The Prognostic Role of Magnetic-Resonance-Imaging-Detected Corpus Invasion in Patients with Cervical Carcinoma Who Underwent Definitive or Adjuvant Pelvic Radiotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Measurement of Uterine Corpus Invasion
2.3. External Beam Radiotherapy
2.4. High-Dose-Rate Brachytherapy
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of the Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Clinical Practice
4.5. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| MRI | Magnetic Resonance Imaging |
| FIGO | International Federation of Gynecology and Obstetrics |
| AJCC | American Joint Committee on Cancer |
| PALN | Para-aortic Lymph Node |
| CCRT | Concurrent Chemoradiotherapy |
| UCI | Uterine Corpus Invasion |
| EFRT | Extended-Field Radiotherapy |
| SCC-Ag | Squamous Cell Carcinoma Antigen |
| CEA | Carcinoembryonic Antigen |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| OS | Overall Survival |
| CSS | Cancer-Specific Survival |
| LR | Local recurrence |
| RR | Regional recurrence |
| LRR | Locoregional Recurrence |
| EPM | Extrapelvic Metastasis |
| PALNR | Para-aortic Lymph Node Recurrence |
| CT | Computed Tomography |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| ROC Curve | Receiver Operating Characteristic Curve |
| AUC | Area Under the Curve |
| EQD2 | Equivalent Dose at 2 Gy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Islami, F.; Fedewa, S.A.; Jemal, A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev. Med. 2019, 123, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J. Clin. 2021, 71, 287–298. [Google Scholar] [CrossRef]
- Narayan, K.; Fisher, R.; Bernshaw, D. Significance of tumor volume and corpus uteri invasion in cervical cancer patients treated by radiotherapy. Int. J. Gynecol. Cancer 2006, 16, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, W.; Lee, M.; Song, E.; Loh, J.J. Tumor volume and uterine body invasion assessed by MRI for prediction of outcome in cervical carcinoma treated with concurrent chemotherapy and radiotherapy. Jpn J. Clin. Oncol. 2007, 37, 858–866. [Google Scholar] [CrossRef][Green Version]
- Narayan, K.; Fisher, R.J.; Bernshaw, D.; Shakher, R.; Hicks, R.J. Patterns of failure and prognostic factor analyses in locally advanced cervical cancer patients staged by positron emission tomography and treated with curative intent. Int. J. Gynecol. Cancer 2009, 19, 912–918. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Blake, E.A.; Takiuchi, T.; Mikami, M.; Roman, L.D. Significance of uterine corpus tumor invasion in early-stage cervical cancer. Eur. J. Surg. Oncol. 2017, 43, 725–734. [Google Scholar] [CrossRef]
