Targeting c-MET Alterations in Cancer: A Review of Genetic Drivers and Therapeutic Implications
Simple Summary
Abstract
1. c-MET Protein Structure
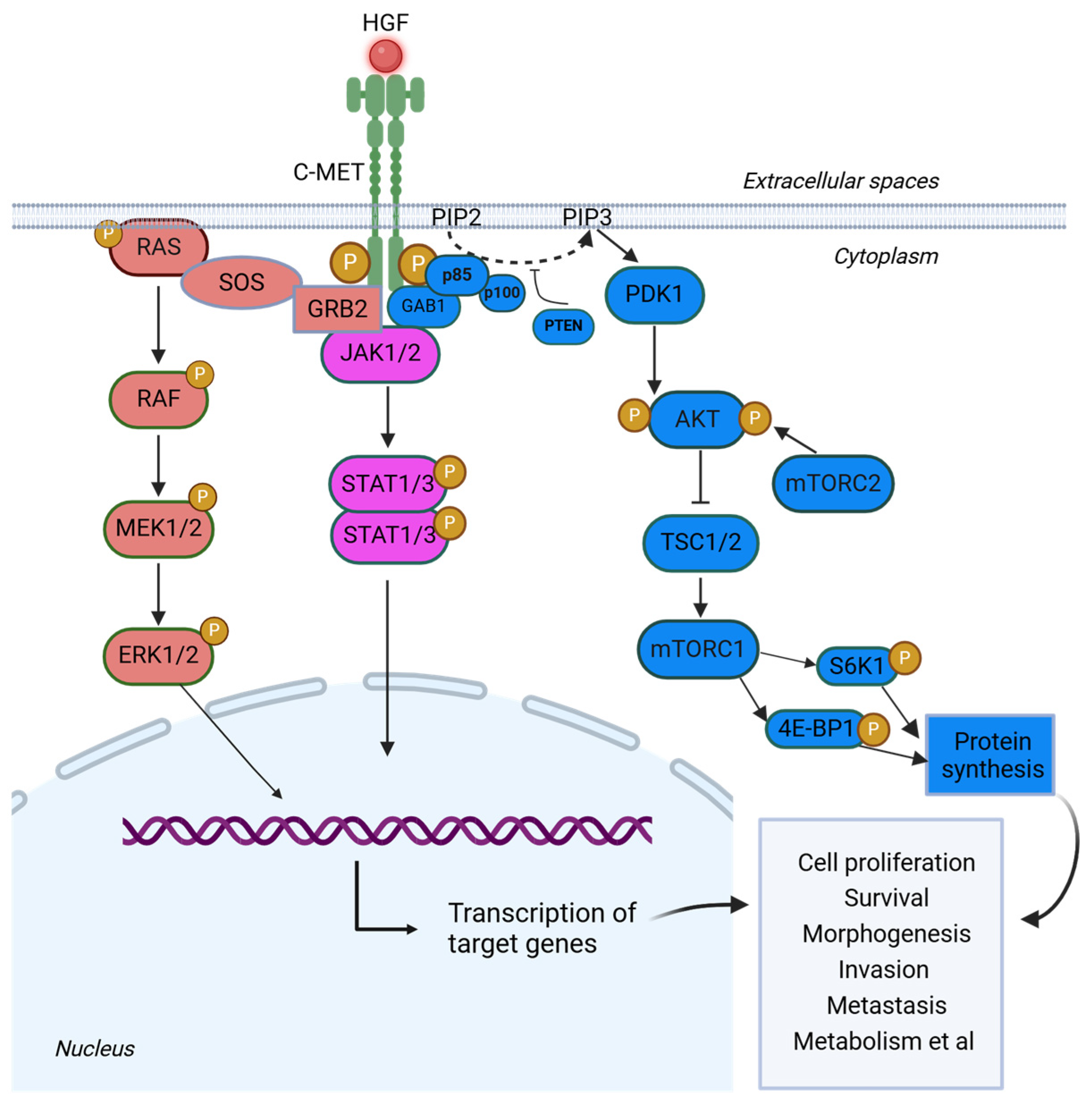
2. c-MET Signaling Pathway
3. Genomic Alterations of MET Gene
3.1. MET Gene Amplification
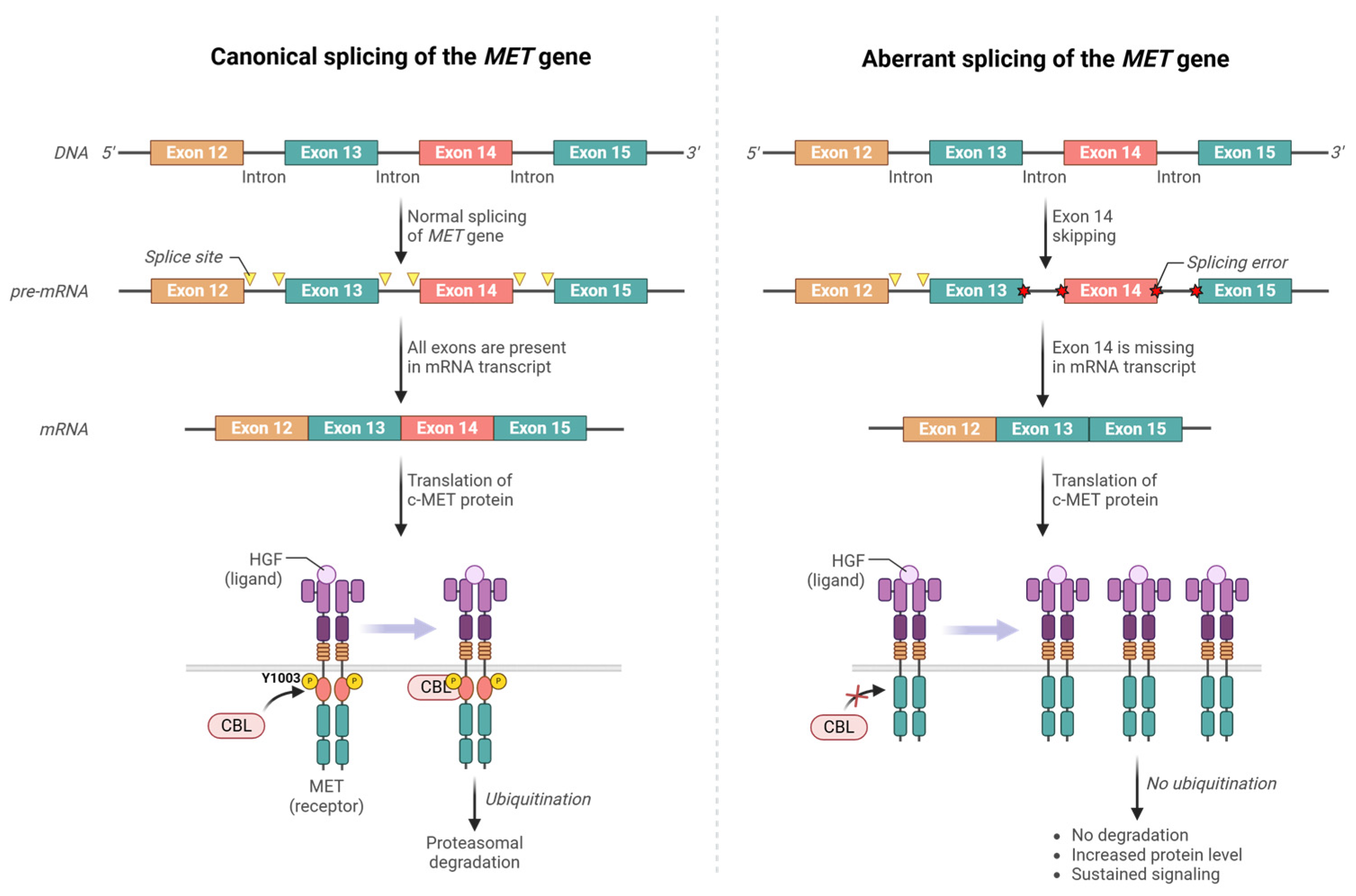
3.2. MET Exon 14 Skipping Mutations
3.3. MET Missense Mutations
3.4. MET Gene Fusions
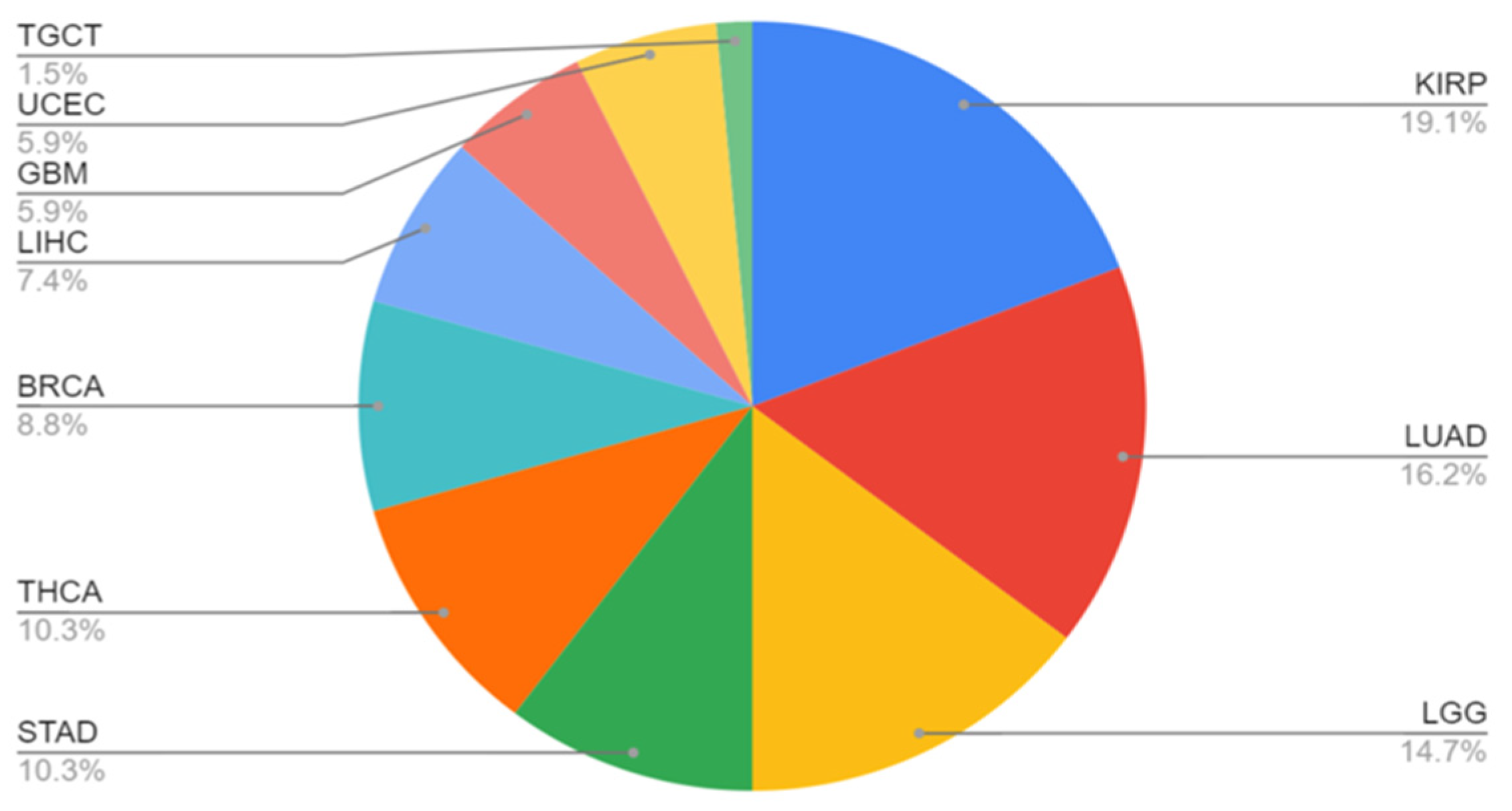
4. Variation in Tumor Profiles
5. Drug Treatment for MET Genomic Alterations
5.1. Crizotinib: A Multitarget Inhibitor
5.2. Capmatinib and Tepotinib: Selective Inhibitors
5.3. Type 2 and 3 Inhibitors
5.4. Antibody-Based Treatments
5.5. Mechanisms of Resistance
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cecchi, F.; Rabe, D.C.; Bottaro, D.P. The Hepatocyte Growth Factor Receptor: Structure, Function and Pharmacological Targeting in Cancer. Curr. Signal Transduct. Ther. 2011, 6, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.S.; Park, M.; Blair, D.G.; Tainsky, M.A.; Huebner, K.; Croce, C.M.; Vande Woude, G.F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984, 311, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, E.; Sandin, S.; Petoukhov, M.V.; Finch, J.; Youles, M.E.; Ofverstedt, L.G.; Miguel, R.N.; Blundell, T.L.; Vande Woude, G.F.; Skoglund, U.; et al. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc. Natl. Acad. Sci. USA 2006, 103, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Naldini, L.; Weidner, K.M.; Sachs, M.; Vigna, E.; Comoglio, P.M.; Birchmeier, W. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc. Natl. Acad. Sci. USA 1992, 89, 11574–11578. [Google Scholar] [CrossRef]
- Uchikawa, E.; Chen, Z.; Xiao, G.Y.; Zhang, X.; Bai, X.C. Structural basis of the activation of c-MET receptor. Nat. Commun. 2021, 12, 4074. [Google Scholar] [CrossRef]
- Antipenko, A.; Himanen, J.P.; van Leyen, K.; Nardi-Dei, V.; Lesniak, J.; Barton, W.A.; Rajashankar, K.R.; Lu, M.; Hoemme, C.; Puschel, A.W.; et al. Structure of the semaphorin-3A receptor binding module. Neuron 2003, 39, 589–598. [Google Scholar] [CrossRef]
- Love, C.A.; Harlos, K.; Mavaddat, N.; Davis, S.J.; Stuart, D.I.; Jones, E.Y.; Esnouf, R.M. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat. Struct. Biol. 2003, 10, 843–848. [Google Scholar] [CrossRef]
- Altintas, D.M.; Gallo, S.; Basilico, C.; Cerqua, M.; Bocedi, A.; Vitacolonna, A.; Botti, O.; Casanova, E.; Rancati, I.; Milanese, C.; et al. The PSI Domain of the MET Oncogene Encodes a Functional Disulfide Isomerase Essential for the Maturation of the Receptor Precursor. Int. J. Mol. Sci. 2022, 23, 12427. [Google Scholar] [CrossRef]
- Basilico, C.; Arnesano, A.; Galluzzo, M.; Comoglio, P.M.; Michieli, P. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J. Biol. Chem. 2008, 283, 21267–21277. [Google Scholar] [CrossRef]
- Vigna, E.; Gramaglia, D.; Longati, P.; Bardelli, A.; Comoglio, P.M. Loss of the exon encoding the juxtamembrane domain is essential for the oncogenic activation of. Oncogene 1999, 18, 4275–4281. [Google Scholar] [CrossRef]
- Cortot, A.B.; Kherrouche, Z.; Descarpentries, C.; Wislez, M.; Baldacci, S.; Furlan, A.; Tulasne, D. Exon 14 Deleted MET Receptor as a New Biomarker and Target in Cancers. Jnci-J. Natl. Cancer Inst. 2017, 109, djw262. [Google Scholar] [CrossRef]
- Nakayama, M.; Sakai, K.; Yamashita, A.; Nakamura, T.; Suzuki, Y.; Matsumoto, K. Met/HGF receptor activation is regulated by juxtamembrane Ser985 phosphorylation in hepatocytes. Cytokine 2013, 62, 446–452. [Google Scholar] [CrossRef]
- Garcia-Guzman, M.; Larsen, E.; Vuori, K. The proto-oncogene c-Cbl is a positive regulator of Met-induced MAP kinase activation: A role for the adaptor protein Crk. Oncogene 2000, 19, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Giordano, S.; Longati, P.; Medico, E.; Campiglio, M.; Comoglio, P.M. Structural and functional domains critical for constitutive activation of the HGF-receptor (Met). Oncogene 1994, 9, 1691–1697. [Google Scholar] [PubMed]
- Finisguerra, V.; Prenen, H.; Mazzone, M. Preclinical and clinical evaluation of MET functions in cancer cells and in the tumor stroma. Oncogene 2016, 35, 5457–5467. [Google Scholar] [CrossRef]
- Bardelli, A.; Longati, P.; Williams, T.A.; Benvenuti, S.; Comoglio, P.M. A peptide representing the carboxyl-terminal tail of the met receptor inhibits kinase activity and invasive growth. J. Biol. Chem. 1999, 274, 29274–29281. [Google Scholar] [CrossRef]
