Predictors in Optic Pathway Gliomas in Neurofibromatosis Type 1: A Single Center Study
Simple Summary
Abstract
1. Introduction
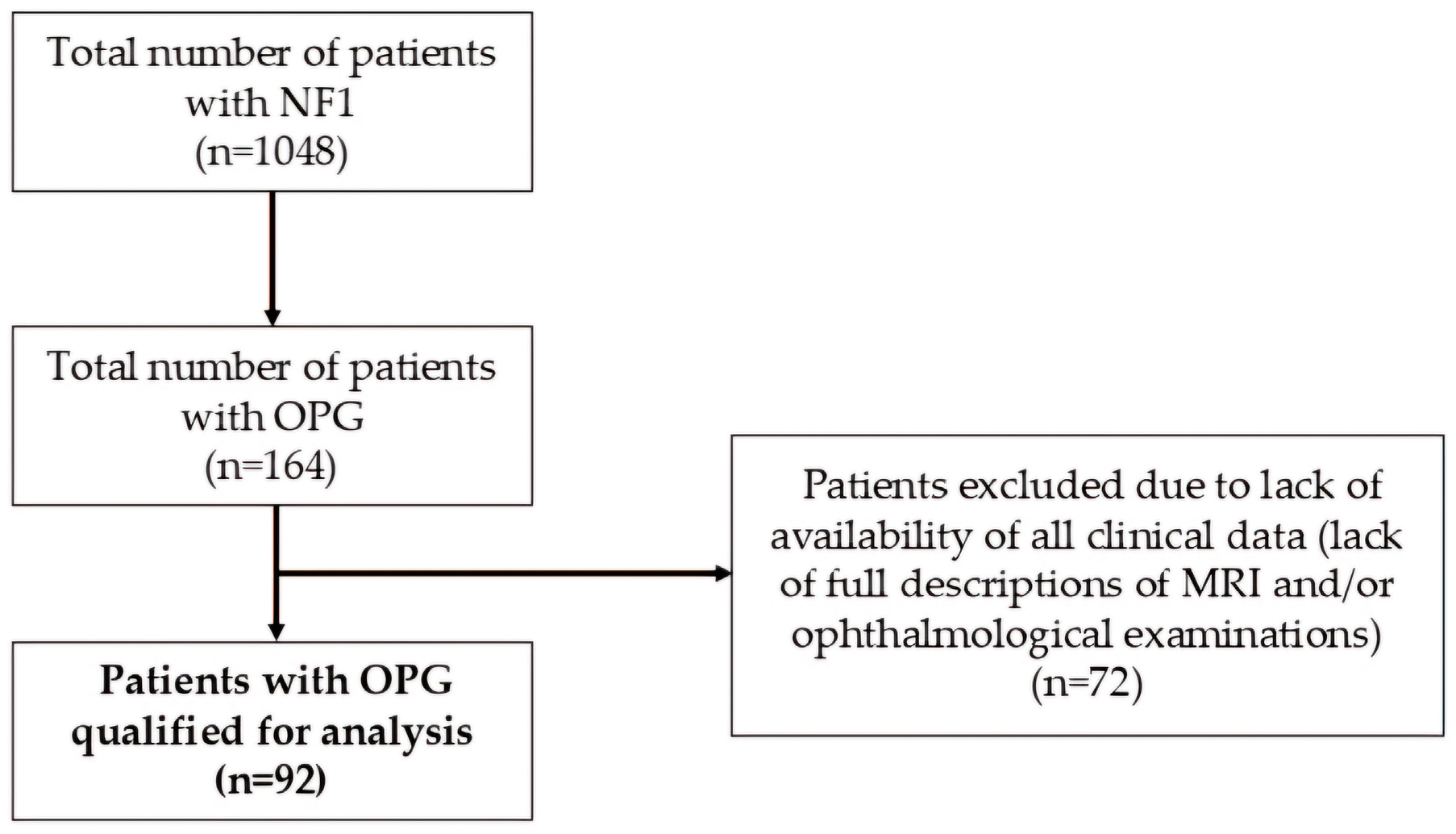
2. Materials and Methods
3. Results
3.1. Demographics and Clinical Profile
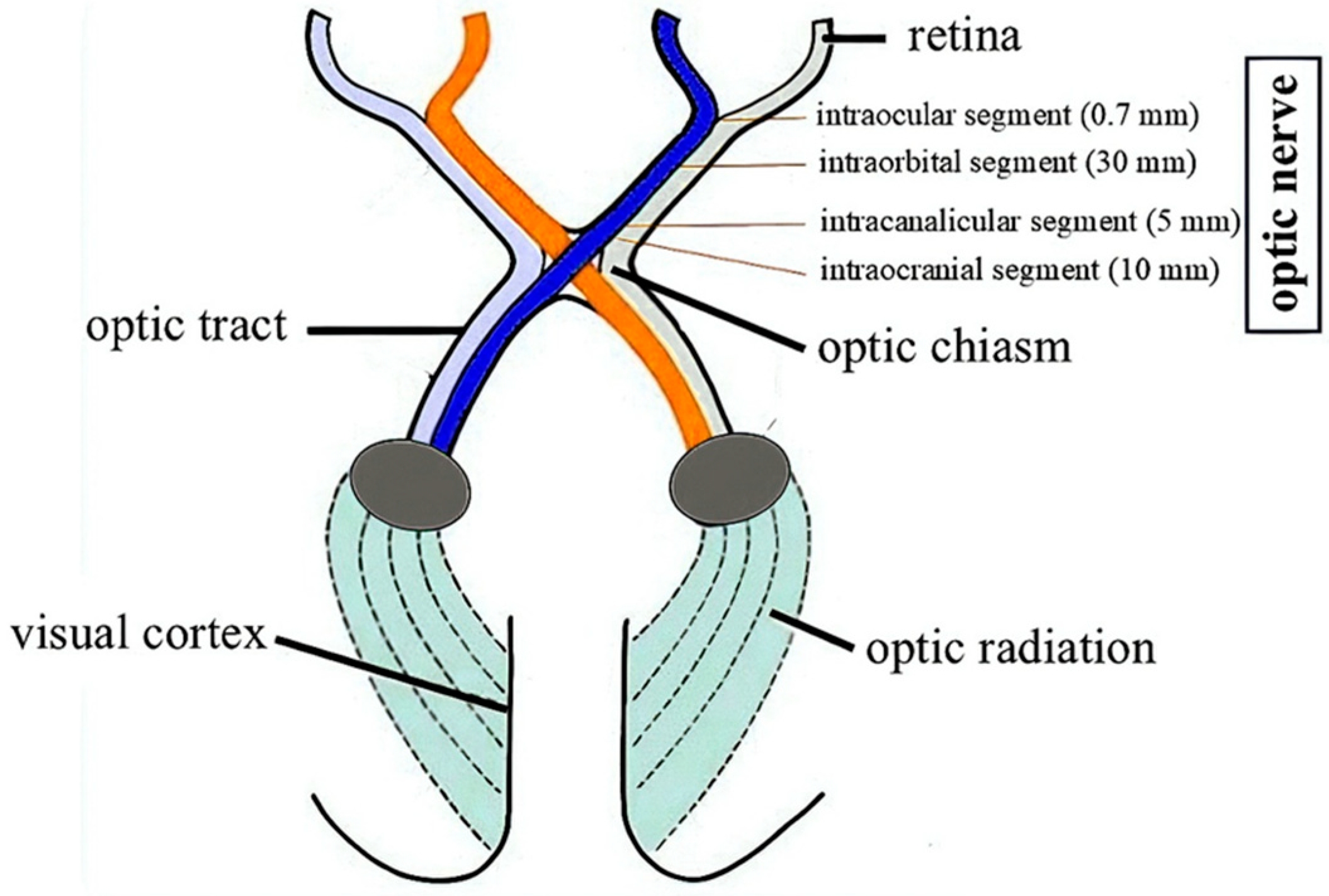
3.2. Characteristics of Optic Pathway Gliomas
3.3. Indications for Oncological Treatment
3.4. Oncological Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OPG | Optic pathway glioma |
| NF1 | Neurofibromatosis type 1 |
| MRI | Magnetic resonance imaging |
| OCT | Optical coherence tomography |
| VEP | Visual evoked potentials |
| CNS | Central nervous system |
| OR | Odds ratio |
| ADHD | Attention deficit hyperactivity disorder |
| PS | Pulmonary stenosis |
| PFO | Patent foramen ovale |
| MR | Mitral regurgitation |
| ASD | Atrial septal defect |
| 95% CI | 95% confidence interval |
| VCR + Carbo | Vincristine + carboplatin |
| VBL | Vinblastine |
| PFS | Progression-free survival |
References
- Carton, C.; Evans, D.G.; Blanco, I.; Friedrich, R.E.; Ferner, R.E.; Farschtschi, S.; Salvador, H.; Azizi, A.A.; Mautner, V.; Rohl, C.; et al. ERN GENTURIS tumour surveillance guidelines for individuals with neurofibromatosis type 1. EClinicalMedicine 2023, 56, 101818. [Google Scholar] [CrossRef]
- De Moraes, C.G. Anatomy of the visual pathways. J. Glaucoma 2013, 22 (Suppl. 5), S2–S7. [Google Scholar] [CrossRef]
- The Visual Pathway of the Brain. Available online: https://www.brainkart.com/article/The-Visual-Pathway-of-the-Brain_19029/ (accessed on 25 February 2025).
- Cassina, M.; Frizziero, L.; Opocher, E.; Parrozzani, R.; Sorrentino, U.; Viscardi, E.; Miglionico, G.; Midena, E.; Clementi, M.; Trevisson, E. Optic Pathway Glioma in Type 1 Neurofibromatosis: Review of Its Pathogenesis, Diagnostic Assessment, and Treatment Recommendations. Cancers 2019, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Koczkowska, M.; Chen, Y.; Callens, T.; Gomes, A.; Sharp, A.; Johnson, S.; Hsiao, M.C.; Chen, Z.; Balasubramanian, M.; Barnett, C.P.; et al. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844-848. Am. J. Hum. Genet. 2018, 102, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Koczkowska, M.; Chen, Y.; Xie, J.; Callens, T.; Gomes, A.; Wimmer, K.; Messiaen, L.M. Analysis of 200 unrelated individuals with a constitutional NF1 deep intronic pathogenic variant reveals that variants flanking the alternatively spliced NF1 exon 31 [23a] cause a classical neurofibromatosis type 1 phenotype while altering predominantly NF1 isoform type II. Hum. Genet. 2023, 142, 849–861. [Google Scholar] [CrossRef]
