1. Introduction
1.1. The Role of Ion Channels in Cellular Physiology
Ion channels are integral membrane proteins that facilitate the selective passage of ions, such as sodium (Na
+), potassium (K
+), calcium (Ca
2+), and chloride (Cl
−), across cellular membranes. Ion channels play critical roles in a variety of biological functions, including nerve signal transmission, heart contraction, temperature sensing, hormone release, and apoptotic control [
1]. Ion channels play an essential role in cell growth and homeostasis [
2]. They are essential in establishing and maintaining the resting membrane potential of cells. Potassium channels allow K
+ ions to flow out of the cell, providing a negative charge inside the cell compared to the outside, creating an electrochemical gradient [
3].
Another essential function is the regulation of intracellular calcium levels. Cells control calcium concentrations in distinct cellular compartments via a variety of ion channels, pumps, and transporters situated at the plasma membrane’s surface and interior organelles [
4]. This involves calcium ion channels, such as those involved in store-operated calcium entry (SOCE) [
5]. The regulation of intracellular Ca
2+ concentrations is critical for various cellular functions, including muscle contraction, secretion, and gene expression [
5]. In addition to being common messengers of biological processes, calcium ions influence long-term metabolic and transcriptional cell reprogramming, which controls cell proliferation, differentiation, and migration [
4].
Ion channels play a pivotal role in signal transduction by allowing the flow of ions that act as secondary messengers in various signaling pathways. Cells produce ion channels accessible to ions, such as calcium, potassium, sodium, and chloride, which control the potential of electricity across biological membranes, allowing for finer control over the initiation of signaling cascades that regulate cellular responses to external stimuli [
4]. Furthermore, mechanosensitive ion channels, such as PIEZO1, regulate cell volume, preventing cellular swelling or shrinkage [
6].
1.2. Tumorigenesis Mechanisms Influenced by Ion Channels
The gain or loss of function of ion channels, caused by mutations or changes in regulation, expression, or oligomerization in biological membranes, disrupts the homeostasis of ion channels and alters the ion signaling network [
4]. Those alterations can lead to various diseases, including neurodegenerative disorders and neoplasms [
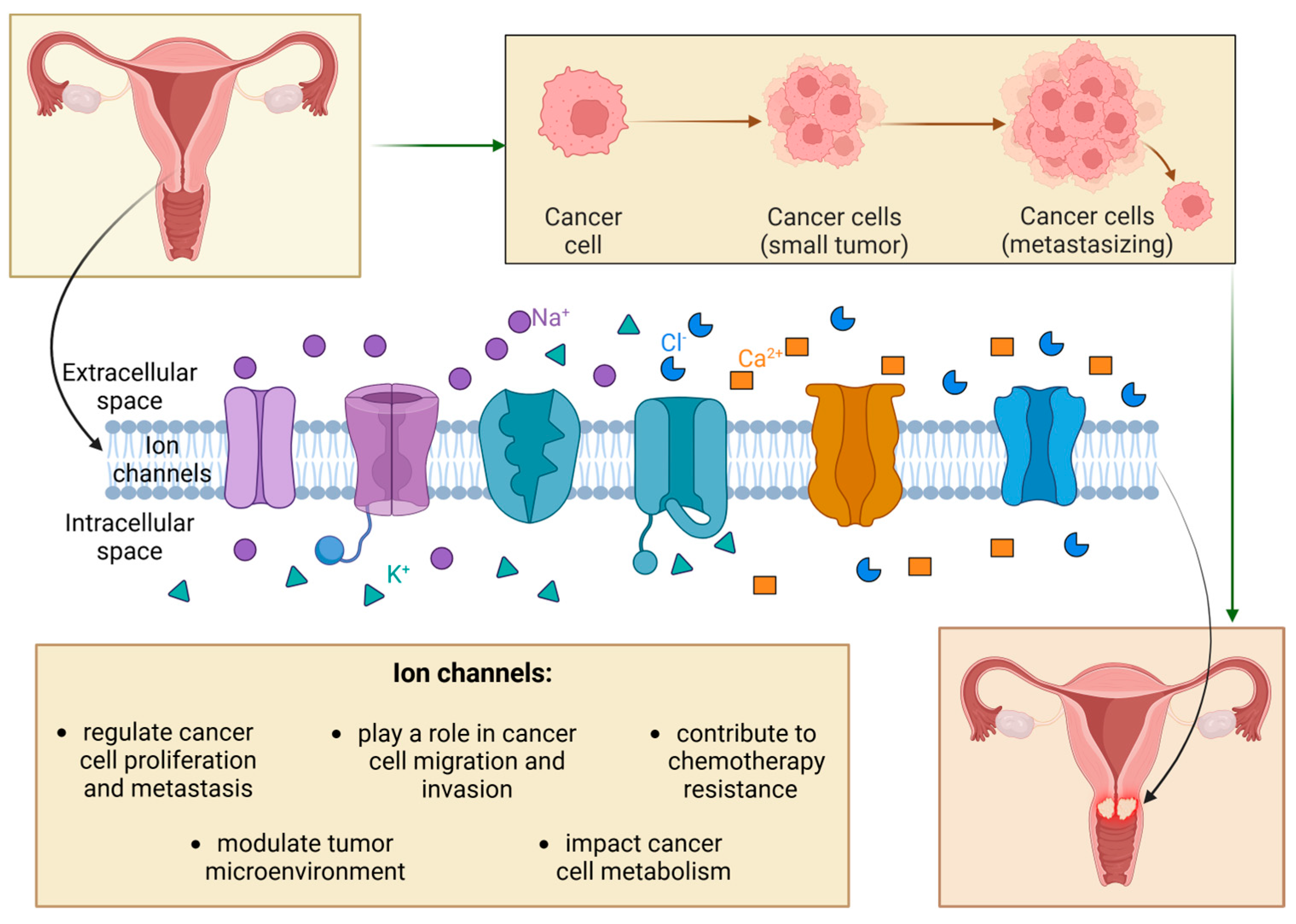
7]. Ion channels play crucial regulatory roles in cancer pathophysiology, performing several functions from the initial transformation of cancers to metastasis. Heterogeneous ion channel distribution is associated with distinct phases of cancer, such as cell proliferation, metastasis, dissemination, and apoptosis [
1]. Cancer cells use ion channels to promote their progress, expand as tumors, and integrate into a microenvironment, including various non-cancerous cells (
Figure 1) [
2].
Many ion channels, especially calcium channels like SOCE and voltage-gated calcium channels, regulate intracellular calcium signaling pathways. Elevated calcium influx activates PI3K/AKT and MAPK pathways, driving cell cycle progression and causing uncontrolled proliferation. Calcium-dependent enzymes, such as matrix metalloproteinases (MMPs), degrade the extracellular matrix (ECM), facilitating cell migration and metastasis [
8]. Moreover, voltage-gated sodium and potassium channels regulate the cancer cell membranes’ electrical and mechanical properties. Sodium channels, such as Nav1.5, increase intracellular calcium, promoting cytoskeletal remodeling and invasiveness. Mechanosensitive channels respond to extracellular forces, enhancing metastatic potential [
9].
Ion channels alter ionic balance and extracellular pH, creating a microenvironment conducive to tumor growth. Hypoxia-induced sodium/hydrogen exchangers and chloride channels lead to acidification, enhancing angiogenesis and ECM degradation [
10]. Mechanosensitive ion channels, like PIEZO1, further respond to tumor mechanical cues, regulating angiogenesis and cell migration [
11]. A higher concentration of hormones may also cause an increased distribution of ion channels, which leads to cancer cell proliferation [
2].
Another significant ability of proteins discussed in this paper is their impact on cancer cell metabolism. Potassium channels maintain membrane potential, which is crucial for ATP synthesis in mitochondria. Dysregulation of sodium and chloride channels affects ionic gradients, influencing glycolysis and oxidative phosphorylation. These metabolic changes allow cancer cells to thrive in hostile microenvironments, including nutrient-deprived and hypoxic conditions, promoting tumor survival [
12]. Moreover, the volume-sensitive chloride channels display osmosensing abilities. Through various interactions, they are able to regulate vital cell processes, affecting the course of the disease [
13]. Additionally, ion channels promote chemotherapy resistance by activating survival pathways and efflux pumps.
Overexpression of the potassium channels hyperpolarizes the membrane, reducing drug uptake. Calcium and sodium channels enhance efflux pump activity, such as P-glycoprotein, and inhibit apoptotic pathways, protecting cancer cells from therapeutic agents [
14].
1.3. Types of Ion Channels Implicated in Cervical Cancer
Cervical cancer is one of the leading health concerns for today’s females. It ranks as the fourth most frequent disease in women worldwide, with about 660,000 new cases and 350,000 deaths in 2022 [
15]. Although vaccination and screening initiatives have lowered the global incidence of CCa by more than 80%, the death prevalence in low-income countries is still high [
16]. The primary etiological agent is human papillomavirus. The cytological Pap smear test is the most commonly used procedure for cervical cancer screening. Despite its effectiveness in identifying precancerous lesions and responding to the disease’s early stages, higher screening and diagnostic reliability, as well as increased specificity and sensitivity, are required [
17]. That is why new methods of detection and therapy based on cancer pathophysiology are being developed. One of the most promising areas is research on the following ion channels.
Table 1 presents the important ion channels and their characteristics.
The aim of this review is to explore the various roles of ion channels in cervical cancer progression. It highlights their significance as molecular biomarkers and therapeutic targets. It examines their involvement in processes such as metastasis, cell migration, and tumor microenvironment interaction, while also discussing diagnostic advancements and the potential of ion channel modulators to improve conventional treatments.
2. Ion Channels as Molecular Biomarkers in Cervical Cancer
The process of carcinogenesis in cervical cancer is complex and undoubtedly depends on persistent infection with the human papillomavirus (HPV). However, it is essential to note that even 80% of women who have been exposed to HPV never develop cervical cancer. Therefore, thorough research of the processes in which individual ion channels are involved is crucial for better understanding the disease’s pathogenesis and for creating modern diagnostic and prognostic methods [
11,
12]. It should also be noted that, just as the process of carcinogenesis depends on various factors, such as viral infections, hormonal influences, or the presence of carcinogens in tobacco smoke, it is equally challenging to demonstrate the direct effect of HPV on gene expression and the function of ion channels [
13]. Among many ion channels that are widely studied, potassium, calcium, and sodium channels seem to be the most promising biomarkers for cervical cancer.
2.1. Potassium Channels
Potassium channels are one of the most studied in gynecological cancers due to their role in cancer cell proliferation, apoptosis, and migration processes. Several types of potassium channels have been linked to tumorigenesis. They could be potentially used as biomarkers—voltage-gated potassium channels (K
V), calcium-activated potassium channels (K
Ca), and ATP-sensitive potassium channels (K
ATP), in particular [
13,
14,
15].