- Rivas, S.; Marín, A.; Samtani, S.; González-Feliú, E.; Armisén, R. MET Signaling Pathways, Resistance Mechanisms, and Opportunities for Target Therapies. Int. J. Mol. Sci. 2022, 23, 13898. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef]
- Bu, R.; Uddin, S.; Bavi, P.; Hussain, A.R.; Al-Dayel, F.; Ghourab, S.; Ahmed, M.; Al-Kuraya, K.S. HGF/c-Met pathway has a prominent role in mediating antiapoptotic signals through AKT in epithelial ovarian carcinoma. Lab. Investig. 2011, 91, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Che, L.; Li, L.; Pilo, M.G.; Cigliano, A.; Ribback, S.; Li, X.; Latte, G.; Mela, M.; Evert, M.; et al. Co-activation of AKT and c-Met triggers rapid hepatocellular carcinoma development via the mTORC1/FASN pathway in mice. Sci. Rep. 2016, 6, 20484. [Google Scholar] [CrossRef]
- Usatyuk, P.V.; Fu, P.; Mohan, V.; Epshtein, Y.; Jacobson, J.R.; Gomez-Cambronero, J.; Wary, K.K.; Bindokas, V.; Dudek, S.M.; Salgia, R.; et al. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J. Biol. Chem. 2014, 289, 13476–13491. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.F.; Gu, X.Y.; Shi, Q.M.; Yuan, X.; Chu, Q.F.; Bao, Z.Y.; Lu, J.; Li, L.J. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Iweala, E.E.J.; Amuji, D.N.; Oluwajembola, A.M.; Ugbogu, E.A. Targeting c-Met in breast cancer: From mechanisms of chemoresistance to novel therapeutic strategies. Curr. Res. Pharmacol. Drug Discov. 2024, 7, 100204. [Google Scholar] [CrossRef] [PubMed]
- Kermorgant, S.; Zicha, D.; Parker, P.J. PKC controls HGF-dependent c-Met traffic, signalling and cell migration. EMBO J. 2004, 23, 3721–3734. [Google Scholar] [CrossRef]
- Kermorgant, S.; Parker, P.J. Receptor trafficking controls weak signal delivery: A strategy used by c-Met for STAT3 nuclear accumulation. J. Cell Biol. 2008, 182, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Barrow-McGee, R.; Kermorgant, S. Met endosomal signalling: In the right place, at the right time. Int. J. Biochem. Cell Biol. 2014, 49, 69–74. [Google Scholar] [CrossRef]
- Ye, F.; Yuan, Z.; Tang, Y.; Li, J.; Liu, X.; Sun, X.; Chen, S.; Ye, X.; Zeng, Z.; Zhang, X.K.; et al. Endocytic activation and exosomal secretion of matriptase stimulate the second wave of EGF signaling to promote skin and breast cancer invasion. Cell Rep. 2024, 43, 114002. [Google Scholar] [CrossRef]
- Chen, M.K.; Du, Y.; Sun, L.; Hsu, J.L.; Wang, Y.H.; Gao, Y.; Huang, J.; Hung, M.C. H2O2 induces nuclear transport of the receptor tyrosine kinase c-MET in breast cancer cells via a membrane-bound retrograde trafficking mechanism. J. Biol. Chem. 2019, 294, 8516–8528. [Google Scholar] [CrossRef]
- Stanislovas, J.; Kermorgant, S. c-Met-integrin cooperation: Mechanisms, tumorigenic effects, and therapeutic relevance. Front. Cell Dev. Biol. 2022, 10, 994528. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kobayashi, R.; Bishop, J.M. Cellular adherence elicits ligand-independent activation of the Met cell-surface receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 8425–8430. [Google Scholar] [CrossRef]
- Wang, R.; Ferrell, L.D.; Faouzi, S.; Maher, J.J.; Bishop, J.M. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J. Cell Biol. 2001, 153, 1023–1034. [Google Scholar] [CrossRef]
- Mitra, A.K.; Sawada, K.; Tiwari, P.; Mui, K.; Gwin, K.; Lengyel, E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 2011, 30, 1566–1576. [Google Scholar] [CrossRef]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 2001, 107, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Minuti, G.; D’Incecco, A.; Salvini, J.; Cappuzzo, F. MET overexpression and gene amplification in NSCLC: A clinical perspective. Lung Cancer 2013, 4, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Danilkovitch-Miagkova, A.; Zbar, B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J. Clin. Investig. 2002, 109, 863–867. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Marchetti, A.; Skokan, M.; Rossi, E.; Gajapathy, S.; Felicioni, L.; Del Grammastro, M.; Sciarrotta, M.G.; Buttitta, F.; Incarbone, M.; et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J. Clin. Oncol. 2009, 27, 1667–1674. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Bean, J.; Brennan, C.; Shih, J.Y.; Riely, G.; Viale, A.; Wang, L.; Chitale, D.; Motoi, N.; Szoke, J.; Broderick, S.; et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA 2007, 104, 20932–20937. [Google Scholar] [CrossRef]