- Wigertz, A.; Lonn, S.; Schwartzbaum, J.; Hall, P.; Auvinen, A.; Christensen, H.C.; Johansen, C.; Klaeboe, L.; Salminen, T.; Schoemaker, M.J.; et al. Allergic conditions and brain tumor risk. Am. J. Epidemiol. 2007, 166, 941–950. [Google Scholar] [CrossRef]
- Chatterjee, J.; Sanapala, S.; Cobb, O.; Bewley, A.; Goldstein, A.K.; Cordell, E.; Ge, X.; Garbow, J.R.; Holtzman, M.J.; Gutmann, D.H. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat. Commun. 2021, 12, 7122. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.J.; Gutmann, D.H. Optic Pathway Gliomas in Neurofibromatosis Type 1. J. Child Neurol. 2018, 33, 73–81. [Google Scholar] [CrossRef]
- Tang, Y.; Gutmann, D.H. Neurofibromatosis Type 1-Associated Optic Pathway Gliomas: Current Challenges and Future Prospects. Cancer Manag. Res. 2023, 15, 667–681. [Google Scholar] [CrossRef]
- Vagge, A.; Camicione, P.; Pellegrini, M.; Gatti, G.; Capris, P.; Severino, M.; Di Maita, M.; Panarello, S.; Traverso, C.E. Role of visual evoked potentials and optical coherence tomography in the screening for optic pathway gliomas in patients with neurofibromatosis type I. Eur. J. Ophthalmol. 2021, 31, 698–703. [Google Scholar] [CrossRef]
- Park, M. Recent Update in Pharmacological Agents for Optic Pathway Glioma. Brain Tumor. Res. Treat. 2022, 10, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Ater, J.; Allen, J.; Phillips, P.; Geyer, R.; Nicholson, H.S.; Jakacki, R.; Kurczynski, E.; Needle, M.; Finlay, J.; et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J. Neurosurg. 1997, 86, 747–754. [Google Scholar] [CrossRef]
- Dal Bello, S.; Martinuzzi, D.; Tereshko, Y.; Veritti, D.; Sarao, V.; Gigli, G.L.; Lanzetta, P.; Valente, M. The Present and Future of Optic Pathway Glioma Therapy. Cells 2023, 12, 2380. [Google Scholar] [CrossRef]