2.1.1. Voltage-Gated Potassium Channels
The K
V1.1 channel (encoded by the KCNA1 gene) functions as a potassium-selective channel, allowing the passage of potassium cations according to the electrochemical gradient. However, significantly higher levels of KCNA1 mRNA have been observed in cancer cells compared to healthy tissue, with the highest expression of this gene found in HeLa cells. One study has shown that overexpression promotes cancer cell growth, proliferation, and migration, ultimately increasing the invasive potential of the tumor. It also established the following correlation: the higher the KCNA1 expression, the shorter the predicted survival and the worse the prognosis. Monitoring KCNA1 expression levels might serve as a marker for evaluating cancer progression and estimating survival outcomes [
16]. The KCNH1 gene could be another promising biomarker. It encodes the Kv10.1 channel that belongs to the Ether-à-go-go (EAG) superfamily, and while it physiologically occurs in tissues such as the brain, placenta, testes, and adrenal glands, its significantly elevated expression has been observed in cervical cancer tissues. Studies have shown that inhibiting KCNH1 suppresses cell proliferation and limits tumor growth [
17]. Although further research is needed, KCNH1 overexpression is suggested to be a useful marker for monitoring tumor progression and potential metastasis [
18].
2.1.2. Calcium-Activated Potassium Channels
Among calcium-activated potassium channels, two of them seem to be particularly important for the development of modern diagnostic methods for cervical cancer. Increased expression of the KCNMA1 gene, which encodes the K
Ca1.1 channel, has been observed both in mice with induced tumors and in cervical biopsy samples. No expression of this gene was detected in non-cancerous tissues, while more than half of LSIL (low-grade squamous intraepithelial lesion) samples showed its presence, and in HSIL (high-grade squamous intraepithelial lesion) and cervical cancer biopsies, KCNMA1 expression was found in all cases [
19]. Monitoring KCNMA1 gene expression could provide valuable data on the progression of dysplastic changes. Moreover, the KCa3.1 channel, encoded by the KCNN4 gene, has shown overexpression in cervical cancer tissues and HeLa cell lines. Its expression level is correlated with the advancement of histopathological changes, which emphasizes its potential as a diagnostic marker [
20].
2.1.3. ATP-Sensitive Potassium Channels
K-ATP channels physiologically occur in various tissues, such as cardiomyocytes, skeletal and smooth muscle cells, the brain, and the pancreas. These channels are heterooctameric membrane complexes composed of four Kir6.X subunits and four sulfonylurea receptor (SUR) subunits. Studies on cell lines and tumor tissue samples have revealed the presence of Kir6.2, SUR1, SUR2a, and SUR2b subunits, which are not characteristic of healthy cervical tissue. It is worth noting that the role of SUR2 subunits remains a topic of debate in the literature. High levels of Kir6.2 expression were observed in both cell lines and cervical squamous cell carcinoma samples at stage IV, as well as in highly invasive tumors [
10,
15]. Furthermore, it was found that the E7 oncoprotein significantly upregulates the expression of the ABCC8 gene, which encodes the SUR1 protein, via a positive feedback mechanism. The highest expression levels were detected in CIN III (cervical intraepithelial neoplasia grade III) samples and cervical squamous cell carcinoma cases, suggesting that K-ATP channels may serve as an additional marker for dysplasia progression and malignancy [
10].
2.2. Calcium Channels
Calcium channels have recently gained significant interest in molecular diagnostics, particularly TRP (transient receptor potential channels) and CRAC (calcium release-activated calcium channels) families, which are characterized the most. Studies have shown that alterations in the expression of specific proteins and channels affect the processes of cancer cell proliferation and their potential for tissue invasion and metastasis. Furthermore, it was found that some of these channels could be a valuable source of information on patient prognosis [
6,
21].
2.2.1. Transient Receptor Potential Channels
The TRP superfamily, which includes TRPC, TRPV, and TRPM channels, plays a substantial role in the development of advanced diagnostic methods. One study has demonstrated an increased expression of the gene encoding the TRPC6 channel in HeLa and SiHa cell lines. Additionally, an important correlation was observed between high expression of this gene and tumor invasion of vascular and lymphatic spaces, highlighting its potential in diagnostics and assessment of progression [
22]. Among the TRPV subfamily, the TRPV1 channel has been identified as a potential biomarker, although the results of different studies are inconsistent. According to Wang and their research team, TRPV1 expression in squamous cell carcinoma was significantly lower compared to healthy tissue. Their studies indicated that this channel plays a role in processes such as cell differentiation, ferroptosis, inflammatory response, and metabolic regulation. Altered TRPV1 expression may contribute to immune evasion by the tumor. Furthermore, patients with low TRPV1 expression were observed to have a poorer overall survival prognosis [
21].
However, different results were reported in the study by Jiahui Lei et al. The article indicated that TRPV1 expression is higher in cervical cancer tissue compared to healthy tissue or tissue with epithelial dysplasia. Moreover, it was observed that high TRPV1 expression and low PTEN (phosphatase and tensin homolog) gene expression may serve as an independent prognostic factor in estimating patient survival time [
6]. The same study also pointed to TRPV6 as a promising biomarker. The levels of mRNA and proteins were significantly lower in cells during the early stages of tumor development, suggesting that gene expression may correlate with tumor characteristics and patient prognosis. Patients with early-stage cervical cancer and low TRPV6 expression had a short progression-free survival time and overall survival [
6,
23]. Lastly, increased expression of the TRPM4 channel, which promotes cancer cell proliferation through the activation of the β-catenin-TCF/LEF pathway, was also linked to unfavorable prognosis [
21,
24].
2.2.2. Calcium Release-Activated Calcium Channels
CRAC channels, particularly STIM1 and Orai1, play crucial roles in the progression of cervical cancer. Their elevated levels were observed in the majority of cervical cancer samples. STIM1 participates in processes such as cancer cell proliferation, migration, and angiogenesis. Its increased expression is associated with a higher risk of metastasis and reduced survival rate, while its inhibition suppresses cell proliferation, that combined, highlights its importance in tumor progression. STIM1 overexpression promotes cervical cancer cell invasion, whereas its knockout decreases its migratory potential [
6]. In HPV-positive cervical cancer, STIM1 overexpression leads to increased VEGF-A (vascular endothelial growth factor) production, which facilitates cancer cell invasion [
13]. That is why elevated STIM1 expression could serve as a valuable biomarker for cervical cancer.
2.3. Sodium Channels
2.3.1. Voltage-Gated Sodium Channels
The voltage-gated sodium channels (VGSC) family consists of nine subtypes (Nav1.1–Nav1.9), encoded by the SCN1A–SCN11A genes [
1,
13]. In cervical cancer tissues, Nav1.2, Nav1.4, Nav1.6, and Nav1.7 were detected, with Nav1.2, Nav1.6, and Nav1.7 being particularly abundant, suggesting their potential as diagnostic markers. By contrast, only Nav1.4 was observed in healthy tissues. Further studies have revealed overexpression of Nav1.6 and Nav1.7b mRNA in HPV16-positive cervical cancer, accompanied by a diffuse distribution of the Nav1.6 protein across all layers of the squamous epithelium. Nav1.6 overexpression was specifically associated with the increased invasive potential of cancer cells [
1,
14]. Additionally, SCN8A overexpression in HeLa, SiHa, and CaSki cell lines increased MMP-2 activity, which promoted tumor invasion and progression. Inhibition of SCN8A significantly reduced the invasive potential of cancerous cells, pointing to the key role of VGSCs in tumor progression [
13].
2.3.2. Epithelial Sodium Channels
Epithelial sodium channels (ENaC) represent another family of sodium channels with potential prognostic significance in cervical cancer. Overexpression of ENaC was associated with favorable patient outcomes, particularly when SCNN1A, SCNN1B, and SCNN1G genes were simultaneously overexpressed. This phenomenon was more frequent in healthy tissues and low-grade cervical cancer, suggesting ENaC might act as a biomarker of good prognosis [
14]. High ENaC expression may indicate less aggressive tumor characteristics, making it a promising tool for stratifying patients based on their risk.
Table 2 below presents a summary regarding ion channels as biomarkers
3. Mechanisms Linking Ion Channels to Cervical Cancer Metastasis
3.1. Role of Ion Channels in Cell Migration, Invasion, and Metastasis
3.1.1. Migration
Cervical cancer cell migration is an essential prerequisite for metastasis. Several types of ion channels have been implicated in regulating cell motility.
Potassium (K
+) Channels: Voltage-gated potassium channels, such as K
V1.3 and K
V10.1, are often upregulated in cancer cells. They are essential for maintaining the resting membrane potential and regulating calcium influx, which is necessary for cell contraction during migration. Inhibiting K
+ channel activity reduces the migratory activity of cervical cancer cells [
25]. K
Ca3.1 channels also play a role, and inhibition of these channels leads to reduced invasion and migration [
26].
Calcium (Ca
2+) Channels: Calcium signaling is critical for various cellular functions, including cell migration. Voltage-gated calcium channels (VGCCs), such as Cav1.2, are important contributors to intracellular calcium levels. Dysregulation of VGCCs can result in increased calcium influx, promoting cytoskeletal remodeling and enhanced migration, as observed in some cervical cancer cell lines [
27]. Calcium channel dysregulation in cancer cells leads to increased invasiveness and metastatic potential. Furthermore, calcium influx in stromal cells can alter their behavior, fostering an immunosuppressive TME that supports tumor growth [
28].
Chloride (Cl
−) Channels: Chloride channels, such as CLCN3 and CLCA2, are involved in cell volume regulation and are crucial for the formation of membrane protrusions during cell migration. Studies have shown that altered chloride channel activity can affect cell volume changes necessary for cell migration and invasion in cervical cancer [
29]. Calcium-activated chloride channels (CaCl) open in response to an increase in cytosolic Ca
2+. Numerous functions, such as afterdepolarization, transepithelial transport, vascular tone control, cellular excitability modulation, epithelial secretion, and cell proliferation in carcinogenesis processes, depend on these channels [
30].
Sodium (Na
+) Channels: Na
V1.5 expression is significantly increased in cervical cancer tissues compared to normal tissues. Silencing Na
V1.5 can lead to reduced cell migration and invasion, and this suggests its role in promoting metastatic behavior through activation of the ERK signaling pathway, which is crucial for cell motility [
31]. Na
V1.5 induces pro-migratory and pro-invasive properties. In particular, Na
V1.5 activity causes the Na
+-H
+ exchanger type 1 (NHE-1) to undergo allosteric regulation, which raises its activity and causes the extracellular space to become more acidic. This favors the pH-dependent activity of proteolytic cysteine cathepsins [
32].
Na
V1.7 is upregulated in metastatic cervical cancer. Inhibition of Na
V1.7 can result in decreasing cell proliferation and migration, implicating this channel in the metastatic cascade [
33]. Na
V1.7 may modulate intracellular calcium levels, affecting the cytoskeletal dynamics essential for cell movement [
34]. Na
V1.6 expression correlates with the advanced stages of cervical cancer. It is involved in boosting the activity of matrix metalloproteinases (MMP2) and Na
+/H
+ exchanger type 1 (NHE-1), further supporting the role of sodium channels in the metastatic process [
35]. Increased sodium influx enhances membrane depolarization, facilitating cytoskeletal reorganization and promoting cellular motility.
3.1.2. Invasion
Invasion involves the penetration of tumor cells into the surrounding tissue and ECM. Ion channels influence this process through the following aspects.
