Musculoskeletal Tumor Care Disparities Prior to Initiation of Treatment Among Newly Diagnosed Adult Patients
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Definition of High-Grade Sarcoma Versus Other Histology
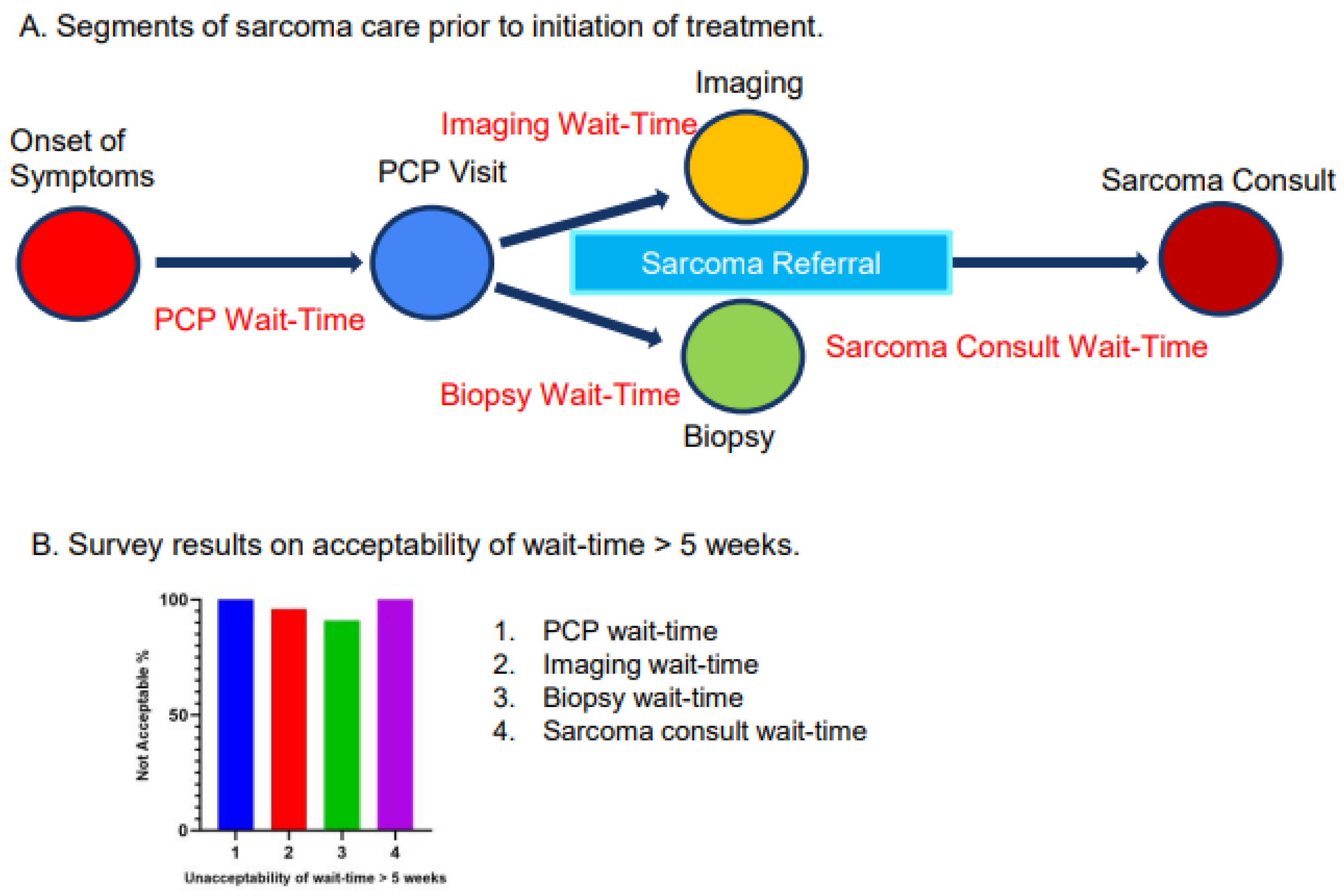
2.3. Definition of Segments of MST Care Prior to Initiation of Treatment
2.4. Insurance Type
2.5. Distances from Stanford Medical Center
2.6. Survey Questionnaires
2.7. Statistical Analysis
3. Results
3.1. Demographics
3.2. Survey Results
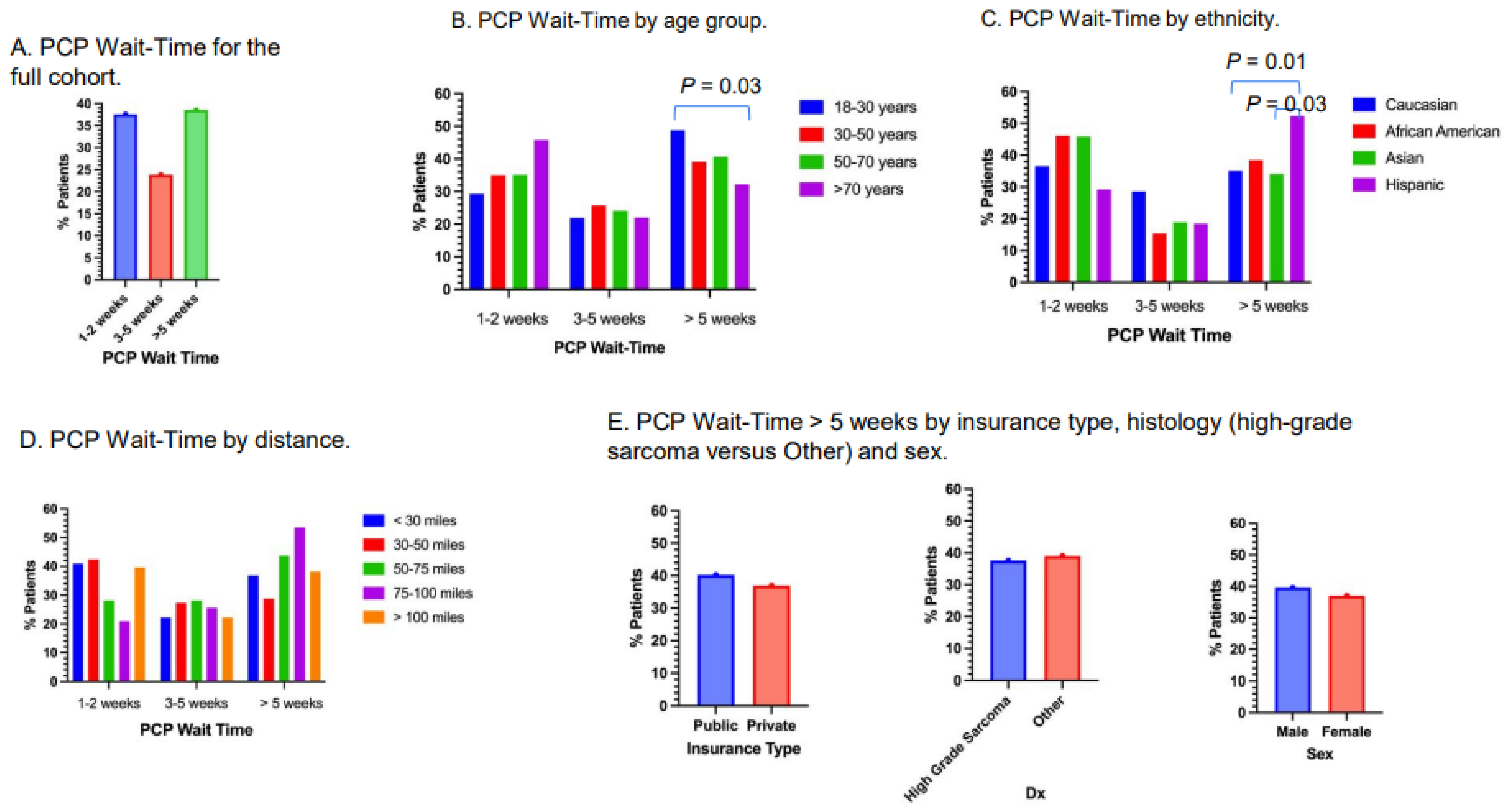
3.3. PCP Wait-Time
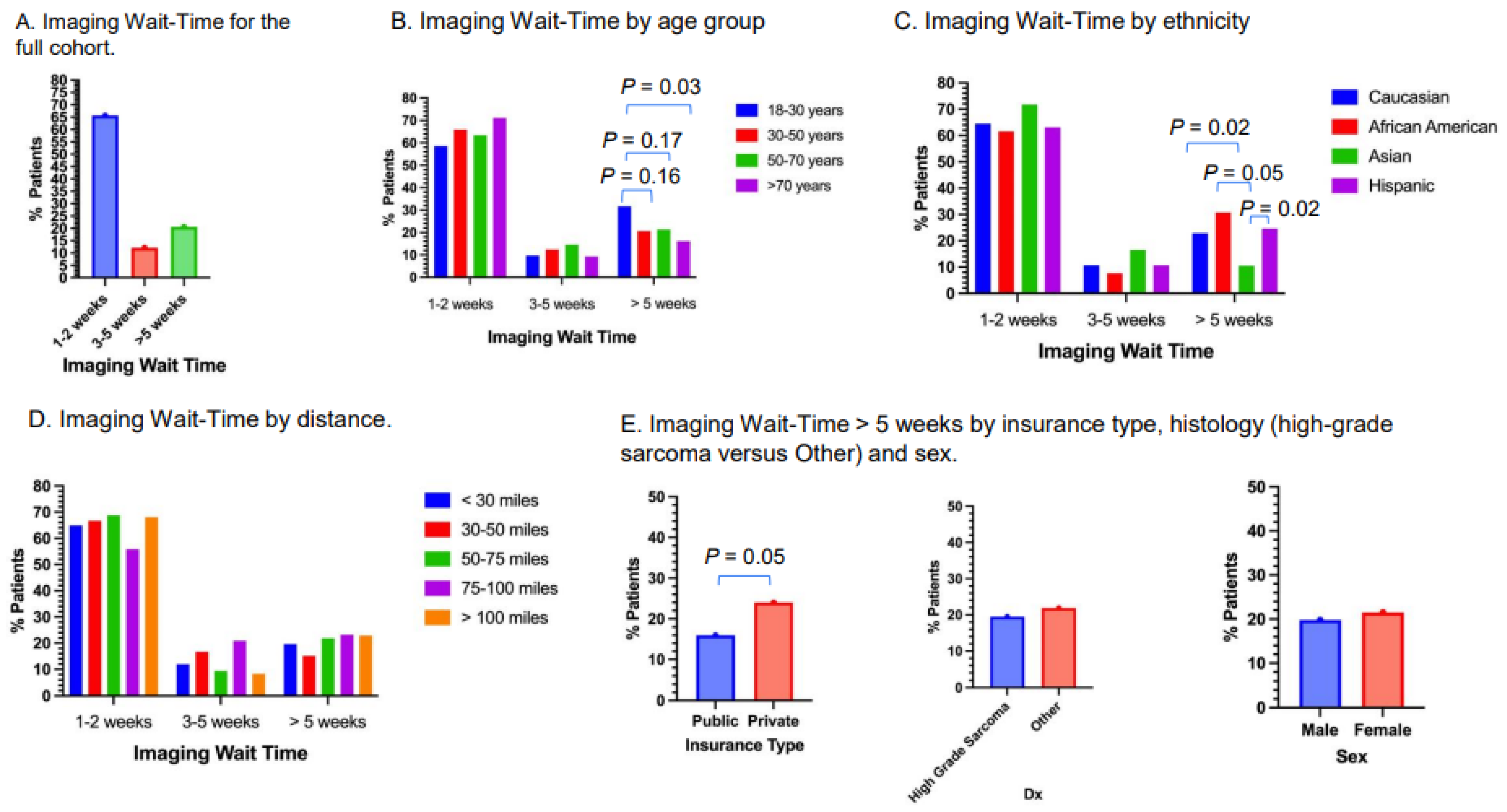
3.4. Imaging Wait-Time
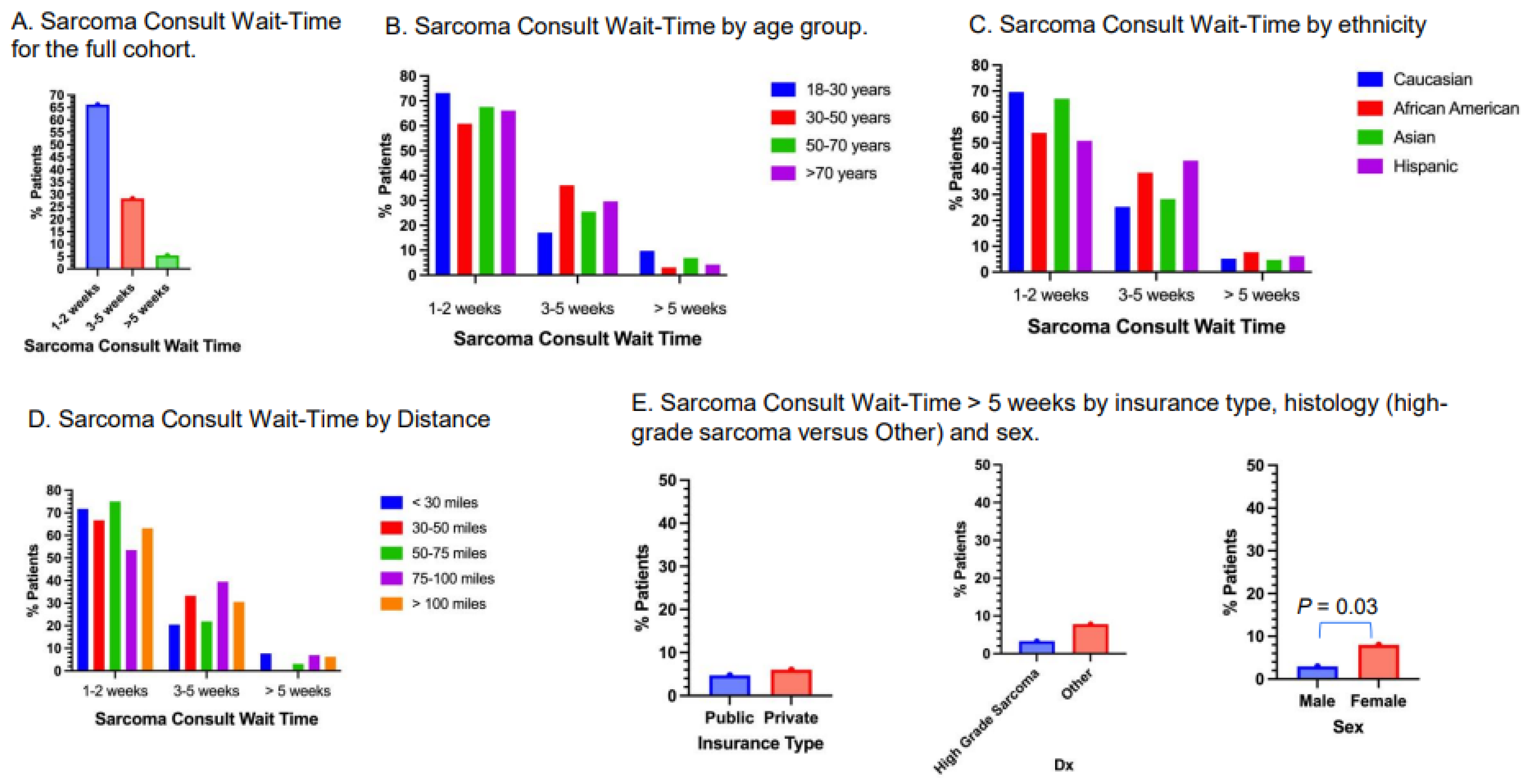
3.5. Sarcoma Consult Wait-Time
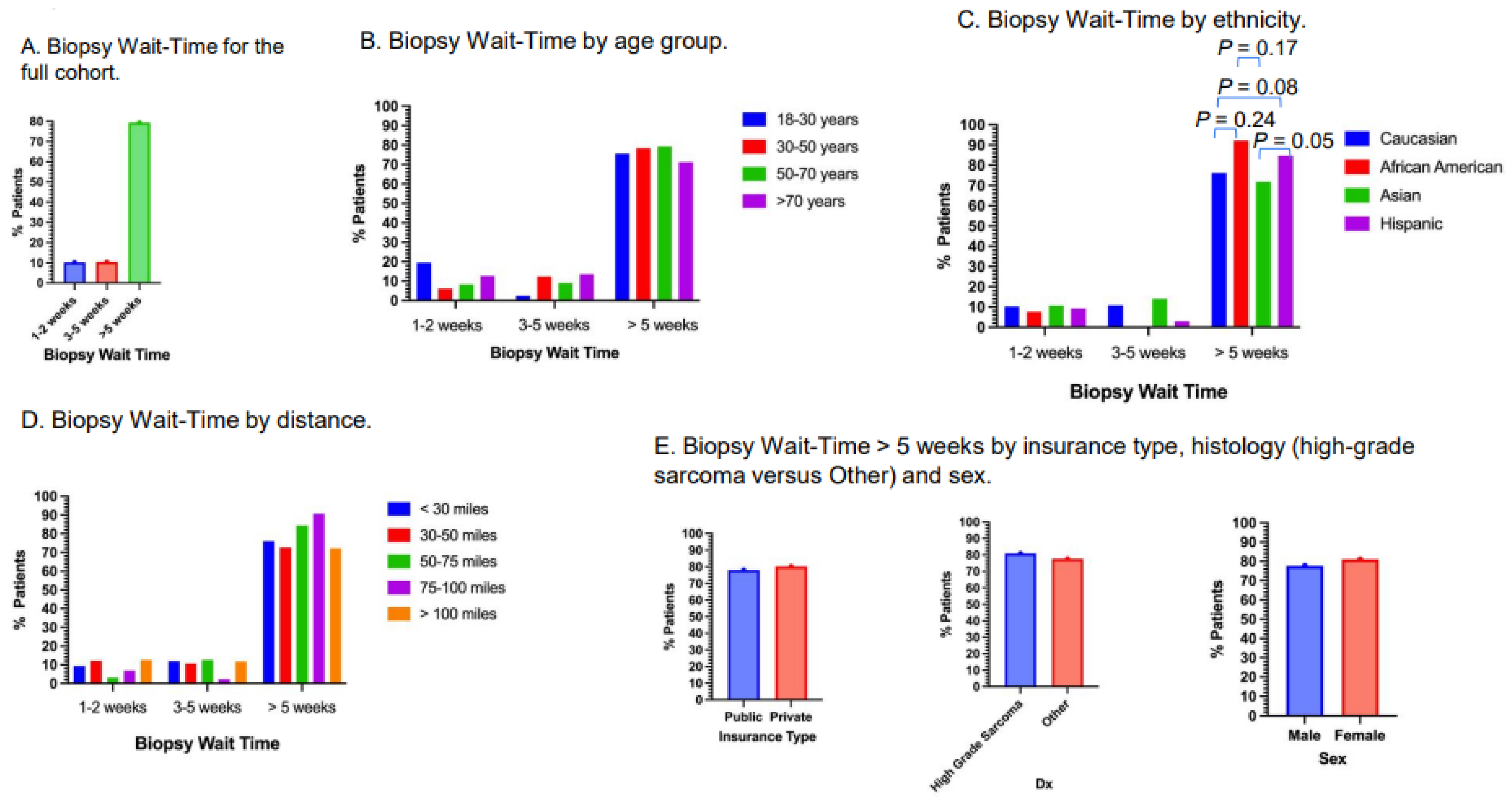
3.6. Biopsy Wait-Time
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcaraz, K.I.; Wiedt, T.L.; Daniels, E.C.; Yabroff, K.R.; Guerra, C.E.; Wender, R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA A Cancer J. Clin. 2020, 70, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Esnaola, N.F.; Ford, M.E. Racial Differences and Disparities in Cancer Care and Outcomes: Where’s the Rub? Surg. Oncol. Clin. N. Am. 2012, 21, 417–437. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Biermann, J.S.; Siegel, G.W.; Chugh, R.; Jacobson, J.; Lucas, D.; Feng, M.; Chang, A.C.; Smith, S.R.; Wong, S.; Hasen, J.L. The multidisciplinary management of bone and soft tissue sarcoma: An essential organizational framework. J. Multidiscip. Health 2015, 8, 109–115. