Well-Differentiated Jejunoileal Neuroendocrine Tumors and Corresponding Liver Metastases: Mesenteric Fibrogenesis and Extramural Vascular Invasion in Tumor Progression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Histology, Special Stains, and Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Demographics
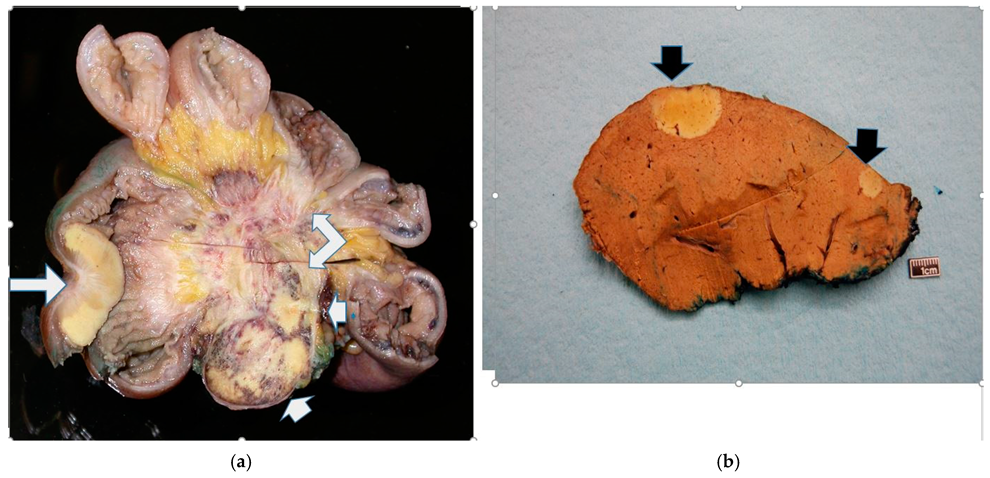
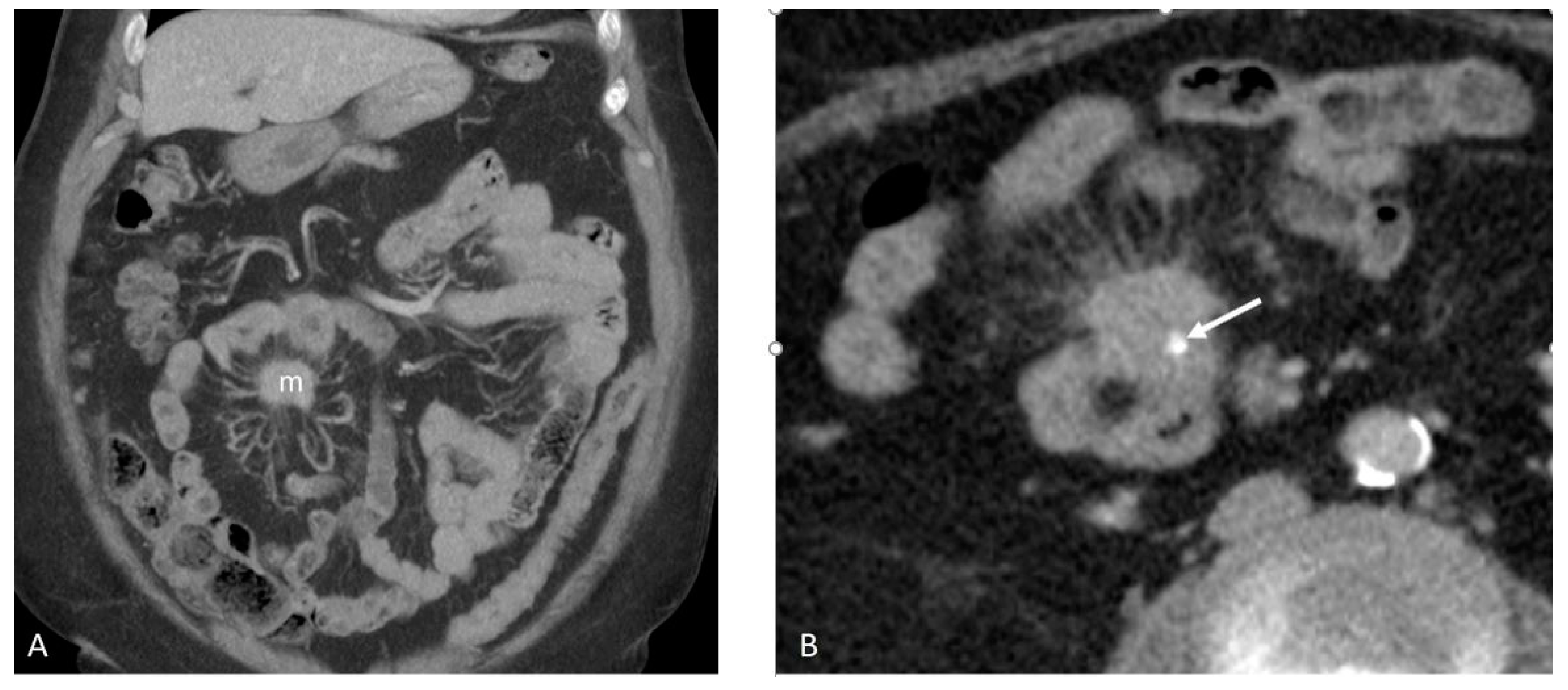
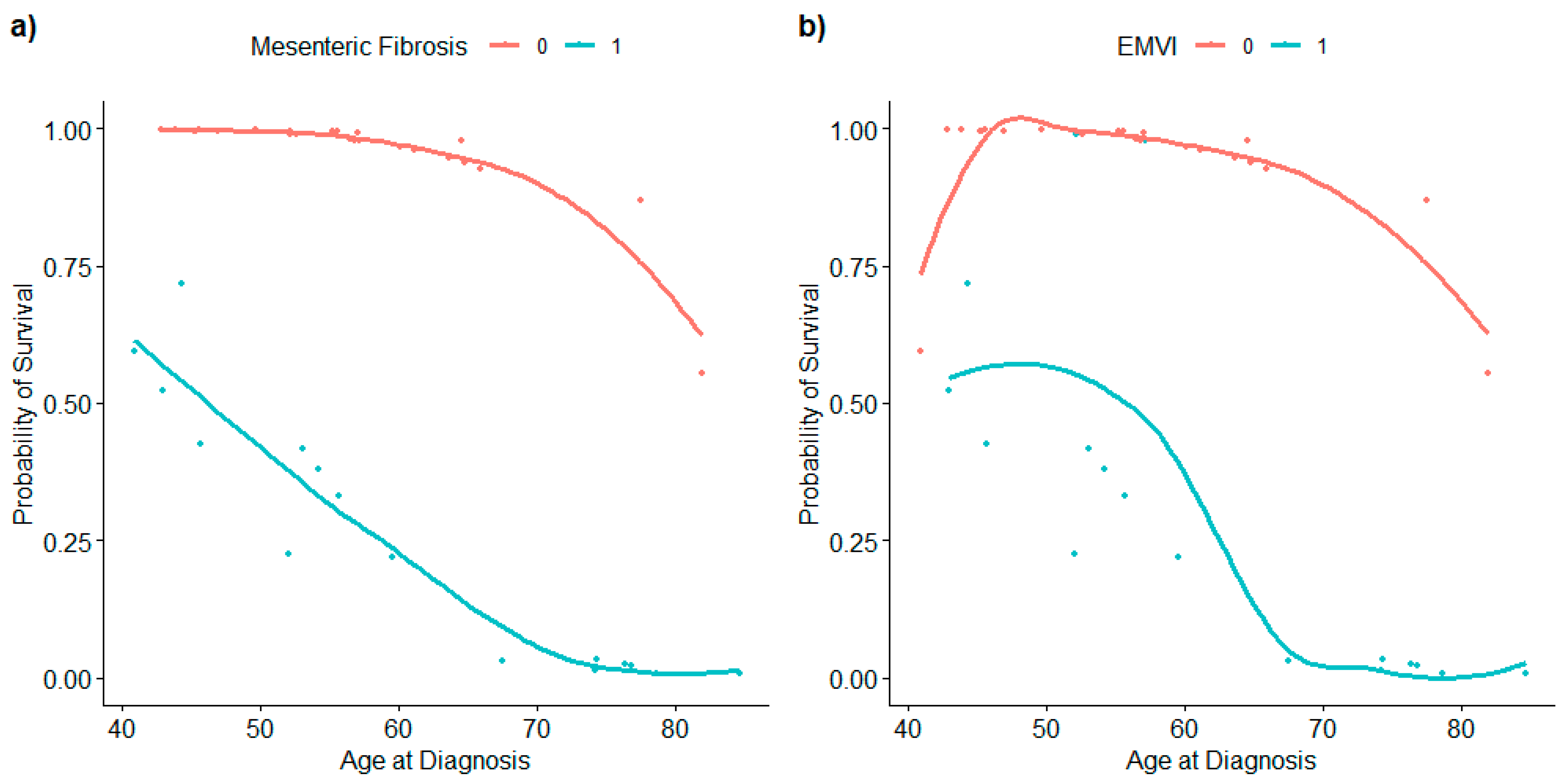
3.2. Mesenteric Fibrogenesis and Extramural Vascular Invasion
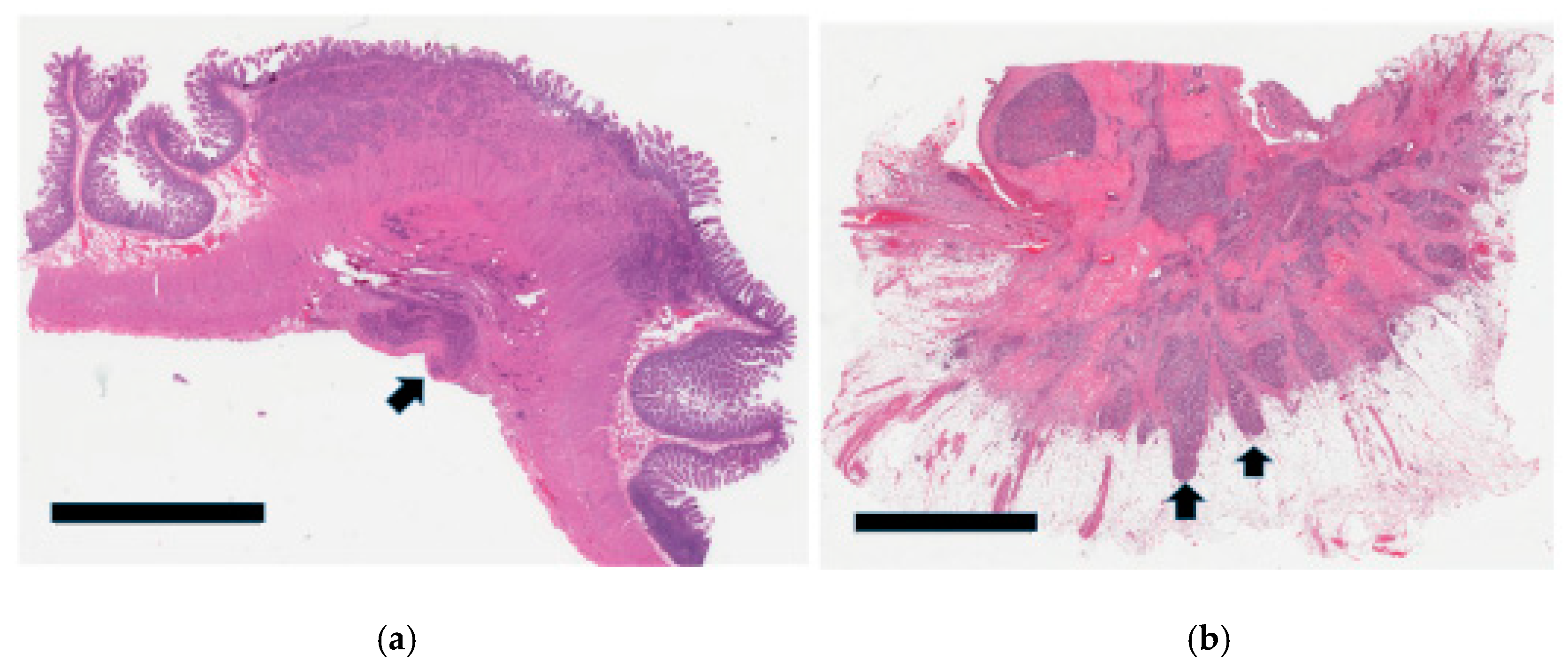
3.3. Tumor Infiltration Pattern
3.4. Tumor Grade
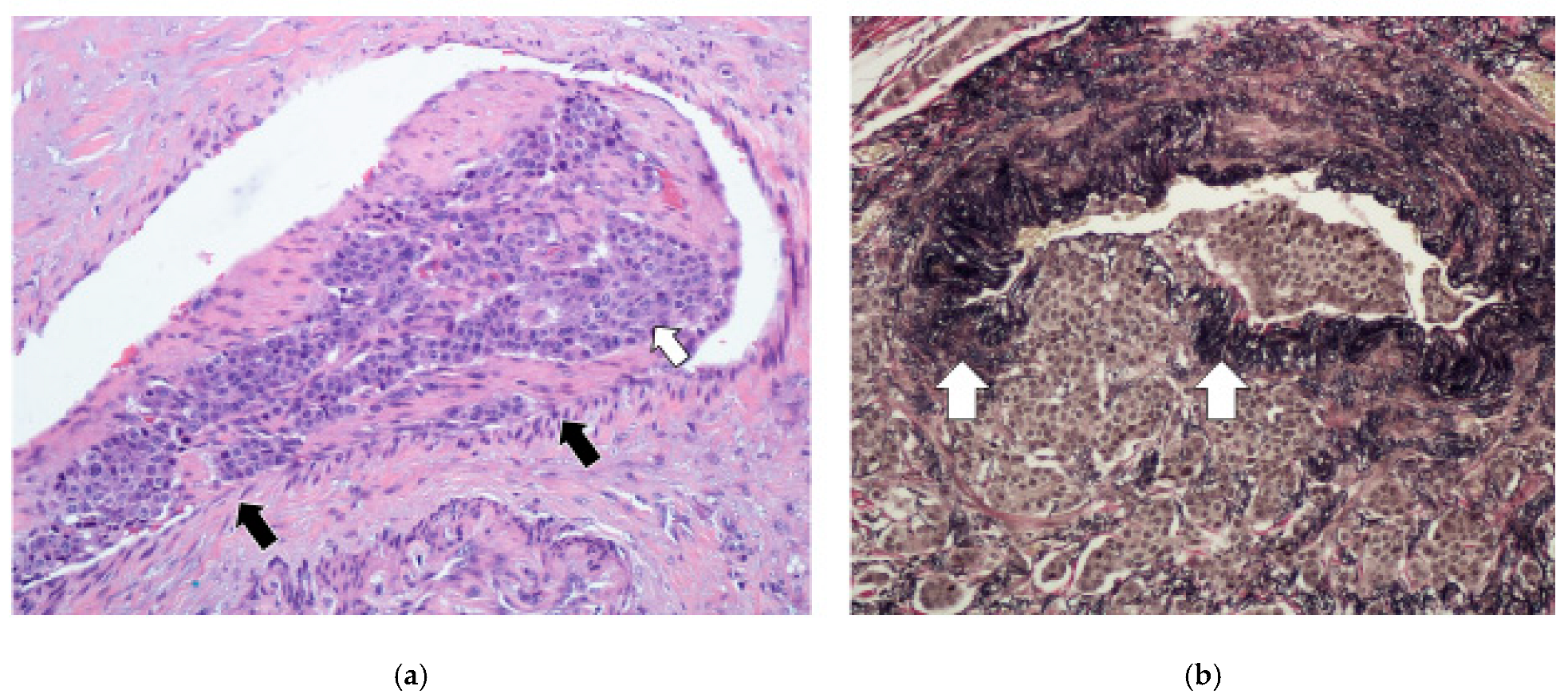
3.5. Lymphovascular Invasion and Perineural Invasion
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| EMVI | Extramural vascular invasion |
| GI | Gastrointestinal |
| IQR | Interquartile range dichroism |
| JI | Jejunoileal |
| LVI | Lymphovascular invasion |
| MF | Mesenteric fibrogenesis |
| NEC | Neuroendocrine carcinoma |
| NEN | Neuroendocrine neoplasm |
| NET | Neuroendocrine tumor |
| OR | Odds ratio |
| PNI | Perineural invasion |
| TME | Tumor microenvironment |
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Morrison, H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011, 3, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.; Klöppel, G.; Krenning, E.; Ahlman, H.; Plöckinger, U.; Wiedenmann, B.; Arnold, R.; Auernhammer, C.J.; Körner, M.; Rindi, G.; et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors-well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology 2008, 87, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Arévalo, S.; Gahete, M.D.; Alors-Pérez, E.; Luque, R.M.; Castaño, J.P. Multilayered heterogeneity as an intrinsic hallmark of neuroendocrine tumors. Rev. Endocr. Metab. Disord. 2018, 19, 179–192. [Google Scholar] [CrossRef]
- Varghese, D.G.; Del Rivero, J.; Bergsland, E. Grade progression and intrapatient tumor heterogeneity as potential contributors to resistance in gastroenteropancreatic neuroendocrine tumors. Cancers 2023, 15, 3712. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.; Harter, P.N.; Battke, F.; Winkelmann, R.; Schneider, M.; Holzer, K.; Koch, C.; Bojunga, J.; Zeuzem, S.; Hansmann, M.L.; et al. Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendocrine tumors. Sci. Rep. 2018, 8, 3811. [Google Scholar] [CrossRef]
- Reid, M.D.; Bagci, P.; Ohike, N.; Saka, B.; Seven, I.E.; Dursun, N.; Balci, S.; Gucer, H.; Jang, K.T.; Tajiri, T.; et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: A comparative analysis of four counting methodologies. Mod. Pathol. 2015, 28, 686–694. [Google Scholar] [CrossRef]
