Porphyromonas gingivalis and Human Cytomegalovirus Co-Infection: A Potential Link Between Periodontal Disease and Oral Cancer Development
Simple Summary
Abstract
1. Introduction
2. P. gingivalis and HCMV Co-Infection in Periodontal Disease
3. Association of Periodontal Disease and Oral Cancer
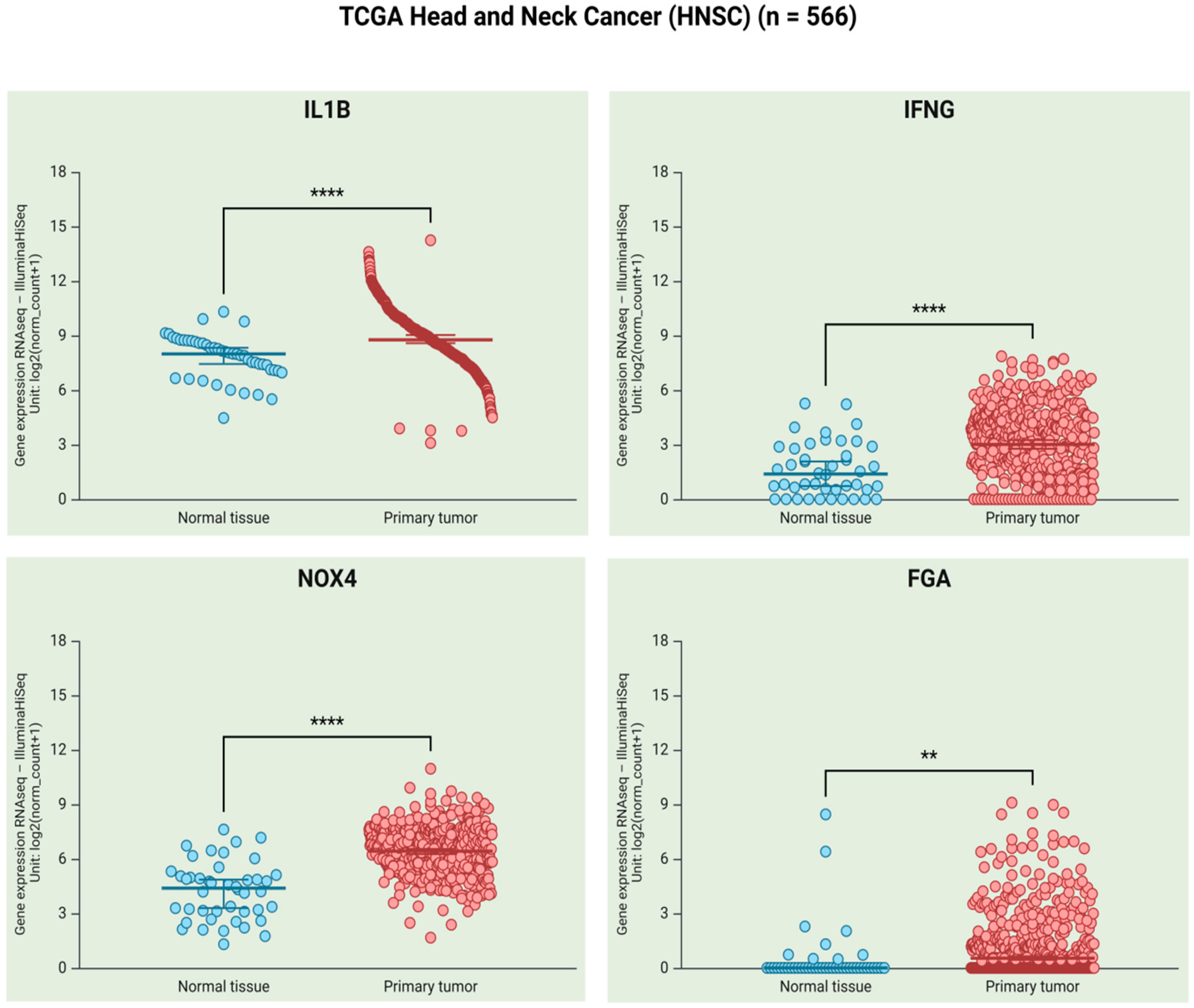
4. Frequency of P. gingivalis and HCMV Infections in OSCC
5. Mechanisms of P. gingivalis and HCMV That Induce Immune Evasion and Chronic Inflammation
5.1. Disruption of Host Immune Response
5.2. ROS Production and Other By-Products Related to Tissue Damage
5.3. Disruption of the Blood Coagulation System
5.4. Interactions of P. gingivalis and Other Viruses
6. Oncogenic Properties of P. gingivalis and HCMV Proteins
6.1. Activation of Signaling Pathways That Control Cell Proliferation
6.2. Regulation of Apoptosis and Cell Survival
6.3. Induction of EMT and Invasiveness
6.4. Promotion of Cell Immortalization and Tumor Growth
6.5. Potential Diagnostic Markers for Co-Infection-Related Oral Cancer
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4NQO | 4-nitroquinoline-1 oxide |
| ABL | Alveolar bone loss |
| aHR | Adjusted hazard ratio |
| aOR | Adjusted odd ratio |
| BOP | Bleeding on probing |
| CAL | Clinical attachment loss |
| CASP | Caspase |
| CCL5 | CC motif ligand 5 |
| CI | Coefficient interval |
| CK | Cytokeratin |
| COX-2 | Cyclooxygenase-2 |
| CXCL2 | CXC motif chemokine ligand 2 |
| CXCL11 | CXC motif chemokine ligand 11 |
| DCs | Dendritic cells |
| DMFT | Decayed, missing, and filled teeth |
| EBV | Epstein-Barr virus |
| EMT | Epithelial–mesenchymal transition |
| EpCAM | Epithelial cell adhesion molecule |
| FISH | Fluorescence in situ hybridization |
| GBD | Global burden of disease |
| H2S | Hydrogen sulfide |
| HDI | Human development index |
| HCMV | Human cytomegalovirus |
| HHV-5 | Human Herpesvirus 5 |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papillomavirus |
| HSV-1 | Herpes simplex virus-1 |
| hTERT | Human telomerase reverse transcriptase |
| HUVECs | Human umbilical vein endothelial cells |
| IFI16 | Gamma-IFN-inducible protein 16 |
| IHC | Immunohistochemistry |
| IHGE | Immortalized human gingival epithelial cells |
| IL | Interleukin |
| INF-γ | Interferon-gamma |
| IRAK IL | 1R-associated kinase |
| IRF | Interferon regulatory factor |
| ISH | in situ hybridization |
| JAK1 | Janus kinase |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen-activated protein kinases |
| MDSCs | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| miR | microRNA |
| MMP | Matrix metalloproteinase |
| MOAP1 | Modulator of apoptosis-1 |
| mtDNA | mitochondrial DNA |
| mtROS | mitochondrial ROS |
| MyD88 | Myeloid differentiation factor 88 |
| Nmi | Human N-myc interactor |
| NLRX1 | Nucleotide-binding oligomerization domain, leucine rich repeat containing X1 |
| OC | Oral cancer |
| OMVs | Outer membrane vesicles |
| OR | Odd ratio |
| OSCC | Squamous cell carcinoma |
| PAI-1 | Plasminogen activator inhibitor type I |
| PD | Periodontal disease |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PPD | Probing pocket depth |
| proMMP | pro matrix metalloproteinase |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SMD | Standardized mean difference |
| TAMs | Tumor-associated macrophages |
| TANs | Tumor-associated neutrophils |
| TCGA | The Cancer Genome Atlas |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| TSP-1 | Thrombospondin-1 |
References
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.C.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63, S91–S99. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.Y.A.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA—Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Global Cancer Observatory. Cancer Tomorrow. Available online: https://gco.iarc.who.int/tomorrow/en (accessed on 3 April 2025).