Regulating Matrix Metalloproteinase (MMP) Activity: MMPs are enzymes that degrade ECM components, facilitating tumor invasion. For instance, some ion channels influence the expression and activity of MMPs by modulating intracellular calcium levels. Activation of calcium channels, like Orai1, can lead to the downstream activation of transcription factors that promote MMP production, hence increasing invasion [
36].
Controlling Cell Volume and Contractility: Cell volume and contractility changes are necessary for cells to navigate through the ECM. Ion channels, especially those involved in regulating water transport, such as aquaporins, indirectly contribute to invasion by facilitating cell deformation and movement through confined spaces. Furthermore, the activity of chloride channels and potassium channels contributes to cell shrinkage and swelling necessary for the invasive process [
37].
Modulating Cell–ECM Interactions: Ion channels can affect the localization and turnover of focal adhesions through changes in intracellular calcium and pH, influencing the ability of cells to adhere to and detach from the ECM during invasion [
16,
38,
39,
40,
41,
42].
3.1.3. Metastasis
Ultimately, metastasis is the culmination of migration and invasion, and ion channels play a vital role in the following processes.
Influencing Survival in Circulation: Ion channel dysregulation could contribute to increased resistance to anoikis (detachment-induced apoptosis), a phenomenon critical for tumor cell survival in circulation. Furthermore, some ion channels may contribute to the ability of cancer cell clusters to establish metastases, as has been shown with K
Ca3.1 and the formation of multicellular spheroids [
43].
3.2. Interaction Between Ion Channels and the Tumor Microenvironment
The tumor microenvironment (TME) refers to the complex environment surrounding a tumor, including various cell types, signaling molecules, blood vessels, and extracellular matrix components [
44,
45,
46,
47,
48,
49,
50,
51,
52,
53]. The TME plays a crucial role in cancer development, progression, and metastasis by influencing tumor cell behavior, immune response, and treatment outcomes [
2]. The intricate relationship between ion channels and the tumor microenvironment has been a subject of growing interest in the field of cancer research. Tumor-associated immune cells, the immune response’s dysfunctional side, may arise as a result of the low pH and changes in the ionic composition of TME [
17]. The activity of ion channels in all tumor tissue cells is significantly impacted by the acidity of inadequately perfused tumor regions [
54].
Elevated concentrations of [K
+] and [Na
+] in the interstitium can cause acidification, severe hypoxia, and mechanical stress, which leads to cell necrosis. It also has an impact on immune cell infiltration [
55,
56]. As a result, the ionic composition of the tumor microenvironment is defined by components like protons that strongly influence channel activity, as well as by changed concentration gradients across the plasma membrane, which correspond to the changed electrochemical driving forces. Crucially, ion channels in stromal, immunological, and cancer cells detect the altered ionic composition and invariably alter their activity [
19].
Altered expression of ion channels in tumor cells can affect their interactions with immune cells. For instance, changes in Ca
2+ signaling can influence the ability of immune cells to recognize and eliminate tumor cells [
57]. Cancer-associated fibroblasts (CAFs) are known to promote metastasis. There is evidence that ion channels, especially those responsive to mechanical forces, may influence the activity of CAFs and their capacity to remodel the ECM and facilitate cancer cell invasion [
20].
As discussed earlier, ion channel activity can indirectly influence ECM remodeling by affecting the release of ECM-degrading enzymes, like MMPs. Moreover, changes in ion flux across cell membranes can lead to the altered activity of enzymes and proteins in the ECM, affecting its stiffness and topography, which in turn influences cell behavior [
58]. Among the numerous effects generated by hypoxia, two—cell migration/invasion and resistance to apoptosis—are significantly associated with cell volume regulation, a complicated process meticulously governed by transporters and ion channels situated on the plasma membrane. Cells must prevent significant changes in volume, which threaten structural integrity and the stability of the intracellular environment. In reaction to hypotonic stress, cells protect themselves by initiating the outflow of osmolytes, including ions and certain organic molecules, together with water, to reduce the volume [
59].
3.3. Ion Channel-Mediated Regulation of Cellular Adhesion and Motility
Cellular adhesion and motility are intricately linked in the metastatic process. Ion channels contribute to their regulation through several mechanisms. The formation and dissolution of focal adhesions are critical for cell migration. Calcium signaling, which is modulated by calcium channels, is essential for the regulation of focal adhesion assembly/disassembly and the activation of integrins, which are key for adhesion [
6].
Intracellular calcium and pH levels, regulated by ion channels, influence cytoskeletal dynamics, which are essential for changes in cell shape and membrane protrusions during cell migration. Thus, ion channels can impact cell contractility, driving cell movement and invasion [
60]. Ion channels can also indirectly influence cell–cell adhesion by regulating the expression and function of adhesion molecules, such as E-cadherin. For example, altered calcium signaling can disrupt cadherin-mediated cell adhesion, leading to increased cell motility [
61].
4. Ion Channels as Therapeutic Targets
The past two decades of research have accumulated evidence that the association between specific ion channels and tumor progression will gradually become an important chapter of oncological treatment. The ion channel-oriented therapeutic approach offers a wide range of possible molecular targets, as the relationship between ion channels and carcinogenesis is multidimensionally complex and includes metabolic pathways, oxidative stress, immune response, and drug synergism. A multitude of cellular phenomena involved in ion channel-mediated neoplastic processes result in a diversity of the anticancer effects of ion channel-targeted therapies. Those effects include the promotion of apoptosis as well as the inhibition of cancer cell proliferation, migration, vascularization, and chemotherapy resistance [
62]. Nevertheless, ion channels play important roles in nearly all physiological aspects of cell functionality, and thus, increasing cancer cell-specificity and avoiding adverse effects on healthy cells remains a challenge [
63].
4.1. Potassium Channels as Therapeutic Targets
The application of potassium channel modulators constitutes a promising therapeutic approach in cervical cancer (CCa). On the one hand, potassium channel inhibitors may decrease the malignant properties of cancer cells; whereas, on the other hand, overexpression of some ion channels can sensitize neoplastic cells to specific cytotoxic drugs. The research on the most significant potassium channel types is presented in
Table 3.
K
V1.1 overexpression was reported in HeLa, SiHa, and C-33A CCa cell lines. Knockdown of this potassium channel decreases cellular levels of Wnt1 and Hedgehog (Hgh) in HeLa cells. It suppresses cell proliferation, migration, and invasion. In an animal model, the knockdown of K
V1.1 results in smaller xenograft tumors in nude mice as well as their prolonged survival. According to clinical observations, higher expression of this ion channel correlates with poor prognosis and reduced survival time [
64]. Migration of the HeLa cells is also suppressed under the influence of BDS-II, which causes K
V3.4 channel blockage. BDS-II also increases PTEN activation and inactivates the AKT pathway [
65]. K
V10.1, also known as the ether-à-go-go-1 (EAG1) channel, is strongly associated with the proliferation of cancer cells, and thus, EAG1 inhibitors are suspected to have significant therapeutic potential. Among these inhibitors, tetrandrine reduces tumor growth in vivo [
66]. In vitro, astemizole increases cancer cell apoptosis and decreases cell proliferation in HeLa, SiHa, CaSki, C-33A, and INBL cell lines [
15]. In keratinocytes, EAG1 expression is conditioned by either the presence of HPV oncogenes or cellular immortalization. The combination of astemizole and imipramine decreases channel expression and increases apoptosis in E6/E7-transfected keratinocytes [
1]. Calcitriol decreases EAG1 mRNA level and inhibits proliferation in the SiHa cell line [
67].
Among intermediate-conductance-Ca
2+ potassium channels, K
Ca1.1 constitutes a potential CCa early marker, as higher immunostaining of the protein is more evident in high-grade dysplasia and cervical cancer tissues, which is of great importance, since detection of CCa in early stages constitutes the basis for effective treatment. What is more, in K14E7 transgenic mice, K
Ca1.1-targeted estradiol treatment increases mRNA level and protein expression [
69]. Following another study, activation of K
Ca3.1 sensitizes cancer cells to the growth suppressant effects of DNA-binding fluorescent cation, Hoechst 33258 (H33258). Thus, K
Ca3.1 channel activators could potentially be used to improve the penetration of small cationic toxins into CCa cells that express the discussed channel [
70]. On the other hand, blocking K
Ca3.1 with siRNA, as well as clotrimazole, results in inhibition of cell proliferation [
71]; however, only siRNA blockage causes an increase in the proportion of apoptotic HeLa cells [
71].
4.2. Sodium Channels as Therapeutic Targets
Sodium channels, widely known for their contribution to cell membrane depolarization in excitable cells, can also be found in non-excitable cells, where they participate in the regulation of proliferation and migration [
80]. As sodium fluxes alter membrane potential, research examining the mutual influence of the discussed potential and cancer-associated factors would be of great relevance and significance. The most important types of sodium channels that were found overexpressed in CCa cells are presented in
Table 3. 4.3. Calcium and TRP Channels
Calcium channel regulation constitutes a vast area of molecular possibilities to inhibit CCa cell proliferation, as specific channel blockers or microRNAs could be administered in combination with fundamental anticancer therapy in order to enhance anti-CCa effectiveness. The most important types of calcium channels expressed in CCa are presented in
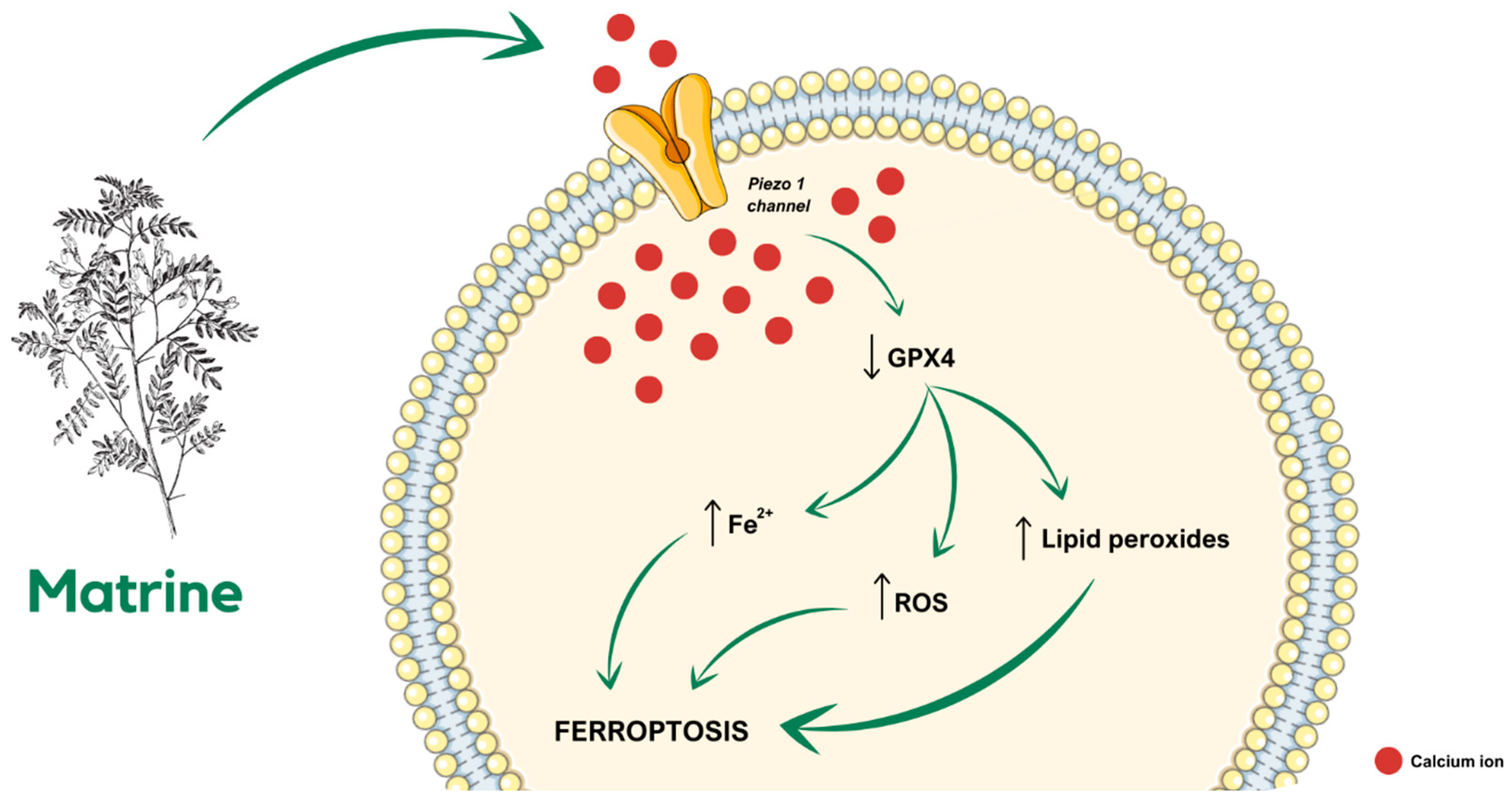
Table 3. Matrine, an alkaloid found in plants from the
Sophora genus, is widely known for its anti-CCa properties. According to the latest research, in a SiHa-derived tumor-bearing mouse model, treatment with matrine inhibits tumor growth in a ferroptosis-involved manner. Matrine administration causes a decrease in glutathione peroxidase 4 (GPX4) cellular level and, simultaneously, an increase in lipid peroxides, reactive oxygen species (ROS), and Fe
2+ levels, eventually leading to cell death. Further investigation of the molecular complexity of the described dependencies revealed that ferroptosis is related to matrine-caused calcium influx through the Piezo1 channel (
Figure 2), as Piezo1 small interfering RNA (siRNA) reverses the influence of matrine in the SiHa cells [
79].