- Hope, A.J.; Saha, P.; Grigsby, P.W. FDG-PET in carcinoma of the uterine cervix with endometrial extension. Cancer 2006, 106, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.; Mckenzie, A.F.; Hicks, R.J.; Fisher, R.; Bernshaw, D.; Bau, S. Relation between FIGO stage, primary tumor volume, and presence of lymph node metastases in cervical cancer patients referred for radiotherapy. Int. J. Gynecol. Cancer 2003, 13, 657. [Google Scholar] [CrossRef]
- Prempree, T.; Patanaphan, V.; Viravathana, T.; Sewchand, W.; Cho, Y.K.; Scott, R.M. Radiation treatment of carcinoma of the cervix with extension into the endometrium: A reappraisal of its significance. Cancer 1982, 49, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Zivnuska, F.; Askin, F.; Kumar, B.; Camel, H.M.; Powers, W.E. Prognostic significance of endometrial extension from primary carcinoma of the uterinecervix. Cancer 1975, 35, 1493–1504. [Google Scholar] [CrossRef]
- He, F.; Li, W.; Liu, P.; Kang, S.; Sun, L.; Zhao, H.; Chen, X.; Yin, L.; Wang, L.; Chen, J.; et al. Influence of uterine corpus invasion on prognosis in stage IA2–IIB cervical cancer: A multicenter retrospective cohort study. Gynecol. Oncol. 2020, 158, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Turan, T.; Kimyon Comert, G.; Boyraz, G.; Kilic, F.; Cakir, C.; Kilic, C.; Yuksel, D.; Unsal, M.; Karalok, A.; Turkmen, O. What is the impact of corpus uterine invasion on oncologic outcomes in surgically treated cervical cancer? J. Obs. Gynaecol. Res. 2021, 47, 3634–3643. [Google Scholar] [CrossRef]
- Roh, H.J.; Kim, K.B.; Lee, J.H.; Kim, H.J.; Kwon, Y.S.; Lee, S.H. Early Cervical Cancer: Predictive Relevance of Preoperative 3-Tesla Multiparametric Magnetic Resonance Imaging. Int. J. Surg. Oncol. 2018, 2018, 9120753. [Google Scholar] [CrossRef]
- Huang, E.Y.; Wang, C.J.; Chen, H.C.; Fang, F.M.; Huang, Y.J.; Wang, C.Y.; Hsu, H.C. Multivariate analysis of para-aortic lymph node recurrence after definitive radiotherapy for stage IB-IVA squamous cell carcinoma of uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 834–842. [Google Scholar] [CrossRef]
- Huang, E.Y.; Huang, Y.J.; Chanchien, C.C.; Lin, H.; Wang, C.J.; Sun, L.M.; Tseng, C.W.; Tsai, C.C.; Ou, Y.C.; Fu, H.C.; et al. Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix. Radiat. Oncol. 2012, 7, 13. [Google Scholar] [CrossRef]
- Hsu, H.C.; Leung, S.W.; Huang, E.Y.; Wang, C.J.; Sun, L.M.; Fang, F.M.; Chen, H.C. Impact of the extent of parametrial involvement in patients with carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 405–410. [Google Scholar] [CrossRef]
- Yoo, W.; Mayberry, R.; Bae, S.; Singh, K.; He, Q.P.; Lillard, J.W., Jr. A Study of Effects of MultiCollinearity in the Multivariable Analysis. Int. J. Appl. Sci. Technol. 2014, 4, 9–19. [Google Scholar] [PubMed]