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Y Cir. Bucal 2018, 23, E23–E29. [Google Scholar] [CrossRef]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Current trends on prevalence, risk factors and prevention of oral cancer. Front. Oral Health 2024, 5, 1505833. [Google Scholar] [CrossRef]
- Prostakishina, E.A.; Sidenko, E.A.; Kolegova, E.S.; Patysheva, M.R.; Kononova, G.A.; Choinzonov, E.L. Premalignant lesions of the oral cavity: A narrative review of factors and mechanisms of transformation into cancer. Int. J. Oral Maxillofac. Surg. 2024. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontology 2000 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Dong, X.Y. Prevalence of periodontal disease in middle-aged and elderly patients and its influencing factors. Am. J. Transl. Res. 2022, 14, 5677–5684. [Google Scholar] [PubMed]
- Albandar, J.M.; Muranga, M.B.; Rams, T.E. Prevalence of aggressive periodontitis in school attendees in Uganda. J. Clin. Periodontol. 2002, 29, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Vongpradith, A.; Dominguez, R.-M.V.; Ma, J.; Albertson, S.B.; Novotney, A.; Khalil, I.A.; Troeger, C.E.; Doxey, M.C.; Ledesma, J.R.; et al. Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990–2021, for 204 countries and territories: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 25, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tuerxun, N.; Maimaitili, G. Periodontitis and the risk of oral cancer: A meta-analysis of case-control studies. Acta Odontol. Scand. 2024, 83, 281–289. [Google Scholar] [CrossRef]
- Ye, L.L.; Jiang, Y.H.; Liu, W.D.; Tao, H.B. Correlation between periodontal disease and oral cancer risk: A meta-analysis. J. Cancer Res. Ther. 2016, 12, 237–240. [Google Scholar] [CrossRef]
- Li, R.; Hou, M.J.; Yu, L.Y.; Luo, W.; Liu, R.H.; Wang, H.Y. Association between periodontal disease and oral squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2023, 61, 394–402. [Google Scholar] [CrossRef]
- Patil, R.T.; Dhadse, P.V.; Salian, S.S.; Punse, S.D. Role of Oxidative Stress in Periodontal Diseases. Cureus J. Med. Sci. 2024, 16, e60779. [Google Scholar] [CrossRef]
- Gào, X.; Schöttker, B. Reduction-oxidation pathways involved in cancer development: A systematic review of literature reviews. Oncotarget 2017, 8, 51888–51906. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Ng, W.; Tonzetich, J. Effect of Hydrogen-Sulfide and Methyl Mercaptan on the Permeability of Oral-Mucosa. J. Dent. Res. 1984, 63, 994–997. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Aleksijevic, L.H.; Aleksijevic, M.; Skrlec, I.; Sram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Chan, A.; Belton, C.M.; Izutsu, K.T.; Vasel, D.; Weinberg, A. Porphyromonas-gingivalis Invasion of Gingival Epithelial-Cells. Infect. Immun. 1995, 63, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Amornchat, C.; Rassameemasmaung, S.; Sripairojthikoon, W.; Swasdison, S. Invasion of Porphyromonas gingivalis into human gingival fibroblasts in vitro. J. Int. Acad. Periodontol. 2003, 5, 98–105. [Google Scholar]
- Rodrigues, P.H.; Progulske-Fox, A. Gene expression profile analysis of Porphyromonas gingivalis during invasion of human coronary artery endothelial cells. Infect. Immun. 2005, 73, 6169–6173. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Ito, T.; Mori, G.; Oda, Y.; Hirano, T.; Sasaki, H.; Honma, S.; Furuya, Y.; Yajima, Y. Clinical evaluation of periodontal pathogen levels by real-time polymerase chain reaction in peri-implantitis patients. Int. J. Implant. Dent. 2021, 7, 1335–1360. [Google Scholar] [CrossRef]
- Puletic, M.; Popovic, B.; Jankovic, S.; Brajovic, G. Detection rates of periodontal bacteria and herpesviruses in different forms of periodontal disease. Microbiol. Immunol. 2020, 64, 815–824. [Google Scholar] [CrossRef]
- Lmbronito, A.V.; Okuda, O.S.; de Freitas, N.M.; Lotufo, R.F.M.; Nunest, F.D. Detection of Herpesviruses and Periodontal Pathogens in Subgingival Plaque of Patients with Chronic Periodontitis, Generalized Aggressive Periodontitis, or Gingivitis. J. Periodontol. 2008, 79, 2313–2321. [Google Scholar] [CrossRef]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Zanghellini, F.; Boppana, S.B.; Emery, V.C.; Griffiths, P.D.; Pass, R.F. Asymptomatic primary cytomegalovirus infection: Virologic and immunologic features. J. Infect. Dis. 1999, 180, 702–707. [Google Scholar] [CrossRef]
- Wreghitt, T.G.; Teare, E.L.; Sule, O.; Devi, R.; Rice, P. Cytomegalovirus infection in immunocompetent patients. Clin. Infect. Dis. 2003, 37, 1603–1606. [Google Scholar] [CrossRef] [PubMed]
- Adland, E.; Klenerman, P.; Goulder, P.; Matthews, P.C. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front. Microbiol. 2015, 6, 1016. [Google Scholar] [CrossRef]
- Pérez-Sola, M.J.; Castón, J.J.; Solana, R.; Rivero, A.; Torre-Cisneros, J. Indirect effects of cytomegalovirus infection in solid organ transplant recipients. Enfermedades Infecc. Y Microbiol. Clin. 2008, 26, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.S.; Pierrotti, L.C.; Abdala, E.; Costa, S.F.; Strabelli, T.M.V.; Campos, S.V.; Ramos, J.F.; Latif, A.Z.A.; Litvinov, N.; Maluf, N.Z.; et al. Cytomegalovirus infection in transplant recipients. Clinics 2015, 70, 515–523. [Google Scholar] [CrossRef]
- Gerna, G.; Kabanova, A.; Lilleri, D. Human Cytomegalovirus Cell Tropism and Host Cell Receptors. Vaccines 2019, 7, 70. [Google Scholar] [CrossRef]
- Park, A.; Ra, E.A.; Lee, T.A.; Choi, H.J.; Lee, E.; Kang, S.; Seo, J.Y.; Lee, S.; Park, B. HCMV-encoded US7 and US8 act as antagonists of innate immunity by distinctively targeting TLR-signaling pathways. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Landais, I.; Pelton, C.; Streblow, D.; DeFilippis, V.; McWeeney, S.; Nelson, J.A. Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway. PLoS Pathog. 2015, 11, e1004881. [Google Scholar] [CrossRef]
- Feng, L.Y.; Sheng, J.X.; Vu, G.P.; Liu, Y.J.; Foo, C.M.; Wu, S.B.; Trang, P.; Paliza-Carre, M.; Ran, Y.H.; Yang, X.P.; et al. Human cytomegalovirus UL23 inhibits transcription of interferon-γ stimulated genes and blocks antiviral interferon-γ responses by interacting with human N-myc interactor protein. PLoS Pathog. 2018, 14, e1006867. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Intern. Med. 2006, 259, 219–246. [Google Scholar] [CrossRef]
- Michalowicz, B.S.; Ronderos, M.; Camara-Silva, R.; Contreras, A.; Slots, J. Human herpesviruses and Porphyromonas gingivalis are associated with juvenile periodontitis. J. Periodontol. 2000, 71, 981–988. [Google Scholar] [CrossRef]
- Nakamura, M.; Shigeishi, H.; Su, C.Y.; Sugiyama, M.; Ohta, K. Oral human cytomegalovirus prevalence and its relationships with periodontitis and Porphyromonas gingivalis in Japanese adults: A cross-sectional study. J. Appl. Oral Sci. 2020, 28, e20200501. [Google Scholar] [CrossRef] [PubMed]