- Luchini, C.; Pantanowitz, L.; Adsay, V.; Asa, S.L.; Antonini, P.; Girolami, I.; Veronese, N.; Nottegar, A.; Cingarlini, S.; Landoni, L.; et al. Ki-67 assessment of pancreatic neuroendocrine neoplasms: Systematic review and meta-analysis of manual vs. digital pathology scoring. Mod. Pathol. 2022, 35, 712–720. [Google Scholar] [CrossRef]
- Bourdeleau, P.; Couvelard, A.; Ronot, M.; Lebtahi, R.; Hentic, O.; Ruszniewski, P.; Cros, J.; de Mestier, L. Spatial and temporal heterogeneity of digestive neuroendocrine neoplasms. Ther. Adv. Med. Oncol. 2023, 15, 17588359231179310. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G.; Lehembre, F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol. Med. 2007, 13, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Samsom, K.G.; van Veenendaal, L.M.; Valk, G.D.; Vriens, M.R.; Tesselaar, M.E.; van den Berg, J.G. Molecular prognostic factors in small-intestinal neuroendocrine tumours. Endocr. Connect. 2019, 8, 906–922. [Google Scholar] [CrossRef]
- Uccella, S. Molecular classification of gastrointestinal and pancreatic neuroendocrine neoplasms: Are we ready for that? Endocr. Pathol. 2024, 35, 91–106. [Google Scholar] [CrossRef]
- Carpizo, D.R.; Harris, C.R. Genetic drivers of ileal neuroendocrine tumors. Cancers 2021, 13, 5070. [Google Scholar] [CrossRef]
- Pozas, J.; Alonso-Gordoa, T.; San Román, M.; Santoni, M.; Thirlwell, C.; Grande, E.; Molina-Cerrillo, J. Novel therapeutic approaches in GEP-NETs based on genetic and epigenetic alterations. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2022, 1877, 188804. [Google Scholar] [CrossRef]
- Webster, A.P.; Thirlwell, C. The molecular biology of midgut neuroendocrine neoplasms. Endocr. Rev. 2024, 45, 343–350. [Google Scholar] [CrossRef]
- McMurry, H.S.; Del Rivero, J.; Chen, E.Y.; Kardosh, A.; Lopez, C.D.; Pegna, G.J. Gastroenteropancreatic neuroendocrine tumors: Epigenetic landscape and clinical implications. Curr. Probl. Cancer 2024, 52, 101131. [Google Scholar] [CrossRef]
- Lopez-Aguiar, A.G.; Ethun, C.G.; Postlewait, L.M.; Zhelnin, K.; Krasinskas, A.; El-Rayes, B.F.; Russell, M.C.; Sarmiento, J.M.; Kooby, D.A.; Staley, C.A.; et al. Redefining the Ki-67 index stratification for low-grade pancreatic neuroendocrine tumors: Improving its prognostic value for recurrence of disease. Ann. Surg. Oncol. 