- De Blank, P.M.K.; Fisher, M.J.; Liu, G.T.; Gutmann, D.H.; Listernick, R.; Ferner, R.E.; Avery, R.A. Optic Pathway Gliomas in Neurofibromatosis Type 1: An Update: Surveillance, Treatment Indications, and Biomarkers of Vision. J. Neuro-Ophthalmol. 2017, 37 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Jakacki, R.; Goldman, S.; Hargrave, D.; Hawkins, C.; Shroff, M.; Hukin, J.; Bartels, U.; Foreman, N.; Kellie, S.; et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J. Clin. Oncol. 2012, 30, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Khan, M.; Lethbridge, R.; Lee, G.; Lee, S.; Dyke, J.; Fabian, V.; McGrath, A.; Taylor, M.; Jacoby, P.; et al. Long-term outcomes of symptomatic optic pathway glioma: 32-year experience at a single Western Australian tertiary pediatric oncology center. Front. Oncol. 2023, 13, 1157909. [Google Scholar] [CrossRef] [PubMed]
- Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch. Neurol. 1988, 45, 575–578. [Google Scholar]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet. Med. 2021, 23, 1506–1513. [Google Scholar] [CrossRef]
- Montagne, A.; Huuskonen, M.T.; Rajagopal, G.; Sweeney, M.D.; Nation, D.A.; Sepehrband, F.; D’Orazio, L.M.; Harrington, M.G.; Chui, H.C.; Law, M.; et al. Undetectable gadolinium brain retention in individuals with an age-dependent blood-brain barrier breakdown in the hippocampus and mild cognitive impairment. Alzheimer’s Dement. 2019, 15, 1568–1575. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Maloney, E.; Stanescu, A.L.; Perez, F.A.; Iyer, R.S.; Otto, R.K.; Leary, S.; Steuten, L.; Phipps, A.I.; Shaw, D.W.W. Surveillance magnetic resonance imaging for isolated optic pathway gliomas: Is gadolinium necessary? Pediatr. Radiol. 2018, 48, 1472–1484. [Google Scholar] [CrossRef] [PubMed]
- Marsault, P.; Ducassou, S.; Menut, F.; Bessou, P.; Havez-Enjolras, M.; Chateil, J.F. Diagnostic performance of an unenhanced MRI exam for tumor follow-up of the optic pathway gliomas in children. Neuroradiology 2019, 61, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Kilian, A.; Aigner, A.; Simon, M.; Salchow, D.J.; Potratz, C.; Thomale, U.W.; Hernaiz Driever, P.; Tietze, A. Tumor load rather than contrast enhancement is associated with the visual function of children and adolescents with optic pathway glioma–A retrospective Magnetic Resonance Imaging study. J. Neurooncol. 2022, 156, 589–597. [Google Scholar] [CrossRef]