- Elamin, A.; Ali, R.W.; Bakken, V. Putative periodontopathic bacteria and herpes viruses interactions in the subgingival plaque of patients with aggressive periodontitis and healthy controls. Clin. Exp. Dent. Res. 2017, 3, 183–190. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Zamanian Azodi, M. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Puig-Silla, M.; Dasí-Fernández, F.; Montiel-Company, J.M.; Almerich-Silla, J.M. Prevalence of fimA genotypes of Porphyromonas gingivalis and other periodontal bacteria in a Spanish population with chronic periodontitis. Med. Oral Patol. Oral Y Cir. Bucal 2012, 17, E1047–E1053. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Chen, Y.; Tao, R.; Zeng, Q.; Liu, Z.; Jiang, L.; Ye, L.; Lin, X. Periodontopathogens and human β-defensin-2 expression in gingival crevicular fluid from patients with periodontal disease in Guangxi, China. J. Periodontal Res. 2015, 50, 403–410. [Google Scholar] [CrossRef]
- Riep, B.; Edesi-Neuss, L.; Claessen, F.; Skarabis, H.; Ehmke, B.; Flemmig, T.F.; Bernimoulin, J.P.; Göbel, U.B.; Moter, A. Are Putative Periodontal Pathogens Reliable Diagnostic Markers? J. Clin. Microbiol. 2009, 47, 1705–1711. [Google Scholar] [CrossRef]
- Kulkarni, P.G.; Gosavi, S.; Haricharan, P.B.; Malgikar, S.; Mudrakola, D.P.; Turagam, N.; Ealla, K.K. Molecular Detection of Porphyromonas gingivalis in Chronic Periodontitis Patients. J. Contemp. Dent. Pract. 2018, 19, 992–996. [Google Scholar]
- Botero, J.E.; Rodriguez-Medina, C.; Jaramillo-Echeverry, A.; Contreras, A. Association between human cytomegalovirus and periodontitis: A systematic review and meta-analysis. J. Periodontal Res. 2020, 55, 551–558. [Google Scholar] [CrossRef]
- Li, F.; Zhu, C.; Deng, F.Y.; Wong, M.C.M.; Lu, H.X.; Feng, X.P. Herpesviruses in etiopathogenesis of aggressive periodontitis: A meta-analysis based on case-control studies. PLoS ONE 2017, 12, e0186373. [Google Scholar] [CrossRef]
- Zhu, C.; Li, F.; Wong, M.C.M.; Feng, X.P.; Lu, H.X.; Xu, W. Association between Herpesviruses and Chronic Periodontitis: A Meta-Analysis Based on Case-Control Studies. PLoS ONE 2015, 10, e0144319. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.M.A.G.; Bharadwaj, R. Role of herpesviruses in chronic periodontitis and their association with clinical parameters and in increasing severity of the disease. Eur. J. Dent. 2017, 11, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.M.; Bhat, K.G.; Katti, S.S.; Kugaji, M.S.; Ingalgi, P.S. Prevalence of Herpesvirus and Correlation with Clinical Parameters in Indian Subjects with Chronic Periodontitis. J. Contemp. Dent. Pract. 2015, 16, 915–920. [Google Scholar] [CrossRef]
- Saygun, I.; Sahin, S.; Özdemir, A.; Kurtis, B.; Yapar, M.; Kubar, A.; Özcan, G. Detection of human viruses in patients with chronic periodontitis and the relationship between viruses and clinical parameters. J. Periodontol. 2002, 73, 1437–1443. [Google Scholar] [CrossRef]
- Botero, J.E.; Parra, B.; Jaramillo, A.; Contreras, A. Subgingival human cytomegalovirus correlates with increased clinical periodontal parameters and bacterial coinfection in periodontitis. J. Periodontol. 2007, 78, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.M.; Bhat, K.G.; Kugaji, M.S.; Ingalagi, P.S. Prevalence of Porphyromonas gingivalis and its relationship with herpesvirus in Indian subjects with chronic periodontitis: A cross-sectional study. J. Int. Clin. Dent. Res. Organ. 2016, 8, 106–110. [Google Scholar] [CrossRef]
- Saygun, I.; Kubar, A.; Ozdemir, A.; Yapar, M.; Slots, J. Herpesviral-bacterial interrelationships in aggressive periodontitis. J. Periodontal Res. 2004, 39, 207–212. [Google Scholar] [CrossRef]
- Herrero-Sánchez, A.; Haroyan-Darbinyan, E. Prevalence of Epstein-Barr Virus, Cytomegalovirus, and Periodontopathic Bacteria in Patients with Periodontitis: A Case-Control Study. Clin. Exp. Dent. Res. 2025, 11, e70084. [Google Scholar] [CrossRef]
- Slots, J.; Kamma, J.J.; Sugar, C. The herpesvirus-Porphyromonas gingivalis-periodontitis axis. J. Periodontal Res. 2003, 38, 318–323. [Google Scholar] [CrossRef]
- Sharma, S.; Tapashetti, R.P.; Patil, S.R.; Kalra, S.M.; Bhat, G.K.; Guvva, S. Revelation of Viral-Bacterial Interrelationship in Aggressive Periodontitis via Polymerase Chain Reaction: A Microbiological Study. J. Int. Oral. Health. 2015, 7, 101–107. [Google Scholar]
- Kousar, J. Comparative Analysis of Filifactor Alocis, Dialister Pneumosintes, Cytomegalovirus and Porphyromonas gingivalis in Subjects with Periodontal Diseases–A Clinico–Microbiological Study. Ph.D. Thesis, Rajiv Gandhi University of Health Sciences, Bangalore, India, 2017. [Google Scholar]
- Passariello, C.; Gigola, P.; Testarelli, L.; Puttini, M.; Schippa, S.; Petti, S. Evaluation of microbiota associated with Herpesviruses in active sites of generalized aggressive periodontitis. Ann. Stomatol. 2017, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kamma, J.J.; Contreras, A.; Slots, J. Herpes viruses and periodontopathic bacteria in early-onset periodontitis. J. Clin. Periodontol. 2001, 28, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Contreras, A.; Slots, J. Aggressive periodontitis in southwestern American Indian adolescents. J. Periodontol. 2024, 95, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Umeda, M.; Chen, C.; Bakker, I.; Morrison, J.L.; Slots, J. Relationship between herpesviruses and adult periodontitis and periodontopathic bacteria. J. Periodontol. 1999, 70, 478–484. [Google Scholar] [CrossRef]
- Narayan, T.V.; Revanna, G.M.; Hallikeri, U.; Kuriakose, M.A. Dental Caries and Periodontal Disease Status in Patients with Oral Squamous Cell Carcinoma: A Screening Study in Urban and Semiurban Population of Karnataka. J. Maxillofac. Oral Surg. 2014, 13, 435–443. [Google Scholar] [CrossRef]
- Shin, Y.J.; Choung, H.W.; Lee, J.H.; Rhyu, I.C.; Kim, H.D. Association of Periodontitis with Oral Cancer: A Case-Control Study. J. Dent. Res. 2019, 98, 526–533. [Google Scholar] [CrossRef]
- Tezal, M.; Sullivan, M.A.; Hyland, A.; Marshall, J.R.; Stoler, D.; Reid, M.E.; Loree, T.R.; Rigual, N.R.; Merzianu, M.; Hauck, L.; et al. Chronic Periodontitis and the Incidence of Head and Neck Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2406–2412. [Google Scholar] [CrossRef]
- Wen, B.W.; Tsai, C.S.; Lin, C.L.; Chang, Y.J.; Lee, C.F.; Hsu, C.H.; Kao, C.H. Cancer risk among gingivitis and periodontitis patients: A nationwide cohort study. QJM—Int. J. Med. 2014, 107, 283–290. [Google Scholar] [CrossRef]
- Moergel, M.; Kämmerer, P.; Kasaj, A.; Armouti, E.; Alshihri, A.; Weyer, V.; Al-Nawas, B. Chronic periodontitis and its possible association with oral squamous cell carcinoma—a retrospective case control study. Head Face Med. 2013, 9, 39. [Google Scholar] [CrossRef]
- Bundgaard, T.; Wildt, J.; Frydenberg, M.; Elbrond, O.; Nielsen, J.E. Case-Control Study of Squamous-Cell Cancer of the Oral Cavity in Denmark. Cancer Causes Control. 1995, 6, 57–67. [Google Scholar] [CrossRef]
