| Histology (Sq/Non-Sq) | Non-Sq | 24 (63.2) |
| Stage | Stage IV | 32 (84.2) |
| Recurrence after surgery | 2 (5.3) | |
| Recurrence after CRT | 4 (10.5) | |
| Smoking | Current or past smoker | 36 (94.7) |
| PS | 0–1 | 38 (100) |
| Sex | Male | 31 (81.6) |
| ICI retreatment regimen | Nivo | 21 (55.2) |
| Pembro | 5 (13.2) | |
| Atezo | 12 (31.6) | |
| Prior chemo-ICI regimen | Platinum-doublet + Pembro | 26 (68.4) |
| Platinum-doublet + Nivo + Bev | 1 (2.6) | |
| Platinum-doublet + Nivo + Ipi | 1 (2.6) | |
| Platinum-doublet + Atezo ± Bev | 6 (15.8) | |
| Carboplatin + PTX + radiation followed by durva | 4 (10.5) | |
| TPS * | <49% | 26 (68.4) |
| ≥50% | 9 (23.6) | |
| CNS metastasis | Positive | 12 (31.6) |
| Liver metastasis | Positive | 4 (10.5) |
| Number of prior treatment lines | Median (range) | 2.5 (1–4) |
| ICI-free interval | Median (range), months | 11.9 (1.0–38.9) |
| Prior history of docetaxel | 33 (86.8) | |
| Prior chemo-ICI cessation | Due to PD | 29 (76.3) |
| Due to non-PD (toxicity/completion of durva) | 8/1 (23.7) |
| All Patients (n = 38) | |
|---|---|
| ORR, % (95% CI) | 10.5 (2.9–24.8) |
| DCR, % (95% CI) | 47.4 (31.0–64.2) |
| CR, n (%) | 1 (2.6) |
| PR, n (%) | 3 (7.9) |
| SD, n (%) | 14 (36.8) |
| PD, n (%) | 17 (44.7) |
| NA, n (%) | 3 (7.9) |
| Patients with an ICI-free interval >11.9 months (n = 19) | |
| ORR, % (95%CI) | 21.1 (6.1–45.6) |
| DCR, % (95%CI) | 63.2 (38.4–83.7) |
| Patients with an ICI-free interval ≤ 11.9 months (n = 19) | |
| ORR, % (95%CI) | 0 (0–17.6) |
| DCR, % (95%CI) | 31.6 (12.6–56.6) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Histology: Non-Sq (vs. Sq) | 0.40 (0.19–0.82) | 0.013 | 0.64 (0.29–1.40) | 0.26 |