- Okuda, K.; Sasaki, H.; Yukiue, H.; Yano, M.; Fujii, Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2008, 99, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Okamoto, I.; Arao, T.; Okamoto, W.; Matsumoto, K.; Taniguchi, H.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K.; et al. amplification as a potential therapeutic target in gastric cancer. Oncotarget 2013, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Kijima, T.; Maulik, G.; Fox, E.A.; Sattler, M.; Griffin, J.D.; Johnson, B.E.; Salgia, R. c-MET mutational analysis in small cell lung cancer: Novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003, 63, 6272–6281. [Google Scholar]
- Ma, P.C.; Jagadeeswaran, R.; Jagadeesh, S.; Tretiakova, M.S.; Nallasura, V.; Fox, E.A.; Hansen, M.; Schaefer, E.; Naoki, K.; Lader, A.; et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005, 65, 1479–1488. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Ou, S.H. MET exon 14 deletion (METex14): Finally, a frequent-enough actionable oncogenic driver mutation in non-small cell lung cancer to lead MET inhibitors out of “40 years of wilderness” and into a clear path of regulatory approval. Transl. Lung Cancer Res. 2015, 4, 820–824. [Google Scholar] [CrossRef]
- Lee, C.C.; Yamada, K.M. Identification of a novel type of alternative splicing of a tyrosine kinase receptor. Juxtamembrane deletion of the c-met protein kinase C serine phosphorylation regulatory site. J. Biol. Chem. 1994, 269, 19457–19461. [Google Scholar] [CrossRef]
- Lee, J.H.; Gao, C.F.; Lee, C.C.; Kim, M.D.; Vande Woude, G.F. An alternatively spliced form of Met receptor is tumorigenic. Exp. Mol. Med. 2006, 38, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, B.; Lee, S.B.; Jeong, Y.; Oh, Y.M.; Song, Y.J.; Jung, S.; Choi, J.; Lee, S.; Cheong, K.H.; et al. Cbl-independent degradation of Met: Ways to avoid agonism of bivalent Met-targeting antibody. Oncogene 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Peschard, P.; Fournier, T.M.; Lamorte, L.; Naujokas, M.A.; Band, H.; Langdon, W.Y.; Park, M. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 2001, 8, 995–1004. [Google Scholar] [CrossRef]
- Abella, J.V.; Peschard, P.; Naujokas, M.A.; Lin, T.; Saucier, C.; Urbe, S.; Park, M. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol. Cell Biol. 2005, 25, 9632–9645. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.H.; Yeung, S.F.; Chan, A.W.H.; Chung, L.Y.; Chau, S.L.; Lung, R.W.M.; Tong, C.Y.; Chow, C.; Tin, E.K.Y.; Yu, Y.H.; et al. Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin. Cancer Res. 2016, 22, 3048–3056. [Google Scholar] [CrossRef]
- Mazieres, J.; Vioix, H.; Pfeiffer, B.M.; Campden, R.I.; Chen, Z.; Heeg, B.; Cortot, A.B. MET Exon 14 Skipping in NSCLC: A Systematic Literature Review of Epidemiology, Clinical Characteristics, and Outcomes. Clin. Lung Cancer 2023, 24, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Mizuuchi, H.; Tomida, S.; Terashima, M.; Hayashi, H.; Nishio, K.; Mitsudomi, T. gene exon 14 deletion created using the CRISPR/Cas9 system enhances cellular growth and sensitivity to a MET inhibitor. Lung Cancer 2015, 90, 590–597. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, S.U.; Cho, H.; Jennings, B.; Gerrard, B.; Dean, M.; Schmidt, L.; Zbar, B.; Vande Woude, G.F. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 2000, 19, 4947–4953. [Google Scholar] [CrossRef] [PubMed]
- Tovar, E.A.; Graveel, C.R. MET in human cancer: Germline and somatic mutations. Ann. Transl. Med. 2017, 5, 205. [Google Scholar] [CrossRef]
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Janne, P.A.; Verma, S.; et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J. Clin. Oncol. 2016, 34, 721–730. [Google Scholar] [CrossRef]
- Kong-Beltran, M.; Seshagiri, S.; Zha, J.P.; Zhu, W.J.; Bhawe, K.; Mendoza, N.; Holcomb, T.; Pujara, K.; Stinson, J.; Fu, L.; et al. Somatic mutations lead to an oncogenic deletion of Met in lung cancer. Cancer Res. 2006, 66, 283–289. [Google Scholar] [CrossRef]
- Mitiushkina, N.V.; Kholmatov, M.M.; Tiurin, V.I.; Romanko, A.A.; Yatsuk, O.S.; Sokolova, T.N.; Ivantsov, A.O.; Kuligina, E.S.; Stepanov, I.A.; Belyaev, A.M.; et al. Comparative analysis of expression of mutant and wild-type alleles is essential for reliable PCR-based detection of MET exon 14 skipping. Biochimie 2019, 165, 267–274. [Google Scholar] [CrossRef]