- Chang, J.S.; Lo, H.I.; Wong, T.Y.; Huang, C.C.; Lee, W.T.; Tsai, S.T.; Chen, K.C.; Yen, C.J.; Wu, Y.H.; Hsueh, W.T.; et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013, 49, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.Z.; Boyle, P.; Hu, H.F.; Duan, J.; Jiang, P.J.; Ma, D.Q.; Shui, L.P.; Niu, S.; Scully, C.; Macmahon, B. Dentition, Oral Hygiene, and Risk of Oral-Cancer—A Case-Control Study in Beijing, Peoples-Republic-of-China. Cancer Causes Control. 1990, 1, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Komlós, G.; Csurgay, K.; Horváth, F.; Pelyhe, L.; Németh, Z. Periodontitis as a risk for oral cancer: A case-control study. BMC Oral Health 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.R.; Geng, F.X.; Shi, X.T.; Li, Y.C.; Zhang, X.; Zhao, X.D.; Pan, Y.P. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404. [Google Scholar] [CrossRef]
- Guo, Z.C.; Jumatai, S.; Jing, S.L.; Hu, L.L.; Jia, X.Y.; Gong, Z.C. Bioinformatics and immunohistochemistry analyses of expression levels and clinical significance of CXCL2 and TANs in an oral squamous cell carcinoma tumor microenvironment of Prophyromonas gingivalis infection. Oncol. Lett. 2021, 21, 189. [Google Scholar] [CrossRef]
- Gadde, S.; Poda, S. Prevalence of Herpes Simplex Virus (HSV) and Cytomegalovirus (CMV) in Oral Squamous Cell Carcinoma patients with a history of Nicotine and Alcohol abuse. Curr. Trends Biotechnol. Pharm. 2023, 17, 873–884. [Google Scholar] [CrossRef]
- Kaliamoorthy, S.; Saranyan, R.; Balakrishnan, J.; Govindasamy, A.; John, B.J.; Mohan, J.; Kannan, S. A Study on the Presence of Porphyromonas gingivalis in Oral Squamous Cell Carcinoma. Ann. Rom. Soc. Cell Biol. 2021, 25, 10279–10284. [Google Scholar]
- Monier, N.M.; Atteya, I.M.; Askar, H.; Helmy, S. Detection of the periodontal pathogen Porphyromonas gingivalis in Oral Squamous Cell Carcinoma. Mansoura J. Dent. 2020, 7, 24–28. [Google Scholar] [CrossRef]
- Katz, J.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef]
- Chen, Q.L.; Shao, Z.; Liu, K.; Zhou, X.C.; Wang, L.; Jiang, E.H.; Luo, T.T.; Shang, Z.J. Salivary Porphyromonas gingivalis predicts outcome in oral squamous cell carcinomas: A cohort study. BMC Oral Health 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.U.; Khan, S.; Ahmed, A.; Lail, A.; Gul, S.; Ahmed, S. Prevalence of EBV, CMV, and HPV in oral squamous cell carcinoma patients in the Pakistani population. J. Med. Virol. 2020, 92, 3880–3883. [Google Scholar] [CrossRef]
- Khashman, B.M. Study the Oncomodulation Potential of Human Cytomegalovirus and its Correlation with TGF-β1 in a Group of Iraqi Patients with OSCC. Int. J. Sci. Res. 2017, 6, 2196–2198. [Google Scholar]
- Ali, S.H.M.; Al Jewari, M.M.M.; Al Huda, N.; Saaed, A.A. Localization of Human Cytomegalovirus-Late Gene DNA, Expression of P53 Gene and CD8-Tumor Infiltrating Lymphocytes in Oral Squamous Cell Carcinoma. Iraqi Postgrad. Med. J. 2013, 12, 296–305. [Google Scholar]
- Delavarian, Z.; Pakfetrat, A.; Falaki, F.; Pazouki, M.; Pazouki, N. The Role of Viruses in Oral Squamous Cell Carcinoma in Young Patients in Khorasan (Northeast of Iran). J. Appl. Sci. 2010, 10, 981–985. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Koh, L.W.; Tsai, J.H.; Tsai, C.H.; Wong, E.F.C.; Lin, S.J.; Yang, C.C. Involvement of viral and chemical factors with oral cancer in Taiwan. Jpn. J. Clin. Oncol. 2004, 34, 176–183. [Google Scholar] [CrossRef]
- Dayyani, F.; Tavakolian, S.; Goudarzi, H.; Biucki, F.Y.; Faghihloo, E. Prevalence of HSV, Varicella-Zoster, CMV, EBV and HPV in the oral cavity and the larynx carcinoma in Iran. Future Virol. 2021, 16, 141–151. [Google Scholar] [CrossRef]
- Saravani, S.; Kadeh, H.; Miri-Moghaddam, E.; Zekri, A.; Sanadgol, N.; Gholami, A. Human Cytomegalovirus in Oral Squamous Cell Carcinoma in Southeast of Iran. Jundishapur J. Microbiol. 2015, 8, e21838. [Google Scholar] [CrossRef]
- Ursu, R.G.; Luchian, I.; Ghetu, N.; Costan, V.V.; Stamatin, O.; Palade, O.D.; Damian, C.; Iancu, L.S.; Porumb-Andrese, E. Emerging Oncogenic Viruses in Head and Neck Cancers from Romanian Patients. Appl. Sci. 2021, 11, 9356. [Google Scholar] [CrossRef]
- Saeed, Z.A.; Saeed, N.A.-H.A.A.; Al-Gharrawi, S.A.R. Epstein Barr Virus and Cytomegalovirus Correlation with Oral Squamous Cell Carcinoma Patients by Using in Situ Hybridization Method in the City of Baghdad. J. Glob. Pharma Technol. 2017, 8, 158–163. [Google Scholar]
- Sachit, H.G.; Almahbobi, T.F.; Ali, Z.M.M.; Ali, S.H.M.; Al-Alwany, S.H.M. A Molecular Implicatory Propositioning Roles for Human Cytomegalovirus and P16 Gene Expression in Oral Squamous Cellular Carcinogenesis. J. Pure Appl. Microbiol. 2019, 13, 2333–2342. [Google Scholar] [CrossRef]
- Coats, S.R.; Jones, J.W.; Do, C.T.; Braham, P.H.; Bainbridge, B.W.; To, T.T.; Goodlett, D.R.; Ernst, R.K.; Darveau, R.P. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1-and 4′-phosphatase activities. Cell. Microbiol. 2009, 11, 1587–1599. [Google Scholar] [CrossRef]
- Domon, H.; Honda, T.; Oda, T.; Yoshie, H.; Yamazaki, K. Early and preferential induction of IL-1 receptor-associated kinase-M in THP-1 cells by LPS derived from Porphyromonas gingivalis. J. Leukoc. Biol. 2008, 83, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Tzach-Nahman, R.; Potempa, J.; Shapira, L.; Nussbaum, G. Porphyromonas gingivalis Gingipains Selectively Reduce CD14 Expression, Leading to Macrophage Hyporesponsiveness to Bacterial Infection. J. Innate Immun. 2015, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, G.; Altman, H.; Kolenbrander, P.E.; Chalmers, N.I.; Gabai-Gutner, M.; Mor, A.; Friedman, M.; Steinberg, D. Resistance of Porphyromonas gingivalis ATCC 33277 to direct killing by antimicrobial peptides is protease independent. Antimicrob. Agents Chemother. 2008, 52, 638–642. [Google Scholar] [CrossRef]
- Popadiak, K.; Potempa, J.; Riesbeck, K.; Blom, A.M. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007, 178, 7242–7250. [Google Scholar] [CrossRef]
- Fletcher, J.; Nair, S.; Poole, S.; Henderson, B.; Wilson, M. Cytokine degradation by biofilms of Porphyromonas gingivalis. Curr. Microbiol. 1998, 36, 216–219. [Google Scholar] [CrossRef]
- Yun, P.L.W.; DeCarlo, A.A.; Hunter, N. Modulation of major histocompatibility complex protein expression by human gamma interferon mediated by cysteine proteinase-adhesin polyproteins of Porphyromonas gingivalis. Infect. Immun. 1999, 67, 2986–2995. [Google Scholar] [CrossRef]
- Calkins, C.C.; Platt, K.; Potempa, J.; Travis, J. Inactivation of tumor necrosis factor-α by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis: Implications of immune evasion. J. Biol. Chem. 1998, 273, 6611–6614. [Google Scholar] [CrossRef]
- Oleksy, A.; Banbula, A.; Bugno, M.; Travis, J.; Potempa, J. Proteolysis of interleukin-6 receptor (IL-6R) by Porphyromonas gingivalis cysteine proteinases (gingipains) inhibits interleukin-6-mediated cell activation. Microb. Pathog. 2002, 32, 173–181. [Google Scholar] [CrossRef]