2018, 25, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Detjen, K.; Hammerich, L.; Özdirik, B.; Demir, M.; Wiedenmann, B.; Tacke, F.; Jann, H.; Roderburg, C. Models of gastroenteropancreatic neuroendocrine neoplasms: Current status and future directions. Neuroendocrinology 2021, 111, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Taskin, O.C.; Reid, M.D.; Bagci, P.; Balci, S.; Armutlu, A.; Demirtas, D.; Pehlivanoglu, B.; Saka, B.; Memis, B.; Bozkurtlar, E.; et al. Infiltration pattern predicts metastasis and progression better than the T-stage and grade in pancreatic neuroendocrine tumors: A proposal for a novel infiltration-based morphologic grading. Mod. Pathol. 2022, 35, 777–785. [Google Scholar] [CrossRef]
- Aysal, A.; Agalar, C.; Egeli, T.; Unek, T.; Oztop, I.; Obuz, F.; Sagol, O. Reconsideration of clinicopathologic prognostic factors in pancreatic neuroendocrine tumors for better determination of adverse prognosis. Endocr. Pathol. 2021, 32, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Luong, T.V.; Watkins, J.; Toumpanakis, C.; Caplin, M.E.; Meyer, T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br. J. Cancer 2013, 108, 1838–1845. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Zhang, M.; Shang, L.; Zhang, P.; Wang, W.; Fang, C.; Li, J.; Xu, T.; Tan, H.; et al. Clinicopathological features and prognostic validity of WHO grading classification of SI-NENs. BMC Cancer 2017, 17, 521. [Google Scholar] [CrossRef]
- Clift, A.K.; Faiz, O.; Goldin, R.; Martin, J.; Wasan, H.; Liedke, M.O.; Schloericke, E.; Malczewska, A.; Rindi, G.; Kidd, M.; et al. Predicting the survival of patients with small bowel neuroendocrine tumours: Comparison of 3 systems. Endocr. Connect. 2017, 6, 71–81. [Google Scholar] [CrossRef]
- Liu, Q.; Polydorides, A.D. Diagnosis and prognostic significance of extramural venous invasion in neuroendocrine tumors of the small intestine. Mod. Pathol. 2020, 33, 2318–2329. [Google Scholar] [CrossRef]
- Izumo, W.; Higuchi, R.; Furukawa, T.; Yazawa, T.; Uemura, S.; Matsunaga, Y.; Shiihara, M.; Takayama, Y.; Tahara, J.; Shimizu, K.; et al. Evaluation of the significance of lymphatic, microvascular and perineural invasion in patients with pancreatic neuroendocrine neoplasms. Cancer Diagn. Progn. 2022, 2, 150–159. [Google Scholar] [CrossRef]
- Kiritani, S.; Arita, J.; Mihara, Y.; Nagata, R.; Ichida, A.; Kawaguchi, Y.; Ishizawa, T.; Akamatsu, N.; Kaneko, J.; Hasegawa, K. Venous invasion and lymphatic invasion are correlated with the postoperative prognosis of pancreatic neuroendocrine neoplasm. Surgery 2023, 173, 365–372. [Google Scholar] [CrossRef]