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA A Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- Helman, L.J.; Meltzer, P. Mechanisms of sarcoma development. Nat. Rev. Cancer 2003, 3, 685–694. [Google Scholar] [CrossRef]
- Llanos, A.A.M.; Ashrafi, A.; Ghosh, N.; Tsui, J.; Lin, Y.; Fong, A.J.; Ganesan, S.; Heckman, C.J. Evaluation of Inequities in Cancer Treatment Delay or Discontinuation Following SARS-CoV-2 Infection. JAMA Netw. Open 2023, 6, e2251165. [Google Scholar] [CrossRef]
- Dunlop, H.M.; Bende, B.; Ruff, S.M.; Kim, A.; Fisher, J.L.; Grignol, V.P.; Contreras, C.M.; Obeng-Gyasi, S.; Konieczkowski, D.J.; Pawlik, T.M.; et al. Disparities in Survival and NCCN Guideline–Concordant Care in Patients with Extremity Soft Tissue Sarcoma. J. Natl. Compr. Cancer Netw. 2024, 22, 26–33. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Seto, T.; Yu, J.; Sidhu, M.; Kim, B.; McCormick, C.; Fang, A.; Song, J.; Morse, L.J.; Peng, P.D.; et al. Feasibility and Value of Establishing a Community-Based Virtual Multidisciplinary Sarcoma Case Conference. JCO Oncol. Pract. 2020, 16, e1143–e1150. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Yu, J.; Sidhu, M.; Seto, T.; Fang, A. Impact of a Virtual Multidisciplinary Sarcoma Case Conference on Treatment Plan and Survival in a Large Integrated Healthcare System. JCO Oncol. Pract. 2021, 17, e1711–e1718. [Google Scholar] [CrossRef] [PubMed]
