Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer
Simple Summary
Abstract
1. Introduction
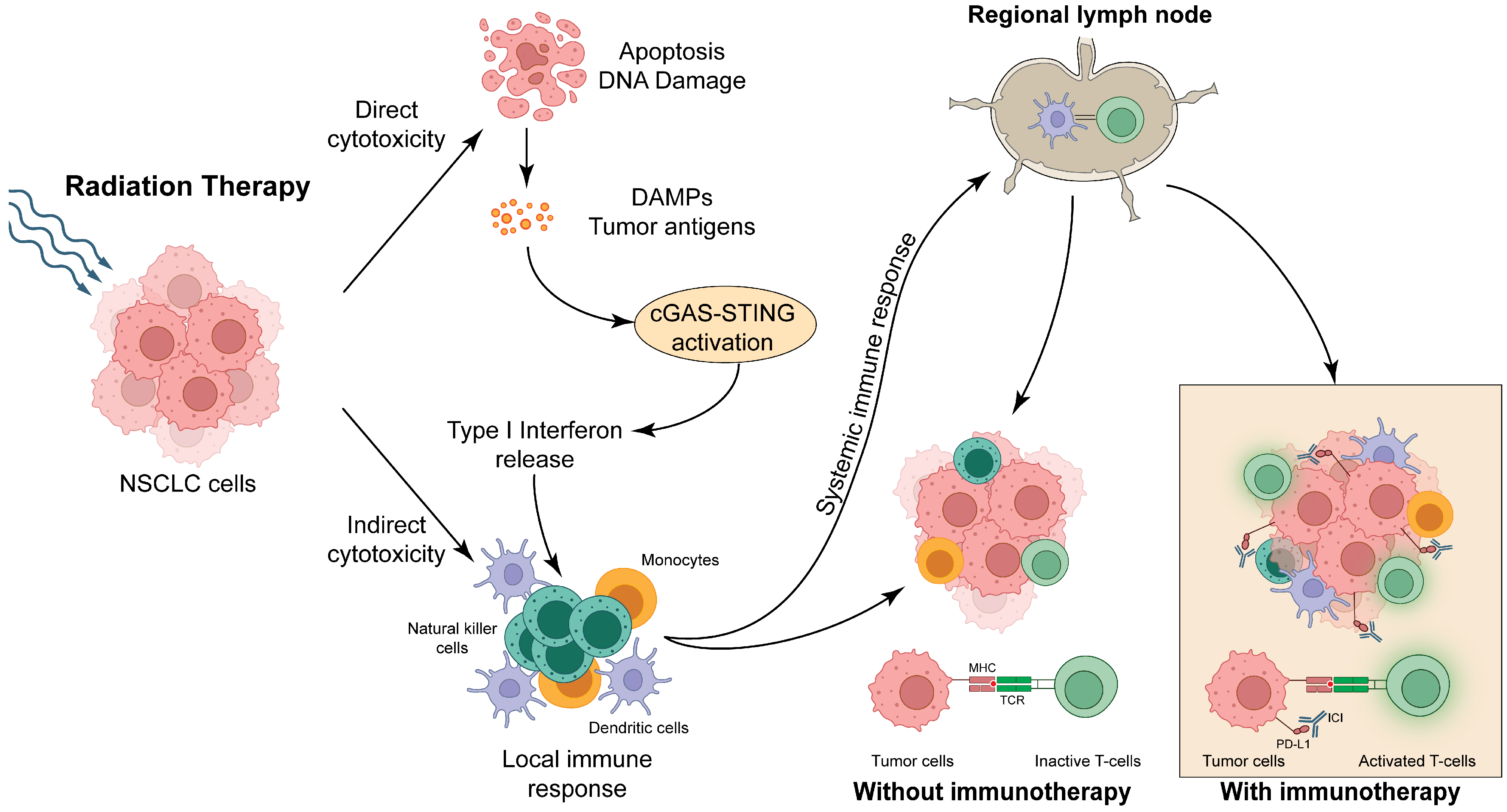
2. Mechanism of Synergy Between Immunotherapy and Radiation
3. Chemotherapy-Free Treatment Regimens for Advanced/Metastatic NSCLC
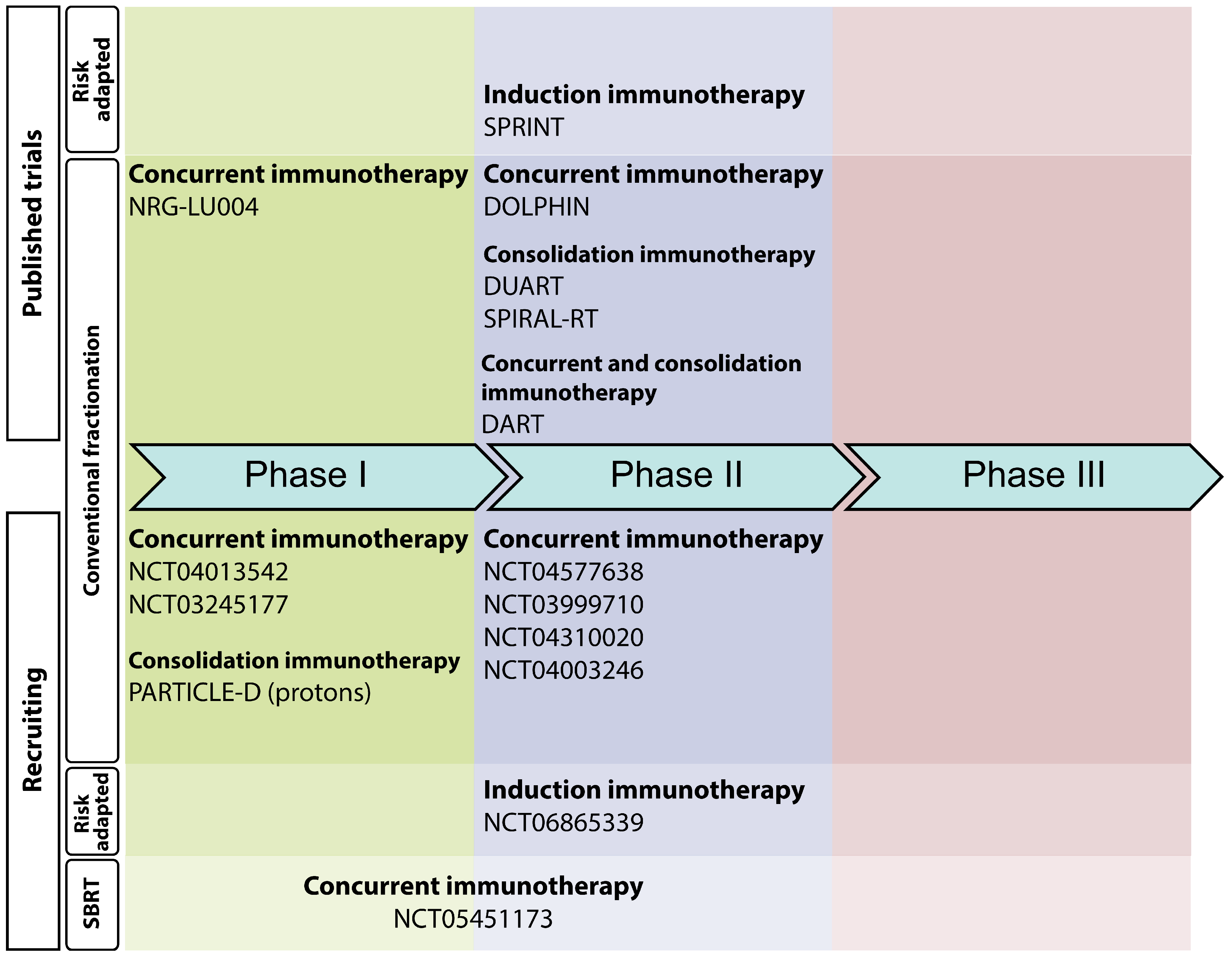
4. Completed Clinical Trials Exploring Chemotherapy-Free Regimens in LA-NSCLC
| Registration #, Trial Name | Sample Size | Phase | Primary Endpoint | Immunotherapy Schedule and Timing | RT Schedule | Patient/Biomarker Selection | Key Findings |
|---|---|---|---|---|---|---|---|
| jRCT2080224763 DOLPHIN [20] | 35 | II | 1-year PFS rate | Durvalumab, every two weeks for one year, starting concurrent with RT | 60 Gy in 30 fractions | PD-L1 TPS ≥ 1% | 1-year PFS = 72% 1-year OS = 94% |