- Schiavo Lena, M.; Partelli, S.; Andreasi, V.; Muffatti, F.; Redegalli, M.; Brunetto, E.; Maghini, B.; Falke, M.; Cangi, M.G.; Perren, A.; et al. Infiltrative Growth Predicts the Risk of Recurrence After Surgery in Well-Differentiated Non-Functioning Pancreatic Neuroendocrine Tumors. Endocr. Pathol. 2023, 34, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Bösch, F.; Bruewer, K.; D’Anastasi, M.; Ilhan, H.; Knoesel, T.; Pratschke, S.; Thomas, M.; Rentsch, M.; Guba, M.; Werner, J.; et al. Neuroendocrine tumors of the small intestine causing a desmoplastic reaction of the mesentery are a more aggressive cohort. Surgery 2018, 164, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Moyana, T.N.; Kendal, W.S.; Chatterjee, A.; Jonker, D.J.; Maroun, J.A.; Grimard, L.; Shabana, W.; Mimeault, R.; Hakim, S.W. Role of fine-needle aspiration in the surgical management of pancreatic neuroendocrine tumors: Utility and limitations in light of the new World Health Organization classification. Arch. Pathol. Lab. Med. 2014, 138, 896–902. [Google Scholar] [CrossRef]
- Dunn, P.K.; Smyth, G.K. Generalized Linear Models with Examples in R; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mansournia, M.A.; Geroldinger, A.; Greenland, S.; Heinze, G. Separation in Logistic Regression: Causes, Consequences, and Control. Am. J. Epidemiol. 2018, 187, 864–870. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Storan, D.; Swan, N.; Swan, K.; Thuillier, R.; Skehan, S.; Gallagher, T.; O’Shea, D.; O’Toole, D. Clinical features and outcomes of appendiceal neuroendocrine tumours: 10 year audit from the Irish NET Centre of Excellence. J. Neuroendocr. 2023, 35, e13329. [Google Scholar] [CrossRef]
- Moyana, T.N.; Safkunam, N. A comparative immunohistochemical study of jejunoileal and appendiceal carcinoids. Implications for histogenesis and pathogenesis. Cancer 1992, 70, 1081–1088. [Google Scholar] [CrossRef]
- Köseoğlu, H.; Duzenli, T.; Sezikli, M. Gastric neuroendocrine neoplasms: A review. World J. Clin. Cases 2021, 9, 7973–7985. [Google Scholar] [CrossRef]
- Niederle, B.; Selberherr, A.; Bartsch, D.K.; Brandi, M.L.; Doherty, G.M.; Falconi, M.; Goudet, P.; Halfdanarson, T.R.; Ito, T.; Jensen, R.T.; et al. Multiple endocrine neoplasia type 1 and the pancreas: Diagnosis and treatment of functioning and non-functioning pancreatic and duodenal neuroendocrine neoplasia within the MEN1 syndrome–an international consensus statement. Neuroendocrinology 2021, 111, 609–630. [Google Scholar] [CrossRef]
- Garbrecht, N.; Anlauf, M.; Schmitt, A.; Henopp, T.; Sipos, B.; Raffel, A.; Eisenberger, C.F.; Knoefel, W.T.; Pavel, M.; Fottner, C.; et al. Somatostatin-producing neuroendocrine tumors of the duodenum and pancreas: Incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr. Relat. Cancer 2008, 15, 229–241. [Google Scholar] [CrossRef]