- Fujino, T.; Suda, K.; Mitsudomi, T. Lung Cancer with MET exon 14 Skipping Mutation: Genetic Feature, Current Treatments, and Future Challenges. Lung Cancer 2021, 12, 35–50. [Google Scholar] [CrossRef]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Dong, S.M.; Kim, S.Y.; Na, E.Y.; Shin, M.S.; Pi, J.H.; Kim, B.J.; Bae, J.H.; Hong, Y.K.; Lee, K.S.; et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999, 59, 307–310. [Google Scholar] [PubMed]
- Ghadjar, P.; Blank-Liss, W.; Simcock, M.; Hegyi, I.; Beer, K.T.; Moch, H.; Aebersold, D.M.; Zimmer, Y. MET Y1253D-activating point mutation and development of distant metastasis in advanced head and neck cancers. Clin. Exp. Metastasis 2009, 26, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Neklason, D.W.; Done, M.W.; Sargent, N.R.; Schwartz, A.G.; Anton-Culver, H.; Griffin, C.A.; Ahnen, D.J.; Schildkraut, J.M.; Tomlinson, G.E.; Strong, L.C.; et al. Activating mutation in MET oncogene in familial colorectal cancer. BMC Cancer 2011, 11, 424. [Google Scholar] [CrossRef]
- Stein, M.N.; Hirshfield, K.M.; Zhong, H.; Singer, E.A.; Ali, S.M.; Ganesan, S. Response to crizotinib in a patient with MET-mutant papillary renal cell cancer after progression on tivantinib. Eur. Urol. 2015, 67, 353–354. [Google Scholar] [CrossRef]
- Lorenzato, A.; Olivero, M.; Patane, S.; Rosso, E.; Oliaro, A.; Comoglio, P.M.; Di Renzo, M.F. Novel somatic mutations of the MET oncogene in human carcinoma metastases activating cell motility and invasion. Cancer Res. 2002, 62, 7025–7030. [Google Scholar]
- Di Renzo, M.F.; Olivero, M.; Martone, T.; Maffe, A.; Maggiora, P.; Stefani, A.D.; Valente, G.; Giordano, S.; Cortesina, G.; Comoglio, P.M. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 2000, 19, 1547–1555. [Google Scholar] [CrossRef]
- Kong-Beltran, M.; Stamos, J.; Wickramasinghe, D. The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell 2004, 6, 75–84. [Google Scholar] [CrossRef]
- Soman, N.R.; Wogan, G.N.; Rhim, J.S. TPR-MET oncogenic rearrangement: Detection by polymerase chain reaction amplification of the transcript and expression in human tumor cell lines. Proc. Natl. Acad. Sci. USA 1990, 87, 738–742. [Google Scholar] [CrossRef]
- Febres-Aldana, C.A.; Vojnic, M.; Odintsov, I.; Zhang, T.; Cheng, R.; Beach, C.Z.; Lu, D.; Mattar, M.S.; Gazzo, A.M.; Gili, L.; et al. Pan-cancer analysis of oncogenic MET fusions reveals distinct pathogenomic subsets with differential sensitivity to MET-targeted therapy. Cancer Discov. 2025. [Google Scholar] [CrossRef]
- International Cancer Genome Consortium PedBrain Tumor Project. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat. Med. 2016, 22, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.; Fassunke, J.; Scheel, A.H.; Scheffler, M.; Heydt, C.; Nogova, L.; Michels, S.; Fischer, R.N.; Eisert, A.; Scharpenseel, H.; et al. MET Fusions in NSCLC: Clinicopathologic Features and Response to MET Inhibition. J. Thorac. Oncol. 2024, 19, 160–165. [Google Scholar] [CrossRef]
- Sun, D.; Wu, W.; Wang, L.; Qu, J.; Han, Q.; Wang, H.; Song, S.; Liu, N.; Wang, Y.; Hou, H. Identification of MET fusions as novel therapeutic targets sensitive to MET inhibitors in lung cancer. J. Transl. Med. 2023, 21, 150. [Google Scholar] [CrossRef]
- Bao, Z.S.; Chen, H.M.; Yang, M.Y.; Zhang, C.B.; Yu, K.; Ye, W.L.; Hu, B.Q.; Yan, W.; Zhang, W.; Akers, J.; et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome Res. 2014, 24, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Peschard, P.; Park, M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 2007, 26, 1276–1285. [Google Scholar] [CrossRef]
- Xiao, G.H.; Jeffers, M.; Bellacosa, A.; Mitsuuchi, Y.; Vande Woude, G.F.; Testa, J.R. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA 2001, 98, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Lee, Y.; Kim, S.; Yu, H.J.; Ji, S.Y.; Bae, J.M.; Won, J.K.; Shin, J.H.; Weinberger, D.R.; Choi, S.H.; et al. A glioneuronal tumor with CLIP2-MET fusion. NPJ Genom. Med. 2020, 5, 24. [Google Scholar] [CrossRef]
- Pfaff, A.L.; Bubb, V.J.; Quinn, J.P.; Koks, S. A Genome-Wide Screen for the Exonisation of Reference SINE-VNTR-Alus and Their Expression in CNS Tissues of Individuals with Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 11548. [Google Scholar] [CrossRef]
- Schrock, A.B.; Frampton, G.M.; Suh, J.; Chalmers, Z.R.; Rosenzweig, M.; Erlich, R.L.; Halmos, B.; Goldman, J.; Forde, P.; Leuenberger, K.; et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J. Thorac. Oncol. 2016, 11, 1493–1502. [Google Scholar] [CrossRef]
- Socinski, M.A.; Pennell, N.A.; Davies, K.D. MET Exon 14 Skipping Mutations in Non-Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021, 5, PO-20. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Mol. Diagn. 2018, 20, 129–159. [Google Scholar] [CrossRef]