- Mitchell, D.G.; Snyder, B.; Coakley, F.; Reinhold, C.; Thomas, G.; Amendola, M.; Schwartz, L.H.; Woodward, P.; Pannu, H.; Hricak, H. Early invasive cervical cancer: Tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J. Clin. Oncol. 2006, 24, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- de Boer, P.; Adam, J.A.; Buist, M.R.; van de Vijver, M.J.; Rasch, C.R.; Stoker, J.; Bipat, S.; Stalpers, L.J. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: Systematic review of diagnostic test accuracy. Eur. J. Radiol. 2013, 82, e422–e428. [Google Scholar] [CrossRef]
- Huang, X.; Chen, K.; Shi, L.; Luo, Y.; Ou-Yang, Y.; Li, J.; Huo, L.; Huang, L.; Chen, F.; Cao, X. Construction of refined staging classification systems integrating FIGO/T-categories and corpus uterine invasion for non-metastatic cervical cancer. Cancer Med. 2023, 12, 15079–15089. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Zhou, Y.; Hu, K.; Zhang, F.; Qiu, J.; Hou, X.; Lian, X.; Yan, J.; Liu, Z.; et al. Prophylactic extended-field irradiation versus pelvic irradiation in patients with cervical cancer with 2018 FIGO Stage IIIC1 disease. Pract. Radiat. Oncol. 2023, 13, e409–e415. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Meng, Q.; Zhang, F.; Hu, K. Prophylactic extended-field irradiation for patients with cervical cancer treated with concurrent chemoradiotherapy: A propensity-score matching analysis. Int. J. Gynecol. Cancer 2018, 28, 1584–1591. [Google Scholar] [CrossRef]
- Chen, C.S.; Wang, Y.M.; Huang, E.Y. Comparative analysis of oncologic outcomes in patients with squamous cell carcinoma of the uterine cervix with high-risk features for para-aortic recurrence: Prophylactic extended-field versus pelvic chemoradiotherapy. Cancer Manag. Res. 2024, 16, 269–279. [Google Scholar] [CrossRef]
- Peters, M.; de Leeuw, A.A.; Nomden, C.N.; Tanderup, K.; Kirchheiner, K.; Lindegaard, J.C.; Kirisits, C.; Haie-Meder, C.; Sturdza, A.; Fokdal, L.; et al. EMBRACE Collaborative Group. Risk factors for nodal failure after radiochemotherapy and image guided brachytherapy in locally advanced cervical cancer: An EMBRACE analysis. Radiother. Oncol. 2021, 163, 150–158. [Google Scholar] [CrossRef]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Gomes, A.J.P.d.S.; et al. ENGOT-cx11/GOG-3047/KEYNOTE-A18 investigators. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Cvek, J.; Randall, L.; de Santana Gomes, A.J.P.; et al. ENGOT-cx11/GOG-3047/KEYNOTE-A18 investigators. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 404, 1321–1332. [Google Scholar] [PubMed]