| NCT04249362 DUART [21,22] (Cohort A) | 53 | II | Safety | Durvalumab, every four weeks for one year, starting after RT | 60 Gy (+/−10%) | Ineligible for chemotherapy | 7 Grade 3–5 adverse events related to study therapy 1-year PFS = 47% 1-year OS = 64% |
| JMA-IIA00434 (jRCT) SPIRAL-RT [17,18] | 33 | II | 1-year PFS rate | Durvalumab, every two weeks for one year, starting after RT | 54 to 66 Gy in 27 to 33 fractions | Ineligible for concurrent chemo-radiotherapy | 1-year PFS = 39% 1-year OS = 72% |
| NCT03523702 SPRINT [19] | 25 | II | 1-year PFS rate | Pembrolizumab, every three weeks, before RT (3 cycles) and after RT (12 cycles) | 48 or 55 Gy in 20 fractions (risk-adapted) | PD-L1 TPS ≥ 50% | 1-year PFS = 76% 1-year OS = 92% |
| NCT03999710 DART [26] | 27 | II | 2-year PFS rate | Durvalumab, every four weeks for one year, starting concurrent with RT | 54–66 Gy in 27–33 fractions | Ineligible for concurrent chemo-radiotherapy | 1-year PFS = 42% 1-year OS = 75% |
