Impact of Infection on Survival Outcomes in High-Grade Gliomas: A Retrospective Analysis of 26 Cases in Our Fifteen-Year Experience—Janus Faced Phenomenon
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection and Treatment Protocol
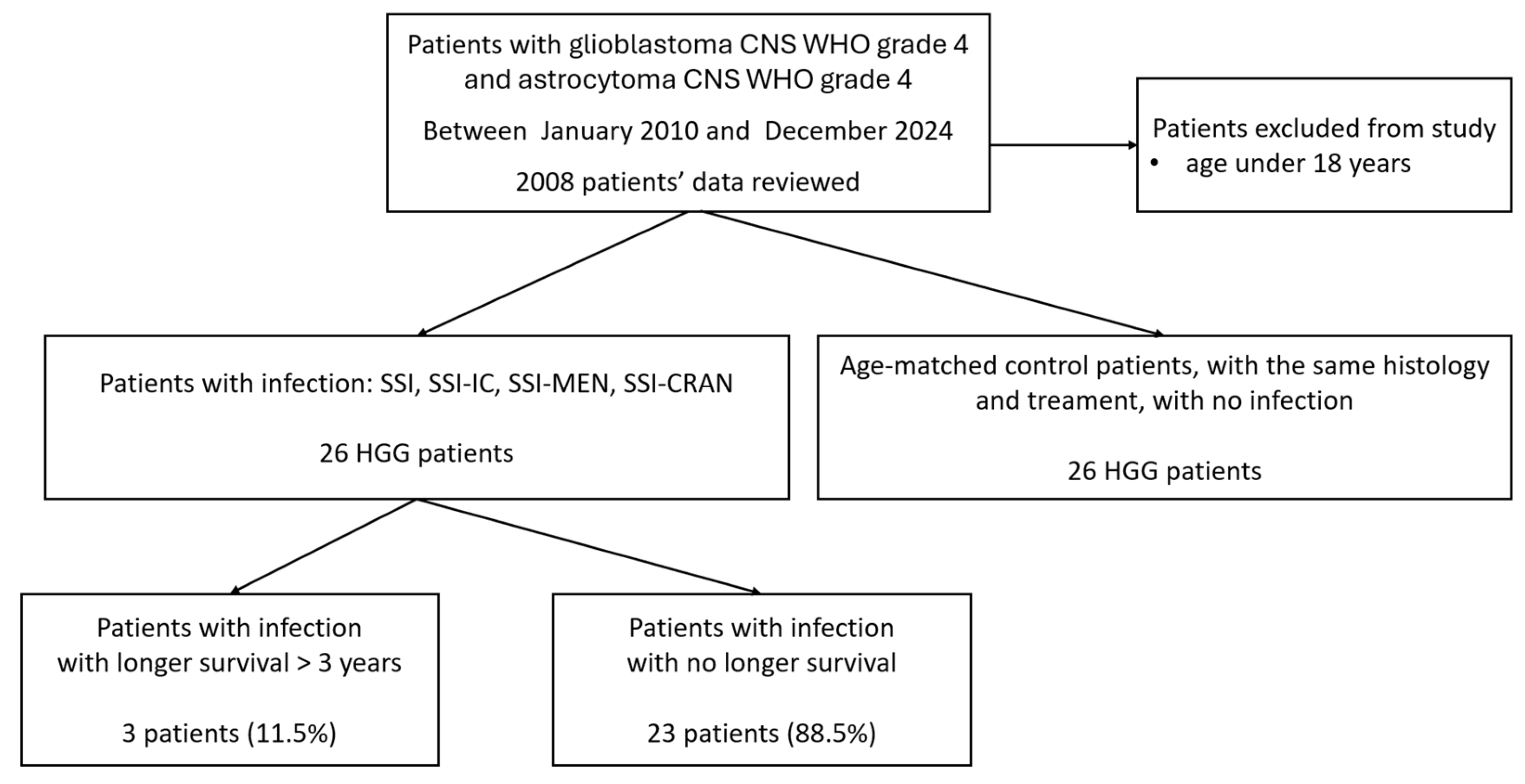
2.2. Data Collection and Patient Cohort