- Alcindor, T.; Dumitra, S.; Albritton, K.; Thomas, D.M. Disparities in Cancer Care: The Example of Sarcoma—In Search of Solutions for a Global Issue. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 405–411. [Google Scholar] [CrossRef]
- Bhatia, S. Disparities in cancer outcomes: Lessons learned from children with cancer. Pediatr. Blood Cancer 2011, 56, 994–1002. [Google Scholar] [CrossRef]
- Zappettini, E. Global Disparities in Surgical Oncology: Bridging the Gap in Cancer Care a Challenge to Overcome. Clin. Surg. Oncol. 2024, 3, 100060. [Google Scholar] [CrossRef]
- Blank, A.T.; Takemoto, R.C.; Patel, N.M.; Baig, A.; Rapp, T.B. Sarcoma Care in an Urban Health-care System: Which Factors Lead to Variance of Care? J. Racial Ethn. Health Disparities 2014, 1, 135–139. [Google Scholar] [CrossRef]
- Pan, M.; Fang, A.; Kavanagh, M.; Kim, B.; Lee, J.D.; McCormick, C.; Morse, L.J.; Nag, S.; Nybbaken, G.; Peng, P.; et al. Improving care quality for patients with soft tissue and bone sarcoma by establishing a national virtual tumor board and electronic consultation platform. J. Clin. Oncol. 2016, 34 (Suppl. S7), 211. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Soibinet, P.; Penel, N.; Bompas, E.; Duffaud, F.; Stoeckle, E.; Mir, O.; Adam, J.; Chevreau, C.; Bonvalot, S.; et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann. Oncol. 2017, 28, 2852–2859. [Google Scholar] [CrossRef]