| NCT04003246 [27] | 10 | II | PFS rate | Durvalumab, every four weeks for one year, starting concurrently with RT. | 54–66 Gy in 27–33 fractions | Medically inoperable disease or unwilling to undergo surgery PD-L1 TPS (any) | 1-year PFS = 20% |
| NCT03801902 NRG-LU004 [28] | 24 | I | Safety | Durvalumab, every four weeks for one year, starting 0–2 weeks before RT start | Cohort 1: 60 Gy in 30 fractions Cohort 2: 60 Gy in 15 fractions | PD-L1 TPS ≥ 50% | No DLTs related to study therapy |
5. Ongoing Chemotherapy-Free Clinical Trials in LA-NSCLC Patients
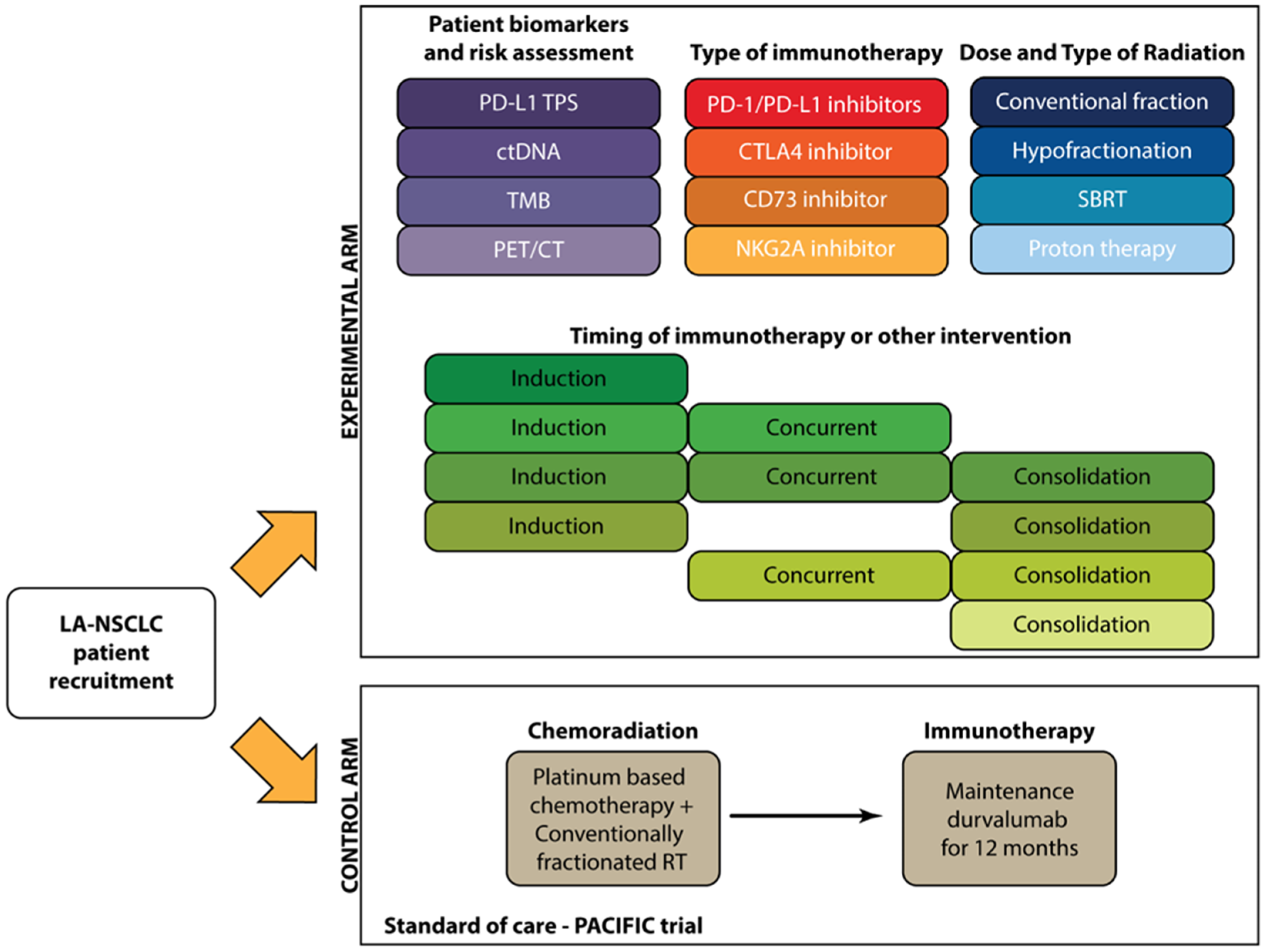
6. Considerations for Future Studies of Chemotherapy-Free Treatment of LA-NSCLC
6.1. Patient Selection
- (1)
- Patients who are deemed to be ineligible for standard chemoradiotherapy
- (2)
- Patients for whom chemotherapy-free treatment is expected to yield superior outcomes compared to standard chemoradiotherapy.