- Barlesi, F.; Mazieres, J.; Merlio, J.P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, L.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016, 387, 1415–1426. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Suda, K.; Yatabe, Y. Surgery for NSCLC in the era of personalized medicine. Nat. Rev. Clin. Oncol. 2013, 10, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Graveel, C.; Su, Y.L.; Koeman, J.; Wang, L.M.; Tessarollo, L.; Fiscella, M.; Birchmeier, C.; Swiatek, P.; Bronson, R.; Woude, G.V. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc. Natl. Acad. Sci. USA 2004, 101, 17198–17203. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, C.; Gong, H.; Tang, Z.; Wen, J.; Wang, S.; Xiao, S. Therapeutic potential of tyrosine-protein kinase MET in osteosarcoma. Front. Mol. Biosci. 2024, 11, 1367331. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Prechtel, D.; Resau, J.H.; Gauger, K.; Welk, A.; Lindemann, K.; Salanti, G.; Richter, T.; Knudsen, B.; Vande Woude, G.F.; et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int. J. Cancer 2005, 113, 678–682. [Google Scholar] [CrossRef]
- Tuck, A.B.; Park, M.; Sterns, E.E.; Boag, A.; Elliott, B.E. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am. J. Pathol. 1996, 148, 225–232. [Google Scholar]
- Jin, L.; Fuchs, A.; Schnitt, S.J.; Yao, Y.; Joseph, A.; Lamszus, K.; Park, M.; Goldberg, I.D.; Rosen, E.M. Expression of scatter factor and c-met receptor in benign and malignant breast tissue. Cancer 1997, 79, 749–760. [Google Scholar] [CrossRef]
- Edakuni, G.; Sasatomi, E.; Satoh, T.; Tokunaga, O.; Miyazaki, K. Expression of the hepatocyte growth factor/c-Met pathway is increased at the cancer front in breast carcinoma. Pathol. Int. 2001, 51, 172–178. [Google Scholar] [CrossRef]
- Kang, J.Y.; Dolled-Filhart, M.; Ocal, I.T.; Singh, B.; Lin, C.Y.; Dickson, R.B.; Rimm, D.L.; Camp, R.L. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003, 63, 1101–1105. [Google Scholar]
- Minuti, G.; Landi, L. MET deregulation in breast cancer. Ann. Transl. Med. 2015, 3, 181. [Google Scholar] [CrossRef] [PubMed]
- de Melo Gagliato, D.; Jardim, D.L.; Falchook, G.; Tang, C.; Zinner, R.; Wheler, J.J.; Janku, F.; Subbiah, V.; Piha-Paul, S.A.; Fu, S.; et al. Analysis of MET genetic aberrations in patients with breast cancer at MD Anderson Phase I unit. Clin. Breast Cancer 2014, 14, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, J.S.; Seo, J.; Song, J.Y.; Lee, S.E.; Kwon, M.J.; Kwon, M.J.; Kundu, J.; Jung, K.; Oh, E.; et al. MET is a potential target for use in combination therapy with EGFR inhibition in triple-negative/basal-like breast cancer. Int. J. Cancer 2014, 134, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, S.; Kherrouche, Z.; Cockenpot, V.; Stoven, L.; Copin, M.C.; Werkmeister, E.; Marchand, N.; Kyheng, M.; Tulasne, D.; Cortot, A.B. MET amplification increases the metastatic spread of EGFR-mutated NSCLC. Lung Cancer 2018, 125, 57–67. [Google Scholar] [CrossRef]
- Garcia, S.; Dales, J.P.; Charafe-Jauffret, E.; Carpentier-Meunier, S.; Andrac-Meyer, L.; Jacquemier, J.; Andonian, C.; Lavaut, M.N.; Allasia, C.; Bonnier, P.; et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum. Pathol. 2007, 38, 830–841. [Google Scholar] [CrossRef]
- Gonçalves, A.; Charafe-Jauffret, E.; Bertucci, F.; Audebert, S.; Toiron, Y.; Esterni, B.; Monville, F.; Tarpin, C.; Jacquemier, J.; Houvenaeghel, G.; et al. Protein profiling of human breast tumor cells identifies novel biomarkers associated with molecular subtypes. Mol. Cell Proteom. 2008, 7, 1420–1433. [Google Scholar] [CrossRef]
- Wu, J.M.; Fackler, M.J.; Halushka, M.K.; Molavi, D.W.; Taylor, M.E.; Teo, W.W.; Griffin, C.; Fetting, J.; Davidson, N.E.; De Marzo, A.M.; et al. Heterogeneity of breast cancer metastases: Comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin. Cancer Res. 2008, 14, 1938–1946. [Google Scholar] [CrossRef]
- Ponzo, M.G.; Lesurf, R.; Petkiewicz, S.; O’Malley, F.P.; Pinnaduwage, D.; Andrulis, I.L.; Bull, S.B.; Chughtai, N.; Zuo, D.; Souleimanova, M.; et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 12903–12908. [Google Scholar] [CrossRef]
- Beviglia, L.; Matsumoto, K.; Lin, C.S.; Ziober, B.L.; Kramer, R.H. Expression of the c-Met/HGF receptor in human breast carcinoma: Correlation with tumor progression. Int. J. Cancer 1997, 74, 301–309. [Google Scholar] [CrossRef]