- Broecker, J.S.; Ethun, C.G.; Postlewait, L.M.; Le, N.; Mcinnis, M.; Russell, M.C.; Sullivan, P.; Kooby, D.A.; Staley, C.A.; Maithel, S.K.; et al. Colon and rectal neuroendocrine tumors: Are they really one disease? A single-institution experience over 15 years. Am. Surg. 2018, 84, 717–726. [Google Scholar] [CrossRef]
- Levy, S.; van Veenendaal, L.M.; Korse, C.M.; Breekveldt, E.C.; Verbeek, W.H.; Vriens, M.R.; Kuhlmann, K.F.; van den Berg, J.G.; Valk, G.D.; Tesselaar, M.E. Survival in patients with neuroendocrine tumours of the small intestine: Nomogram validation and predictors of survival. J. Clin. Med. 2020, 9, 2502. [Google Scholar] [CrossRef] [PubMed]
- Laskaratos, F.M.; Walker, M.; Wilkins, D.; Tuck, A.; Ramakrishnan, S.; Phillips, E.; Gertner, J.; Megapanou, M.; Papantoniou, D.; Shah, R.; et al. Evaluation of clinical prognostic factors and further delineation of the effect of mesenteric fibrosis on survival in advanced midgut neuroendocrine tumours. Neuroendocrinology 2018, 107, 292–304. [Google Scholar] [CrossRef]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; Debraud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 380–387. [Google Scholar] [CrossRef]
- Bennett, S.; Coburn, N.; Law, C.; Mahar, A.; Zhao, H.; Singh, S.; Zuk, V.; Myrehaug, S.; Gupta, V.; Levy, J.; et al. Upfront small bowel resection for small bowel neuroendocrine tumors with synchronous metastases: A propensity-score matched comparative population-based analysis. Ann. Surg. 2022, 276, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Koumarianou, A.; Alexandraki, K.I.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Pathogenesis and clinical management of mesenteric fibrosis in small intestinal neuroendocine neoplasms: A systematic review. J. Clin. Med. 2020, 9, 1777. [Google Scholar] [CrossRef]
- Bagante, F.; Spolverato, G.; Merath, K.; Postlewait, L.M.; Poultsides, G.A.; Mullen, M.G.; Bauer, T.W.; Fields, R.C.; Lamelas, J.; Marques, H.P.; et al. Neuroendocrine liver metastasis: The chance to be cured after liver surgery. J. Surg. Oncol. 2017, 115, 687–695. [Google Scholar] [CrossRef]
- Gudmundsdottir, H.; Habermann, E.B.; Vierkant, R.A.; Starlinger, P.; Thiels, C.A.; Warner, S.G.; Smoot, R.L.; Truty, M.J.; Kendrick, M.L.; Halfdanarson, T.R.; et al. Survival and symptomatic relief after cytoreductive hepatectomy for neuroendocrine tumor liver metastases: Long-term follow-up evaluation of more than 500 patients. Ann. Surg. Oncol. 2023, 30, 4840–4851. [Google Scholar] [CrossRef] [PubMed]
- Maspero, M.; Rossi, R.E.; Sposito, C.; Coppa, J.; Citterio, D.; Mazzaferro, V. Long-term outcomes of resection versus transplantation for neuroendocrine liver metastases meeting the Milan criteria. Am. J. Transplant. 2022, 22, 2598–2607. [Google Scholar] [CrossRef]