6.2. Identification of LA-NSCLC Patients Most Likely to Benefit from Immunotherapy
6.3. Radiation Type and Dose
6.4. Safety and Toxicity
6.5. Type and Order of Immunotherapy
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Sugawara, S.; Lee, K.H.H.; Ostoros, G.; Demirkazik, A.; Zemanova, M.; Sriuranpong, V.; Gelatti, A.; Menezes, J.; Zurawski, B.; et al. LBA1 Durvalumab in Combination with Chemoradiotherapy for Patients with Unresectable Stage III NSCLC: Final Results from PACIFIC-2. ESMO Open 2024, 9, 102986. [Google Scholar] [CrossRef]
- Peters, S.; Tan, D.S.W.; Gerber, D.E.; Urbanic, J.; Ramalingam, S.S.; Yu, J.; Xing, L.; Rittmeyer, A.; Ciuleanu, T.; Menezes, J.; et al. CheckMate 73L: Phase 3 Study Comparing Nivolumab (N) + Concurrent Chemoradiotherapy (CCRT) Followed by N_Ipilimumab (I) v CCRT Followed by Durvalumab (D) for Previously Untreated, Locally Advanced Stage (Stg) III NSCLC. Ann. Oncol. 2024, 24, 100808. [Google Scholar] [CrossRef]
- Eichkorn, T.; Bozorgmehr, F.; Regnery, S.; Dinges, L.A.; Kudak, A.; Bougatf, N.; Weber, D.; Christopoulos, P.; Muley, T.; Kobinger, S.; et al. Consolidation Immunotherapy After Platinum-Based Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Cross-Sectional Study of Eligibility and Administration Rates. Front. Oncol. 2020, 10, 586449. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Ito, K.; Furuhashi, K.; Nakamura, Y.; Suzuki, Y.; Nishii, Y.; Taguchi, O.; Hataji, O. Patients with Unresectable Stage III Non-Small Cell Lung Cancer Eligible to Receive Consolidation Therapy with Durvalumab in Clinical Practice Based on PACIFIC Study Criteria. Respir. Investig. 2019, 57, 466–471. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, M.; Jiang, O.; Pan, Y.; Hu, D.; Lin, Q.; Wu, G.; Cui, J.; Chang, J.; Cheng, Y.; et al. Sugemalimab versus Placebo after Concurrent or Sequential Chemoradiotherapy in Patients with Locally Advanced, Unresectable, Stage III Non-Small-Cell Lung Cancer in China (GEMSTONE-301): Interim Results of a Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 209–219. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.-W.; Costa, E.C.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Modi, C.; Berim, L.; Isserow, L.; Malhotra, J.; Patel, M.; Langenfeld, J.; Aisner, J.; Almeldin, D.; Jabbour, S.K. Combining Radiation Therapy and Immunotherapy for Lung Cancers: A Narrative Review. Shanghai Chest 2021, 5, 10. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory Microenvironment Remodelling by Tumour Cells after Radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Z.; Bai, M.; Yan, Y.; Yu, J.; Xu, Y. Radiation Combined with Immune Checkpoint Inhibitors for Unresectable Locally Advanced Non-Small Cell Lung Cancer: Synergistic Mechanisms, Current State, Challenges, and Orientations. Cell Commun. Signal. 2023, 21, 119. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Giobbie-Hurder, A.; Ranasinghe, S.; Kao, K.Z.; Lako, A.; Tsuji, J.; Liu, Y.; Brennick, R.C.; Gentzler, R.D.; Lee, C.; et al. Durvalumab plus Tremelimumab Alone or in Combination with Low-Dose or Hypofractionated Radiotherapy in Metastatic Non-Small-Cell Lung Cancer Refractory to Previous PD(L)-1 Therapy: An Open-Label, Multicentre, Randomised, Phase 2 Trial. Lancet Oncol. 2022, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.M.E.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.G.J.V.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without Radiotherapy for Metastatic Non-Small-Cell Lung Cancer: A Pooled Analysis of Two Randomised Trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 391, 1313–1327. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Yamada, T.; Uchino, J.; Chihara, Y.; Shimamoto, T.; Iwasaku, M.; Tamiya, N.; Kaneko, Y.; Kiyomi, F.; Takayama, K. Rationale and Design of a Phase II Trial of Durvalumab Treatment in Patients with NSCLC Ineligible for Stage III Chemoradiotherapy Following Radiation Monotherapy (SPIRAL-RT Study). Ther. Adv. Med. Oncol. 2020, 12, 1758835920927841. [Google Scholar] [CrossRef]