- Hochgrafe, F.; Zhang, L.; O’Toole, S.A.; Browne, B.C.; Pinese, M.; Porta Cubas, A.; Lehrbach, G.M.; Croucher, D.R.; Rickwood, D.; Boulghourjian, A.; et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res. 2010, 70, 9391–9401. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, S.; Comoglio, P.M.; Trusolino, L. The Met oncogene and basal-like breast cancer: Another culprit to watch out for? Breast Cancer Res. 2010, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, M.; Massafra, M.; Gebbia, V.; D’Aquino, A.; Garipoli, C.; Altavilla, G.; Rosell, R. A narrative review of MET inhibitors in non-small cell lung cancer with MET exon 14 skipping mutations. Transl. Lung Cancer Res. 2021, 10, 1536–1556. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhang, J.; Heymach, J.V.; Le, X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835921992976. [Google Scholar] [CrossRef]
- Remon, J.; Hendriks, L.E.L.; Mountzios, G.; Garcia-Campelo, R.; Saw, S.P.L.; Uprety, D.; Recondo, G.; Villacampa, G.; Reck, M. MET alterations in NSCLC-Current Perspectives and Future Challenges. J. Thorac. Oncol. 2023, 18, 419–435. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Wei, Z.; Mehra, R.; Shaw, A.T.; Lieu, C.H.; Forde, P.M.; Drilon, A.E.; Mitchell, E.P.; Wright, J.J.; Takebe, N.; et al. Crizotinib in patients with tumors harboring ALK or ROS1 rearrangements in the NCI-MATCH trial. NPJ Precis. Oncol. 2022, 6, 13. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Oxnard, G.R.; Elkin, S.; Sullivan, E.K.; Carter, J.L.; Barbie, D.A. Response to Crizotinib in a Patient With Lung Adenocarcinoma Harboring a MET Splice Site Mutation. Clin. Lung Cancer 2015, 16, E101–E104. [Google Scholar] [CrossRef]
- Waqar, S.N.; Morgensztern, D.; Sehn, J. MET Mutation Associated with Responsiveness to Crizotinib. J. Thorac. Oncol. 2015, 10, e29–e31. [Google Scholar] [CrossRef]
- Drilon, A. Exon 14 Alterations in Lung Cancer: Exon Skipping Extends Half-Life. Clin. Cancer Res. 2016, 22, 2832–2834. [Google Scholar] [CrossRef]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef]
- Drilon, A.E.; Camidge, D.R.; Ou, S.H.I.; Clark, J.W.; Socinski, M.A.; Weiss, J.; Riely, G.J.; Winter, M.; Wang, S.C.; Monti, K.; et al. Efficacy and safety of crizotinib in patients (pts) with advanced MET exon 14-altered non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2016, 34, 108. [Google Scholar] [CrossRef]
- Dong, Y.T.; Xu, J.C.; Sun, B.Y.; Wang, J.; Wang, Z.J. MET-Targeted Therapies and Clinical Outcomes: A Systematic Literature Review. Mol. Diagn. Ther. 2022, 26, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Ho-Yen, C.M.; Jones, J.L.; Kermorgant, S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Markham, A. Tepotinib: First Approval. Drugs 2020, 80, 829–833. [Google Scholar] [CrossRef]
- Desai, A.; Cuellar, S. The Current Landscape for METex14 Skipping Mutations in Non-Small Cell Lung Cancer. J. Adv. Pract. Oncol. 2022, 13, 539–544. [Google Scholar] [CrossRef]
- Bahcall, M.; Paweletz, C.P.; Kuang, Y.; Taus, L.J.; Sim, T.; Kim, N.D.; Dholakia, K.H.; Lau, C.J.; Gokhale, P.C.; Chopade, P.R.; et al. Combination of Type I and Type II MET Tyrosine Kinase Inhibitors as Therapeutic Approach to Prevent Resistance. Mol. Cancer Ther. 2022, 21, 322–335. [Google Scholar] [CrossRef]
- Grullich, C. Cabozantinib: Multi-kinase Inhibitor of MET, AXL, RET, and VEGFR2. Recent. Results Cancer Res. 2018, 211, 67–75. [Google Scholar] [CrossRef]
- Schlumberger, M.; Elisei, R.; Muller, S.; Schoffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; Powles, T.; Scheffold, C.; Choueiri, T.K. Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. Br. J. Cancer 2018, 118, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Maroto, P.; Porta, C.; Capdevila, J.; Apolo, A.B.; Viteri, S.; Rodriguez-Antona, C.; Martin, L.; Castellano, D. Cabozantinib for the treatment of solid tumors: A systematic review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221107112. [Google Scholar] [CrossRef] [PubMed]
- Gerendash, B.S.; Creel, P.A. Practical management of adverse events associated with cabozantinib treatment in patients with renal-cell carcinoma. Onco Targets Ther. 2017, 10, 5053–5064. [Google Scholar] [CrossRef]
- Apolo, A.B.; Nadal, R.; Girardi, D.M.; Niglio, S.A.; Ley, L.; Cordes, L.M.; Steinberg, S.M.; Sierra Ortiz, O.; Cadena, J.; Diaz, C.; et al. Phase I Study of Cabozantinib and Nivolumab Alone or With Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors. J. Clin. Oncol. 2020, 38, 3672–3684. [Google Scholar] [CrossRef] [PubMed]