- Vincents, B.; Guentsch, A.; Kostolowska, D.; von Pawel-Ramminger, U.; Eick, S.; Potempa, J.; Abrahamson, M. Cleavage of IgG1 and IgG3 by gingipain K from Porphyromonas gingivalis may compromise host defense in progressive periodontitis. FASEB J. 2011, 25, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Herath, T.D.K.; Darveau, R.P.; Seneviratne, C.J.; Wang, C.Y.; Wang, Y.; Jin, L.J. Tetra- and Penta-Acylated Lipid A Structures of Porphyromonas gingivalis LPS Differentially Activate TLR4-Mediated NF-κB Signal Transduction Cascade and Immuno-Inflammatory Response in Human Gingival Fibroblasts. PLoS ONE 2013, 8, e58496. [Google Scholar] [CrossRef]
- Liang, S.; Krauss, J.L.; Domon, H.; McIntosh, M.L.; Hosur, K.B.; Qu, H.C.; Li, F.G.; Tzekou, A.; Lambris, J.D.; Hajishengallis, G. The C5a Receptor Impairs IL-12-Dependent Clearance of Porphyromonas gingivalis and Is Required for Induction of Periodontal Bone Loss. J. Immunol. 2011, 186, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Na, H.S.; Song, Y.R.; Shin, S.Y.; Kim, Y.M.; Chung, J. Activation of NLRP3 and AIM2 Inflammasomes by Porphyromonas gingivalis Infection. Infect. Immun. 2014, 82, 112–123. [Google Scholar] [CrossRef]
- Xu, X.; Cai, J.W.; Wang, X.M.; Lu, Y.T.; Guo, B.H.; Lai, M.M.; Lan, L.H.; Peng, Y.; Zheng, X.Q. Human cytomegalovirus infection activates NLRP3 inflammasome by releasing mtDNA into the cytosol in human THP-1 cells. Microbiol. Immunol. 2023, 67, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.J.; Pooley, J.C.; Walsh, D.J.; Vercellotti, G.M.; Weber, M.L.; Kovacs, A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. Transplantation 1999, 67, 137–144. [Google Scholar] [CrossRef]
- Srisatjaluk, R.; Doyle, R.J.; Justus, D.E. Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-γ-mediated MHC class II expression by human vascular endothelial cells. Microb. Pathog. 1999, 27, 81–91. [Google Scholar] [CrossRef]
- Stern, J.; Shai, E.; Zaks, B.; Halabi, A.; Houri-Haddad, Y.; Shapira, L.; Palmon, A. Reduced expression of gamma interferon in serum and marked lymphoid depletion induced by Porphyromonas gingivalis increase murine morbidity and mortality due to cytomegalovirus infection. Infect. Immun. 2004, 72, 5791–5798. [Google Scholar] [CrossRef]
- Tada, H.; Sugawara, S.; Nemoto, E.; Imamura, T.; Potempa, J.; Travis, J.; Shimauchi, H.; Takada, H. Proteolysis of ICAM-1 on human oral epithelial cells by gingipains. J. Dent. Res. 2003, 82, 796–801. [Google Scholar] [CrossRef]
- Ren, J.L.; Han, X.; Lohner, H.; Hoyle, R.G.; Li, J.; Liang, S.; Wang, H.Z. P. gingivalis Infection Upregulates PD-L1 Expression on Dendritic Cells, Suppresses CD8+ T-cell Responses, and Aggravates Oral Cancer. Cancer Immunol. Res. 2023, 11, 290–305. [Google Scholar] [CrossRef]
- Yuan, Q.; Fan, Z.S.; Huang, W.Q.; Huo, X.P.; Yang, X.P.; Ran, Y.H.; Chen, J.; Li, H.J. Human cytomegalovirus UL23 exploits PD-L1 inhibitory signaling pathway to evade T cell-mediated cytotoxicity. Mbio 2024, 15, e01191-24. [Google Scholar] [CrossRef]
- Jones, T.R.; Sun, L. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 1997, 71, 2970–2979. [Google Scholar] [CrossRef]
- Tomazin, R.; Boname, J.; Hegde, N.R.; Lewinsohn, D.M.; Altschuler, Y.; Jones, T.R.; Cresswell, P.; Nelson, J.A.; Riddell, S.R.; Johnson, D.C. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 1999, 5, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, P.K.; Buchkovich, N.J. Human Cytomegalovirus Decreases Major Histocompatibility Complex Class II by Regulating Class II Transactivator Transcript Levels in a Myeloid Cell Line. J. Virol. 2020, 94, e01901-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Zhou, H.X.; Duan, X.X.; Jotwani, R.; Vuddaraju, H.; Liang, S.; Scott, D.A.; Lamont, R.J. Porphyromonas gingivalis-Induced Reactive Oxygen Species Activate JAK2 and Regulate Production of Inflammatory Cytokines through c-Jun. Infect. Immun. 2014, 82, 4118–4126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.X.; Zheng, M.; Luan, Q.X. Mitochondrial reactive oxygen species mediate the lipopolysaccharide-induced pro-inflammatory response in human gingival fibroblasts. Exp. Cell Res. 2016, 347, 212–221. [Google Scholar] [CrossRef]
- Su, W.Q.; Li, J.W.; Jiang, L.S.; Lei, L.; Li, H.X. Hexokinase 2-mediated glycolysis supports inflammatory responses to Porphyromonas gingivalis in gingival fibroblasts. BMC Oral Health 2023, 23, 103. [Google Scholar] [CrossRef]
- Speir, E.; Shibutani, T.; Yu, Z.X.; Ferrans, V.; Epstein, S.E. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ. Res. 1996, 79, 1143–1152. [Google Scholar] [CrossRef]
- Shibutani, T.; Johnson, T.M.; Yu, Z.X.; Ferrans, V.J.; Moss, J.; Epstein, S.E. Pertussis toxin-sensitive G proteins as mediators of the signal transduction pathways activated by cytomegalovirus infection of smooth muscle cells. J. Clin. Investig. 1997, 100, 2054–2061. [Google Scholar] [CrossRef]
- Xiao, J.; Deng, J.; Lv, L.P.; Kang, Q.; Ma, P.; Yan, F.; Song, X.; Gao, B.; Zhang, Y.Y.; Xu, J.B. Hydrogen Peroxide Induce Human Cytomegalovirus Replication through the Activation of p38-MAPK Signaling Pathway. Viruses 2015, 7, 2816–2833. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Bostanghadiri, N.; Kouhzad, M.; Taki, E.; Elahi, Z.; Khoshbayan, A.; Navidifar, T.; Darban-Sarokhalil, D. Oral microbiota and metabolites: Key players in oral health and disorder, and microbiota-based therapies. Front. Microbiol. 2024, 15, 1431785. [Google Scholar] [CrossRef]
- Kashyap, B.; Kullaa, A. Salivary Metabolites Produced by Oral Microbes in Oral Diseases and Oral Squamous Cell Carcinoma: A Review. Metabolites 2024, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Nakano, Y.; Yamashita, Y.; Oho, T.; Saito, T.; Koga, T. Formation of methyl mercaptan from L-methionine by Porphyromonas gingivalis. Infect. Immun. 2000, 68, 6912–6916. [Google Scholar] [CrossRef]
- Johnson, P.W.; Ng, W.; Tonzetich, J. Modulation OF Human Gingival Fibroblast Cell-Metabolism by Methyl Mercaptan. J. Periodontal Res. 1992, 27, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Tan, L.P.; Wu, L.Z.; Li, J.Y.; Zhang, Y.; Shen, Z.S.; Zhang, C.; Zhao, C.J.; Gao, L. Regulation of tryptophan-indole metabolic pathway in Porphyromonas gingivalis virulence and microbiota dysbiosis in periodontitis. NPJ Biofilms Microbiomes 2025, 11, 37. [Google Scholar] [CrossRef]
- Martin, M.; Kumar, R.; Buchkovich Nicholas, J.; Norbury Christopher, C. HCMV infection downregulates GPX4 and stimulates lipid peroxidation but does not induce ferroptosis. J. Virol. 2025, 99, e01851-24. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chen, H.S.; Chen, S.L.; Ho, Y.P.; Ho, K.Y.; Wu, Y.M.; Hung, C.C. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J. Periodontal Res. 2005, 40, 378–384. [Google Scholar] [CrossRef]
- Amratia, P.S.; Kerr-Jones, L.E.; Chapman, L.; Marsden, M.; Clement, M.; Stanton, R.J.; Humphreys, I.R. Cytomegalovirus-induced peroxynitrite promotes virus entry and contributes to pathogenesis in a murine model of infection. Mbio 2024, 15, e03152-23. [Google Scholar] [CrossRef]