- Höckel, M.; Horn, L.-C.; Fritsch, H. Association between the mesenchymal compartment of uterovaginal organogenesis and local tumour spread in stage IB-IIB cervical carcinoma: A prospective study. Lancet Oncol. 2005, 6, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Horn, L.-C.; Manthey, N.; Braumann, U.D.; Wolf, U.; Teichmann, G.; Frauenschläger, K.; Dornhöfer, N.; Einenkel, J. Resection of the embryologically defined uterovaginal (Müllerian) compartment and pelvic control in patients with cervical cancer: A prospective analysis. Lancet Oncol. 2009, 10, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, F.; Liu, P.; Duan, H.; Ni, Y.; Wang, S.; Lin, L.; Yin, Z.; Chen, X.; Yin, L.; et al. Uterine corpus invasion in cervical cancer: A multicenter retrospective case-control study. Arch. Gynecol. Obstet. 2021, 303, 777–785. [Google Scholar] [CrossRef]
- Saunders, N.; Anderson, D.; Sheridan, E.; Gilbert, L.; Sharp, F. Endoscopic localization of the squamocolumnar junction before cervical cone biopsy in 284 patients. Cancer 1990, 65, 1312–1317. [Google Scholar] [CrossRef]
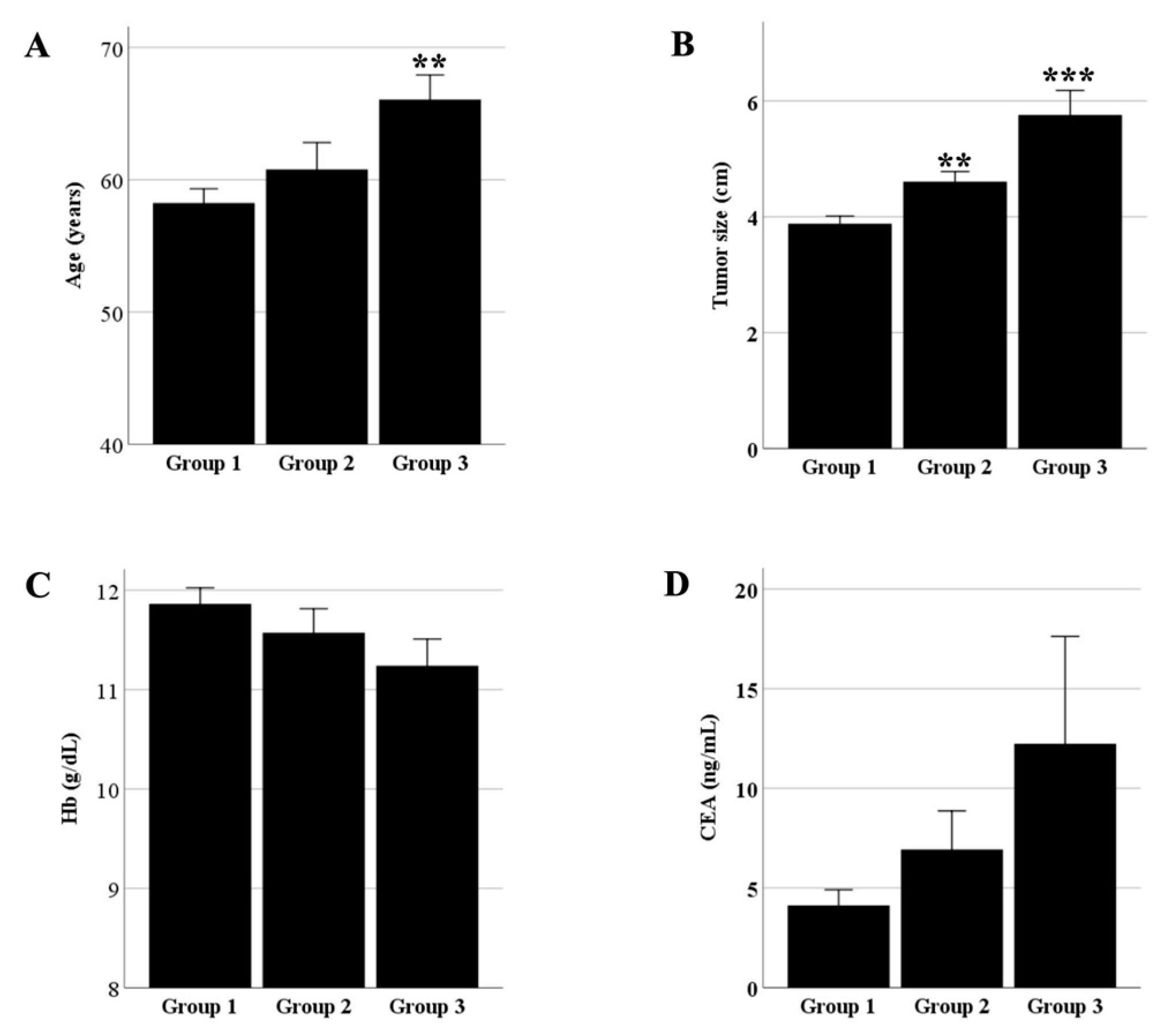
| Group 1 (n = 171) | Group 2 (n = 49) | Group 3 (n = 39) | Total (n = 259) | p-Value | |
|---|---|---|---|---|---|
| Parameter | n (%) | n (%) | n (%) | n (%) | |
| Age (y) | 0.002 | ||||
| <65 | 114 (66.7) | 30 (61.2) | 15 (38.5) | 159 (61.4) | |
| ≥65 | 57 (33.3) | 19 (38.8) | 24 (61.5) | 100 (38.6) | |
| Size (cm) | <0.001 | ||||
| <4 | 79 (46.2) | 12 (24.5) | 7 (17.9) | 98 (37.8) | |
| ≥4 | 64 (37.4) | 34 (69.4) | 30 (76.9) | 128 (49.4) | |
| Unknown | 28 (16.4) | 3 (6.1) | 2 (5.1) | 33 (12.7) | |
| Stage | <0.001 | ||||
| I | 68 (39.8) | 9 (18.4) | 6 (15.4) | 83 (32.0) | |
| II | 86 (50.3) | 30 (61.2) | 25 (64.1) | 141 (54.4) | |
| III | 14 (8.2) | 8 (16.3) | 5 (12.8) | 27 (10.4) | |
| IV | 3 (1.8) | 2 (4.1) | 3 (7.7) | 8 (3.1) | |
| PM score | 0.001 | ||||
| 0–3 | 144 (84.2) | 37 (75.5) | 24 (61.5) | 205 (79.2) | |
| 4–6 | 13 (7.6) | 8 (16.3) | 8 (20.5) | 29 (11.2) | |
| Unknown | 14 (8.2) | 4 (8.2) | 7 (17.9) | 25 (9.7) | |
| Pathology | 0.534 | ||||
| SCC | 144 (84.2) | 43 (87.8) | 34 (87.2) | 221 (85.3) | |
| Non-SCC | 27 (15.8) | 6 (12.2) | 5 (12.8) | 38 (14.7) | |
| Hemoglobin (g/dL) | 0.026 | ||||
| <12 | 67 (39.2) | 25 (51.0) | 23 (59.0) | 115 (44.4) | |
| ≥12 | 92 (53.8) | 22 (44.9) | 15 (38.5) | 129 (49.8) | |
| Unknown | 12 (7.0) | 2 (4.1) | 1 (2.6) | 15 (5.8) | |
| Hypertension | 0.046 | ||||
| No | 119 (69.6) | 30 (61.2) | 21 (53.8) | 170 (65.6) | |
| Yes | 52 (30.4) | 19 (38.8) | 18 (46.2) | 89 (34.4) | |
| Diabetes | 0.667 | ||||
| No | 142 (83.0) | 44 (89.8) | 30 (76.9) | 216 (83.4) | |
| Yes | 29 (17.0) | 5 (10.2) | 9 (23.1) | 43 (16.6) | |
| Pelvic lymphadenopathy on MRI | 0.062 | ||||
| Negative | 142 (83.0) | 36 (73.5) | 28 (71.8) | 206 (79.5) | |
| Positive | 29 (17.0) | 13 (26.5) | 11 (28.2) | 53 (20.5) | |
| Hysterectomy | 0.761 | ||||
| No | 162 (94.7) | 47 (95.9) | 38 (97.4) | 247 (95.4) | |
| Yes | 9 (5.3) | 2 (4.1) | 1 (2.6) | 12 (4.6) | |
| Concurrent chemotherapy (cycles) | 0.419 | ||||
| 0 | 49 (28.7) | 11 (23.4) | 9 (23.1) | 69 (26.6) | |