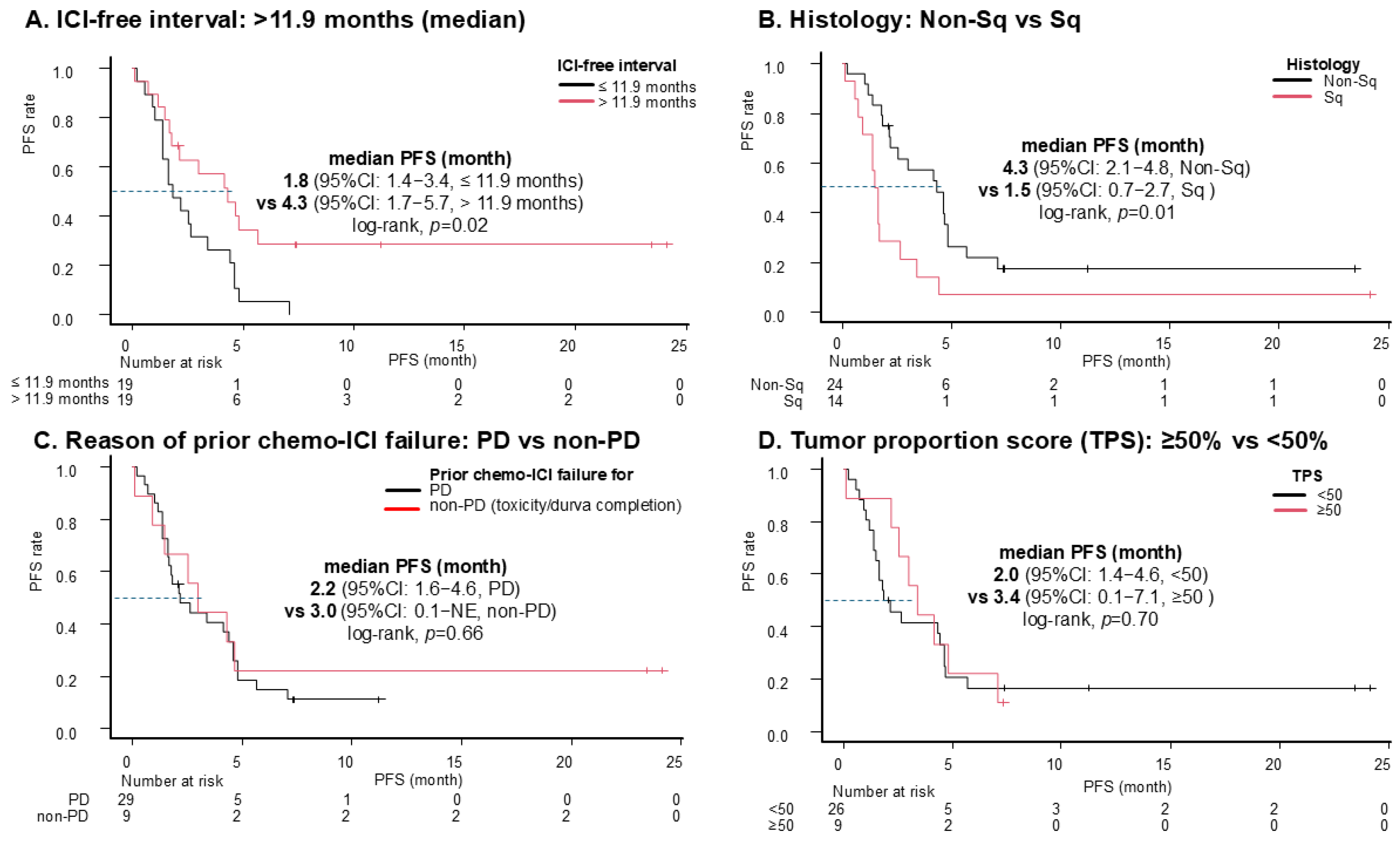
| ICI-free interval: >11.9 months (vs. ≤11.9 months) | 0.42 (0.20–0.87) | 0.019 | 0.33 (0.15–0.76) | 0.009 |
| Prior ICI: anti-PD-L1 (vs. PD-1) | 0.41 (0.18–0.97) | 0.042 | 0.33 (0.13–0.84) | 0.020 |
| Retreatment ICI: anti-PD-L1 (vs. PD-1) | 1.85 (0.86–4.01) | 0.12 | ||
| Liver metastasis: Positive | 2.80 (0.96–8.15) | 0.06 | ||
| Brain metastasis: Positive | 1.02 (0.47–2.21) | 0.96 | ||
| TPS ≥ 50% (vs. <50%) | 0.85 (0.38–1.93) | 0.70 | ||
| Age, years ≥ 75 (vs. <75) | 0.89 (0.36–2.18) | 0.80 | ||
| Prior ICI discontinuation due to non-PD (vs. PD) | 0.83 (0.36–1.92) | 0.66 | ||
| Serum Alb (g/L) ≥ 3.5 | 0.96 (0.48–1.92) | 0.91 | ||
| Serum NLR ≥ 5 | 0.91 (0.45–1.84) | 0.79 | ||
| G1 | G2 | G3 | G4 | All Grade | |
|---|---|---|---|---|---|
| Adverse events reported as irAEs (%) | |||||
| Colitis | 0 | 2.6 | 0 | 0 | 2.6 |
| Rash | 2.6 | 2.6 | 2.6 | 0 | 7.9 |
| Pneumonitis | 0 | 2.6 | 0 | 0 | 2.6 |
| Thyroid dysfunction | 0 | 2.6 | 0 | 0 | 2.6 |
| Myositis | 0 | 0 | 2.6 | 0 | 2.6 |
| Fever | 5.3 | 0 | 0 | 0 | 5.3 |
| Adverse events reported as non-irAEs (%) | |||||
| Anemia | 2.6 | 2.6 | 0 | 0 | 5.3 |
| Creatinine increased | 0 | 2.6 | 0 | 0 | 2.6 |
| Lung infection | 0 | 2.6 | 0 | 0 | 2.6 |
| AST increased | 2.6 | 0 | 0 | 0 | 2.6 |
| Hyperglycemia | 0 | 2.6 | 0 | 0 | 2.6 |
| Anorexia | 0 | 2.6 | 0 | 0 | 2.6 |
| Malaise | 2.6 | 0 | 0 | 0 | 2.6 |
| Shingles | 0 | 2.6 | 0 | 0 | 2.6 |