- Lazarides, A.L.; Visgauss, J.D.; Nussbaum, D.P.; Green, C.L.; Blazer, D.G.; Brigman, B.E.; Eward, W.C. Race is an independent predictor of survival in patients with soft tissue sarcoma of the extremities. BMC Cancer 2018, 18, 488. [Google Scholar] [CrossRef]
- Alamanda, V.K.; Song, Y.; Schwartz, H.S.; Holt, G.E. Racial Disparities in Extremity Soft-Tissue Sarcoma Outcomes: A Nationwide Analysis. Am. J. Clin. Oncol. 2015, 38, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.J.; Lindholm, E.B.; Levy, C.F.; Fish, J.D.; Glick, R.D. Racial and ethnic disparities in treatment and survival of pediatric sarcoma. J. Surg. Res. 2017, 219, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.R.; Robbins, A.S.; Meyers, F.J.; Bold, R.J.; Khatri, V.P.; Goodnight, J.E. Racial and ethnic differences in treatment and survival among adults with primary extremity soft-tissue sarcoma. Cancer 2008, 112, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Nilbert, M. Difficult journeys in sarcoma care; socioeconomic disparity added to the multiple challenges of a rare tumor diagnosis. Acta Oncol. 2020, 59, 125–126. [Google Scholar] [CrossRef]
- Dhir, A.; Rahul, R.; Liu, Q.; Pham, D.; Kronenfeld, R.; Koru-Sengul, T.; Pinheiro, P.S. Disparities in incidence and survival for patients with Ewing sarcoma in Florida. Cancer Med. 2024, 13, e7151. [Google Scholar] [CrossRef]