In CCa cell lines, calcium signaling alterations and disruptions occur commonly, and thus, aberrations in calcium channel expression are related to cancer prognosis [
81]. Transient receptor potential vanilloid type 1 (TRPV1) is commonly overexpressed in CCa tissues in comparison to cervical intraepithelial neoplasia and normal epithelial tissue. Interestingly, TRPV1 expression is negatively correlated with phosphatase and tension homolog (PTEN) expression. Thus, the high TRPV1/low PTEN expression is considered a prognostic biomarker for overall CCa survival. At the cellular level, TRPV1 overexpression is associated with increased cell viability and colony formation and could be a biomarker for the chemoradiation response prediction. Transient receptor potential vanilloid subfamily member 6 (TRPV6) constitutes an element of the PRMT5/STC1/TRPV6/JNK axis. Trichostatin A (TSA) significantly suppresses TRPV6 level, as well as protein arginine methyltransferase 5 (PRMT5) level, simultaneously enhancing stanniocalcin 1 (STC1) and phosphorylated c-Jun N-terminal kinase (p-JNK) levels. In a described way, in the HeLa and CaSki cell lines, TSA suppresses cell proliferation and induces apoptosis and autophagy through regulation of the PRMT5/STC1/TRPV6/JNK axis [
14].
Another widely overexpressed TRP channel in CCa is the transient receptor potential cation channel subfamily M member 4 (TRPM4) [
1], which regulates the β-catenin pathway. In the HeLa cell line, the reduction of TRPM4 expression caused a decrease in cell proliferation. Accordingly, TRPM4 silencing caused a reduction of β-catenin/TCF/LEF-dependent transcription. On the contrary, TRPM4 overexpression in T-REx™ 293 cells increases the β-catenin level, leading to activation of the β-catenin/TCF/LEF pathway. The described chain of molecular events results in the facilitation of cell proliferation and invasion [
82].
According to bioinformatic analyses, the transient receptor potential cation channel, subfamily M, member 7 (TRPM7) is correlated with varied types of microRNAs that modulate gene expression, such as miR-192-5p and miR-543 [
77,
78]. Overexpression of miR-192-5p in SiHa and CaSki cell lines reduces proliferation and migration, simultaneously facilitating apoptosis [
77]. Similarly, miR-543 overexpression reduces proliferation and invasion in CCa cells [
78]. TRPM7 overexpression decreases the tumor suppressive effects of miR-192-5p and miR-543 [
77,
78].
5. Innovative Diagnostic Tools Based on Ion Channel Activity
5.1. Development of Diagnostic Assays for Ion Channel Activity in Cervical Cancer
Evaluation of ion channel activity can be possible via diagnostic assays. Their development can offer valuable observation into cervical cancer progression and strengthen clinical decision-making. The above-mentioned assays may utilize various techniques, including fluorescence-based ion sensing, patch-clamp electrophysiology, and high-throughput screening platforms, to evaluate ion channel function in cervical cancer cell lines, patient-derived samples, or animal models [
83]. Previous studies have shown that integrating ion channel-based diagnostic approaches with other clinicopathological variables, including tumor stage, HPV status, and patient demographics, allows a more comprehensive and accurate evaluation of cervical cancer risk and prognosis [
84,
85]. The potential of ion channels as diagnostic tools comes from their ability to serve as biomarkers for early detection, disease staging, and prognostic assessment of cervical cancer. The studies so far have emphasized the abnormal expression and dysregulation of various ion channels in cervical cancer tissues and cell lines, highlighting their promise as diagnostic and prognostic [
86,
87].
A recent study has identified the presence of functional voltage-gated sodium channels in primary cultures of human cervical cancer cells [
41]. Using the whole-cell patch-clamp technique, the researchers were able to isolate and characterize a voltage-gated sodium current as the main component of the inward current in all the investigated cells. These findings suggest that voltage-gated sodium channels may play a crucial role in the pathogenesis of cervical cancer and could potentially serve as therapeutic targets or biomarkers for this disease [
41].
Furthermore, high-throughput screening platforms have the potential to facilitate the discovery of novel ion channel modulators and their application in targeted cancer therapies. These automated screening systems enable the rapid evaluation of large chemical libraries, accelerating the identification of new pharmacological agents that can selectively modulate ion channel activity and potentially be developed into targeted cancer therapeutics [
72,
81].
5.2. Imaging Techniques and Biosensors for Ion Channel Detection
Investigators have directed their focus to utilize imaging techniques and biosensors to detect and monitor ion channel activity within cervical cancer cells. Molecular imaging techniques, including fluorescent biosensors, have been employed to map the localization and temporal dynamics of particular ion channels within the cervical tumor microenvironment [
88]. The literature review shows that these methods facilitate real-time observation of ion channel activity, offering valuable insights into their involvement in the pathogenesis of cervical cancer. Electrochemical biosensors have emerged as a promising tool for the label-free detection and quantification of ion channels. They can measure changes in ion channel activity, which can serve as biomarkers for early cancer detection and disease monitoring [
86,
89,
90]. Furthermore, those devices offer the ability to quantify ion channel activity and identify potential therapeutic targets [
88,
89,
90].
Several studies have indicated that complex interactions between ion channels and diverse signaling cascades involved in cervical cancer, including those associated with vascular endothelial growth factor (VEGF) and its receptors, are significant for understanding the underlying molecular mechanisms [
72,
86,
91]. The literature review shows that systems biology has been essential in understanding the complex regulatory pathways that control the development and progression of cervical cancer.
It has been established that utilizing these advanced imaging methods and biosensor technologies allows researchers to develop more effective diagnostic and therapeutic strategies that acquire an improved understanding of the role of ion channels in cervical cancer [
41,
92]. As has been previously reported in the literature, fluorescent imaging techniques have been utilized to monitor the activity of calcium channels, which are critical regulators of angiogenesis and tumor vascularization [
86]. Furthermore, electrochemical biosensors have emerged as a valuable tool for the detection and quantification of ion channels, such as the sodium–boron cotransporter 1, a membrane-bound protein that plays a pivotal role in maintaining boron homeostasis [
93]. Some studies have highlighted the promising applications of carbon dots, a class of fluorescent nanomaterials, in targeted cancer imaging and treatment [
94,
95]. These engineered nanoparticles can selectively localize within tumor tissues, providing a multifaceted platform for both diagnostic and therapeutic purposes [
10,
96,
97].
5.3. Potential for Non-Invasive Diagnostic Techniques
Cervical cancer is a prevalent form of gynecological cancer, and early detection is crucial for effective treatment and management. Traditional diagnostic techniques, such as colposcopy and biopsy, may cause patient discomfort [
98,
99]. Prior research has investigated the potential of non-invasive diagnostic techniques, with particular attention to the role of ion channels in cervical cancer [
87]. The abnormal expression and dysregulation of ion channels can contribute to the hallmarks of cancer, such as dysregulated proliferation, evasion of apoptosis, and enhanced metastasis [
100,
101].
A series of recent studies of ion channels in cervical cancer has prompted investigations into non-invasive diagnostic approaches, including liquid biopsy and wearable technology. A liquid biopsy, involving the analysis of circulating tumor cells, cell-free tumor DNA, or extracellular vesicles, may offer a non-invasive method to detect and monitor the presence of ion channel-associated biomarkers in cervical cancer patients [
87,
101,
102]. Unfortunately, those non-invasive methods may encounter some difficulties before being applied to clinical use, due to their low sensitivity and specificity. Sensitivity ensures the accurate identification of individuals with cervical cancer, minimizing false-negative results, while specificity focuses on excluding those without the disease, reducing false-positive findings. Overcoming those challenges may be possible through advancements in analytical techniques and biomarker detection [
4,
103,
104].
A better understanding of ion channel dysregulation seems to be the key to enhancing the detection of cervical cancer. The development of non-invasive diagnostic tools may also be crucial for risk evaluation and personalized treatment planning, ultimately leading to improved patient outcomes.
6. Combination Therapies Involving Ion Channel Modulation
6.1. Synergistic Effects of Ion Channel Modulators with Chemotherapy, Radiation, or Immunotherapy
Dysregulated ion channels significantly contribute to cancer cell chemoresistance by altering drug uptake and efflux, thereby reducing the effectiveness of chemotherapeutic agents. Ion channel modulators can be used in combination with more standard cancer therapies, such as chemotherapy, radiation, or immunotherapy, and have shown potential to improve these treatments. Ion channel modulators, for example, can sensitize cancer cells to certain chemotherapeutics, thereby enhancing their efficacy. Likewise, their conjugation with radiation therapy can enhance the radiation exposure damage to neoplastic cells causing an improved treatment efficacy [
105]. For example, matrine has been shown to have an effect on the treatment of cervical cancer by promoting ferroptosis. Matrine was found to inhibit cancer cell proliferation with no apparent side effects. The treatment led to a reduction in GPX4 protein levels and an increase in lipid peroxides and Fe
2+ content, indicating the induction of ferroptosis. Additionally, matrine upregulated the expression of the Piezo1 channel, facilitating calcium influx, and further promoting ferroptosis. These findings suggest that matrine exerts its anti-cancer effects by activating the Piezo1 channel and inducing ferroptosis, offering a promising therapeutic approach for cervical cancer [
79].