- Mezad-Koursh, D.; Bachar Zipori, A.; Zur, D.; Degabli, L.; Ben-Dov, M.; Klein, A. Visual function tests including the role of optical coherence tomography in neurofibromatosis 1. Childs Nerv. Syst. 2020, 36, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Neuroophthalmological management of optic pathway gliomas. Neurosurg. Focus 2007, 23, E1. [Google Scholar] [CrossRef]
- Duran, M.; Aykac, S.; Eliacik, S. Evaluation of ganglion cell complex and retinal nerve fiber layer thinning in epilepsy patients. Indian J. Ophthalmol. 2023, 71, 3053–3058. [Google Scholar] [CrossRef]
- Hilton, E.J.; Hosking, S.L.; Betts, T. The effect of antiepileptic drugs on visual performance. Seizure 2004, 13, 113–128. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, W.; Lu, L.; Wu, X.; Cao, L.; Chen, J.; Xiao, Y.; Sander, J.W.; Wu, B.; Zhou, D. The thickness of the retinal nerve fiber layer, macula, and ganglion cell-inner plexiform layer in people with drug-resistant epilepsy. Epilepsia Open 2024, 9, 1783–1792. [Google Scholar] [CrossRef]
- Amirian, E.S.; Zhou, R.; Wrensch, M.R.; Olson, S.H.; Scheurer, M.E.; Il’yasova, D.; Lachance, D.; Armstrong, G.N.; McCoy, L.S.; Lau, C.C.; et al. Approaching a Scientific Consensus on the Association between Allergies and Glioma Risk: A Report from the Glioma International Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 282–290. [Google Scholar] [CrossRef]
- Samoliński, B.; Raciborski, F.; Lipiec, A.; Tomaszewska, A.; Krzych-Fałta, E.; Samel-Kowalik, P.; Walkiewicz, A.; Lusawa, A.; Borowicz, J.; Komorowski, J.; et al. Epidemiology of allergic diseases in Poland. Pol. J. Allergol. 2014, 1, 10–18. [Google Scholar]
- Lasaletta, A.; Scheinemann, K.; Zelcer, S.M.; Hukin, J.; Wilson, B.A.; Jabado, N.; Carret, A.S.; Bouffet, E.; Lafay-Kuzynka, L.; Larouche, V.; et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: A canadian pediatric brain tumor consortium study. J. Clin. Oncol. 2016, 34, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Kebudi, R.; Yildirim, I.M.; İribaş, A.; Tuncer, S. Optic pathway gliomas in children: Clinical characteristics, treatment, and outcome of 95 patients in a single center over a 31-year period. Can we avoid radiotherapy? Pediatr. Blood Cancer 2024, 71, e31337. [Google Scholar] [CrossRef]
