Role of Denosumab in Patients with Intermediate Spinal Instability Neoplastic Score (SINS)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Outcome Assessment
2.3. Propensity Matching and Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
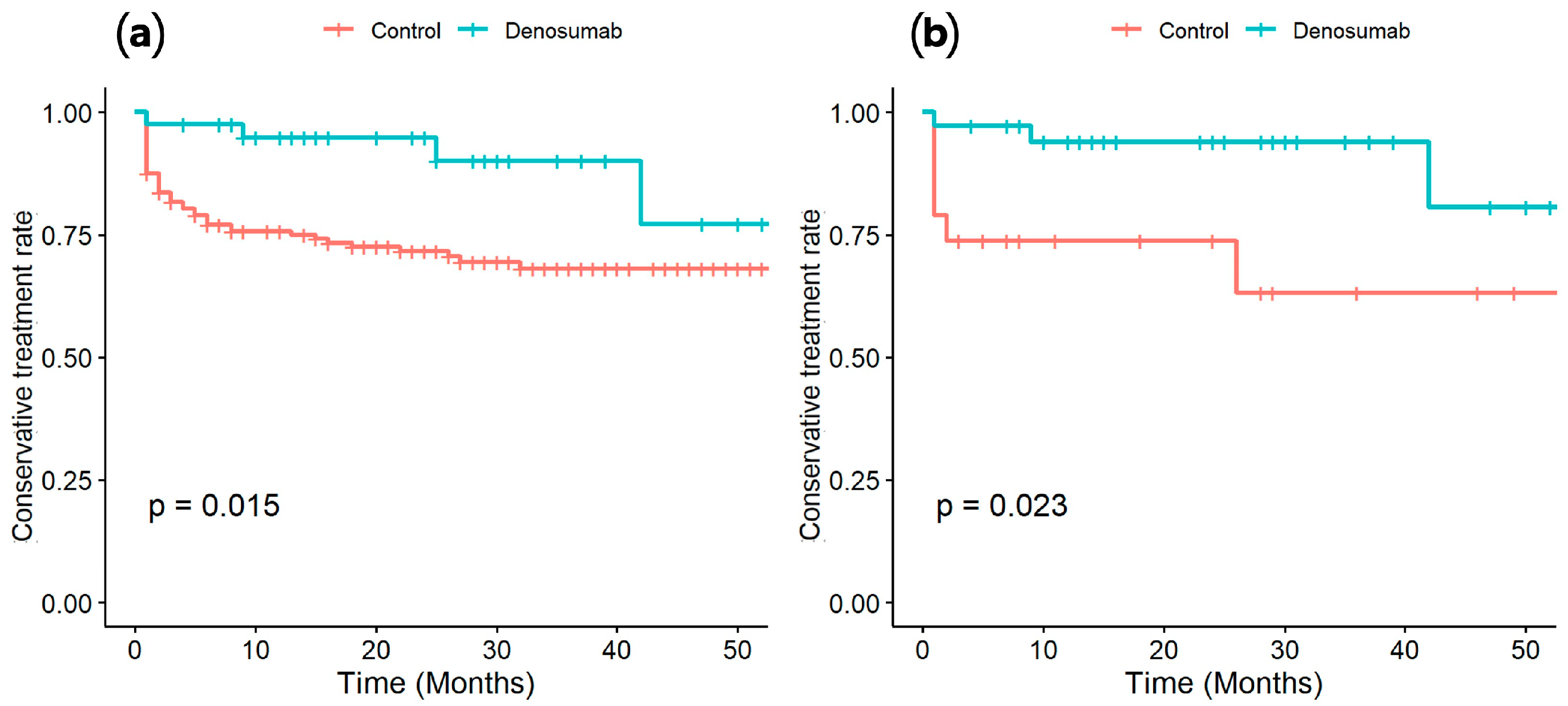
3.2. Comparison of Conversion-to-Surgery Rate
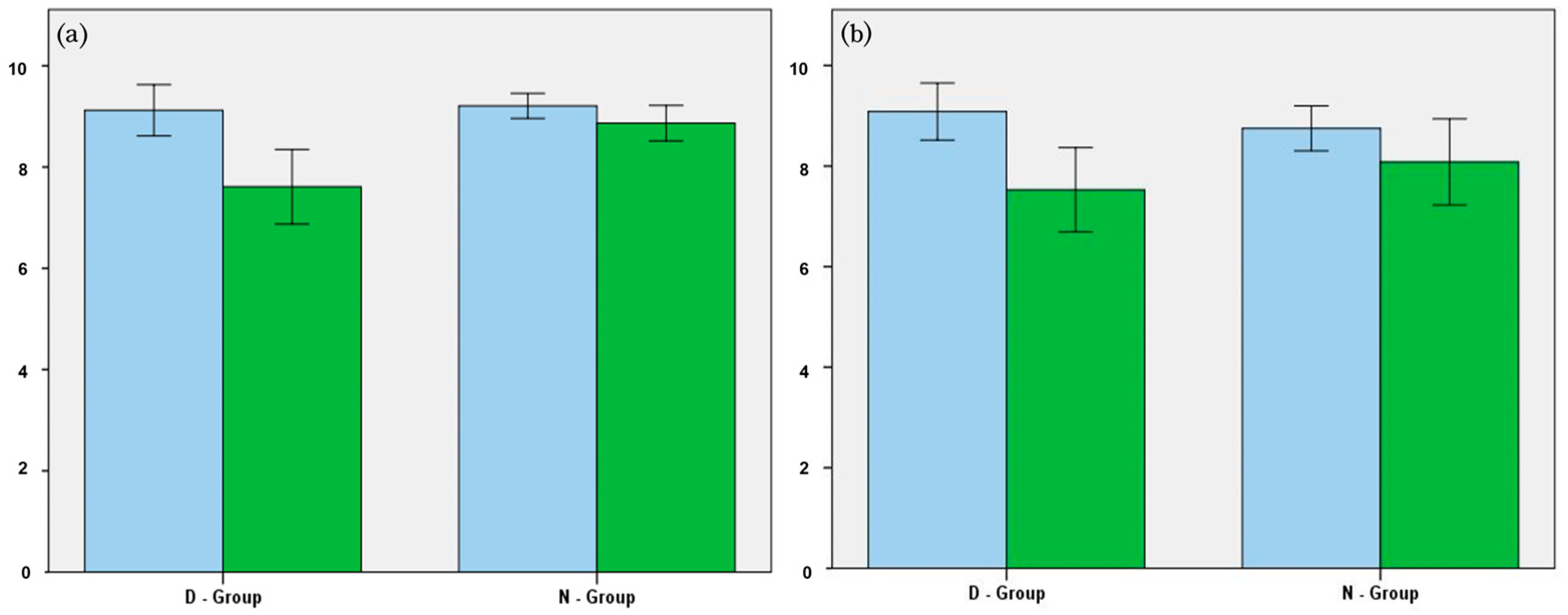
3.3. Changes in SINS and HU
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| SINS | Spinal instability neoplastic score |
| HU | Hounsfield unit |
| PLC | Posterior ligamentous complex |
| RNKL | Receptor Activator of Nuclear Factor Kappa-B ligand |
| SREs | Skeletal-related events |
| PSM | Propensity score matching |
| VCF | Vertebral compression fracture |
References
- World Health Organization. Who Report on Cancer: Setting Priorities, Investing Wesely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Van den Brande, R.; Cornips, E.M.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J. Bone Oncol. 2022, 35, 100446. [Google Scholar] [CrossRef]
- Hong, S.H.; Chang, B.S.; Kim, H.; Kang, D.H.; Chang, S.Y. An Updated Review on the Treatment Strategy for Spinal Metastasis from the Spine Surgeon’s Perspective. Asian Spine J. 2022, 16, 799–811. [Google Scholar] [CrossRef]
- Truong, V.T.; Al-Shakfa, F.; Roberge, D.; Masucci, G.L.; Tran, T.P.Y.; Dib, R.; Yuh, S.J.; Wang, Z. Assessing the Performance of Prognostic Scores in Patients with Spinal Metastases from Lung Cancer Undergoing Non-surgical Treatment. Asian Spine J. 2023, 17, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Ahmed, A.K.; Westbroek, E.M.; Cottrill, E.; Lubelski, D.; Goodwin, M.L.; Sciubba, D.M. SINS Score and Stability: Evaluating the Need for Stabilization Within the Uncertain Category. World Neurosurg. 2019, 128, e1034–e1047. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.; Chang, S.Y.; Kim, H.; Chang, B.-S. Treatment Strategy for Impending Instability in Spinal Metastases. Clin. Orthop. Surg. 2020, 12, 337–342. [Google Scholar] [CrossRef]
- Kwan, W.C.; Zuckerman, S.L.; Fisher, C.G.; Laufer, I.; Chou, D.; O’Toole, J.E.; Schultheiss, M.; Weber, M.H.; Sciubba, D.M.; Pahuta, M.; et al. What is the Optimal Management of Metastatic Spine Patients With Intermediate Spinal Instability Neoplastic Scores: To Operate or Not to Operate? Glob. Spine J. 2025, 15, 132s–142s. [Google Scholar] [CrossRef]