Immunotherapy can also benefit from the inclusion of ion channel modulators [
105,
106]. Enhanced calcium signaling is closely linked to the development of tumors, promoting the malignant behaviors of cancer cells, activating various oncogenes and pathways, driving the creation and release of exosomes, and weakening the immune response by regulating immune checkpoints. Consequently, inhibiting calcium signaling could lower exosomal PD-L1 expression by reducing exosome secretion from cancer cells, presenting a promising strategy for cancer treatment through immune checkpoint blockade therapy. Therefore, all calcium-permeable ion channels and pumps emerge as natural therapeutic targets [
107].
6.2. Potential for Ion Channel Inhibitors to Enhance Treatment Response
Na
v channels have been demonstrated to play a key role in glioblastoma multiforme (GBM) progression and stemness regulation, demonstrating a strong association between their expression and poor patient outcomes, particularly in the proneural subtype. A therapeutic strategy that combines Na
v channel inhibitors with standard treatments and repurposing antiepileptic drugs has shown significant potential for clinical application. These findings not only advance our understanding of the pathophysiology of GBM but also point to innovative approaches to more effectively treat this aggressive cancer [
108]. Moreover, the voltage-gated sodium channel modulator DPI-201-106 has been shown to reduce glioblastoma cell viability by inducing cell cycle arrest and apoptosis [
95]. Similar approaches can be applied to cervical cancer, where a transient receptor potential vanilloid 1 (TRPV1) inhibitor may enhance the efficacy of existing treatments and potentially overcome resistance to cisplatin therapy [
96]. The combination of ion channel inhibitors with DNA damage response inhibitors has demonstrated synergistic effects in preclinical models, suggesting that these inhibitors can be effectively integrated into combination therapy strategies [
95].
Researchers have demonstrated that K
ATP channels are active in HPV-positive cells and necessary for HPV oncoprotein expression. The regulatory subunit SUR1 of the K
ATP channel complex was upregulated in HPV-positive cervical cancer cells and patient samples, dependent on the E7 oncoprotein. Inhibition or knockdown of SUR1 significantly reduced cell proliferation by inducing G1 cell cycle arrest, confirmed in both in vitro and in vivo assays. The study suggests that K
ATP channels drive cell proliferation through the MAPK/AP-1 signaling pathway. These findings highlight the potential of targeting KATP channels with clinically available inhibitors as a novel therapy for HPV-driven cervical cancer [
10].
Nifedipine has been shown to reduce PD-L1 expression in colorectal cancer cells and PD-1 expression in CD8+ T cells, thereby reactivating immune surveillance and potentially enhancing the efficacy of PD-1-based immunotherapy [
97]. Similarly, calcium channel blockers, such as amlodipine, felodipine, manidipine, and cilnidipine, have demonstrated significant efficacy in inhibiting filopodia formation, directional cell migration, and invasive behavior in breast and pancreatic cancers [
91,
98]. Notably, amlodipine selectively induces the autophagic degradation of PD-L1 in a calcium-dependent manner, reducing PD-1 binding, increasing T-cell cytotoxicity, suppressing tumor growth, and improving the effectiveness of anti-PD-L1 therapy in preclinical models [
99]. Furthermore, the T-type calcium channel blocker KTt-45 has been identified as a potential anticancer agent with promising efficacy against HeLa cervical cancer cells. As can be seen from the results of the cited study, KTt-45 has cytotoxic activity on numerous cancer cell lines, and it is very specific for HeLa cells. Its mechanism involves inducing mitochondrial-dependent apoptosis, as evidenced by cytoplasmic vacuole accumulation, chromatin condensation, nuclear fragmentation, and the activation of caspase-9 and caspase-3 [
100]. Uncovering the full anticancer therapeutic potential of calcium channel antagonists requires further research.
A fact worth noting is that certain ion channels play a critical role in cancer cell migration and invasion, making them attractive targets for reducing metastatic potential [
101,
102]. The mechanosensitive ion channel Piezo1 has been identified as a potential prognostic and therapeutic target in cervical cancer. Elevated expression of Piezo1 was observed in cervical cancer tissues and cells, particularly in patients with lymph node metastasis. Silencing Piezo1 significantly reduced cervical cancer cell invasion and migration, while activation with the selective agonist enhanced these processes. It was found that activation of Piezo1 is capable of regulating the release of extracellular ATP, which can enhance invasion, migration, and pseudopodium formation in cervical cancer. The in vivo analysis also strengthened the findings on the importance of Piezo1 in tumor progression. The results of the cited study suggest that Piezo1 contributes to cervical cancer progression by facilitating ATP release and could serve as a promising target for therapeutic and prognostic applications [
4].
Ion channels play a major role in modulating the tumor microenvironment, influencing factors like pH and hypoxia that are closely associated with treatment resistance. Targeting these channels can potentially improve the effectiveness of already approved therapies [
103]. Recent studies have also found a close correlation between high sodium concentrations within the tumor microenvironment and adverse clinical outcomes, with several types of Na
+ channels acting as important regulatory factors. These novel findings open the door for therapeutic innovation, which is already being exploited in the clinic as reflected in ongoing clinical trials aimed at targeting this altered sodium microenvironment [
104].
6.3. Clinical Trials and Studies Exploring Combination Strategies
A venom-derived peptide, SOR-C13, has been identified as a potent TRPV6 blocker, effectively inhibiting tumor growth in ovarian and breast cancer models. Following successful preclinical studies and safety validation, SOR-C13 entered phase 1 clinical trials, demonstrating safety, tolerability, and anti-tumor activity, particularly in pancreatic cancer. Encouraged by these results, Phase 1b trials are underway, and SOR-C13 has been granted orphan drug status by the FDA for ovarian and pancreatic cancer treatment [
105].
Chemotherapy-induced peripheral neuropathy, a common side effect of drugs like oxaliplatin, often causes hypersensitivity with few effective treatments. TRPA1, an ion channel linked to chemotherapy-induced peripheral neuropathy, is modulated by the Sigma-1 receptor. Sigma-1 receptor antagonists disrupt this interaction, reducing TRPA1 activity and preventing painful symptoms in a mouse model of oxaliplatin neuropathy. These findings highlight the Sigma-1 receptor antagonists as a potential strategy for treating chemotherapy-induced peripheral neuropathy and neuropathic pain [
106].
The presence of the Kir6.2/SUR2 potassium channel could be a positive indicator for the prognosis of gynecologic cancers. Using minoxidil to stimulate this channel effectively halts tumor growth in a xenograft model of ovarian cancer. Minoxidil changes the metabolic and oxidative state of cancer cells, leading to mitochondrial disruption and significant DNA damage, which triggers a cell death pathway independent of caspase-3. These findings suggest that repurposing FDA-approved K
+ channel activators could offer a novel, safe adjuvant therapeutic approach to traditional chemotherapy for treating gynecologic cancers [
107].
The phytochemical α-mangostin exhibits powerful antineoplastic effects, making it a promising candidate to use as an adjunct to standard therapies for the treatment of cervical neoplasia. It potently inhibits cell proliferation in a dose-dependent fashion in cell lines containing increased HPV16 copy numbers. Notably, α-mangostin greatly reduces the expression of HPV oncogenes E6 and E7 as well as the KCNH1 gene both in vitro and in vivo. Additionally, it modulates cytokine profiles, decreases Ki-67 levels, and suppresses tumor growth in xenografted mice. These findings underscore the potential of α-mangostin as a therapeutic agent for both the prevention and treatment of cervical cancer [
108].
7. Challenges and Future Directions
Ion channels are essential for many cell functions. Their distribution varies depending on the cell type and disease stage. So far, scientists have been able to identify several profiles of ion channels that correlate with tumor behavior, drug resistance, and clinical outcome. This makes them promising targets for cancer detection and therapy. However, there are still many challenges. Ion channels are responsible for the regulation of many processes, making it hard to isolate their influence on cancer progression. We also need a more profound understanding to fully distinguish tumor-promoting from tumor-suppressing effects.
Another challenge is the lack of selectivity of the discussed proteins. Despite some ion channels being recognized as potential biomarkers, their specificity, and reliability in identifying cervical cancer cells are limited. Moreover, the expression patterns of ion channels vary significantly among cervical cancer patients. This restricts the determination of common biomarkers and treatment targets. It also leads to different treatment outcomes. This shows a need for more clinical trials in order to validate the efficacy, safety, and optimal use of the discussed methods. Additionally, the development of standardized assays to measure ion channel activity in clinical samples is essential. While promising, ion channel-based diagnostic tools require refinement to achieve clinical-grade sensitivity, specificity, and cost-effectiveness. Innovative diagnostic tools should be further developed and validated for the proper monitoring of cervical cancer progress. The development of biosensors and imaging agents that target ion channels could improve early detection, particularly when combined with other diagnostic workflows.
Due to the complexity of ion channel physiology, resistance to treatment may occur. Cells may display compensatory pathways or activation of alternative ion channels. Moreover, the presence of ion channels in every cell increases the risk of targeting healthy tissues, which may lead to unexpected complications. The integration of genomics, transcriptomics, and proteomics data may be crucial in the development of more selective modulators. Advances in molecular modeling, drug design, and nano-technology-based delivery systems may play a key role in creating more personalized medicine. For such tailored therapies, we also need patient-specific data, such as ion channel expression profiles and genetic mutations. This shows the great importance of creating accurate predictive models and biomarker-driven clinical trials.
Ion channel modulators have the potential to be used in combination therapies to enhance the effectiveness of conventional treatments, such as chemotherapy, radiotherapy, and immunotherapy. It may also help in overcoming the resistance of the cancer cells. To optimize these effects, more research into synergistic effects and optimal sequencing is required. Conducting large-scale, longitudinal studies across diverse populations is critical to validate the clinical utility of ion channel biomarkers and therapies. Such studies can also uncover regional and demographic differences in ion channel-related cancer biology.
8. Conclusions
Despite significant advances in understanding the factors contributing to the development of cervical cancer, as well as the implementation of preventive actions, such as HPV vaccination and numerous screening programs, this cancer remains a significant epidemiological, diagnostic, and therapeutic challenge. Therefore, it is crucial to conduct advanced research, including molecular diagnostics, which will not only deepen our understanding of the disease’s pathogenesis but enable us to develop modern diagnostic methods and effective targeted therapies.
Ion channels seem to be promising targets for such research, due to their critical role in the progression of cervical cancer. Their diverse functions, including influencing the processes of cancer cell proliferation, migration, and invasion, make them important potential biomarkers for cancer detection and disease progression assessment. Additionally, the evaluation of gene expression may be significant in predicting responses to traditional anticancer treatments and determining patient prognosis.