| AEs leading to ICI discontinuation: 2.6% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Kawashima, Y.; Tanaka, H.; Yoshimura, N.; Tsukita, Y.; Saito, R.; Nakagawa, T.; Inomata, M.; Nagashima, H.; Sugawara, S. Prospective Multi-Institutional Observational Study of Retreatment with Anti-PD-1/PD-L1 Antibodies in Patients with Non-Small Cell Lung Cancer Previously Treated with Anti-PD-1/PD-L1 Plus Chemotherapy: NJLCG (North Japan Lung Cancer Group) Trial 1901. Cancers 2025, 17, 1551. https://doi.org/10.3390/cancers17091551
Saito S, Kawashima Y, Tanaka H, Yoshimura N, Tsukita Y, Saito R, Nakagawa T, Inomata M, Nagashima H, Sugawara S. Prospective Multi-Institutional Observational Study of Retreatment with Anti-PD-1/PD-L1 Antibodies in Patients with Non-Small Cell Lung Cancer Previously Treated with Anti-PD-1/PD-L1 Plus Chemotherapy: NJLCG (North Japan Lung Cancer Group) Trial 1901. Cancers. 2025; 17(9):1551. https://doi.org/10.3390/cancers17091551
Chicago/Turabian StyleSaito, Shin, Yosuke Kawashima, Hisashi Tanaka, Naruo Yoshimura, Yoko Tsukita, Ryota Saito, Taku Nakagawa, Minehiko Inomata, Hiromi Nagashima, and Shunichi Sugawara. 2025. "Prospective Multi-Institutional Observational Study of Retreatment with Anti-PD-1/PD-L1 Antibodies in Patients with Non-Small Cell Lung Cancer Previously Treated with Anti-PD-1/PD-L1 Plus Chemotherapy: NJLCG (North Japan Lung Cancer Group) Trial 1901" Cancers 17, no. 9: 1551. https://doi.org/10.3390/cancers17091551
APA StyleSaito, S., Kawashima, Y., Tanaka, H., Yoshimura, N., Tsukita, Y., Saito, R., Nakagawa, T., Inomata, M., Nagashima, H., & Sugawara, S. (2025). Prospective Multi-Institutional Observational Study of Retreatment with Anti-PD-1/PD-L1 Antibodies in Patients with Non-Small Cell Lung Cancer Previously Treated with Anti-PD-1/PD-L1 Plus Chemotherapy: NJLCG (North Japan Lung Cancer Group) Trial 1901. Cancers, 17(9), 1551. https://doi.org/10.3390/cancers17091551