- Yamada, T.; Goto, Y.; Tanaka, H.; Kimura, H.; Minato, K.; Gyotoku, H.; Honda, T.; Watanabe, S.; Morimoto, K.; Kiyomi, F.; et al. A Phase 2 Trial of Durvalumab Treatment Following Radiation Monotherapy in Patients with Non-Small Cell Lung Cancer Ineligible for Stage III Chemoradiotherapy: The SPIRAL-RT Study. Eur. J. Cancer 2023, 195, 113373. [Google Scholar] [CrossRef]
- Ohri, N.; Jolly, S.; Cooper, B.T.; Kabarriti, R.; Bodner, W.R.; Klein, J.; Guha, C.; Viswanathan, S.; Shum, E.; Sabari, J.K.; et al. Selective Personalized RadioImmunotherapy for Locally Advanced Non–Small-Cell Lung Cancer Trial (SPRINT). J. Clin. Oncol. 2024, 42, 562–570. [Google Scholar] [CrossRef]
- Tachihara, M.; Tsujino, K.; Ishihara, T.; Hayashi, H.; Sato, Y.; Kurata, T.; Sugawara, S.; Shiraishi, Y.; Teraoka, S.; Azuma, K.; et al. Durvalumab Plus Concurrent Radiotherapy for Treatment of Locally Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2023, 9, 1505–1513. [Google Scholar] [CrossRef]
- Filippi, A.R.; Dziadziuszko, R.; Campelo, M.R.G.; Paoli, J.-B.; Sawyer, W.; Pérez, I.E.D. DUART: Durvalumab after Radiotherapy in Patients with Unresectable, Stage III NSCLC Who Are Ineligible for Chemotherapy. Future Oncol. 2021, 17, 4657–4663. [Google Scholar] [CrossRef]
- Filippi, A.R.R.; García-Campelo, M.R.; Paoli, J.-B.; Kowalski, D.; Bennati, C.; Borghetti, P.; Cortinovis, D.L.; Delmonte, A.; Genova, C.; Hulst, S.V.; et al. LBA62 Durvalumab after Radiotherapy (RT) in Patients with Unresectable Stage III NSCLC Ineligible for Chemotherapy (CT): Primary Results from the DUART Study. Ann. Oncol. 2023, 34, S1302. [Google Scholar] [CrossRef]
- Filippi, A.R.; Campelo, M.R.G.; Paoli, J.-B.; Kowalski, D.; Bennati, C.; Borghetti, P.; Cortinovis, D.; Delmonte, A.; Genova, C.; Mroz, R.; et al. 66MO Durvalumab after Radiotherapy (RT) in Patients (Pts) with Unresectable Stage III NSCLC Ineligible for Chemotherapy (CT): Final Analysis of the Phase II DUART Study. Immuno-Oncol. Technol. 2024, 24, 100809. [Google Scholar] [CrossRef]
- Filippi, A.R.; Garcia-Campelo, M.R.; Paoli, J.-B.; Kowalski, D.M.; Bennati, C.; Borghetti, P.; Cortinovis, D.L.; Delmonte, A.; Genova, C.; Mroz, R.M.; et al. LBA51 Circulating Tumor DNA (CtDNA) Dynamics and Treatment Responses in Chemotherapy-Ineligible Patients (Pts) with Unresectable Stage III NSCLC from the Phase II DUART Trial. Ann. Oncol. 2024, 35, S1241. [Google Scholar] [CrossRef]
- Lebow, E.S.; Fitzgerald, K.J.; Shaverdian, N.; Kotecha, R.; Gomez, D.R.; Wu, A.J.; Gelblum, D.; Shepherd, A.F.; Simone, C.B.; Zhang, Z.; et al. Phase II Trial of Concurrent Durvalumab and Radiation Therapy for Locally Advanced Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S57–S58. [Google Scholar] [CrossRef]
- Rimner, A.; Fitzgerald, K.; Iqbal, A.N.; Shepherd, A.F.; Gomez, D.R.; Shin, J.Y.; Gelblum, D.Y.; Hajj, C.; Albrecht, F.; Kotecha, R.R.; et al. EP05.01-025 Planned Interim Analysis of a Phase II Trial of Concurrent Durvalumab and Radiation Therapy for Locally Advanced Lung Cancer. J. Thorac. Oncol. 2022, 17, S278. [Google Scholar] [CrossRef]
- Zhang, Y.; Iyengar, P.; Montalvo, S.; Westover, K.D.; Rashdan, S.; Donthireddy, K.; Kim, J.; Dowell, J.E.; Drapkin, B.; Bhalla, S.; et al. Concerning Safety and Efficacy of Concurrent and Consolidative Durvalumab with Thoracic Radiation Therapy in PDL1-Unselected Stage III Non-Small Cell Lung Cancer: Brief Report. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 68–74. [Google Scholar] [CrossRef]
- Lin, S.H.; Pugh, S.L.; Tsao, A.S.; Edelman, M.J.; Doemer, A.; Simone, C.B.; Gandhi, S.; Bikkina, S.; Karim, N.F.A.; Shen, X.; et al. Safety Results of NRG-LU004: Phase I Trial of Accelerated or Conventionally Fractionated Radiotherapy Combined with Durvalumab in PD-L1–High Locally Advanced Non-Small Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 8513. [Google Scholar] [CrossRef]