- Graboyes, E.M.; Kompelli, A.R.; Neskey, D.M.; Brennan, E.; Nguyen, S.; Sterba, K.R.; Warren, G.W.; Hughes-Halbert, C.; Nussenbaum, B.; Day, T.A. Association of Treatment Delays with Survival for Patients with Head and Neck Cancer. Arch. Otolaryngol. Neck Surg. 2019, 145, 166–177. [Google Scholar] [CrossRef]
- Murphy, C.T.; Galloway, T.J.; Handorf, E.A.; Egleston, B.L.; Wang, L.S.; Mehra, R.; Flieder, D.B.; Ridge, J.A. Survival Impact of Increasing Time to Treatment Initiation for Patients with Head and Neck Cancer in the United States. J. Clin. Oncol. 2016, 34, 169–178. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Urbach, D.R.; Austin, P.C.; Fleshner, N.E.; Laupacis, A. Longer Wait Times Increase Overall Mortality in Patients with Bladder Cancer. J. Urol. 2009, 182, 1318–1324. [Google Scholar] [CrossRef]
- Eaglehouse, Y.L.; Georg, M.W.; Shriver, C.D.; Zhu, K. Time-to-surgery and overall survival after breast cancer diagnosis in a universal health system. Breast Cancer Res. Treat. 2019, 178, 441–450. [Google Scholar] [CrossRef]
- Bleicher, R.J.; Ruth, K.; Sigurdson, E.R.; Beck, J.R.; Ross, E.; Wong, Y.-N.; Patel, S.A.; Boraas, M.; Chang, E.I.; Topham, N.S.; et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016, 2, 330–339. [Google Scholar] [CrossRef]