- Okamoto, T.; Akaike, T.; Sawa, T.; Miyamoto, Y.; van der Vliet, A.; Maeda, H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar] [CrossRef]
- Katakwar, P.; Metgud, R.; Naik, S.; Mittal, R. Oxidative stress marker in oral cancer: A review. J. Cancer Res. Ther. 2016, 12, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kesarwala, A.H.; Krishna, M.C.; Mitchell, J.B. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Keller, T.T.; van Gorp, E.; ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Banbula, A.; Pereira, P.J.B.; Travis, J.; Potempa, J. Activation of human prothrombin by arginine-specific cysteine proteinases (gingipains R) from Porphyromonas gingivalis. J. Biol. Chem. 2001, 276, 18984–18991. [Google Scholar] [CrossRef]
- Imamura, T.; Potempa, J.; Tanase, S.; Travis, J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J. Biol. Chem. 1997, 272, 16062–16067. [Google Scholar] [CrossRef]
- Imamura, T.; Tanase, S.; Hamamoto, T.; Potempa, J.; Travis, J. Activation of blood coagulation factor IX by gingipains R, arginine-specific cysteine proteinases from Porphyromonas gingivalis. Biochem. J. 2001, 353, 325–331. [Google Scholar] [CrossRef]
- Neilands, J.; Kinnby, B. Porphyromonas gingivalis initiates coagulation and secretes polyphosphates—A mechanism for sustaining chronic inflammation? Microb. Pathog. 2022, 162, 104648. [Google Scholar] [CrossRef]
- Silva, L.M.; Divaris, K.; Bugge, T.H.; Moutsopoulos, N.M. Plasmin-Mediated Fibrinolysis in Periodontitis Pathogenesis. J. Dent. Res. 2023, 102, 972–978. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Rahbar, A.; Söderberg-Nauclér, C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J. Virol. 2005, 79, 2211–2220. [Google Scholar] [CrossRef]
- Grundy, J.E.; Downes, K.L. Up-Regulation of LFA-3 and ICAM-1 on the Surface of Fibroblasts Infected with Cytomegalovirus. Immunology 1993, 78, 405–412. [Google Scholar] [PubMed]
- Grundy, J.E.; Pahal, G.S.; Akbar, A.N. Increased Adherence OF CD2 Peripheral-Blood Lymphocytes to Cytomegalovirus-Infected Fibroblasts is Blocked by Anti-LFA-3 Antibody. Immunology 1993, 78, 413–420. [Google Scholar] [PubMed]
- Javaid, M.; Bi, J.; Biddle, C.; Tsai, C.M.; Häkkinen, L.; Kim, H. Platelet factor 4 upregulates matrix metalloproteinase-1 production in gingival fibroblasts. J. Periodontal Res. 2017, 52, 787–792. [Google Scholar] [CrossRef]
- Gokyu, M.; Kobayashi, H.; Nanbara, H.; Sudo, T.; Ikeda, Y.; Suda, T.; Izumi, Y. Thrombospondin-1 Production Is Enhanced by Porphyromonas gingivalis Lipopolysaccharide in THP-1 Cells. PLoS ONE 2014, 9, e115107. [Google Scholar] [CrossRef] [PubMed]
- Angabo, S.; Pandi, K.; David, K.; Steinmetz, O.; Makkawi, H.; Farhat, M.; Eli-Berchoer, L.; Darawshi, N.; Kawasaki, H.; Nussbaum, G. CD47 and thrombospondin-1 contribute to immune evasion by Porphyromonas gingivalis. Proc. Natl. Acad. Sci. USA 2024, 121, e2405534121. [Google Scholar] [CrossRef]
- Liu, X.X.; Jin, J.; Liu, Y.J.; Shen, Z.G.; Zhao, R.Q.; Ou, L.L.; Xing, T. Targeting TSP-1 decreased periodontitis by attenuating extracellular matrix degradation and alveolar bone destruction. Int. Immunopharmacol. 2021, 96, 107618. [Google Scholar] [CrossRef]
- Woodroffe, S.B.; Kuan, S. Human cytomegalovirus infection induces mRNA expression and secretion of plasminogen inhibitor type-1 in endothelial cells. J. Med. Virol. 1998, 55, 268–271. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef]
- Roth, G.A.; Moser, B.; Huang, S.J.; Brandt, J.S.; Huang, Y.; Papapanou, P.N.; Schmidt, A.M.; Lalla, E. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J. Thromb. Haemost. 2006, 4, 2256–2261. [Google Scholar] [CrossRef]
- Song, L.T.; Tada, H.; Nishioka, T.; Nemoto, E.; Imamura, T.; Potempa, J.; Li, C.Y.; Matsushita, K.; Sugawara, S. Porphyromonas gingivalis Gingipains-Mediated Degradation of Plasminogen Activator Inhibitor-1 Leads to Delayed Wound Healing Responses in Human Endothelial Cells. J. Innate Immun. 2021, 14, 306–319. [Google Scholar] [CrossRef]
- Goldman, M.; Craft, B.; Swatloski, T.; Cline, M.; Morozova, O.; Diekhans, M.; Haussler, D.; Zhu, J.C. The UCSC Cancer Genomics Browser: Update 2015. Nucleic Acids Res. 2015, 43, D812–D817. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.H.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Shigeishi, H.; Oka, I.; Su, C.Y.; Hamada, N.; Nakamura, M.; Nishimura, R.; Sugiyama, M.; Ohta, K. Prevalence of oral Epstein-Barr virus and Porphyromonas gingivalis and their association with periodontal inflamed surface area: A cross-sectional study. Medicine 2022, 101, e31282. [Google Scholar] [CrossRef]
- Imai, K.; Inoue, H.; Tamura, M.; Cueno, M.E.; Inoue, H.; Takeichi, O.; Kusama, K.; Saito, I.; Ochiai, K. The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie 2012, 94, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Takeichi, O.; Imai, K.; Okano, M.; Inoue, S.; Yasukawa, T.; Suzuki, Y. Reactivation of Epstein-Barr virus by n-butyric acid from Pseudoramibacter alactolyticus induces inflammatory cytokines in periapical granulomas. J. Oral Biosci. 2025, 67, 100569. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Thomas, W.A.; Haigh, T.A.; Fitzsimmons, L.; Long, H.M.; Hislop, A.D.; Taylor, G.S.; Rowe, M. Epstein-Barr virus evades CD4+ T cell responses in lytic cycle through BZLF1-mediated downregulation of CD74 and the cooperation of vBcl-2. PLoS Pathog. 2011, 7, e1002455. [Google Scholar] [CrossRef]
- Glasspoole, D.L.C. The Role of Periodontal Bacteria and Epigenetic Modifications on Human Papillomavirus Pathogenicity. Ph.D. Thesis, The University of North Carolina at Chapel Hill, Chapel Hill, CA, USA, 2019. [Google Scholar]
- Luo, X.; Donnelly, C.R.; Gong, W.; Heath, B.R.; Hao, Y.; Donnelly, L.A.; Moghbeli, T.; Tan, Y.S.; Lin, X.; Bellile, E.; et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Investig. 2020, 130, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017, 231, 21–33. [Google Scholar] [CrossRef]
- Lo Cigno, I.; Calati, F.; Girone, C.; Catozzo, M.; Gariglio, M. High-risk HPV oncoproteins E6 and E7 and their interplay with the innate immune response: Uncovering mechanisms of immune evasion and therapeutic prospects. J. Med. Virol. 2024, 96, e29685. [Google Scholar] [CrossRef]
- Song, Y.; Liu, N.; Gao, L.; Yang, D.; Liu, J.; Xie, L.; Dan, H.; Chen, Q. Association between human herpes simplex virus and periodontitis: Results from the continuous National Health and Nutrition Examination Survey 2009–2014. BMC Oral Health 2023, 23, 675. [Google Scholar] [CrossRef]
- Yadalam, P.K.; Neelakandan, A.; Arunraj, R.; Anegundi, R.V.; Ardila, C.M. Exploring the interplay between Porphyromonas gingivalis KGP gingipain, herpes virus MicroRNA-6, and Icp4 transcript in periodontitis: Computational and clinical insights. PLoS ONE 2024, 19, e0312162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lo, K.; Wang, C.; Zhou, G.; Feng, X.; Ni, J.; Chen, X. Herpes simplex virus-induced upregulation of inflammatory cytokines in human gingival fibroblasts. Virol. J. 2024, 21, 323. [Google Scholar] [CrossRef]