- Vargas, E.; Lockney, D.T.; Mummaneni, P.V.; Haddad, A.F.; Rivera, J.; Tan, X.; Jamieson, A.; Mahmoudieh, Y.; Berven, S.; Braunstein, S.E.; et al. An analysis of tumor-related potential spinal column instability (Spine Instability Neoplastic Scores 7-12) eventually requiring surgery with a 1-year follow-up. Neurosurg. Focus. 2021, 50, E6. [Google Scholar] [CrossRef]
- Body, J.J.; Greipp, P.; Coleman, R.E.; Facon, T.; Geurs, F.; Fermand, J.P.; Harousseau, J.L.; Lipton, A.; Mariette, X.; Williams, C.D.; et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 2003, 97, 887–892. [Google Scholar] [CrossRef]
- Gul, G.; Sendur, M.A.; Aksoy, S.; Sever, A.R.; Altundag, K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr. Med. Res. Opin. 2016, 32, 133–145. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kaya, M.; Yanagisawa, Y.; Yamamoto, K.; Takayashiki, N.; Ukita, H.; Nagura, M.; Sugiue, K.; Kitajima, M.; Hirano, K.; et al. Denosumab-induced hypocalcemia in patients with solid tumors and renal dysfunction: A multicenter, retrospective, observational study. BMC Cancer 2024, 24, 218. [Google Scholar] [CrossRef]
- Leone, A.; Cianfoni, A.; Zecchi, V.; Cortese, M.C.; Rumi, N.; Colosimo, C. Instability and impending instability in patients with vertebral metastatic disease. Skelet. Radiol. 2019, 48, 195–207. [Google Scholar] [CrossRef]
- Murotani, K.; Fujibayashi, S.; Otsuki, B.; Shimizu, T.; Sono, T.; Onishi, E.; Kimura, H.; Tamaki, Y.; Tsubouchi, N.; Ota, M.; et al. Prognostic Factors after Surgical Treatment for Spinal Metastases. Asian Spine J. 2024, 18, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Mok, S.; Park, S.C.; Kim, H.; Chang, B.S. Treatment Strategy for Metastatic Spinal Tumors: A Narrative Review. Asian Spine J. 2020, 14, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Peddi, P.; Lopez-Olivo, M.A.; Pratt, G.F.; Suarez-Almazor, M.E. Denosumab in patients with cancer and skeletal metastases: A systematic review and meta-analysis. Cancer Treat. Rev. 2013, 39, 97–104. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Oudard, S.; Shore, N.; Fizazi, K.; Sieber, P.; Tombal, B.; Damiao, R.; Marx, G.; Miller, K.; et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: Exploratory analyses by baseline prostate-specific antigen doubling time. J. Clin. Oncol. 2013, 31, 3800–3806. [Google Scholar] [CrossRef]