Advances made in both in vivo studies and animal models have shown promising results. However, challenges such as the complexity of ion channel interactions and the risk of adverse effects still require extensive research. Diagnostic methods under development also need further refinement to ensure the highest sensitivity and specificity, as well as the ability to detect cancer in its early stages. To meet these goals, future work must rigorously address current limitations—small patient cohorts, model heterogeneity, off-target safety concerns, and drug-delivery hurdles—so that the most promising ion-channel targets can advance from proof-of-concept to well-designed clinical trials and, ultimately, everyday clinical practice. Thus, continued research will be crucial to fully harness the potential of ion channels in cervical cancer diagnostics and treatment.
Author Contributions
Conceptualization—J.K. and C.K.; methodology, data curation and investigation—E.B., M.C., K.R. (Katarzyna Rakoczy), M.F., K.R. (Katarzyna Rekiel), and I.S.; writing—original draft preparation—E.B., M.C., K.R. (Katarzyna Rakoczy), M.F., K.R. (Katarzyna Rekiel), and I.S.; writing—review and editing—J.K. and C.K., visualization—E.B. and J.K.; supervision, project administration and funding acquisition—J.K. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
Scientific Students’ Group no. 148 of the Department of Molecular and Cellular Biology, Wroclaw Medical University, and partially by the Statutory Subsidy Funds of the Department of Molecular and Cellular Biology, no. SUBZ.D260.25.027.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Diaz, D.; Delgadillo, D.M.; Hernández-Gallegos, E.; Ramírez-Domínguez, M.E.; Hinojosa, L.M.; Ortiz, C.S.; Berumen, J.; Camacho, J.; Gomora, J.C. Functional Expression of Voltage-gated Sodium Channels in Primary Cultures of Human Cervical Cancer. J. Cell. Physiol. 2007, 210, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.J.; Song, M.S.; Lee, S.Y. Kv3 Channels Contribute to Cancer Cell Migration via Vimentin Regulation. Biochem. Biophys. Res. Commun. 2021, 551, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiao, C.; Li, Q.; Zhu, X.; Zhao, R.; Peng, X. Research Progress in the Relationship Between P2X7R and Cervical Cancer. Reprod. Sci. 2023, 30, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Li, Y.; Liu, T.; Deng, J.; Liang, H.; Shen, F. The Activation of Piezo1 Channel Promotes Invasion and Migration via the Release of Extracellular ATP in Cervical Cancer. Pathol.-Res. Pract. 2024, 260, 155426. [Google Scholar] [CrossRef]
- Litan, A.; Langhans, S.A. Cancer as a Channelopathy: Ion Channels and Pumps in Tumor Development and Progression. Front. Cell. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef]
- Lei, J.; Deng, F.; Ding, H.; Fu, M.; Xu, T.; Ji, B.; Feng, L.; Li, M.; Qiu, J.; Gao, Q. Recent Developments on the Roles of Calcium Signals and Potential Therapy Targets in Cervical Cancer. Cells 2022, 11, 3003. [Google Scholar] [CrossRef]
- Huang, X.; Li, Q.; Zheng, X.; Jiang, C. TTYH3 Promotes Cervical Cancer Progression by Activating the Wnt/β-Catenin Signaling Pathway. Cancer Investig. 2024, 42, 726–739. [Google Scholar] [CrossRef]
- Wang, B.; Wang, W.; Zhou, W.; Zhao, Y.; Liu, W. Cervical Cancer-specific Long Non-coding RNA Landscape Reveals the Favorable Prognosis Predictive Performance of an Ion-channel-related Signature Model. Cancer Med. 2024, 13, e7389. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, X.; Wu, Q.; Zheng, J.; Cui, Z.; Cai, X.; Li, Y.; Zheng, C.; Sun, Y. Transient Receptor Potential Channels’ Genes Forecast Cervical Cancer Outcomes and Illuminate Its Impact on Tumor Cells. Front. Genet. 2024, 15, 1391842. [Google Scholar] [CrossRef]
- Scarth, J.A.; Wasson, C.W.; Patterson, M.R.; Evans, D.; Barba-Moreno, D.; Carden, H.; Cassidy, R.; Whitehouse, A.; Mankouri, J.; Samson, A.; et al. Exploitation of ATP-Sensitive Potassium Ion (KATP) Channels by HPV Promotes Cervical Cancer Cell Proliferation by Contributing to MAPK/AP-1 Signalling. Oncogene 2023, 42, 2558–2577. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Shen, R. The Important Role of Ion Transport System in Cervical Cancer. Int. J. Mol. Sci. 2021, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Chiliquinga, A.J.; Acosta, B.; Ogonaga-Borja, I.; Villarruel-Melquiades, F.; De La Garza, J.; Gariglio, P.; Ocádiz-Delgado, R.; Ramírez, A.; Sánchez-Pérez, Y.; García-Cuellar, C.M.; et al. Ion Channels as Potential Tools for the Diagnosis, Prognosis, and Treatment of HPV-Associated Cancers. Cells 2023, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Ogonaga-Borja, I.; Acosta, B.; Chiliquinga, A.J.; de la Garza, J.; Gariglio, P.; Ocádiz-Delgado, R.; Bañuelos, C.; Camacho, J. Ion Channels and Personalized Medicine in Gynecological Cancers. Pharmaceuticals 2023, 16, 800. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, A.Y.; Hinojosa, L.M.; Parraguirre-Martínez, S.; González, A.; Morales, F.; Montalvo, G.; Vera, E.; Hernández-Gallegos, E.; Camacho, J. Expression of KATP Channels in Human Cervical Cancer: Potential Tools for Diagnosis and Therapy. Oncol. Lett. 2018, 15, 6302–6308. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zhang, Q.; Li, C. Silencing of KCNA1 Suppresses the Cervical Cancer Development via Mitochondria Damage. Channels 2019, 13, 321–330. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Li, J.; Guo, S.; Lin, X.; Zhang, H.; Zhan, Y.; An, H. Tetrandrine, a Novel Inhibitor of Ether-à-Go-Go-1 (Eag1), Targeted to Cervical Cancer Development. J. Cell. Physiol. 2019, 234, 7161–7173. [Google Scholar] [CrossRef]
- Pardo, L.A.; Contreras-Jurado, C.; Zientkowska, M.; Alves, F.; Stühmer, W. Role of Voltage-Gated Potassium Channels in Cancer. J. Membr. Biol. 2005, 205, 115–124. [Google Scholar] [CrossRef]
- Ramírez, A.; Vera, E.; Gamboa-Domínguez, A.; Lambert, P.; Gariglio, P.; Camacho, J. Calcium-Activated Potassium Channels as Potential Early Markers of Human Cervical Cancer. Oncol. Lett. 2018, 15, 7249–7254. [Google Scholar] [CrossRef]
- Liu, L.; Zhan, P.; Nie, D.; Fan, L.; Lin, H.; Gao, L.; Mao, X. Intermediate-Conductance-Ca2-Activated K Channel IKCa1 Is Upregulated and Promotes Cell Proliferation in Cervical Cancer. Med. Sci. Monit. Basic Res. 2017, 23, 45–57. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, J.; Tian, W.; Qiao, S.; Wang, H. Role of TRPV1 Ion Channel in Cervical Squamous Cell Carcinoma Genesis. Front. Mol. Biosci. 2022, 9, 980262. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.P.; Chen, Y.L.; Zheng, A. Pharmacological Targeting Transient Receptor Potential Canonical Channel 6 Modulates Biological Behaviors for Cervical Cancer HeLa and SiHA Cell. Cancer Cell Int. 2022, 22, 145. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.M. TRPV6 as a Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Borgström, A.; Peinelt, C.; Stokłosa, P. TRPM4 in Cancer—A New Potential Drug Target. Biomolecules 2021, 11, 229. [Google Scholar] [CrossRef]
- Aissaoui, D.; Mlayah-Bellalouna, S.; Jebali, J.; Abdelkafi-Koubaa, Z.; Souid, S.; Moslah, W.; Othman, H.; Luis, J.; ElAyeb, M.; Marrakchi, N.; et al. Functional Role of Kv1.1 and Kv1.3 Channels in the Neoplastic Progression Steps of Three Cancer Cell Lines, Elucidated by Scorpion Peptides. Int. J. Biol. Macromol. 2018, 111, 1146–1155. [Google Scholar] [CrossRef]
- Todesca, L.M.; Maskri, S.; Brömmel, K.; Thale, I.; Wünsch, B.; Koch, O.; Schwab, A. Targeting Kca3.1 Channels in Cancer. Cell. Physiol. Biochem. 2021, 55, 131–144. [Google Scholar] [CrossRef]
- Martínez-Delgado, G.; Felix, R. Emerging Role of CaV1.2 Channels in Proliferation and Migration in Distinct Cancer Cell Lines. Oncology 2017, 93, 1–10. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef]
- Guan, Y.; Luan, Y.; Xie, Y.; Zhou, H.; Li, W.; Zhang, X.; Shen, X.; Chen, Y.; Xu, L.; Lin, Z.; et al. Chloride Channel-3 Is Required for Efficient Tumour Cell Migration and Invasion in Human Cervical Squamous Cell Carcinoma. Gynecol. Oncol. 2019, 153, 661–669. [Google Scholar] [CrossRef]
- Hartzell, C.; Putzier, I.; Arreola, J. Calcium-Activated Chloride Channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Poisson, L.; Benouna, O.; Lemoine, R.; Chadet, S.; Pétereau, A.; Lahlou, W.; Guyétant, S.; Ouaissi, M.; Pukkanasut, P.; et al. Voltage-Gated Sodium Channel NaV1.5 Controls NHE−1−Dependent Invasive Properties in Colon Cancer Cells. Cancers 2023, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Gillet, L.; Roger, S.; Besson, P.; Lecaille, F.; Gore, J.; Bougnoux, P.; Lalmanach, G.; Le Guennec, J.-Y. Voltage-Gated Sodium Channel Activity Promotes Cysteine Cathepsin-Dependent Invasiveness and Colony Growth of Human Cancer Cells. J. Biol. Chem. 2009, 284, 8680–8691. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Plata, E.; Ortiz, C.S.; Marquina-Castillo, B.; Medina-Martinez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of NaV 1.6 Channels Is Associated with the Invasion Capacity of Human Cervical Cancer. Int. J. Cancer 2012, 130, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Pukkanasut, P.; Whitt, J.; Guenter, R.; Lynch, S.E.; Gallegos, C.; Rosendo-Pineda, M.J.; Gomora, J.C.; Chen, H.; Lin, D.; Sorace, A.; et al. Voltage-Gated Sodium Channel NaV1.7 Inhibitors with Potent Anticancer Activities in Medullary Thyroid Cancer Cells. Cancers 2023, 15, 2806. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Pukkanasut, P.; Velu, S.E.; Brackenbury, W.J.; Hales, T.G.; Besson, P.; Gomora, J.C.; Roger, S. Pharmacological and Nutritional Targeting of Voltage-Gated Sodium Channels in the Treatment of Cancers. iScience 2021, 24, 102270. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, J.; Liu, K.; Xie, C.; Chen, H.; Guo, Z.; Guo, S.; Chen, Y. Orai1-Mediated Store-Operated Ca2+ Entry Promotes Cervical Cancer Progression through IL-6 Signaling. Front. Mol. Biosci. 2022, 9, 1041674. [Google Scholar] [CrossRef]