- Iyengar, P.; Zhang-Velten, E.; Court, L.; Westover, K.; Yan, Y.; Lin, M.-H.; Xiong, Z.; Patel, M.; Rivera, D.; Chang, J.; et al. Accelerated Hypofractionated Image-Guided vs Conventional Radiotherapy for Patients with Stage II/III Non–Small Cell Lung Cancer and Poor Performance Status. JAMA Oncol. 2021, 7, 1497. [Google Scholar] [CrossRef]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T.; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus Chemotherapy with or without Surgical Resection for Stage III Non-Small-Cell Lung Cancer: A Phase III Randomised Controlled Trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef]
- Ortland, I.; Ott, M.M.; Kowar, M.; Sippel, C.; Jaehde, U.; Jacobs, A.H.; Ko, Y.-D. Comparing the Performance of the CARG and the CRASH Score for Predicting Toxicity in Older Patients with Cancer. J. Geriatr. Oncol. 2020, 11, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; Wiel, B.A.V.D.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non–Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Chae, Y.K.; Pan, A.; Davis, A.A.; Raparia, K.; Mohindra, N.A.; Matsangou, M.; Giles, F.J. Biomarkers for PD-1/PD-L1 Blockade Therapy in Non–Small-Cell Lung Cancer: Is PD-L1 Expression a Good Marker for Patient Selection? Clin. Lung Cancer 2016, 17, 350–361. [Google Scholar] [CrossRef]
- Willis, C.; Bauer, H.; Au, T.H.; Menon, J.; Unni, S.; Tran, D.; Rivers, Z.; Akerley, W.; Schabath, M.B.; Badin, F.; et al. Real-World Survival Analysis by Tumor Mutational Burden in Non-Small Cell Lung Cancer: A Multisite U.S. Study. Oncotarget 2022, 13, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Carbone, D.P.; Li, G.; Schrock, A.; Graf, R.P.; Zhang, L.; Murugesan, K.; Ross, J.S.; Tolba, K.; Sands, J.; et al. Durable Responders in Advanced NSCLC with Elevated TMB and Treated with 1L Immune Checkpoint Inhibitor: A Real-World Outcomes Analysis. J. Immunother. Cancer 2023, 11, e005801. [Google Scholar] [CrossRef]
- Lebow, E.S.; Shepherd, A.; Eichholz, J.E.; Offin, M.; Gelblum, D.Y.; Wu, A.J.; Simone, C.B.; Schoenfeld, A.J.; Jones, D.R.; Rimner, A.; et al. Analysis of Tumor Mutational Burden, Progression-Free Survival, and Local-Regional Control in Patents with Locally Advanced Non–Small Cell Lung Cancer Treated with Chemoradiation and Durvalumab. JAMA Netw. Open 2023, 6, e2249591. [Google Scholar] [CrossRef]
- Li, N.; Wan, Z.; Lu, D.; Chen, R.; Ye, X. Long-Term Benefit of Immunotherapy in a Patient with Squamous Lung Cancer Exhibiting Mismatch Repair Deficient/High Microsatellite Instability/High Tumor Mutational Burden: A Case Report and Literature Review. Front. Immunol. 2023, 13, 1088683. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T.; Wang, J.; Wang, J.; Xu, Y.; Zhao, X.; Ou, Q.; Shao, Y.; Wang, X.; Wu, Y.; et al. The Clinical Utility of Dynamic CtDNA Monitoring in Inoperable Localized NSCLC Patients. Mol. Cancer 2022, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Lebow, E.S.; Shaverdian, N.; Eichholz, J.E.; Kratochvil, L.B.; McCune, M.; Murciano-Goroff, Y.R.; Jee, J.; Eng, J.; Chaft, J.E.; Kris, M.G.; et al. CtDNA-Based Detection of Molecular Residual Disease in Stage I-III Non-Small Cell Lung Cancer Patients Treated with Definitive Radiotherapy. Front. Oncol. 2023, 13, 1253629. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.; Lebow, E.S.; Yeh, R.; Das, J.P.; Namakydoust, A.; Paik, P.K.; Chaft, J.E.; Jayakumaran, G.; Brannon, A.R.; Benayed, R.; et al. Overall Survival with Circulating Tumor DNA-Guided Therapy in Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 2353–2363. [Google Scholar] [CrossRef]
- Moding, E.J.; Liu, Y.; Nabet, B.Y.; Chabon, J.J.; Chaudhuri, A.A.; Hui, A.B.; Bonilla, R.F.; Ko, R.B.; Yoo, C.H.; Gojenola, L.; et al. Circulating Tumor DNA Dynamics Predict Benefit from Consolidation Immunotherapy in Locally Advanced Non-Small-Cell Lung Cancer. Nat. Cancer 2020, 1, 176–183. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Martin, T.K.; Dinerman, A.; Sudhaman, S.; Budde, G.; Palsuledesai, C.C.; Krainock, M.; Liu, M.C.; Smith, E.; Tapias, L.; Podgaetz, E.; et al. Early Real-World Experience Monitoring Circulating Tumor DNA in Resected Early-Stage Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2024, 168, 1349–1359.e2. [Google Scholar] [CrossRef]
- Bradley, J.D.; Hu, C.; Komaki, R.R.; Masters, G.A.; Blumenschein, G.R.; Schild, S.E.; Bogart, J.A.; Forster, K.M.; Magliocco, A.M.; Kavadi, V.S.; et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 706–714. [Google Scholar] [CrossRef]