- Martin, M.; Bell, R.; Bourgeois, H.; Brufsky, A.; Diel, I.; Eniu, A.; Fallowfield, L.; Fujiwara, Y.; Jassem, J.; Paterson, A.H.; et al. Bone-related complications and quality of life in advanced breast cancer: Results from a randomized phase III trial of denosumab versus zoledronic acid. Clin. Cancer Res. 2012, 18, 4841–4849. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; Garcia-Sanz, R.; Durie, B.; Legiec, W.; Krejci, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Kouloulias, V.; Liakouli, Z.; Zygogianni, A.; Mystakidou, K.; Kouvaris, J.R. Bone Density as a Marker of Response to Radiotherapy in Bone Metastatic Lesions: A Review of the Published Data. Int. J. Mol. Sci. 2016, 17, 1391. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, H.; Dohzono, S.; Sasaoka, R.; Takamatsu, K.; Hoshino, M.; Nakamura, H. Computed tomography Hounsfield unit values as a treatment response indicator for spinal metastatic lesions in patients with non-small-cell lung cancer: A retrospective study in Japan. Asian Spine J. 2025, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, S.; Tseng, C.L.; Whyne, C.; Alghamdi, M.; Wilson, J.; Myrehaug, S.; Soliman, H.; Lee, Y.; Maralani, P.; Yang, V.; et al. Vertebral Compression Fracture After Spine Stereotactic Body Radiation Therapy: A Review of the Pathophysiology and Risk Factors. Neurosurgery 2018, 83, 314–322. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 286) | D-Group (n = 41) | N-Group (n = 245) | p Value |
|---|---|---|---|---|
| Age (means) | 68.0 ± 12.6 | 65.2 ± 14.1 | 68.5 ± 12.3 | 0.128 |
| Male–female | 151:135 | 10:31 | 141:104 | 0.001 |
| Follow-up (month) | 37.1 ± 26.5 | 24.6 ± 15.1 | 39.2 ± 27.4 | 0.001 |
| Primary cancers (n, %) | ||||
| Lung | 85 (29.7%) | 6 (14.6%) | 79 (32.2%) | 0.022 |
| Breast | 58 (20.3%) | 18 (43.9%) | 40 (16.3%) | 0.001 |
| Liver | 39 (13.6%) | 1 (2.4%) | 38 (15.5%) | 0.024 |
| Kidney | 24 (8.4%) | 2 (4.9%) | 22 (9.0%) | 0.380 |
| Prostate | 24 (8.4%) | 3 (7.3%) | 21 (8.6%) | 0.788 |