- Kasai, Y.; Ito, T.; Masui, T.; Nagai, K.; Anazawa, T.; Uchida, Y.; Ishii, T.; Umeshita, K.; Eguchi, S.; Soejima, Y.; et al. Liver transplantation for gastroenteropancreatic neuroendocrine liver metastasis: Optimal patient selection and perioperative management in the era of multimodal treatments. J. Gastroenterol. 2024, 60, 1–9. [Google Scholar] [CrossRef]
- Halle-Smith, J.M.; Powell-Brett, S.; Roberts, K.; Chatzizacharias, N.A. Resection of isolated liver oligometastatic disease in pancreatic ductal adenocarcinoma: Is there a survival benefit? A systematic review. World J. Gastrointest. Surg. 2023, 15, 1512–1521. [Google Scholar] [CrossRef]
- Clements, N.; Gaskins, J.; Martin, R.C. Surgical Outcomes in Stage IV Pancreatic Cancer with Liver Metastasis Current Evidence and Future Directions: A Systematic Review and Meta-Analysis of Surgical Resection. Cancers 2025, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; Kirsch, R.; Driman, D.K.; Messenger, D.E.; Assarzadegan, N.; Riddell, R.H. Optimizing the detection of venous invasion in colorectal cancer: The Ontario, Canada, experience and beyond. Front. Oncol. 2015, 4, 354. [Google Scholar] [CrossRef][Green Version]
- Mc Entee, P.D.; Shokuhi, P.; Rogers, A.C.; Mehigan, B.J.; McCormick, P.H.; Gillham, C.M.; Kennedy, M.J.; Gallagher, D.J.; Ryan, C.E.; Muldoon, C.B.; et al. Extramural venous invasion (EMVI) in colorectal cancer is associated with increased cancer recurrence and cancer-related death. Eur. J. Surg. Oncol. 2022, 48, 1638–1642. [Google Scholar] [CrossRef]
- Lord, A.C.; Knijn, N.; Brown, G.; Nagtegaal, I.D. Pathways of spread in rectal cancer: A reappraisal of the true routes to distant metastatic disease. Eur. J. Cancer 2020, 128, 1–6. [Google Scholar] [CrossRef]
- Spyroglou, A.; Violetis, O.; Iliakopoulos, K.; Vezakis, A.; Alexandraki, K. Mesenteric Fibrosis in Neuroendocrine Neoplasms: A Systematic Review of New Thoughts on Causation and Potential Treatments. Curr. Oncol. Rep. 2025, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cuny, T.; De Herder, W.; Barlier, A.; Hofland, L.J. Role of the tumor microenvironment in digestive neuroendocrine tumors. Endocr. Relat. Cancer 2018, 25, R519–R544. [Google Scholar] [CrossRef] [PubMed]
- Blažević, A.; Iyer, A.M.; van Velthuysen, M.L.; Hofland, J.; van Koestveld, P.M.; Franssen, G.J.; Feelders, R.A.; Zajec, M.; Luider, T.M.; de Herder, W.W.; et al. Aberrant tryptophan metabolism in stromal cells is associated with mesenteric fibrosis in small intestinal neuroendocrine tumors. Endocr. Connect. 2022, 11, e220020. [Google Scholar] [CrossRef]