- Jin, J.-Y.; Hu, C.; Xiao, Y.; Zhang, H.; Paulus, R.; Ellsworth, S.G.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Kavadi, V.S.; et al. Higher Radiation Dose to the Immune Cells Correlates with Worse Tumor Control and Overall Survival in Patients with Stage III NSCLC: A Secondary Analysis of RTOG0617. Cancers 2021, 13, 6193. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, G.; Ye, L.; Shi, S.; Du, S.; Zeng, Z.; He, J. Treatment-Duration Is Related to Changes in Peripheral Lymphocyte Counts during Definitive Radiotherapy for Unresectable Stage III NSCLC. Radiat. Oncol. 2019, 14, 86. [Google Scholar] [CrossRef]
- Chen, K.; Kalaghchi, B.; Caruthers, D.; Robinson, C.G.; Waqar, S.; Morgensztern, D.; Govindan, R.; Huang, Y.; Bergom, C.; Samson, P.; et al. Treatment-Associated Lymphopenia from Hypofractionated vs. Conventional Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, e12. [Google Scholar] [CrossRef]
- Ohri, N.; Bodner, W.R.; Kabarriti, R.; Shankar, V.; Gucalp, R.; Cheng, H.; Halmos, B. Randomized Evaluation of the PET-Adjusted IMRT for NSCLC Trial (REPAINT). Int. J. Radiat. Oncol. Biol. Phys. 2025, in press. [Google Scholar] [CrossRef]
- Mauguen, A.; Péchoux, C.L.; Saunders, M.I.; Schild, S.E.; Turrisi, A.T.; Baumann, M.; Sause, W.T.; Ball, D.; Belani, C.P.; Bonner, J.A.; et al. Hyperfractionated or Accelerated Radiotherapy in Lung Cancer: An Individual Patient Data Meta-Analysis. J. Clin. Oncol. 2012, 30, 2788–2797. [Google Scholar] [CrossRef]
- Baumann, M.; Herrmann, T.; Koch, R.; Matthiessen, W.; Appold, S.; Wahlers, B.; Kepka, L.; Marschke, G.; Feltl, D.; Fietkau, R.; et al. Final Results of the Randomized Phase III CHARTWEL-Trial (ARO 97-1) Comparing Hyperfractionated-Accelerated versus Conventionally Fractionated Radiotherapy in Non-Small Cell Lung Cancer (NSCLC). Radiother. Oncol. 2011, 100, 76–85. [Google Scholar] [CrossRef]
- Belani, C.P.; Wang, W.; Johnson, D.H.; Wagner, H.; Schiller, J.; Veeder, M.; Mehta, M.; Group, E.C.O. Phase III Study of the Eastern Cooperative Oncology Group (ECOG 2597): Induction Chemotherapy Followed by Either Standard Thoracic Radiotherapy or Hyperfractionated Accelerated Radiotherapy for Patients with Unresectable Stage IIIA and B Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 3760–3767. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, J.; Uitterhoeve, L.; Zandwijk, N.V.; Belderbos, H.; Rodrigus, P.; van de Vaart, P.; Price, A.; van Walree, N.; Legrand, C.; Dussenne, S.; et al. Randomised Trial of Sequential versus Concurrent Chemo-Radiotherapy in Patients with Inoperable Non-Small Cell Lung Cancer (EORTC 08972-22973). Eur. J. Cancer 2007, 43, 114–121. [Google Scholar] [CrossRef]
- Said, B.I.; Geng, Y.; Badiyan, S.N.; Bang, A.; Bezjak, A.; Chua, K.L.M.; Faivre-Finn, C.; Kong, F.-M.; Przybysz, D.; Putora, P.M.; et al. Accelerated Hypofractionated Radiotherapy for Locally-Advanced Non-Small Cell Lung Cancer: A Systematic Review from the International Association for the Study of Lung Cancer (IASLC) Advanced Radiation Technology (ART) Subcommittee. J. Thorac. Oncol. 2024, 20, 39–51. [Google Scholar] [CrossRef]
- Simone, C.B.; Hu, C.; Heinzerling, J.H.; Mileham, K.; Higgins, K.A.; Lin, L.; Abazeed, M.; Ohri, N.; Bradley, J.D. NRG LU008: Phase III Prospective Randomized Trial of Primary Lung Tumor Stereotactic Body Radiation Therapy (SBRT) Followed by Concurrent Mediastinal Chemoradiation for Locally-Advanced Non-Small Cell Lung Cancer (LA-NSCLC). Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, e63. [Google Scholar] [CrossRef]