| 1–3 | 7 (4.1) | 1 (2.0) | 3 (7.7) | 11 (4.2) | |
| 4–6 | 105 (61.4) | 34 (69.4) | 24 (61.5) | 163 (62.9) | |
| >6 | 10 (5.8) | 3 (6.1) | 3 (7.7) | 16 (6.2) | |
| SCC-Ag level (ng/mL) | 0.285 | ||||
| <20 | 150 (87.7) | 41 (85.1) | 35 (89.7) | 226 (87.3) | |
| 20–40 | 12 (7.0) | 3 (6.1) | 0 (0.0) | 15 (5.8) | |
| ≥40 | 3 (1.8) | 3 (6.1) | 3 (7.7) | 9 (3.5) | |
| Unknown | 6 (3.5) | 2 (4.1) | 1 (2.6) | 9 (3.5) | |
| CEA level (ng/mL) | |||||
| <10 | 157 (91.8) | 41 (83.7) | 31 (79.5) | 229 (88.4) | 0.005 |
| ≥10 | 8 (4.7) | 6 (12.2) | 7 (17.9) | 21 (8.1) | |
| Unknown | 6 (3.5) | 2 (4.1) | 1 (2.6) | 9 (3.5) | |
| Risk group | 0.110 | ||||
| Low | 101 (59.1) | 23 (46.9) | 14 (35.9) | 138 (53.3) | |
| Intermediate | 28 (16.4) | 10 (20.4) | 9 (23.1) | 47 (18.1) | |
| High | 19 (13.5) | 10 (20.4) | 10 (25.6) | 39 (15.1) | |
| Unknown | 23 (13.5) | 6 (12.2) | 6 (15.4) | 35 (13.5) | |
| EQD2 (Gy) | 0.306 | ||||
| 61–69.9 | 9 (5.6) | 1 (2.1) | 4 (10.5) | 14 (5.7) | |
| 70–79.9 | 19 (80.2) | 36 (76.6) | 26 (68.4) | 192 (77.7) | |
| >80 | 23 (14.2) | 10 (21.3) | 8 (21.1) | 41 (16.6) |
| Parameter | Age | Stage | Size | PM Score | Hb | SCC | CEA |
|---|---|---|---|---|---|---|---|
| Age | 1 | - | - | - | - | - | - |
| Stage | 0.130 (0.037) | 1 | - | - | - | - | - |
| Size | −0.088 (0.186) | 0.502 (<0.001) | 1 | - | - | - | - |
| PM score | 0.072 (0.274) | 0.766 (<0.001) | 0.558 (<0.001) | 1 | - | - | - |
| Hb | 0.037 (0.564) | −0.305 (<0.001) | −0.368 (<0.001) | −0.273 (<0.001) | 1 | - | - |
| SCC | 0.207 (0.001) | 0.432 (<0.001) | 0.418 (<0.001) | 0.459 (<0.001) | −0.288 (<0.001) | 1 | - |
| CEA | 0.235 (<0.001) | 0.208 (0.001) | 0.122 (0.068) | 0.267 (<0.001) | −0.189 (0.004) | 0.222 (0.001) | 1 |
| Corpus invasion | 0.174 (0.005) | 0.245 (<0.001) | 0.332 (<0.001) | 0.248 (<0.001) | −0.165 (0.010) | 0.152 (0.016) | 0.214 (0.001) |
| Parameter | OS | p-Value | CSS | p-Value | LRR | p-Value | EPM | p-Value | PALNR | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 0.006 | 0.707 | 0.459 | 0.526 | 0.072 | |||||
| <65 | 81.9 | 83.4 | 14.7 | 21.2 | 14.9 | |||||
| ≥65 | 70.7 | 86.7 | 13.7 | 21.6 | 7.9 | |||||
| Size (cm) | 0.030 | 0.004 | 0.132 | 0.016 | 0.011 | |||||
| <4 | 83.3 | 92.4 | 11.8 | 17.4 | 8.8 | |||||
| ≥4 | 70.4 | 74.5 | 18.0 | 28.3 | 18.0 | |||||
| Stage | <0.001 | <0.001 | 0.001 | 0.016 | 0.002 | |||||
| I | 87.8 | 95.9 | 7.2 | 9.7 | 7.1 | |||||
| II | 76.3 | 82.7 | 14.0 | 24.3 | 14.6 | |||||
| III | 68.2 | 73.1 | 25.7 | 32.1 | 8.6 | |||||
| IV | 25.0 | 28.6 | 51.1 | 55.6 | 55.6 | |||||
| PM score [20] | 0.030 | 0.001 | 0.019 | 0.006 | 0.002 | |||||
| 0–3 | 81.8 | 89.7 | 12.3 | 16.6 | 9.3 | |||||
| 4–6 | 58.2 | 61.7 | 28.8 | 44.3 | 32.6 | |||||
| Pathology | 0.605 | 0.122 | 0.024 | 0.068 | 0.458 | |||||
| SCC | 77.6 | 85.3 | 12.8 | 19.6 | 12.4 | |||||