- Trevisson, E.; Cassina, M.; Opocher, E.; Vicenzi, V.; Lucchetta, M.; Parrozzani, R.; Miglionico, G.; Mardari, R.; Viscardi, E.; Midena, E.; et al. Natural history of optic pathway gliomas in a cohort of unselected patients affected by Neurofibromatosis 1. J. Neurooncol. 2017, 134, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Prada, C.E.; Hufnagel, R.B.; Hummel, T.R.; Lovell, A.M.; Hopkin, R.J.; Saal, H.M.; Schorry, E.K. The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type 1. J. Pediatr. 2015, 167, 851–856. [Google Scholar] [CrossRef]
- Blazo, M.A.; Lewis, R.A.; Chintagumpala, M.M.; Frazier, M.; McCluggage, C.; Plon, S.E. Outcomes of systematic screening for optic pathway tumors in children with Neurofibromatosis Type 1. Am. J. Med. Genet.-Part A 2004, 127, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.A.; Walker, D.A.; Liu, J.F.; Sehested, A.; Jaspan, T.; Pemp, B.; Simmons, I.; Ferner, R.; Grill, J.; Hargrave, D.; et al. NF1 optic pathway glioma: Analyzing risk factors for visual outcome and indications to treat. Neuro. Oncol. 2021, 23, 100–111. [Google Scholar] [CrossRef]
- Fisher, M.J.; Avery, R.A.; Allen, J.C.; Ardern-Holmes, S.L.; Bilaniuk, L.T.; Ferner, R.E.; Gutmann, D.H.; Listernick, R.; Martin, S.; Ullrich, N.J.; et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology 2013, 81 (Suppl. 1), S15–S24. [Google Scholar] [CrossRef]
| No | Mutation | Optic Pathway Glioma | Additional Information |
|---|---|---|---|
| 1 | c.4600C>T p.Arg1534* |
|
|
| 2 | c.2325G>A p.Glu775= |
|
|
| 3 | c.4250delG p.Gly1417fs |
|
|
| 4 | c.1392+1G>A/- p.[?] |
|
|
| 5 | c.1392+1G>A/- p.[?] |
|
|
| 6 | c.1392+1G>A/- p.[?] |
|
|
| 7 | c.4084C>T p.Arg1362Ter |
|
|
| 8 | c.1756_1759del p.Thr586Valfs*18 |
|
|
| 9 | c.5804T>G p.Leu1935Arg |
|
|
| 10 | c.7458-2A>G p.[?] |
|
|
| 11 | c.3721C>T p.Arg1241* |
|
|
| 12 | c.3721C>T p.Arg1241Ter |
|
|
| 13 | c.6349C>T p.Gln2117* |
|
|
| 14 | c.1318C>T p.Arg440-* |
|
|
| 15 | c.4368-2A>G p.[?] |
|
|
| 16 | c.1381C>T p.Arg461Ter |
|
|
| 17 | c.1061_1062+13delinsGA p.Lys354ARG |
|
|
| 18 | c.79>T ??? p.Gln27Ter |
|
|
| 19 | c.1541_1542del p.Gln514Argfs*43/- |
|
|
| 20 | c.2146G>T p.Glu716Ter |
|
|
| 21 | c.3721C>T p.Arg1241* |
|
|
| Disorder | Frequency |
|---|---|
| Delayed psychomotor development | 33.7% (31/92) |