- Liao, Z.; Lee, J.J.; Komaki, R.; Gomez, D.R.; O’Reilly, M.S.; Fossella, F.V.; Jr, G.R.B.; Heymach, J.V.; Vaporciyan, A.A.; Swisher, S.G.; et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 1813–1822. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy Combined with Immunotherapy: The Dawn of Cancer Treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Kumagai, S.; Togashi, Y.; Kamada, T.; Sugiyama, E.; Nishinakamura, H.; Takeuchi, Y.; Vitaly, K.; Itahashi, K.; Maeda, Y.; Matsui, S.; et al. The PD-1 Expression Balance between Effector and Regulatory T Cells Predicts the Clinical Efficacy of PD-1 Blockade Therapies. Nat. Immunol. 2020, 21, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; Menezes, J.J.D.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Majem, M.; Barlesi, F.; Carcereny, E.; Chu, Q.; Monnet, I.; Sanchez-Hernandez, A.; Dakhil, S.; Camidge, D.R.; Winzer, L.; et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination with Oleclumab or Monalizumab in Patients with Unresectable, Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; Kar, G.; Spicer, J.D.; Garcia-Campelo, R.; Weder, W.; Daniel, D.B.; Spigel, D.R.; Hussein, M.; Mazieres, J.; Oliveira, J.; et al. Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase 2 NeoCOAST Platform Trial. Cancer Discov. 2023, 13, 2394–2411. [Google Scholar] [CrossRef]
- Liveringhouse, C.L.; Latifi, K.; Asous, A.G.; Lam, N.B.; Rosenberg, S.A.; Dilling, T.J.; MacMillan, G.V.; Chiappori, A.A.; Haura, E.B.; Creelan, B.; et al. Dose-Limiting Pulmonary Toxicity in a Phase 1/2 Study of Radiation and Chemotherapy with Ipilimumab Followed by Nivolumab for Patients with Stage 3 Unresectable Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 837–848. [Google Scholar] [CrossRef]
| Trial Name | Phase | Primary Endpoint(s) | Immunotherapy Schedule and Timing | RT Schedule | Patient/Biomarker Selection | Status |
|---|---|---|---|---|---|---|
| NCT04013542 | I | Safety and feasibility | Nivolumab, every three weeks for up to 8 cycles, and ipilimumab every six weeks for up to 4 cycles, both starting with RT. | 60 Gy in 30 fractions | Ineligible for concurrent chemoradiotherapy | Active, not recruiting |
| NCT03818776 (PARTICLE-D) | I | Safety | Durvalumab, every four weeks for one year, starting one week before RT | Arm 1: 60–69 CGyE in 30 fractions (proton radiotherapy) | Ineligible for concurrent chemoradiotherapy | Terminated |
| NCT05451173 | I/II | Safety, PFS | Durvalumab, every four weeks for one year, starting concurrently with RT. | 3.5–4.0 Gy × 15 fractions | PD-L1 TPS ≥ 1% (“preferred”) | Not yet recruiting |
| NCT04577638(AIRING) | II | DCR * | Nivolumab, every two weeks for six months, starting concurrently with RT. | 66 Gy in 24 fractions | ≥1 of several “fragility criteria”, which include ECOG performance status 2 and age > 74 | Recruiting |
| NCT04310020(SWOG S1933) | II | PFS | Atezolizumab, every three weeks for one year, starting after RT | 60 Gy in 15 fractions | Ineligible for concurrent chemoradiotherapy | Recruiting |
| NCT04351256(Trade-HYPO) | II | Safety and feasibility | Durvalumab, every four weeks for one year, starting concurrently with RT. | 55 Gy in 20 fractions | Ineligible for concurrent chemoradiotherapy | Recruitiing |
| NCT06865339 | II | ORR † to induction immuno-therapy | Cemiplimab and Fianlimab, every three weeks, before RT (3 cycles) and after RT (13 cycles) | Risk-adapted conventional fractionation | Low PD-L1 TPS (<50%) | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozair, M.Z.; Halmos, B.; D’Aiello, A.; Yun, J.; Filippi, A.R.; Rimner, A.; Lin, S.H.; Simone, C.B., II; Ohri, N. Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer. Cancers 2025, 17, 1524. https://doi.org/10.3390/cancers17091524
Ozair MZ, Halmos B, D’Aiello A, Yun J, Filippi AR, Rimner A, Lin SH, Simone CB II, Ohri N. Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer. Cancers. 2025; 17(9):1524. https://doi.org/10.3390/cancers17091524
Chicago/Turabian StyleOzair, M. Zeeshan, Balazs Halmos, Angelica D’Aiello, Jaewon Yun, Andrea R. Filippi, Andreas Rimner, Steven H. Lin, Charles B. Simone, II, and Nitin Ohri. 2025. "Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer" Cancers 17, no. 9: 1524. https://doi.org/10.3390/cancers17091524
APA StyleOzair, M. Z., Halmos, B., D’Aiello, A., Yun, J., Filippi, A. R., Rimner, A., Lin, S. H., Simone, C. B., II, & Ohri, N. (2025). Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer. Cancers, 17(9), 1524. https://doi.org/10.3390/cancers17091524