- Ozaki, S.; Mikami, K.; Kunieda, T.; Tanaka, J. Chloride Intracellular Channel Proteins (CLICs) and Malignant Tumor Progression: A Focus on the Preventive Role of CLIC2 in Invasion and Metastasis. Cancers 2022, 14, 4890. [Google Scholar] [CrossRef]
- Yao, M.; Tijore, A.; Cheng, D.; Li, J.V.; Hariharan, A.; Martinac, B.; Van Nhieu, G.T.; Cox, C.D.; Sheetz, M. Force- and Cell State-Dependent Recruitment of Piezo1 Drives Focal Adhesion Dynamics and Calcium Entry. Sci. Adv. 2022, 8, eabo1461. [Google Scholar] [CrossRef]
- Rodat-Despoix, L.; Chamlali, M.; Ouadid-Ahidouch, H. Ion Channels as Key Partners of Cytoskeleton in Cancer Disease. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188627. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Liu, L.; Wang, M. β-Catenin: Oncogenic Role and Therapeutic Target in Cervical Cancer. Biol. Res. 2020, 53, 33. [Google Scholar] [CrossRef]
- Ramírez, A.; García-Quiroz, J.; Aguilar-Eslava, L.; Sánchez-Pérez, Y.; Camacho, J. Novel Therapeutic Approaches of Ion Channels and Transporters in Cancer. In Targets of Cancer Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2020; Volume 183, pp. 45–101. [Google Scholar] [CrossRef]
- Toplak, Ž.; Hendrickx, L.A.; Abdelaziz, R.; Shi, X.; Peigneur, S.; Tomašič, T.; Tytgat, J.; Peterlin-Mašič, L.; Pardo, L.A. Overcoming Challenges of HERG Potassium Channel Liability through Rational Design: Eag1 Inhibitors for Cancer Treatment. Med. Res. Rev. 2022, 42, 183–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jan, L.Y. Targeting Potassium Channels in Cancer. J. Cell Biol. 2014, 206, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2020, 221, 107753. [Google Scholar] [CrossRef]
- Girault, A.; Ahidouch, A.; Ouadid-Ahidouch, H. Roles for Ca2+ and K+ Channels in Cancer Cells Exposed to the Hypoxic Tumour Microenvironment. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118644. [Google Scholar] [CrossRef]
- Pethő, Z.; Najder, K.; Carvalho, T.; McMorrow, R.; Todesca, L.M.; Rugi, M.; Bulk, E.; Chan, A.; Löwik, C.W.G.M.; Reshkin, S.J.; et al. PH-Channeling in Cancer: How PH-Dependence of Cation Channels Shapes Cancer Pathophysiology. Cancers 2020, 12, 2484. [Google Scholar] [CrossRef]
- He, W.; Xu, J.; Mu, R.; Li, Q.; Lv, D.L.; Huang, Z.; Zhang, J.; Wang, C.; Dong, L. High-Salt Diet Inhibits Tumour Growth in Mice via Regulating Myeloid-Derived Suppressor Cell Differentiation. Nat. Commun. 2020, 11, 1732. [Google Scholar] [CrossRef]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J.; et al. Ionic Immune Suppression within the Tumour Microenvironment Limits T Cell Effector Function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef]
- Hofschröer, V.; Najder, K.; Rugi, M.; Bouazzi, R.; Cozzolino, M.; Arcangeli, A.; Panyi, G.; Schwab, A. Ion Channels Orchestrate Pancreatic Ductal Adenocarcinoma Progression and Therapy. Front. Pharmacol. 2021, 11, 586599. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Liu, J.; Chen, J.; Wang, J.; Hua, H.; Jiang, Y. CAMP-PKA/EPAC Signaling and Cancer: The Interplay in Tumor Microenvironment. J. Hematol. Oncol. 2024, 17, 5. [Google Scholar] [CrossRef]
- Karska, J.; Kowalski, S.; Saczko, J.; Moisescu, M.G.; Kulbacka, J. Mechanosensitive Ion Channels and Their Role in Cancer Cells. Membranes 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Monteith, G.R. Plasma Membrane Ion Channels and Epithelial to Mesenchymal Transition in Cancer Cells. Endocr.-Relat. Cancer 2016, 23, R517–R525. [Google Scholar] [CrossRef] [PubMed]
- De Guadalupe Chávez-López, M.; Hernández-Gallegos, E.; Vázquez-Sánchez, A.Y.; Gariglio, P.; Camacho, J. Antiproliferative and Proapoptotic Effects of Astemizole on Cervical Cancer Cells. Int. J. Gynecol. Cancer 2014, 24, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Ceja-Ochoa, I.; Restrepo-Angulo, I.; Larrea, F.; Avila-Chávez, E.; García-Becerra, R.; Borja-Cacho, E.; Barrera, D.; Ahumada, E.; Gariglio, P.; et al. Estrogens and Human Papilloma Virus Oncogenes Regulate Human Ether-à-Go-Go-1 Potassium Channel Expression. Cancer Res. 2009, 69, 3300–3307. [Google Scholar] [CrossRef]
- Avila, E.; García-Becerra, R.; Rodríguez-Rasgado, J.A.; Díaz, L.; Ordaz-Rosado, D.; Zügel, U.; Steinmeyer, A.; Barrera, D.; Halhali, A.; Larrea, F.; et al. Calcitriol Down-Regulates Human Ether a Go-Go 1 Potassium Channel Expression in Cervical Cancer Cells. Anticancer Res. 2010, 30, 2667–2672. [Google Scholar]
- Bukhari, M.; Deng, H.; Sipes, D.; Ruane-Foster, M.; Purdy, K.; Woodworth, C.D.; Sur, S.; Samways, D.S.K. KCa3.1-Dependent Uptake of the Cytotoxic DNA-Binding Dye Hoechst 33258 into Cancerous but Not Healthy Cervical Cells. J. Biol. Chem. 2021, 296, 100084. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. Noncanonical Roles of Voltage-Gated Sodium Channels. Neuron 2013, 80, 280–291. [Google Scholar] [CrossRef]
- Jin, J.; Fan, Z.; Long, Y.; Li, Y.; He, Q.; Yang, Y.; Zhong, W.; Lin, D.; Lian, D.; Wang, X.; et al. Matrine Induces Ferroptosis in Cervical Cancer through Activation of Piezo1 Channel. Phytomedicine 2024, 122, 155165. [Google Scholar] [CrossRef]
- Liu, J.H.; Cao, Y.M.; Rong, Z.P.; Ding, J.; Pan, X. Trichostatin A Induces Autophagy in Cervical Cancer Cells by Regulating the PRMT5-STC1-TRPV6-JNK Pathway. Pharmacology 2021, 106, 60–69. [Google Scholar] [CrossRef]
- Narayan, G.; Bourdon, V.; Chaganti, S.; Arias-Pulido, H.; Nandula, S.V.; Rao, P.H.; Gissmann, L.; Dürst, M.; Schneider, A.; Pothuri, B.; et al. Gene Dosage Alterations Revealed by CDNA Microarray Analysis in Cervical Cancer: Identification of Candidate Amplified and Overexpressed Genes. Genes. Chromosomes Cancer 2007, 46, 373–384. [Google Scholar] [CrossRef]
- Armisén, R.; Marcelain, K.; Simon, F.; Tapia, J.C.; Toro, J.; Quest, A.F.G.; Stutzin, A. TRPM4 Enhances Cell Proliferation through Up-Regulation of the β-Catenin Signaling Pathway. J. Cell. Physiol. 2011, 226, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.F.; Zhuang, Y.J.; Wang, Y.; Zhang, Z.Y.; Xu, X.Z.; Mao, Y.R.; Yu, J.J. Tumor Suppressor MiR-192-5p Targets TRPM7 and Inhibits Proliferation and Invasion in Cervical Cancer. Kaohsiung J. Med. Sci. 2021, 37, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gan, L.; Zhang, J. MiR-543 Inhibites Cervical Cancer Growth and Metastasis by Targeting TRPM7. Chem.-Biol. Interact. 2019, 302, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.S.; Montante-Montes, D.; Saqui-Salces, M.; Hinojosa, L.M.; Gamboa-Dominguez, A.; Hernández-Gallegos, E.; Martínez-Benítez, B.; Solís-Pancoatl, M.D.R.; Garcia-Villa, E.; Ramírez, A.; et al. Eag1 Potassium Channels as Markers of Cervical Dysplasia. Oncol. Rep. 2011, 26, 1377–1383. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, L.; Nie, D.; Liu, Y.; Mao, X. Effect of Inhibition of Intermediate-Conductance-Ca2+-Activated K+ Channels on HeLa Cell Proliferation. J. Cancer Res. Ther. 2018, 14, S41–S45. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Espinosa, A.M.; Alfaro, A.; Herrera-Carrillo, Z.; Ramirez-Cordero, B.E.; Cortes-Reynosa, P.; Perez Salazar, E.; Berumen, J.; Gomora, J.C. The Invasiveness of Human Cervical Cancer Associated to the Function of NaV1.6 Channels Is Mediated by MMP-2 Activity. Sci. Rep. 2018, 8, 12995. [Google Scholar] [CrossRef]
- Altamura, C.; Greco, M.R.; Carratù, M.R.; Cardone, R.A.; Desaphy, J.F. Emerging Roles for Ion Channels in Ovarian Cancer: Pathomechanisms and Pharmacological Treatment. Cancers 2021, 13, 668. [Google Scholar] [CrossRef]
- Han, G.H.; Chay, D.B.; Nam, S.; Cho, H.; Chung, J.Y.; Kim, J.H. The Combination of Transient Receptor Potential Vanilloid Type 1 (TRPV1) and Phosphatase and Tension Homolog (PTEN) Is an Effective Prognostic Biomarker in Cervical Cancer. Int. J. Gynecol. Pathol. 2021, 40, 214–223. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, L.; Jang, X.X.; Xiong, Y.; Li, Q.; Yue, X.J.; Wei, Y.J.; Wei, Y.X.; Ma, Y.L.; Yu, Y.H. TRPV6 Is a Prognostic Marker in Early-Stage Cervical Squamous Cell Carcinoma. Tumor Biol. 2016, 37, 15743–15751. [Google Scholar] [CrossRef]
- McManus, O.B.; Garcia, M.L.; Weaver, D.; Bryant, M.; Titus, S.; Herrington, J.B. Ion Channel Screening. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2012; pp. 1–25. [Google Scholar]