- Soto, C.; Bugueño, I.; Hoare, A.; Gonzalez, S.; Venegas, D.; Salinas, D.; Melgar-Rodríguez, S.; Vernal, R.; Gamonal, J.; Quest, A.F.G.; et al. The Porphyromonas gingivalis O antigen is required for inhibition of apoptosis in gingival epithelial cells following bacterial infection. J. Periodontal Res. 2016, 51, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Kuboniwa, M.; Hasegawa, Y.; Mao, S.; Shizukuishi, S.; Amano, A.; Lamont, R.J.; Yilmaz, Ö. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Xu, X.; Tan, L.; Lin, L.; Pan, Y. The effects of Porphyromonas gingivalis on the cell cycle progression of human gingival epithelial cells. Oral Dis. 2014, 20, 100–108. [Google Scholar] [CrossRef]
- Iwahori, S.; Hakki, M.; Chou, S.; Kalejta, R.F. Molecular Determinants for the Inactivation of the Retinoblastoma Tumor Suppressor by the Viral Cyclin-dependent Kinase UL97. J. Biol. Chem. 2015, 290, 19666–19680. [Google Scholar] [CrossRef]
- Iwahori, S.; Umaña, A.C.; VanDeusen, H.R.; Kalejta, R.F. Human cytomegalovirus-encoded viral cyclin-dependent kinase (v-CDK) UL97 phosphorylates and inactivates the retinoblastoma protein-related p107 and p130 proteins. J. Biol. Chem. 2017, 292, 6583–6599. [Google Scholar] [CrossRef]
- Poma, E.E.; Kowalik, T.F.; Zhu, L.A.; Sinclair, J.H.; Huang, E.S. The human cytomegalovirus IE1-72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J. Virol. 1996, 70, 7867–7877. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Huong, S.M.; Wang, X.; Huang, D.Y.; Huang, E.S. Interactions between human cytomegalovirus IE1-72 and cellular p107: Functional domains and mechanisms of up-regulation of cyclin E/cdk2 kinase activity. J. Virol. 2003, 77, 12660–12670. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.P.; Valmary-Degano, S.; et al. The Human Cytomegalovirus Strain DB Activates Oncogenic Pathways in Mammary Epithelial Cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef]
- Chang, C.; Wang, H.; Liu, J.; Pan, C.; Zhang, D.; Li, X.; Pan, Y. Porphyromonas gingivalis infection promoted the proliferation of oral squamous cell carcinoma cells through the miR-21/PDCD4/AP-1 negative signaling pathway. ACS Infect. Dis. 2019, 5, 1336–1347. [Google Scholar] [CrossRef]
- Oseguera, C.A.V.; Spencer, J.V. cmvIL-10 Stimulates the Invasive Potential of MDA-MB-231 Breast Cancer Cells. PLoS ONE 2014, 9, e88708. [Google Scholar] [CrossRef] [PubMed]
- Huxiao, L.I.; Xiaotian, L.I.; Xuri, Z.; Huanyu, Z.; Wei, Z.; Zhongchen, S. Effects of gingipain extract on the biological characteristics of oral squamous cell carcinoma cell HN6. Shanghai Jiao Tong Da Xue Xue Bao Yi Xue Ban 2024, 44, 161–168. [Google Scholar]
- Nakhjiri, S.F.; Park, Y.; Yilmaz, O.; Chung, W.O.; Watanabe, K.; El-Sabaeny, A.; Park, K.; Lamont, R.J. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol. Lett. 2001, 200, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.; Jungas, T.; Verbeke, P.; Ojcius David, M. Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway Contributes to Survival of Primary Epithelial Cells Infected with the Periodontal Pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef]
- Mao, S.; Park, Y.; Hasegawa, Y.; Tribble, G.D.; James, C.E.; Handfield, M.; Stavropoulos, M.F.; Yilmaz, Ö.; Lamont, R.J. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell. Microbiol. 2007, 9, 1997–2007. [Google Scholar] [CrossRef]
- Yao, L.; Jermanus, C.; Barbetta, B.; Choi, C.; Verbeke, P.; Ojcius, D.M.; Yilmaz, Ö. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010, 25, 89–101. [Google Scholar] [CrossRef]
- Blanco, R.; Muñoz, J.P. Molecular Insights into HR-HPV and HCMV Co-Presence in Cervical Cancer Development. Cancers 2025, 17, 582. [Google Scholar] [CrossRef]
- Skaletskaya, A.; Bartle, L.M.; Chittenden, T.; McCormick, A.L.; Mocarski, E.S.; Goldmacher, V.S. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 2001, 98, 7829–7834. [Google Scholar] [CrossRef]
- Goldmacher, V.S.; Bartle, L.M.; Skaletskaya, A.; Dionne, C.A.; Kedersha, N.L.; Vater, C.A.; Han, J.-w.; Lutz, R.J.; Watanabe, S.; McFarland, E.D.C.; et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 1999, 96, 12536–12541. [Google Scholar] [CrossRef]
- Poncet, D.; Larochette, N.; Pauleau, A.L.; Boya, P.; Jalil, A.A.; Cartron, P.F.; Vallette, F.; Schnebelen, C.; Bartle, L.M.; Skaletskaya, A.; et al. An anti-apoptotic viral protein that recruits bax to mitochondria. J. Biol. Chem. 2004, 279, 22605–22614. [Google Scholar] [CrossRef]
- Kim, S.; Seo, D.; Kim, D.; Hong, Y.; Chang, H.; Baek, D.; Kim, V.N.; Lee, S.; Ahn, K. Temporal Landscape of MicroRNA-Mediated Host-Virus Crosstalk during Productive Human Cytomegalovirus Infection. Cell Host Microbe 2015, 17, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, A.; Khalko, R.K.; Mishra, A.; Singh, N.; Singh, S.; Saha, S.; Yadav, S.; Saxena, S.; Gosipatala, S.B. Human Cytomegalovirus miR-UL70-3p Downregulates the H2O2-Induced Apoptosis by Targeting the Modulator of Apoptosis-1 (MOAP1). Int. J. Mol. Sci. 2022, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Hong, W.Q.; Wei, X.W. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.N.; Ghoneim, S.M.; Ahmed, E.R.; El-Farouk Abdel Salam, L.O.; Anis Saleh, S.M. Cadherin switching in oral squamous cell carcinoma: A clinicopathological study. J. Oral Biol. Craniofacial Res. 2023, 13, 486–494. [Google Scholar] [CrossRef]
- Ha, N.H.; Woo, B.H.; Kim, D.J.; Ha, E.S.; Choi, J.I.; Kim, S.J.; Park, B.S.; Lee, J.H.; Park, H.R. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumor Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef]
- Teo, W.H.; Chen, H.P.; Huang, J.C.; Chan, Y.J. Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells. Int. J. Oncol. 2017, 51, 1415–1426. [Google Scholar] [CrossRef]
- Nehme, Z.; Pasquereau, S.; Ahmad, S.H.; El Baba, R.; Herbein, G. Polyploid giant cancer cells, EZH2 and Myc upregulation in mammary epithelial cells infected with high-risk human cytomegalovirus. Ebiomedicine 2022, 80, 104056. [Google Scholar] [CrossRef]
- Houle, M.A.; Grenier, D.; Plamondon, P.; Nakayama, K. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. Fems Microbiol. Lett. 2003, 221, 181–185. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczanska, B.; Lacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT-Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Cavanaugh, C.M.; Betsinger, C.N.; Katchur, N.; Zhang, S.; Yang, K.; Nogalski, M.; Cristea, I.M.; Notterman, D. Effect of host telomerase inhibition on human cytomegalovirus. J. Virol. 2025, 99, e01578-24. [Google Scholar] [CrossRef] [PubMed]
- Straat, K.; Liu, C.; Rahbar, A.; Zhu, Q.J.; Liu, L.; Wolmer-Solberg, N.; Lou, F.L.; Liu, Z.X.; Shen, J.; Jia, J.H.; et al. Activation of Telomerase by Human Cytomegalovirus. J. Natl. Cancer Inst. 2009, 101, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Guyon, J.; Ahmad, S.H.; El Baba, R.; Le Quang, M.; Bikfalvi, A.; Daubon, T.; Herbein, G. Generation of glioblastoma in mice engrafted with human cytomegalovirus-infected astrocytes. Cancer Gene Ther. 2024, 31, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Geder, L.; Sanford, E.J.; Rohner, T.J.; Rapp, F. Cytomegalovirus and cancer of the prostate: In vitro transformation of human cells. Cancer Treat. Rep. 1977, 61, 139–146. [Google Scholar]