- Keegan, T.H.; Ries, L.A.; Barr, R.D.; Geiger, A.M.; Dahlke, D.V.; Pollock, B.H.; Bleyer, W.A.; National Cancer Institute Next Steps for Adolescent and Young Adult Oncology Epidemiology Working Group. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer 2016, 122, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Seibel, N.L.; Altekruse, S.F.; Ries, L.A.; Melbert, D.L.; O’Leary, M.; Smith, F.O.; Reaman, G.H. Outcomes for Children and Adolescents with Cancer: Challenges for the Twenty-First Century. J. Clin. Oncol. 2010, 28, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Merchant, M.; Morse, L.J.; Fang, A.; Song, M.-N.; A Russell, E.; Pan, M. Pain as Initial Presenting Symptom Is Associated with Delay to Seeking Medical Attention, Higher Risk of Relapse, and Shorter Survival in Patients with Early-Stage Extremity or Trunk Synovial Sarcoma. Perm. J. 2022, 26, 94–102. [Google Scholar] [CrossRef]
- Pan, M.; Merchant, M. Risk Factors Including Age, Stage and Anatomic Location that Impact the Outcomes of Patients with Synovial Sarcoma. Med. Sci. 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Zhou, M.Y.; Jiang, C.; Zhang, Z.; Bui, N.Q.; Bien, J.; Siy, A.; Achacoso, N.; Solorzano, A.V.; Tse, P.; et al. Sex-dependent Prognosis of Patients with Advanced Soft Tissue Sarcoma. Clin. Cancer Res. 2023, 30, 413–419. [Google Scholar] [CrossRef]