| Non-SCC | 77.2 | 79.8 | 22.0 | 29.3 | 13.1 | |||||
| Hemoglobin (g/dL) | <0.001 | 0.054 | 0.019 | 0.005 | 0.124 | |||||
| <12 | 68.1 | 78.1 | 20.1 | 31.2 | 16.6 | |||||
| ≥12 | 87.5 | 88.3 | 9.3 | 14.6 | 10.1 | |||||
| Pelvic lymphadenopathy | 0.028 | 0.002 | 0.003 | 0.178 | 0.011 | |||||
| Negative | 80.6 | 88.3 | 11.1 | 19.5 | 9.8 | |||||
| Positive | 65.7 | 69.9 | 26.3 | 27.3 | 23.1 | |||||
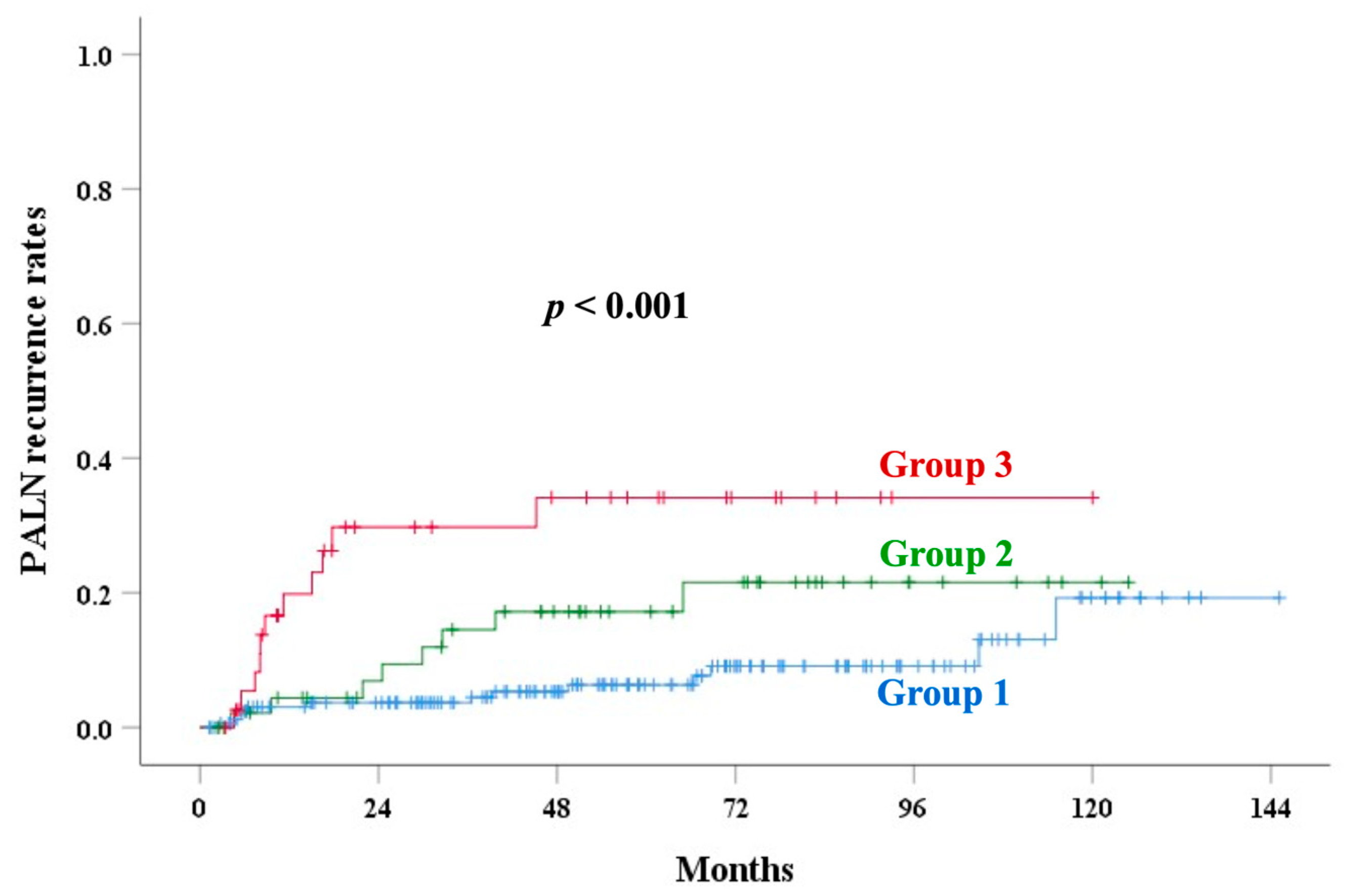
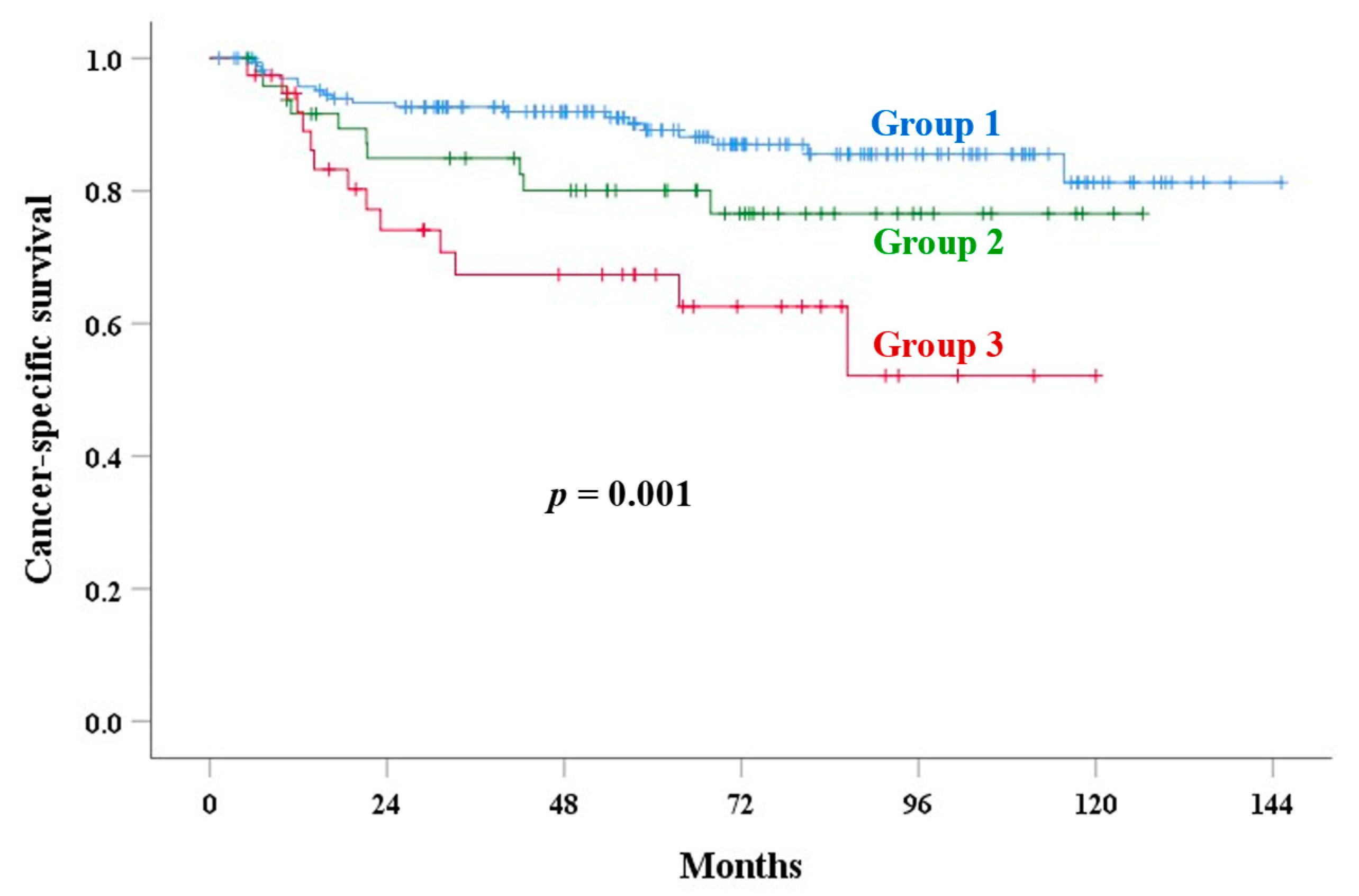
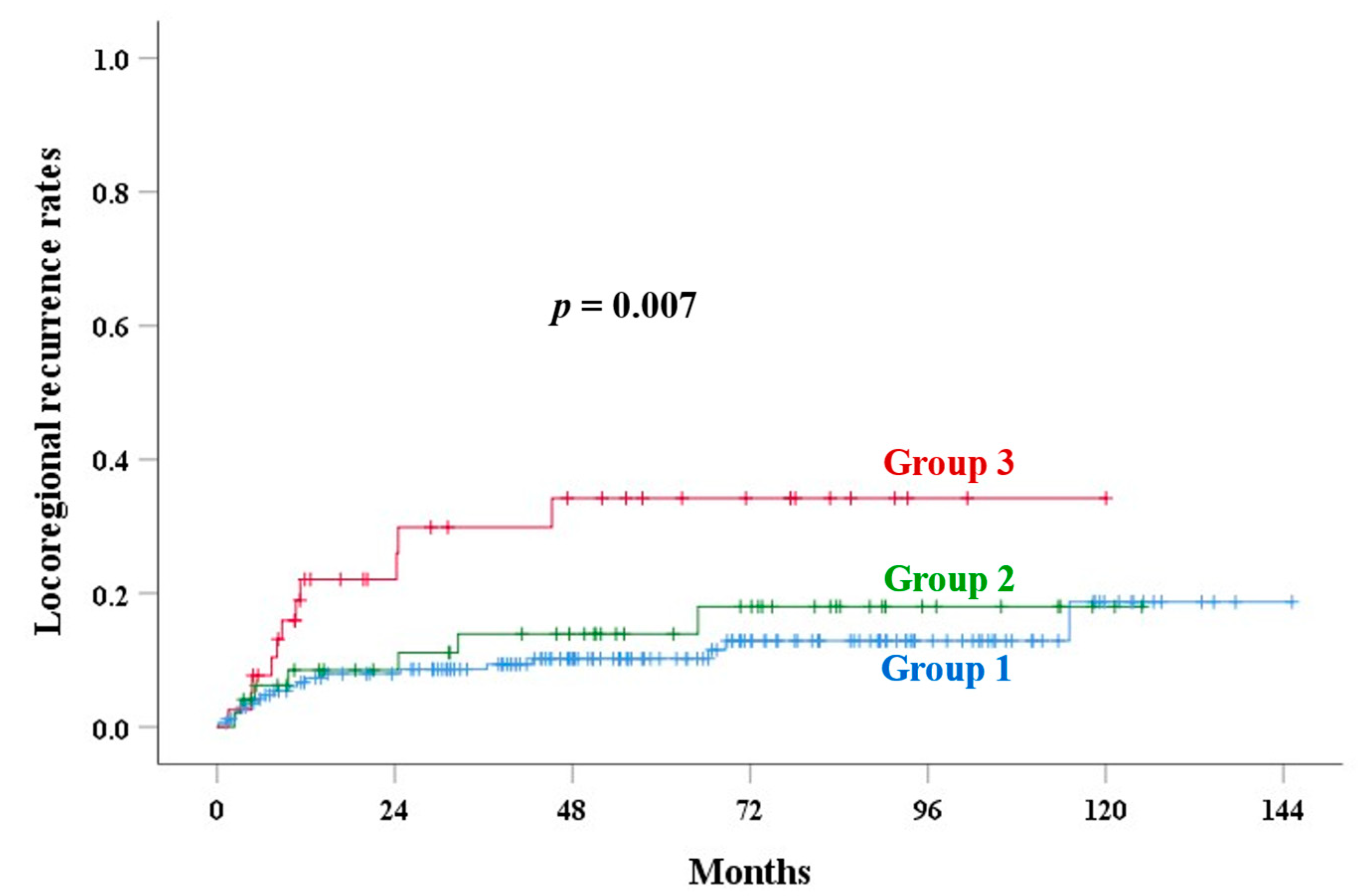
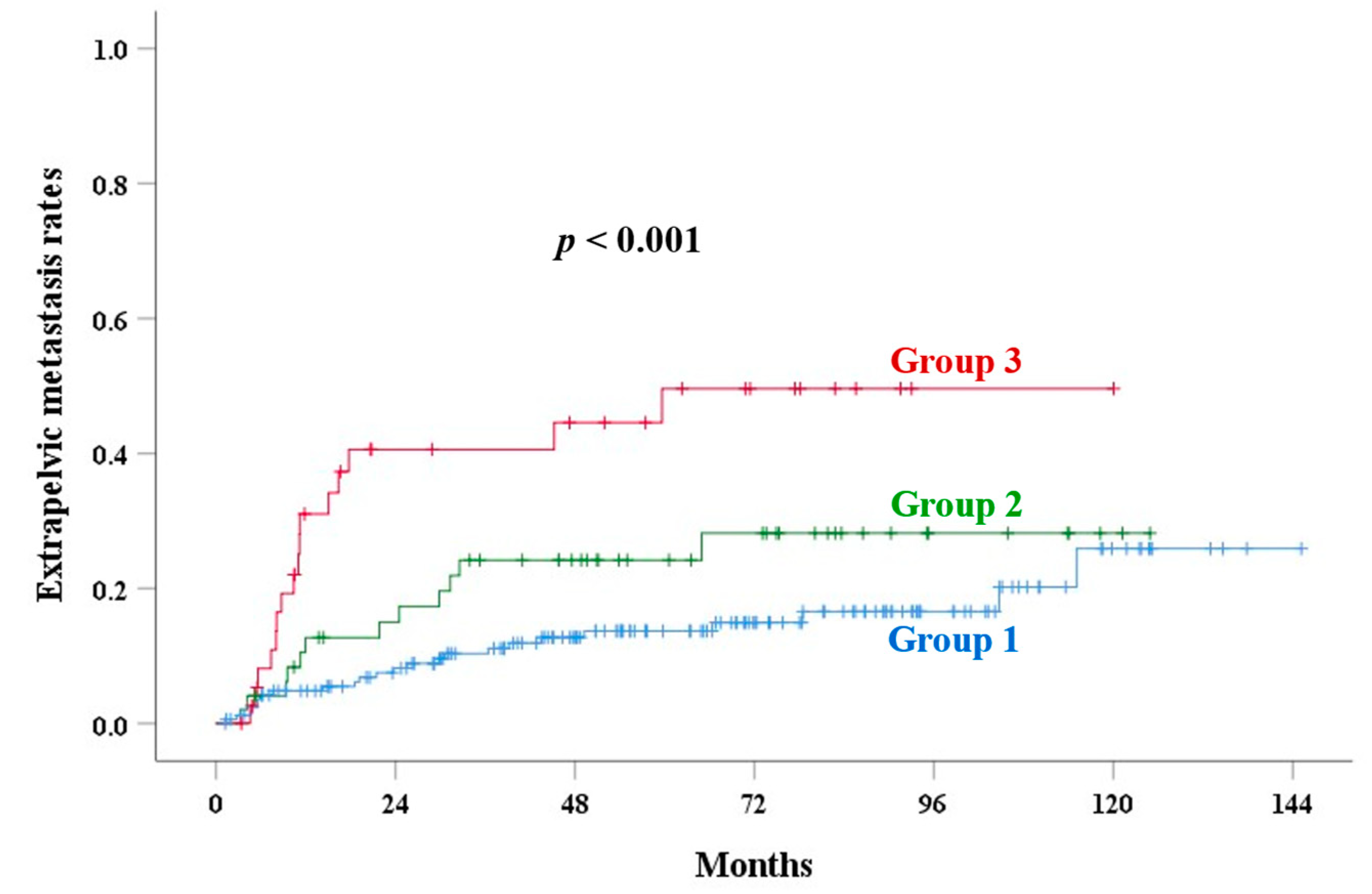
| Corpus invasion | <0.001 | 0.001 | 0.007 | <0.001 | <0.001 | |||||
| Group 1 | 82.0 | 89.2 | 10.2 | 13.7 | 6.3 | |||||
| Group 2 | 77.6 | 80.1 | 13.9 | 24.2 | 17.2 | |||||
| Group 3 | 57.3 | 67.4 | 34.2 | 49.6 | 34.2 | |||||
| SCC-Ag level (ng/mL) | 0.360 | 0.001 | 0.051 | 0.607 | 0.132 | |||||
| <20 | 79.1 | 85.8 | 12.4 | 20.3 | 11.5 | |||||
| 20–40 | 71.4 | 78.6 | 21.2 | 21.4 | 9.1 | |||||
| ≥40 | 66.7 | 75.0 | 37.5 | 37.5 | 37.5 | |||||
| CEA level (ng/mL) | 0.324 | 0.338 | 0.009 | 0.075 | 0.081 | |||||
| <10 | 77.4 | 84.3 | 13.3 | 20.3 | 12.0 | |||||
| ≥10 | 67.4 | 77.1 | 28.6 | 31.9 | 25.0 |
| Parameter | CSS HR (95% CI) | p-Value | LRR HR (95% CI) | p-Value | EPM HR (95% CI) | p-Value | PALNR HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Pathology | 0.218 | 0.049 | 0.088 | 0.524 | ||||
| Non-SCC | Reference | Reference | Reference | Reference | ||||
| SCC | 0.607 (0.274–1.344) | 0.435 (0.190–0.997) | 0.534 (0.260–1.098) | 0.727 (0.272–1.941) | ||||
| Extent of corpus invasion | 0.002 | 0.010 | <0.001 | 0.001 | ||||
| Exocervical | Reference | Reference | Reference | Reference | ||||
| Endocervical | 1.564 (0.715–3.422) | 0.263 | 1.163 (0.478–2.830) | 0.739 | 1.729 (0.853–3.506) | 0.129 | 1.858 (0.749–4.607) | 0.181 |
| Uterine corpus | 3.743 (1.820–7.700) | <0.001 | 3.259 (1.491–7.124) | 0.003 | 4.369 (2.271–8.406) | <0.001 | 5.089 (2.211–11.709) | <0.001 |
| Pelvic lymph node | 0.019 | 0.037 | 0.517 | 0.080 | ||||
| Negative | Reference | Reference | Reference | Reference | ||||
| Positive | 2.205 (1.138–4.275) | 2.163 (1.049–4.460) | 1.239 (0.648–2.371) | 2.005 (0.919–4.732) | ||||
| SCC-Ag level | 0.257 | 0.071 | 0.672 | 0.391 | ||||
| 0–20 ng/mL | Reference | Reference | Reference | Reference | ||||
| 20–40 ng/mL | 2.606 (0.873–7.778) | 0.086 | 3.582 (1.168–10.983) | 0.026 | 1.736 (0.517–5.831) | 0.372 | 1.916 (0.424–8.653) | 0.398 |
| ≥40 ng/mL | 1.385 (0.318–6.025) | 0.664 | 2.263 (0.637–8.031) | 0.206 | 1.696 (0.502–5.732) | 0.395 | 2.391 (0.677–8.446) | 0.176 |
| Unknown | 2.004 (0.537–7.474) | 0.301 | 2.094 (0.555–7.903) | 0.276 | 1.308 (0.359–4.770) | 0.684 | 1.960 (0.407–9.449) | 0.402 |
| Parameter | OS HR (95% CI) | p-Value | RR HR (95% CI) | p-Value | LR HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Pathology | 0.856 | 0.079 | 0.157 | |||