| Allergy | 14.1% (13/92) |
| Plexiform neurofibroma of any location | 14.1% (13/92) |
| Attention deficit hyperactivity disorder (ADHD) | 7.6% (7/92) |
| Epilepsy | 7.6% (7/92) |
| Hydrocephalus | 6.5% (6/92) |
| Congenital heart disease: PS (n = 2), PFO (n = 1), MR (n = 1), ASD (n = 1) | 5.4% (5/92) |
| Short stature | 4.3% (4/92) |
| Autism spectrum disorders | 3.3% (3/92) |
| Precocious puberty | 3.3% (3/92) |
| Pseudarthrosis | 1.1% (1/92) |
| Acoustic neuroma | 1.1% (1/92) |
| Parameter | p | OR | 95% CI |
|---|---|---|---|
| Thickness of the optic nerve ≥ 8 mm | <0.001 | 7.63 | 2.28–25.5 |
| Proptosis | <0.001 | 16.4 | 3.50–77.4 |
| Strabismus | <0.001 | 7.63 | 2.29–25.5 |
| Amblyopia | <0.001 | 10.7 | 2.74–41.6 |
| Epilepsy | 0.008 | 8.97 | 1.77–45.6 |
| Bilateral involvement of the visual pathway by OPG | 0.020 | 4.31 | 1.26–14.8 |
| Involvement of intracranial segment by OPG | 0.028 | 5.71 | 1.21–27.0 |
| Involvement of intracanalicular segment by OPG | 0.031 | 3.87 | 1.13–13.2 |
| Involvement of chiasm by OPG | 0.041 | 3.26 | 1.05–10.1 |
| Post-contrast enhancement of OPG in MRI | 0.099 | 3.71 | 0.78–17.7 |
| Involvement of optic tracts by OPG | 0.115 | 2.71 | 0.79–9.34 |
| Endocrinological disorders | 0.161 | 2.96 | 0.65–13.5 |
| Delayed psychomotor development | 0.250 | 1.93 | 0.63–5.94 |
| Hypertension | 0.260 | 2.81 | 0.47–16.9 |
| Attention deficit hyperactivity disorder (ADHD) | 0.371 | 2.22 | 0.39–12.7 |
| Coincidence of Lisch nodules | 0.403 | 1.61 | 0.53–4.88 |
| Autism spectrum disorders | 0.434 | 2.68 | 0.23–31.6 |
| Familial NF1 | 0.454 | 1.53 | 0.50–4.67 |
| Involvement of intraocular segment by OPG | 0.823 | 1.13 | 0.19–6.50 |
| Allergy | 0.923 | 0.92 | 0.18–4.66 |
| Involvement of optic nerve by OPG | 0.980 | 0.97 | 0.11–8.97 |
| Involvement of intraorbital segment by OPG | 0.999 | ND | ND |
| Plexiform neurofibroma of any location | 0.999 | ND | ND |
| Congenital heart disease | 0.999 | ND | ND |
| Hydrocephalus | 0.999 | ND | ND |
| Pseudarthrosis | 0.999 | ND | ND |
| Parameter | p | OR | 95% CI |
|---|---|---|---|
| Amblyopia | 0.003 | 9.56 | 2.19–41.7 |
| Proptosis | 0.004 | 13.9 | 2.37–82.1 |
| Strabismus | 0.106 | 3.88 | 0.75–20.1 |
| Bilateral involvement of the visual pathway by OPG | 0.122 | 3.29 | 0.73–14.9 |