- Diessner, B.J.; Weigel, B.J.; Murugan, P.; Zhang, L.; Poynter, J.N.; Spector, L.G. Associations of Socioeconomic Status, Public vs Private Insurance, and Race/Ethnicity with Metastatic Sarcoma at Diagnosis. JAMA Netw. Open 2020, 3, e2011087. [Google Scholar] [CrossRef]
- Johnson, G.D.; Smith, G.; Dramis, A.; Grimer, R.J. Delays in Referral of Soft Tissue Sarcomas. Sarcoma 2008, 2008, 378574. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Reiter, A.; Korthaus, A.; Frosch, K.-H.; Schlickewei, C.W.; Priemel, M.H. Diagnostic delay in soft tissue tumors: A single-center study of a university cancer center with a focus on health services research. BMC Health Serv. Res. 2022, 22, 452. [Google Scholar] [CrossRef]
- Weaver, R.; O’connor, M.; Smith, R.C.; Halkett, G.K. The complexity of diagnosing sarcoma in a timely manner: Perspectives of health professionals, patients, and carers in Australia. BMC Health Serv. Res. 2020, 20, 711. [Google Scholar] [CrossRef]
| All Patients (N = 402) | Percentage of All Patients (%) | ||
|---|---|---|---|
| Sex | |||
| Male | 202 | 50.25 | |
| Female | 200 | 49.75 | |
| Insurance Type | |||
| Public | 169 | 42.0 | |
| Private | 233 | 58.0 | |
| Histology | |||
| High-grade | 210 | 52.2 | |
| Other | Low-grade | 136 | 33.8 |
| Benign | 56 | 13.9 | |
| Age (years) | |||
| 18–30 | 40 | 9.9 | |
| 31–50 | 94 | 23.4 | |
| 51–70 | 139 | 34.6 | |
| >70 | 129 | 32.1 | |
| Ethnicity | |||