- Graf, S.D.; Keber, C.U.; Hattesohl, A.; Teply-Szymanski, J.; Hattesohl, S.; Guder, M.; Gercke, N.; Di Fazio, P.; Slater, E.P.; Jesinghaus, M.; et al. Mesenteric fibrosis in patients with small intestinal neuroendocrine tumors is associated with enrichment of alpha-smooth muscle actin-positive fibrosis and COMP-expressing stromal cells. J. Neuroendocr. 2024, 36, e13364. [Google Scholar] [CrossRef]



| Variables | Frequency (Percentage) * | ||
|---|---|---|---|
| Overall (N = 43) | Alive (N = 29) | Dead (N = 14) | |
| Age at follow-up | |||
| Median (IQR) | 63.70 (58.80, 74.14) | 61.73 (57.63, 69.23) | 73.18 (63.57, 84.64) |
| Mean (SD) | 66.32 (12.18) | 63.30 (9.80) | 72.57 (14.49) |
| (Min, Max) | (43.70, 97.53) | (43.70, 83.29) | (46.45, 97.53) |
| Age at diagnosis | |||
| Median (IQR) | 56.53 (50.93, 66.77) | 54.72 (45.64, 61.20) | 70.91 (56.06, 75.94) |
| Mean (SD) | 59.08 (12.21) | 55.48 (10.68) | 66.55 (12.14) |
| (Min, Max) | (40.91, 84.73) | (40.91, 81.93) | (45.66, 84.73) |
| Follow-up time | |||
| Median (IQR) | 5.81 (4.42, 9.44) | 6.32 (4.61, 9.87) | 6.02 (3.68) |
| Mean (SD) | 7.23 (5.16) | 7.82 (5.70) | 5.59 (3.01, 8.74) |
| (Min, Max) | (0.11, 20.99) | (0.11, 20.99) | (0.79, 12.80) |
| Sex | |||
| Female | 18 (41.86) | 10 (34.48) | 8 (57.14) |
| Male | 25 (58.14) | 19 (65.52) | 6 (942.86) |
| Tumor Stage ** | |||
| 1 | 2 (4.76) | 0 (0) | 2 (14.29) |
| 2 | 4 (9.52) | 3 (10.71) | 1 (7.14) |
| 3 | 10 (23.81) | 7 (25.00) | 3 (21.43) |
| 4 | 26 (61.90) | 18 (64.29) | 8 (57.14) |
| Tumor Size: mean (SD) | 1.82 (0.70) | 1.72 (0.50) | 2.02 (0.99) |
| Lymph node stage ** | |||
| 0 | 0 (0) | 0 (0) | 0 (0) |
| 1 | 25 (59.52) | 17 (60.71) | 8 (57.14) |
| 2 | 17 (40.48) | 11 (39.29) | 6 (42.86) |
| Mesenteric fibrosis ** | |||
| No | 24 (58.54) | 23 (82.14) | 1 (7.14) |
| Yes | 17 (41.46) | 4 (14.29) | 13 (92.86) |
| EMVI ** | |||
| No | 23 (56.10) | 23 (85.19) | 0 (0) |
| Yes | 18 (43.90) | 4 (14.81) | 14 (100) |
| Perineural invasion ** | |||
| No | 6 (14.63) | 2 (7.41) | 4 (30.77) |
| Yes | 34 (82.93) | 25 (92.59) | 9 (69.23) |
| Potential Predictor Variables | Unadjusted OR [95% CI] | p-Value |
|---|---|---|
| Sex (Female) | 0.39 [0.10, 1.44] | 0.1632 |
| Age at diagnosis (in years) | 0.92 [0.86, 0.98] | 0.0089 * |
| Age at follow-up (in years) | 0.93 [0.87, 0.99] | 0.0262 * |
| Presence of mesenteric fibrosis | 0.013 [0.001, 0.093] | 0.0002 * |
| Tumor stage | 1.53 [0.73, 3.35] | 0.2580 |
| Tumor size | 0.54 [0.20, 1.36] | 0.1949 |
| Lymph node stage | 0.86 [0.23, 3.26] | 0.8240 |
| Perineural invasion | 5.56 [0.92, 45.39] | 0.0708 |
| Primary Ki67 | 5.62 [0.86, 111.89] | 0.1260 |
| Liver mitosis | 0.76 [0.16, 4.26] | 0.7410 |