| Non-SCC | Reference | Reference | Reference | |||
| SCC | 0.935 (0.451–1.937) | 0.285 (0.070–1.159) | 0.495 (0.187–1.312) | |||
| Extent of corpus invasion | <0.001 | 0.062 | 0.078 | |||
| Exocervical | Reference | Reference | Reference | |||
| Endocervical | 1.165 (0.591–2.299) | 0.659 | 1.563 (0.353–6.930) | 0.557 | 0.931 (0.302–2.873) | 0.901 |
| Uterine corpus | 3.321 (1.858–5.937) | <0.001 | 4.829 (1.279–18.234) | 0.020 | 2.775 (1.096–7.028) | 0.031 |
| Pelvic lymph node | 0.073 | 0.058 | 0.069 | |||
| Negative | Reference | Reference | Reference | |||
| Positive | 1.684 (0.953–2.974) | 3.332 (0.959–11.577) | 2.208 (0.941–5.180) | |||
| SCC-Ag level | 0.246 | 0.048 | 0.727 | |||
| 0–20 ng/mL | Reference | Reference | Reference | |||
| 20–40 ng/mL | 2.084 (0.804–5.399) | 0.131 | 3.209 (0.340–30.331) | 0.309 | 3.426 (0.949–12.366) | 0.060 |
| ≥40 ng/mL | 1.486 (0.448–4.929) | 0.517 | 8.153 (1.724–38.548) | 0.008 | <0.001 (<0.001–) | 0.977 |
| Unknown | 2.097 (0.675–6.511) | 0.200 | 2.473 (0.249–24.561) | 0.440 | 1.898 (0.376–9.566) | 0.438 |
| Parameter | CSS AUC (95% CI) | p-Value | LRR AUC (95% CI) | p-Value | EPM AUC (95% CI) | p-Value | PALNR AUC (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Age | 0.427 (0.333–0.522) | 0.131 | 0.417 (0.321–0.514) | 0.108 | 0.434 (0.349–0.519) | 0.139 | 0.399 (0.299–0.499) | 0.065 |
| Pathology | 0.449 (0.350–0.547) | 0.287 | 0.428 (0.322–0.534) | 0.161 | 0.447 (0.356–0.538) | 0.241 | 0.477 (0.367–0.586) | 0.670 |
| SCC | 0.621 (0.527–0.714) | 0.016 | 0.592 (0.491–0.693) | 0.084 | 0.592 (0.505–0.678) | 0.047 | 0.594 (0.486–0.702) | 0.095 |
| CEA | 0.581 (0.483–0.679) | 0.095 | 0.637 (0.540–0.735) | 0.008 | 0.605 (0.515–0.695) | 0.021 | 0.607 (0.502–0.713) | 0.050 |
| Hb | 0.583 (0.488–0.679) | 0.087 | 0.632 (0.534–0.729) | 0.013 | 0.617 (0.530–0.704) | 0.010 | 0.584 (0.476–0.692) | 0.125 |
| Size | 0.642 (0.547–0.738) | 0.005 | 0.565 (0.458–0.672) | 0.230 | 0.625 (0.539–0.711) | 0.008 | 0.634 (0.536–0.731) | 0.020 |
| LN | 0.600 (0.502–0.699) | 0.038 | 0.601 (0.496–0.706) | 0.048 | 0.540 (0.450–0.630) | 0.368 | 0.597 (0.485–0.709) | 0.075 |
| Stage | 0.654 (0.562–0.746) | 0.001 | 0.610 (0.507–0.712) | 0.033 | 0.595 (0.511–0.679) | 0.035 | 0.547 (0.441–0.654) | 0.385 |
| PM score | 0.654 (0.552–0.756) | 0.003 | 0.590 (0.478–0.701) | 0.095 | 0.639 (0.551–0.727) | 0.003 | 0.615 (0.499–0.730) | 0.046 |
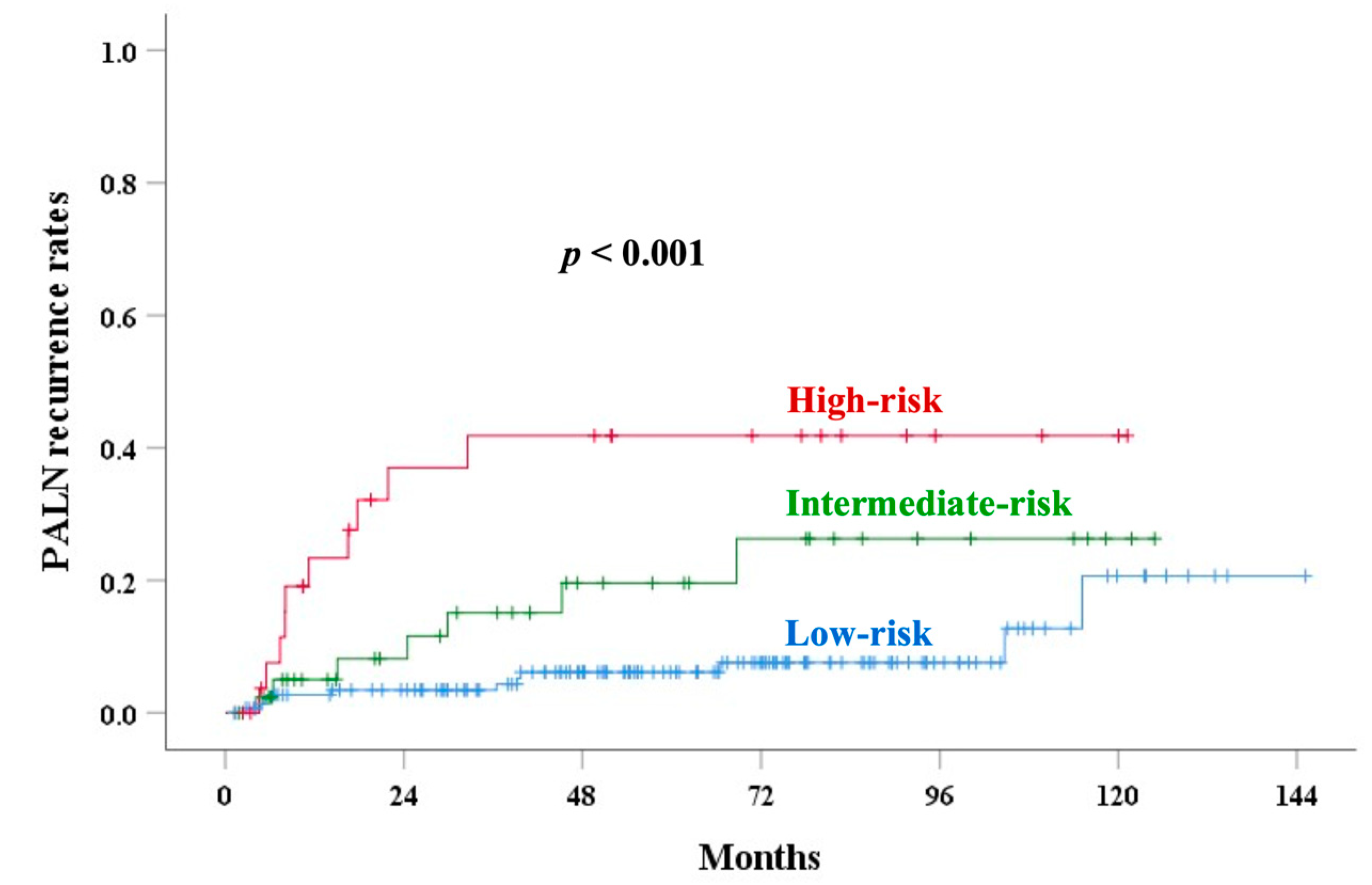
| Extent of corpus invasion | 0.630 (0.534–0.727) | 0.007 | 0.602 (0.497–0.707) | 0.048 | 0.639 (0.550–0.728) | 0.002 | 0.661 (0.553–0.768) | 0.003 |
| Old risk scoring | 0.658 (0.556–0.7861) | 0.003 | 0.698 (0.595–0.801) | <0.001 | 0.593 (0.497–0.690) | 0.053 | 0.655 (0.544–0.765) | 0.008 |