| Caucasians | 218 | 54.2 | |
| Asian | 83 | 20.6 | |
| Black | 13 | 3.2 | |
| Hispanic | 66 | 16.4 | |
| Other | 22 | 5.5 | |
| Distance (miles) | |||
| <30 | 118 | 29.3 | |
| 31–50 | 66 | 16.4 | |
| 51–75 | 32 | 8.0 | |
| 76–100 | 43 | 10.7 | |
| >100 | 143 | 35.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.J.; Bui, N.; Moding, E.J.; Steffner, R.; Mohler, D.; Ganjoo, K.; Pan, M. Musculoskeletal Tumor Care Disparities Prior to Initiation of Treatment Among Newly Diagnosed Adult Patients. Cancers 2025, 17, 1519. https://doi.org/10.3390/cancers17091519
Li LJ, Bui N, Moding EJ, Steffner R, Mohler D, Ganjoo K, Pan M. Musculoskeletal Tumor Care Disparities Prior to Initiation of Treatment Among Newly Diagnosed Adult Patients. Cancers. 2025; 17(9):1519. https://doi.org/10.3390/cancers17091519
Chicago/Turabian StyleLi, Lauren J., Nam Bui, Everett J. Moding, Robert Steffner, David Mohler, Kristen Ganjoo, and Minggui Pan. 2025. "Musculoskeletal Tumor Care Disparities Prior to Initiation of Treatment Among Newly Diagnosed Adult Patients" Cancers 17, no. 9: 1519. https://doi.org/10.3390/cancers17091519
APA StyleLi, L. J., Bui, N., Moding, E. J., Steffner, R., Mohler, D., Ganjoo, K., & Pan, M. (2025). Musculoskeletal Tumor Care Disparities Prior to Initiation of Treatment Among Newly Diagnosed Adult Patients. Cancers, 17(9), 1519. https://doi.org/10.3390/cancers17091519