| Epilepsy | 0.196 | 4.59 | 0.46–6.24 |
| Involvement of intracanalicular segment by OPG | 0.222 | 2.67 | 0.55–12.9 |
| Thickness of the optic nerve ≥ 8 mm | 0.350 | 2.14 | 0.43–10.5 |
| Post-contrast enhancement of OPG in MRI | 0.66 | 5.80 | 0.89–37.9 |
| Involvement of intracranial segment by OPG | 0.689 | 1.59 | 0.16–15.7 |
| Involvement of chiasm by OPG | 0.699 | 0.71 | 0.12–4.12 |
| Parameter | p | OR | 95%CI |
|---|---|---|---|
| Thickness of the optic nerve ≥ 8 mm | <0.001 | 5.90 | 2.21–15.8 |
| Strabismus | <0.001 | 5.90 | 2.21–15.8 |
| Delayed psychomotor development | 0.002 | 4.25 | 1.70–10.7 |
| Involvement of chiasm by OPG | 0.006 | 3.69 | 1.45–9.36 |
| Bilateral involvement of the visual pathway by OPG | 0.007 | 3.41 | 1.40–8.32 |
| Involvement of intracranial segment by OPG | 0.015 | 3.23 | 1.26–8.32 |
| Epilepsy | 0.021 | 12.8 | 1.48–112 |
| Involvement of intracanalicular segment by OPG | 0.048 | 2.41 | 1.01–5.76 |
| Familial NF1 | 0.049 | 2.42 | 1.01–5.83 |
| Proptosis | 0.056 | 4.15 | 0.96–17.9 |
| Endocrinological disorders | 0.056 | 4.15 | 0.96–17.7 |
| Allergy | 0.153 | 2.38 | 0.73–7.79 |
| Involvement of optic tracts by OPG | 0.290 | 1.78 | 0.61–5.17 |
| Post-contrast enhancement of OPG in MRI | 0.301 | 0.62 | 0.25–1.53 |
| Involvement of intraorbital segment by OPG | 0.328 | 0.65 | 0.27–1.56 |
| Involvement of intraocular segment by OPG | 0.459 | 0.43 | 0.05–4.01 |
| Congenital heart disease | 0.459 | 0.43 | 0.05–4.01 |
| Hypertension | 0.461 | 1.87 | 0.36–9.82 |
| Hydrocephalus | 0.461 | 1.87 | 0.36–9.82 |
| Plexiform neurofibroma of any location | 0.680 | 0.77 | 0.18–2.71 |
| Attention deficit hyperactivity disorder (ADHD) | 0.689 | 1.38 | 0.29–6.55 |
| Coincidence of Lisch nodules | 0.879 | 0.94 | 0.39–2.21 |
| Involvement of optic nerve by OPG | 0.893 | 1.13 | 0.19–6.51 |
| Autism spectrum disorders | 0.999 | ND | ND |
| Pseudarthrosis | 0.999 | ND | ND |
| Parameter | p | OR | 95% CI |
|---|---|---|---|
| Strabismus | 0.002 | 8.24 | 2.22–30.6 |
| Bilateral involvement of the visual pathway by OPG | 0.010 | 4.96 | 1.46–16.9 |
| Proptosis | 0.004 | 13.96 | 2.37–82.1 |
| Epilepsy | 0.016 | 22.1 | 1.77–276 |
| Thickness of the optic nerve ≥ 8 mm | 0.033 | 3.48 | 1.11–10.9 |