- He, A.R.; Cohen, R.B.; Denlinger, C.S.; Sama, A.; Birnbaum, A.; Hwang, J.; Sato, T.; Lewis, N.; Mynderse, M.; Niland, M.; et al. First-in-Human Phase I Study of Merestinib, an Oral Multikinase Inhibitor, in Patients with Advanced Cancer. Oncologist 2019, 24, e930–e942. [Google Scholar] [CrossRef]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.; Orlova, R.; Cutsem, E.V.; Adeva, J.; Chen, L.; Obermannova, R.; et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: A randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021, 22, e472. [Google Scholar] [CrossRef]
- Glodde, N.; Bald, T.; van den Boorn-Konijnenberg, D.; Nakamura, K.; O’Donnell, J.S.; Szczepanski, S.; Brandes, M.; Eickhoff, S.; Das, I.; Shridhar, N.; et al. Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity 2017, 47, 789–802.e9. [Google Scholar] [CrossRef]
- Poulsen, T.T.; Grandal, M.M.; Skartved, N.J.O.; Hald, R.; Alifrangis, L.; Koefoed, K.; Lindsted, T.; Frohlich, C.; Pollmann, S.E.; Eriksen, K.W.; et al. Sym015: A Highly Efficacious Antibody Mixture against MET-Amplified Tumors. Clin. Cancer Res. 2017, 23, 5923–5935. [Google Scholar] [CrossRef]
- Lombardi, A.M.; Sangiolo, D.; Vigna, E. MET Oncogene Targeting for Cancer Immunotherapy. Int. J. Mol. Sci. 2024, 25, 6109. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.; Moiseenko, F.; Filippova, E.; Cicin, I.; Ciuleanu, T.; Daaboul, N.; Liu, C.; et al. Telisotuzumab Vedotin Monotherapy in Patients with Previously Treated c-Met Protein-Overexpressing Advanced Nonsquamous EGFR-Wildtype Non-Small Cell Lung Cancer in the Phase II LUMINOSITY Trial. J. Clin. Oncol. 2024, 42, 3000–3011. [Google Scholar] [CrossRef]
- Krebs, M.; Spira, A.I.; Cho, B.C.; Besse, B.; Goldman, J.W.; Janne, P.A.; Ma, Z.Y.; Mansfield, A.S.; Minchom, A.R.; Ou, S.H.I.; et al. Amivantamab in patients with NSCLC with MET exon 14 skipping mutation: Updated results from the CHRYSALIS study. J. Clin. Oncol. 2022, 40, 9008. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, Y.; Wu, L.; Hu, J.; Wang, Z.H.; Chen, J.H.; Fan, Y.; Lin, G.; Wang, Q.M.; Yao, Y.; et al. Vebreltinib for Advanced Non-Small Cell Lung Cancer Harboring c-Met Exon 14 Skipping Mutation: A Multicenter, Single-Arm, Phase II KUNPENG Study. J. Clin. Oncol. 2024, 42, 3680–3691. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, L.D.; Aranda, R.; Lee, M.; Tovar, E.A.; Essenburg, C.J.; Madaj, Z.; Chiang, H.; Briere, D.; Hallin, J.; Lopez-Casas, P.P.; et al. Glesatinib Exhibits Antitumor Activity in Lung Cancer Models and Patients Harboring MET Exon 14 Mutations and Overcomes Mutation-mediated Resistance to Type I MET Inhibitors in Nonclinical Models. Clin. Cancer Res. 2017, 23, 6661–6672. [Google Scholar] [CrossRef]
- Bahcall, M.; Sim, T.; Paweletz, C.P.; Patel, J.D.; Alden, R.S.; Kuang, Y.; Sacher, A.G.; Kim, N.D.; Lydon, C.A.; Awad, M.M.; et al. Acquired METD1228V Mutation and Resistance to MET Inhibition in Lung Cancer. Cancer Discov. 2016, 6, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Recondo, G.; Che, J.; Janne, P.A.; Awad, M.M. Targeting MET Dysregulation in Cancer. Cancer Discov. 2020, 10, 922–934. [Google Scholar] [CrossRef]
- Pruis, M.A.; Paats, M.S.; Geurts, W.R.R.; Dubbink, H.J.; Dingemans, A.C. Overcoming Acquired Resistance Mutation MET D1228N to Crizotinib With Cabozantinib in NSCLC With MET Exon 14 Skipping Mutation. JCO Precis. Oncol. 2021, 5, 849–853. [Google Scholar] [CrossRef]
- Parsons, B.M.; Meier, D.R.; Richmond, C.S.; Gurda, G.T.; Lofgren, K.A.; Burkard, M.E.; Deming, D.A.; Kenny, P.A. Acquisition of Cabozantinib-Sensitive MET D1228N Mutation During Progression on Crizotinib in MET-Amplified Triple-Negative Breast Cancer. Clin. Breast Cancer 2020, 20, e433–e438. [Google Scholar] [CrossRef]
- Recondo, G.; Bahcall, M.; Spurr, L.F.; Che, J.; Ricciuti, B.; Leonardi, G.C.; Lo, Y.C.; Li, Y.Y.; Lamberti, G.; Nguyen, T.; et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin. Cancer Res. 2020, 26, 2615–2625. [Google Scholar] [CrossRef]
- Vuong, H.G.; Ho, A.T.N.; Altibi, A.M.A.; Nakazawa, T.; Katoh, R.; Kondo, T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer—A systematic review and meta-analysis. Lung Cancer 2018, 123, 76–82. [Google Scholar] [CrossRef]