| Liver Ki67 | 0.47 [0.11, 1.67] | 0.2566 |
| Potential Predictor Variables | Adjusted OR [95% CI] | p-Value |
|---|---|---|
| Sex (Female) | 3.64 [0.25, 119.84] | 0.3972 |
| Age at diagnosis | 0.86 [0.70, 0.96] | 0.0441 * |
| Mesenteric fibrosis | 0.002 [0.000, 0.059] | 0.0091 * |
| Tumor Size | 1.50 [0.19, 13.18] | 0.6901 |
| Markers | Frequency (Percentage) | ||
|---|---|---|---|
| Overall | Alive | Dead | |
| Primary infiltration | |||
| 1 | 6 (14.29) | 6 (21.43) | 0 (0) |
| 2 | 7 (16.67) | 7 (25.00) | 0 (0) |
| 3 | 29 (69.05) | 15 (53.57) | 14 (100) |
| Primary mitosis | |||
| 1 | 38 (95.00) | 26 (92.86) | 12 (100) |
| 2 | 2 (5.00) | 2 (7.14) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) |
| Primary Ki67 | |||
| 1 | 25 (69.44) | 16 (61.54) | 9 (90.00) |
| 2 | 11 (30.56) | 10 (38.46) | 1 (10.00) |
| 3 | 0 (0) | 0 (0) | 0 (0) |
| Liver infiltration | |||
| 1 | 22 (59.46) | 22 (84.62) | 0 (0) |
| 2 | 12 (32.43) | 4 (15.38) | 8 (72.73) |
| 3 | 3 (8.11) | 0 (0) | 3 (27.27) |
| Liver mitosis | |||
| 1 | 35 (81.40) | 24 (82.76) | 11 (78.57) |
| 2 | 8 (18.60) | 5 (17.24) | 3 (21.43) |
| 3 | 0 (0) | 0 (0) | 0 (0) |
| Liver Ki67 | |||
| 1 | 17 (42.50) | 13 (46.43) | 4 (33.33) |
| 2 | 22 (55.00) | 15 (53.57) | 7 (58.33) |
| 3 | 1 (2.50) | 0 (0) | 1 (8.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranot, J.M.; Hamid, J.S.; Montazeri, A.; Harper, K.; McCudden, C.; Moyana, T.N. Well-Differentiated Jejunoileal Neuroendocrine Tumors and Corresponding Liver Metastases: Mesenteric Fibrogenesis and Extramural Vascular Invasion in Tumor Progression. Cancers 2025, 17, 1486. https://doi.org/10.3390/cancers17091486
Ranot JM, Hamid JS, Montazeri A, Harper K, McCudden C, Moyana TN. Well-Differentiated Jejunoileal Neuroendocrine Tumors and Corresponding Liver Metastases: Mesenteric Fibrogenesis and Extramural Vascular Invasion in Tumor Progression. Cancers. 2025; 17(9):1486. https://doi.org/10.3390/cancers17091486
Chicago/Turabian StyleRanot, Jacob M., Jemila S. Hamid, Azita Montazeri, Kelly Harper, Christopher McCudden, and Terence N. Moyana. 2025. "Well-Differentiated Jejunoileal Neuroendocrine Tumors and Corresponding Liver Metastases: Mesenteric Fibrogenesis and Extramural Vascular Invasion in Tumor Progression" Cancers 17, no. 9: 1486. https://doi.org/10.3390/cancers17091486
APA StyleRanot, J. M., Hamid, J. S., Montazeri, A., Harper, K., McCudden, C., & Moyana, T. N. (2025). Well-Differentiated Jejunoileal Neuroendocrine Tumors and Corresponding Liver Metastases: Mesenteric Fibrogenesis and Extramural Vascular Invasion in Tumor Progression. Cancers, 17(9), 1486. https://doi.org/10.3390/cancers17091486