- Guo, Z.C.; Jing, S.L.; Jumatai, S.; Gong, Z.C. Porphyromonas gingivalis promotes the progression of oral squamous cell carcinoma by activating the neutrophil chemotaxis in the tumour microenvironment. Cancer Immunol. Immunother. 2023, 72, 1523–1539. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhou, X.D.; Peng, X.; Li, M.Y.; Ren, B.; Cheng, G.; Cheng, L. Porphyromonas gingivalis Promotes Immunoevasion of Oral Cancer by Protecting Cancer from Macrophage Attack. J. Immunol. 2020, 205, 282–289. [Google Scholar] [CrossRef]
- Damgaard, C.; Danielsen, A.K.; Enevold, C.; Massarenti, L.; Nielsen, C.H.; Holmstrup, P.; Belstrøm, D. Porphyromonas gingivalis in saliva associates with chronic and aggressive periodontitis. J. Oral. Microbiol. 2019, 11, 1653123. [Google Scholar] [CrossRef]
- Ozbek, S.M.; Ozbek, A.; Yavuz, M.S. Detection of human cytomegalovirus and Epstein-Barr Virus in symptomatic and asymptomatic apical periodontitis lesions by real-time PCR. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e811–e816. [Google Scholar] [CrossRef]
- Serbanescu, M.A.; Oveisi, M.; Sun, C.; Fine, N.; Bosy, A.; Glogauer, M. Metronidazole enhances killing of Porphyromonas gingivalis by human PMNs. Front. Oral Health 2022, 3, 933997. [Google Scholar] [CrossRef]
- Sunde, P.T.; Olsen, I.; Enersen, M.; Grinde, B. Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J. Clin. Virol. 2008, 42, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, T.; Zhang, B.; Chen, Y.; Li, Z. Porphyromonas gingivalis Vaccine: Antigens and Mucosal Adjuvants. Vaccines 2024, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Permar, S.R.; Schleiss, M.R.; Plotkin, S.A. A vaccine against cytomegalovirus: How close are we? J. Clin. Investig. 2025, 135, e182317. [Google Scholar] [CrossRef] [PubMed]

| Reference | Disease | Results |
|---|---|---|
| Nakamura et al. (2020) [44] | Aggressive periodontitis | The co-presence of P. gingivalis and HCMV was associated with periodontal pockets ≥ 4 mm deep with BOP (p = 0.03). |
| Michalowicz et al. (2020) [43] | Juvenile periodontitis | The presence of P. gingivalis was significantly associated with HCMV infection (OR = 3.6, 95% CI: 1.5–8.8; p = 0.0054). P. gingivalis/HCMV co-infection was associated with the extent of attachment loss (p < 0.05). |
| Joshi et al. (2016) [58] | Chronic periodontitis | An increased level of P. gingivalis was evidenced by culture in HCMV-positive patients (p = 0.023). |
| Botero et al. (2007) [57] | Aggressive periodontitis | Co-infection with HCMV and periodontopathic bacteria, including P. gingivalis, increases the risk of periodontitis development (OR = 7.6, 95% CI: 6.31–9.28; p < 0.01). |
| Elamin et al. (2017) [45] | Aggressive periodontitis | P. gingivalis and HCMV co-infection increases the risk of developing aggressive periodontitis (OR = 29.3, 95% CI: 3.1–278.8; p = 0.003). |
| Imbronito et al. (2008) [31] | Aggressive periodontitis | P. gingivalis and HCMV co-detection was more frequent in aggressive periodontitis sites than in healthy controls without statistically significant difference among groups |
| Saygun et al. (2004) [59] | Aggressive periodontitis | HCMV and P. gingivalis co-infection occurred in 77.8% of patients with aggressive periodontitis and was significantly more frequent than in healthy individuals (p < 0.004). |
| Herrero-Sánchez et al. (2025) [60] | Stage I-II Periodontitis | No significant association was found between the presence of P. gingivalis and HCMV in aggressive periodontitis patients (p = 0.732). |
| Slots et al. (2003) [61] | Aggressive periodontitis | HCMV and HSV were significant predictors of subgingival P. gingivalis presence. Their co-detection was associated with increased periodontitis disease activity (p < 0.001). |
| Sharma et al. (2015) [62] | Aggressive periodontitis | Co-infection with P. gingivalis and HCMV/EBV-I was observed in 40% and 50% of patients, respectively, but without significant association. However, a strong association (77.78%, p = 0.0002) was observed between Aa and HCMV. |
| Kousar et al. (2017) [63] | Chronic periodontitis | P. gingivalis was prevalent in 50–60% of moderate and severe chronic periodontitis patients, but HCMV was not detected in any clinical group. |
| Passariello et al. (2017) [64] | Aggressive periodontitis | HCMV was inversely associated with P. gingivalis, Tannerella forsythia, and Fusobacterium periodonticum (p < 0.05). This suggests that HCMV may influence the subgingival biofilm composition in a virus-specific manner, possibly reducing P. gingivalis prevalence. |
| Kamma et al. (2001) [65] | Early-onset periodontitis | HCMV was detected in 59.4% of active sites and 12.5% of stable sites (p < 0.001). P. gingivalis was present in 71.9% of active sites and 37.5% of stable sites (p = 0.01). Co-infection with HCMV and P. gingivalis (along with D. pneumosintes) occurred in 60% of herpesvirus-positive sites and only 10.3% of virus-negative sites (p = 0.03). |
| Rams et al. (2024) [66] | Aggressive periodontitis (Ag/MI) | P. gingivalis was found in 63.6% of Ag/MI periodontitis patients. Herpesvirus co-infection (CMV + EBV-1) was present in generalized Ag/MI periodontitis cases and absent in healthy controls. Herpesvirus presence showed a 3.5-fold increased odds of Ag/MI periodontitis. |
| Contreras et al. (1999) [67] | Adult periodontitis | HCMV was associated with co-infections involving P. gingivalis, especially with P. nigrescens and T. denticola (ORs = 2.59–3.23). Mixed viral infections increased odds of severe periodontitis (OR = 4.36) and were associated with P. gingivalis (OR = 2.27) and various bacterial combinations (ORs up to 2.91). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, R.; Muñoz, J.P. Porphyromonas gingivalis and Human Cytomegalovirus Co-Infection: A Potential Link Between Periodontal Disease and Oral Cancer Development. Cancers 2025, 17, 1525. https://doi.org/10.3390/cancers17091525
Blanco R, Muñoz JP. Porphyromonas gingivalis and Human Cytomegalovirus Co-Infection: A Potential Link Between Periodontal Disease and Oral Cancer Development. Cancers. 2025; 17(9):1525. https://doi.org/10.3390/cancers17091525
Chicago/Turabian StyleBlanco, Rancés, and Juan P. Muñoz. 2025. "Porphyromonas gingivalis and Human Cytomegalovirus Co-Infection: A Potential Link Between Periodontal Disease and Oral Cancer Development" Cancers 17, no. 9: 1525. https://doi.org/10.3390/cancers17091525
APA StyleBlanco, R., & Muñoz, J. P. (2025). Porphyromonas gingivalis and Human Cytomegalovirus Co-Infection: A Potential Link Between Periodontal Disease and Oral Cancer Development. Cancers, 17(9), 1525. https://doi.org/10.3390/cancers17091525