- Rozenblum, A.B.; Ilouze, M.; Dudnik, E.; Dvir, A.; Soussan-Gutman, L.; Geva, S.; Peled, N. Clinical Impact of Hybrid Capture-Based Next-Generation Sequencing on Changes in Treatment Decisions in Lung Cancer. J. Thorac. Oncol. 2017, 12, 258–268. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, K.A.; Lee, C.Y.; Kim, S.; Chang, S.; Cho, B.C.; Shim, H.S. Molecular Diagnostic Assays and Clinicopathologic Implications of MET Exon 14 Skipping Mutation in Non-small-cell Lung Cancer. Clin. Lung Cancer 2019, 20, e123–e132. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Wang, L.; Arcila, M.E.; Balasubramanian, S.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.; Lipson, D.; Miller, V.A.; Kris, M.G.; et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin. Cancer Res. 2015, 21, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non-Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Yu, T.M.; Morrison, C.; Gold, E.J.; Tradonsky, A.; Layton, A.J. Multiple Biomarker Testing Tissue Consumption and Completion Rates With Single-gene Tests and Investigational Use of Oncomine Dx Target Test for Advanced Non-Small-cell Lung Cancer: A Single-center Analysis. Clin. Lung Cancer 2019, 20, 20–29.e8. [Google Scholar] [CrossRef] [PubMed]


| Fusion Gene | Exons of Partner Gene | Exons of MET | Number of Cases |
|---|---|---|---|
| BAIAP2L1-MET * | 9–14 | 15–21 | 3 |
| BAZ1B-MET * | 4–20 | 20–21 | 1 |
| C8orf34-MET * | 7–10 | 12–21 | 4 |
| MET-C8orf34 * | 3–14 | 14–21 | 4 |
| CAPZA2-MET * | 7–10 | 12–21 | 7 |
| MET-CAPZA2 * | 2–10 | 10–21 | 2 |
| KIF5B-MET * | 24–26 | 14–21 | 2 |
| MET-CAV1 * | 3 | 1–21 | 7 |
| MET-CFTR * | 23 | 1–21 | 4 |
| MET-CNTNQAP * | 3–24 | 2–21 | 1 |
| MET-DYNC1I1 * | 2–17 | 3–21 | 1 |
| MET-TES * | 2–7 | 2–21 | 1 |
| TES-MET * | 1–7 | 2–21 | 2 |
| MET-TFG * | 6–8 | 14–21 | 3 |
| TFG-MET* | 5–8 | 15–21 | 3 |
| MET-WNT2 * | 4–5 | 16–21 | 4 |
| OXR1-MET * | 11–16 | 13–21 | 4 |
| PTPRZ1-MET * [74] | 1–30 | 2–21 | 9 |
| ST7-MET * | 1–7 | 2–21 | 5 |
| TPR-MET * [75] | 1–30 | 15–21 | 16 |
| TRK-MET * [76] | 1–7 | 15–21 | 3 |
| CLIP2-MET * [77] | 1–11 | 15–21 | 2 |
| Drug Name | Type Inhibitor | General Mechanism/Notes |
|---|---|---|
| Crizotinib | Type 1a | Inhibits ALK phosphorylation by interacting with the Y1230 residue, leading to G1/S-phase cell cycle arrest and apoptosis. More effective in patients with high MET copy numbers [104,106]. |
| Capmatinib | Type 1b | A selective small-molecule inhibitor that blocks c-MET phosphorylation by binding to the ATP-binding pocket of the active kinase, thereby preventing downstream signaling [112]. |
| Tepotinib | Type 1b | Similar to capmatinib; selectively inhibits c-MET phosphorylation by binding to its ATP-binding pocket in the active conformation [112]. |
| Bozitinib | Type 1b | A highly selective and specific c-MET inhibitor currently in Phase 2 clinical trials [132]. |
| Cabozantinib | Type 2 | Multi-kinase; binds MET in inactive conformation, approved by the FDA in 2012; targets c-MET along with other receptor tyrosine kinases [119]. |
| Merestinib | Type 2 | Type 2 inhibitor with antitumor and antiproliferative effects against MET, also designed to be active against other receptor tyrosine kinases [125] |
| Sym-015 | Antibody-based (IgG1 mixture) | A combination of two humanized IgG1 antibodies targeting the SEMA domain of c-MET to prevent HGF binding [127]. |
| Telisotuzumab vedotin | Antibody-drug conjugate | Delivers monomethyl auristatin E (MMAE) to the cytosol, causing G2/M phase cell cycle arrest [127,130]. |
| Amivantamab | Bispecific antibody | A human bispecific antibody targeting both EGFR and c-MET; induces receptor degradation and immune cell-mediated cytotoxicity. Under investigation for MET exon 14 alterations [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.; Ganesan, S.; Xia, B.; Huo, Y. Targeting c-MET Alterations in Cancer: A Review of Genetic Drivers and Therapeutic Implications. Cancers 2025, 17, 1493. https://doi.org/10.3390/cancers17091493
Ji M, Ganesan S, Xia B, Huo Y. Targeting c-MET Alterations in Cancer: A Review of Genetic Drivers and Therapeutic Implications. Cancers. 2025; 17(9):1493. https://doi.org/10.3390/cancers17091493
Chicago/Turabian StyleJi, Michelle, Shridar Ganesan, Bing Xia, and Yanying Huo. 2025. "Targeting c-MET Alterations in Cancer: A Review of Genetic Drivers and Therapeutic Implications" Cancers 17, no. 9: 1493. https://doi.org/10.3390/cancers17091493
APA StyleJi, M., Ganesan, S., Xia, B., & Huo, Y. (2025). Targeting c-MET Alterations in Cancer: A Review of Genetic Drivers and Therapeutic Implications. Cancers, 17(9), 1493. https://doi.org/10.3390/cancers17091493