| New risk scoring | 0.713 (0.626–0.800) | <0.001 | 0.714 (0.620–0.807) | <0.001 | 0.640 (0.547–0.733) | 0.004 | 0.692 (0.577–0.807) | 0.001 |
| Parameter | OS AUC (95% CI) | p-Value | RR AUC (95% CI) | p-Value | LR AUC (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Age | 0.600 (0.513–0.688) | 0.016 | 0.449 (0.288–0.609) | 0.532 | 0.381 (0.270–0.492) | 0.047 |
| Pathology | 0.483 (0.401–0.566) | 0.688 | 0.415 (0.243–0.587) | 0.303 | 0.432 (0.308–0.555) | 0.255 |
| SCC | 0.636 (0.559–0.712) | 0.002 | 0.698 (0.543–0.853) | 0.020 | 0.503 (0.388–0.618) | 0.963 |
| CEA | 0.603 (0.522–0.685) | 0.014 | 0.631 (0.478–0.784) | 0.112 | 0.614 (0.492–0.735) | 0.058 |
| Hb | 0.650 (0.570–0.730) | <0.001 | 0.746 (0.625–0.867) | 0.003 | 0.585 (0.463–0.706) | 0.173 |
| Size | 0.565 (0.475–0.654) | 0.143 | 0.553 (0.357–0.749) | 0.573 | 0.597 (0.478–0.716) | 0.113 |
| LN | 0.561 (0.478–0.645) | 0.142 | 0.676 (0.510–0.841) | 0.033 | 0.579 (0.456–0.701) | 0.188 |
| Stage | 0.616 (0.536–0.696) | 0.005 | 0.582 (0.415–0.749) | 0.318 | 0.613 (0.497–0.730) | 0.058 |
| Pm score | 0.599 (0.512–0.686) | 0.024 | 0.557 (0.364–0.750) | 0.506 | 0.582 (0.454–0.710) | 0.198 |
| Extent of corpus invasion | 0.615 (0.531–0.699) | 0.006 | 0.665 (0.502–0.828) | 0.045 | 0.562 (0.438–0.685) | 0.302 |
| Old risk group | 0.588 (0.499–0.677) | 0.049 | 0.724 (0.556–0.892) | 0.012 | 0.667 (0.548–0.786) | 0.010 |
| New risk group | 0.662 (0.582–0.742) | <0.001 | 0.735 (0.571–0.899) | 0.009 | 0.675 (0.571–0.779) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-C.; Cheng, J.-Y.; Chen, C.-S.; Wang, C.-J.; Huang, E.-Y. The Prognostic Role of Magnetic-Resonance-Imaging-Detected Corpus Invasion in Patients with Cervical Carcinoma Who Underwent Definitive or Adjuvant Pelvic Radiotherapy. Cancers 2025, 17, 1449. https://doi.org/10.3390/cancers17091449
Huang K-C, Cheng J-Y, Chen C-S, Wang C-J, Huang E-Y. The Prognostic Role of Magnetic-Resonance-Imaging-Detected Corpus Invasion in Patients with Cervical Carcinoma Who Underwent Definitive or Adjuvant Pelvic Radiotherapy. Cancers. 2025; 17(9):1449. https://doi.org/10.3390/cancers17091449
Chicago/Turabian StyleHuang, Kuan-Ching, Jen-Yu Cheng, Chung-Shih Chen, Chong-Jong Wang, and Eng-Yen Huang. 2025. "The Prognostic Role of Magnetic-Resonance-Imaging-Detected Corpus Invasion in Patients with Cervical Carcinoma Who Underwent Definitive or Adjuvant Pelvic Radiotherapy" Cancers 17, no. 9: 1449. https://doi.org/10.3390/cancers17091449
APA StyleHuang, K.-C., Cheng, J.-Y., Chen, C.-S., Wang, C.-J., & Huang, E.-Y. (2025). The Prognostic Role of Magnetic-Resonance-Imaging-Detected Corpus Invasion in Patients with Cervical Carcinoma Who Underwent Definitive or Adjuvant Pelvic Radiotherapy. Cancers, 17(9), 1449. https://doi.org/10.3390/cancers17091449