| Sarcoma | 23 (8.0%) | 2 (4.9%) | 21 (8.6%) | 0.421 |
| Thyroid | 20 (7.0%) | 7 (17.1%) | 13 (5.3%) | 0.006 |
| Other | 13 (4.6%) | 2 (4.9%) | 11 (4.5%) | 0.912 |
| D-Group | N-Group | p Value | |
|---|---|---|---|
| Location | 0.173 | ||
| 1 | 6 | 13 | |
| 2 | 13 | 10 | |
| 3 | 17 | 13 | |
| Pain | 0.066 | ||
| 0 | 3 | 0 | |
| 1 | 7 | 3 | |
| 3 | 26 | 33 | |
| Bone lesion | 0.334 | ||
| 0 | 1 | 4 | |
| 1 | 13 | 10 | |
| 2 | 22 | 22 | |
| Radiographic alignment | 0.643 | ||
| 0 | 34 | 33 | |
| 2 | 2 | 3 | |
| Body collapse | 0.046 | ||
| 0 | 11 | 9 | |
| 1 | 4 | 11 | |
| 2 | 14 | 15 | |
| 3 | 7 | 1 | |
| Posterolateral involvement | 0.161 | ||
| 0 | 16 | 14 | |
| 1 | 7 | 14 | |
| 3 | 13 | 8 |
| D-Group | N-Group | |||||
|---|---|---|---|---|---|---|
| Initial | Final | p-Value | Initial | Final | p-Value | |
| Location | 2.3 | 2.3 | 1.000 | 2.1 | 2.1 | 1.000 |
| Pain | 2.3 | 1.0 | 0.000 | 2.7 | 2.0 | 0.000 |
| Bone lesion | 1.6 | 1.3 | 0.048 | 1.7 | 1.6 | 0.241 |
| Alignment | 0.1 | 0.2 | 0.649 | 0.2 | 0.4 | 0.033 |
| Body collapse | 1.4 | 1.5 | 0.768 | 1.3 | 1.6 | 0.004 |
| Posterolateral involvement | 1.3 | 1.3 | 1.000 | 1.1 | 1.1 | 0.669 |
| Total SINS | 9.1 | 7.6 | 0.001 | 9.2 | 8.9 | 0.669 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Chang, B.-S.; Kim, H.; Kim, S.T.; Jang, S.; Chang, S.Y. Role of Denosumab in Patients with Intermediate Spinal Instability Neoplastic Score (SINS). Cancers 2025, 17, 1539. https://doi.org/10.3390/cancers17091539
Lee J, Chang B-S, Kim H, Kim ST, Jang S, Chang SY. Role of Denosumab in Patients with Intermediate Spinal Instability Neoplastic Score (SINS). Cancers. 2025; 17(9):1539. https://doi.org/10.3390/cancers17091539
Chicago/Turabian StyleLee, JunYeop, Bong-Soon Chang, Hyoungmin Kim, Sung Taeck Kim, Seonpyo Jang, and Sam Yeol Chang. 2025. "Role of Denosumab in Patients with Intermediate Spinal Instability Neoplastic Score (SINS)" Cancers 17, no. 9: 1539. https://doi.org/10.3390/cancers17091539
APA StyleLee, J., Chang, B.-S., Kim, H., Kim, S. T., Jang, S., & Chang, S. Y. (2025). Role of Denosumab in Patients with Intermediate Spinal Instability Neoplastic Score (SINS). Cancers, 17(9), 1539. https://doi.org/10.3390/cancers17091539