- Del-Río-ibisate, N.; Granda-Díaz, R.; Rodrigo, J.P.; Menéndez, S.T.; García-Pedrero, J.M. Ion Channel Dysregulation in Head and Neck Cancers: Perspectives for Clinical Application. Rev. Physiol. Biochem. Pharmacol. 2020, 181, 375–427. [Google Scholar] [CrossRef]
- Delpire, E.; Gagnon, K.B. Water Homeostasis and Cell Volume Maintenance and Regulation. Curr. Top. Membr. 2018, 81, 3–52. [Google Scholar] [CrossRef] [PubMed]
- Rico, P.; Rodrigo-Navarro, A.; de la Peña, M.; Moulisová, V.; Costell, M.; Salmerón-Sánchez, M. Simultaneous Boron Ion-Channel/Growth Factor Receptor Activation for Enhanced Vascularization. Adv. Biosyst. 2019, 3, 1800220. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Shin, G.H.; Kim, J.T. Carbon Dots: Opportunities and Challenges in Cancer Therapy. Pharmaceutics 2023, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Aung, Y.Y.; Kristanti, A.N.; Lee, H.V.; Fahmi, M.Z. Boronic-Acid-Modified Nanomaterials for Biomedical Applications. ACS Omega 2021, 6, 17750–17765. [Google Scholar] [CrossRef]
- Lin, M.; Ye, M.; Zhou, J.; Wang, Z.P.; Zhu, X. Recent Advances on the Molecular Mechanism of Cervical Carcinogenesis Based on Systems Biology Technologies. Comput. Struct. Biotechnol. J. 2019, 17, 241–250. [Google Scholar] [CrossRef]
- Fan, J.J.; Huang, X. Ion Channels in Cancer: Orchestrators of Electrical Signaling and Cellular Crosstalk. In Targets of Cancer Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2020; Volume 183, pp. 103–133. [Google Scholar] [CrossRef]
- Yuan, C.; Yao, Y.; Cheng, B.; Cheng, Y.; Li, Y.; Li, Y.; Liu, X.; Cheng, X.; Xie, X.; Wu, J.; et al. The Application of Deep Learning Based Diagnostic System to Cervical Squamous Intraepithelial Lesions Recognition in Colposcopy Images. Sci. Rep. 2020, 10, 11639. [Google Scholar] [CrossRef]
- Dasari, S.; Wudayagiri, R.; Valluru, L. Cervical Cancer: Biomarkers for Diagnosis and Treatment. Clin. Chim. Acta 2015, 445, 7–11. [Google Scholar] [CrossRef]
- Peruzzo, R.; Biasutto, L.; Szabò, I.; Leanza, L. Impact of Intracellular Ion Channels on Cancer Development and Progression. Eur. Biophys. J. 2016, 45, 685–707. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Fraser, S.P.; Brackenbury, W.J. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 2019, 11, 1675. [Google Scholar] [CrossRef]
- Kher, C.; Kumar, S. The Application of Nanotechnology and Nanomaterials in Cancer Diagnosis and Treatment: A Review. Cureus 2022, 14, e29059. [Google Scholar] [CrossRef]
- Shen, C.L.; Liu, H.R.; Lou, Q.; Wang, F.; Liu, K.K.; Dong, L.; Shan, C.X. Recent Progress of Carbon Dots in Targeted Bioimaging and Cancer Therapy. Theranostics 2022, 12, 2860–2893. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Nanotechnology Based Therapeutic Application in Cancer Diagnosis and Therapy. 3 Biotech. 2019, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Soutter, W.P. Advances in The Imaging and Detection of Cervical Intra-Epithelial Neoplasia. Future Oncol. 2009, 5, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bijoy, M.B.; Shilimkar, V.; Jayaraj, P.B. Detecting Cervix Type Using Deep Learning and GPU. In Proceedings of the IEEE Region 10 Humanitarian Technology Conference, R10-HTC 2018, Malambe, Sri Lanka, 6–8 December 2018. [Google Scholar] [CrossRef]
- Ren, A.H.; Fiala, C.A.; Diamandis, E.P.; Kulasingam, V. Pitfalls in Cancer Biomarker Discovery and Validation with Emphasis on Circulating Tumor DNA. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2568–2574. [Google Scholar] [CrossRef]
- Kelly, H.; Benavente, Y.; Pavon, M.A.; De Sanjose, S.; Mayaud, P.; Lorincz, A.T. Performance of DNA Methylation Assays for Detection of High-Grade Cervical Intraepithelial Neoplasia (CIN2+): A Systematic Review and Meta-Analysis. Br. J. Cancer 2019, 121, 954–965. [Google Scholar] [CrossRef]
- Shah, S.S.; Senapati, S.; Klacsmann, F.; Miller, D.L.; Johnson, J.J.; Chang, H.C.; Stack, M.S. Current Technologies and Recent Developments for Screening of HPV-Associated Cervical and Oropharyngeal Cancers. Cancers 2016, 8, 85. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, Z.; Yang, H.; Xu, L.; Xia, J.; Gu, J.; Chen, M.; Wang, Y.; Zhao, X.; Liao, Z.; et al. Targeting Ion Channels: Innovative Approaches to Combat Cancer Drug Resistance. Theranostics 2025, 15, 521–545. [Google Scholar] [CrossRef]
- Liang, X.; Wang, D.; Zhao, Y.; Wang, X.; Yao, S.; Huang, W.; Yang, Y.; Dong, X.; Zhang, L.; Yang, J. Tumor Microenvironment-Responsive Manganese-Based Nano-Modulator Activate the CGAS-STING Pathway to Enhance Innate Immune System Response. J. Nanobiotechnol. 2024, 22, 535. [Google Scholar] [CrossRef]
- Alam, M.R.; Rahman, M.M.; Li, Z. The Link between Intracellular Calcium Signaling and Exosomal PD-L1 in Cancer Progression and Immunotherapy. Genes Dis. 2024, 11, 321–334. [Google Scholar] [CrossRef]
- Giammello, F.; Biella, C.; Priori, E.C.; Filippo, M.A.D.S.; Leone, R.; D’Ambrosio, F.; Paterno’, M.; Cassioli, G.; Minetti, A.; Macchi, F.; et al. Modulating Voltage-Gated Sodium Channels to Enhance Differentiation and Sensitize Glioblastoma Cells to Chemotherapy. Cell Commun. Signal. 2024, 22, 434. [Google Scholar] [CrossRef]
- Dewdney, B.; Miranda, P.J.; Kuchibhotla, M.; Palanisamy, R.; Richworth, C.; Milligan, C.J.; Ng, Y.; Ursich, L.; Petrou, S.; Fletcher, E.V.; et al. Ion Channel Modulator DPI-201-106 Significantly Enhances Antitumor Activity of DNA Damage Response Inhibitors in Glioblastoma. Neuro-Oncol. Adv. 2024, 6, vdae187. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lim, J.Y.; Son, M.K.; Ahn, J.H.; Song, K.H.; Lee, H.J.; Kim, S.; Cho, E.H.; Chung, J.Y.; Cho, H.; et al. TRPV1 Inhibition Overcomes Cisplatin Resistance by Blocking Autophagy-Mediated Hyperactivation of EGFR Signaling Pathway. Nat. Commun. 2023, 14, 2691. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, W.; Liao, Q.; Wang, H.; Lin, C.; Tang, L.; Lian, W.; Chen, Z.; Li, K.; Xu, L.; et al. Calcium Channel Blocker Nifedipine Suppresses Colorectal Cancer Progression and Immune Escape by Preventing NFAT2 Nuclear Translocation. Cell Rep. 2020, 33, 108327. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Baghirov, H.; Georgiadou, M.; Sihto, H.; Peuhu, E.; Cettour-Janet, P.; He, T.; Perälä, M.; Kronqvist, P.; Joensuu, H.; et al. L-Type Calcium Channels Regulate Filopodia Stability and Cancer Cell Invasion Downstream of Integrin Signalling. Nat. Commun. 2016, 7, 13297. [Google Scholar] [CrossRef]
- Li, C.; Yao, H.; Wang, H.; Fang, J.Y.; Xu, J. Repurposing Screen Identifies Amlodipine as an Inducer of PD-L1 Degradation and Antitumor Immunity. Oncogene 2020, 40, 1128–1146. [Google Scholar] [CrossRef]
- Du, N.H.; Ngoc, T.T.B.; Cang, H.Q.; Luyen, N.T.T.; Thuoc, T.L.; Le Quan, T.; Thao, D.T.P. KTt-45, a T-Type Calcium Channel Blocker, Acts as an Anticancer Agent by Inducing Apoptosis on HeLa Cervical Cancer Cell Line. Sci. Rep. 2023, 13, 22092. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, N.; Gao, J.; Liang, J.; Liang, Y.; Xie, Y.; Zhou, S.; Tang, X. ASIC1a Stimulates the Resistance of Human Hepatocellular Carcinoma by Promoting EMT via the AKT/GSK3β/Snail Pathway Driven by TGFβ/Smad Signals. J. Cell. Mol. Med. 2022, 26, 2777–2792. [Google Scholar] [CrossRef]
- Xu, X.; Dai, Y.; Feng, L.; Zhang, H.; Hu, Y.; Xu, L.; Zhu, X.; Jiang, Y. Knockdown of Nav1.5 Inhibits Cell Proliferation, Migration and Invasion via Wnt/β-Catenin Signaling Pathway in Oral Squamous Cell Carcinoma. Acta Biochim. Biophys. Sin. 2020, 52, 527–535. [Google Scholar] [CrossRef]
- Zhao, J.; Li, M.; Xu, J.; Cheng, W. The Modulation of Ion Channels in Cancer Chemo-Resistance. Front. Oncol. 2022, 12, 945896. [Google Scholar] [CrossRef]
- Leslie, T.K.; Brackenbury, W.J. Sodium Channels and the Ionic Microenvironment of Breast Tumours. J. Physiol. 2023, 601, 1543–1553. [Google Scholar] [CrossRef]
- Bell, D.C.; Leanza, L.; Gentile, S.; Sauter, D.R. News and Views on Ion Channels in Cancer: Is Cancer a Channelopathy? Front. Pharmacol. 2023, 14, 1258933. [Google Scholar] [CrossRef]
- Marcotti, A.; Fernández-Trillo, J.; González, A.; Vizcaíno-Escoto, M.; Ros-Arlanzón, P.; Romero, L.; Vela, J.M.; Gomis, A.; Viana, F.; De La Peña, E. TRPA1 Modulation by Sigma-1 Receptor Prevents Oxaliplatin-Induced Painful Peripheral Neuropathy. Brain 2023, 146, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Fukushiro-Lopes, D.; Hegel, A.D.; Russo, A.; Senyuk, V.; Liotta, M.; Beeson, G.C.; Beeson, C.C.; Burdette, J.; Potkul, R.K.; Gentile, S. Repurposing Kir6/SUR2 Channel Activator Minoxidil to Arrests Growth of Gynecologic Cancers. Front. Pharmacol. 2020, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Bernadez-Vallejo, S.V.; Vargas-Castro, R.; Avila, E.; Gómez-Ceja, K.A.; García-Becerra, R.; Segovia-Mendoza, M.; Prado-Garcia, H.; Lara-Sotelo, G.; Camacho, J.; et al. The Phytochemical α-Mangostin Inhibits Cervical Cancer Cell Proliferation and Tumor Growth by Downregulating E6/E7-HPV Oncogenes and KCNH1 Gene Expression. Int. J. Mol. Sci. 2023, 24, 3055. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).