| Involvement of chiasm by OPG | 0.284 | 1.99 | 0.57–7.03 |
| Familial NF1 | 0.416 | 1.60 | 0.52–4.97 |
| Strabismus | 0.350 | 2.14 | 0.43–10.5 |
| Involvement of intracanalicular segment by OPG | 0.568 | 1.38 | 0.46–4.19 |
| Involvement of intracranial segment by OPG | 0.585 | 0.66 | 0.15–2.90 |
| NF1-OPG Related Data | Our Results | Comparable Data in Other Authors’ Analyses | |
|---|---|---|---|
| 1 | The typical age of OPG formation is between 2 and 8 years of age. |
| Trevisson et al., 2017 [34] Prada et al., 2015 [35] |
| 2 | More aggressive OPGs are more common in females than males. |
| Trevisson et al., 2017 [34] Prada et al., 2015 [35] Blazo et al., 2004 [36] |
| 3 | The type of mutation in the Nf1 gene may influence the increased risk of developing OPG, therefore performing genetic testing even in patients meeting clinical criteria is important to better understand the genotype-phenotype correlation. |
| Koczkowska et al., 2018 [5] Campen et al., 2018 [9] |
| 4 | OPGs may induce pituitary hormonal dysfunction. |
| Azizi et al., 2021 [37] |
| 5 | Therapeutic decisions are mainly influenced by the patient’s progressive vision loss. |
| Fisher et al., 2013 [38] Campen et al., 2018 [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marjańska, A.; Styczyńska, J.; Jatczak-Gaca, A.; Stachura, J.; Marjański, M.; Styczyński, J. Predictors in Optic Pathway Gliomas in Neurofibromatosis Type 1: A Single Center Study. Cancers 2025, 17, 1404. https://doi.org/10.3390/cancers17091404
Marjańska A, Styczyńska J, Jatczak-Gaca A, Stachura J, Marjański M, Styczyński J. Predictors in Optic Pathway Gliomas in Neurofibromatosis Type 1: A Single Center Study. Cancers. 2025; 17(9):1404. https://doi.org/10.3390/cancers17091404
Chicago/Turabian StyleMarjańska, Agata, Jagoda Styczyńska, Agnieszka Jatczak-Gaca, Joanna Stachura, Michał Marjański, and Jan Styczyński. 2025. "Predictors in Optic Pathway Gliomas in Neurofibromatosis Type 1: A Single Center Study" Cancers 17, no. 9: 1404. https://doi.org/10.3390/cancers17091404
APA StyleMarjańska, A., Styczyńska, J., Jatczak-Gaca, A., Stachura, J., Marjański, M., & Styczyński, J. (2025). Predictors in Optic Pathway Gliomas in Neurofibromatosis Type 1: A Single Center Study. Cancers, 17(9), 1404. https://doi.org/10.3390/cancers17091404