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the HGG Patients with Infection and Age-Matched Case–Control Population
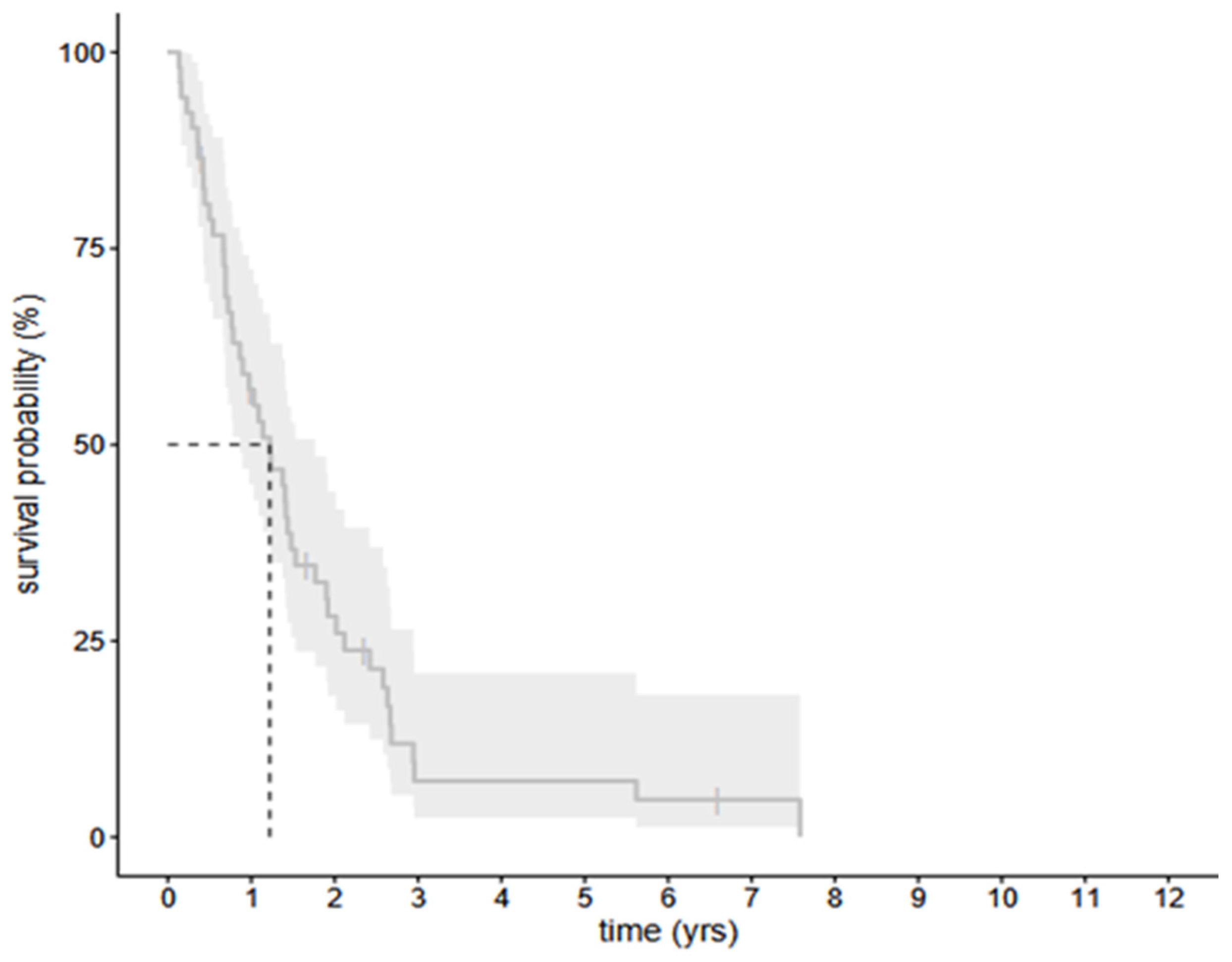
3.2. The Survival Data of All the Selected 56 Cohort Patients with HGG—The Infected and Uninfected Groups Together
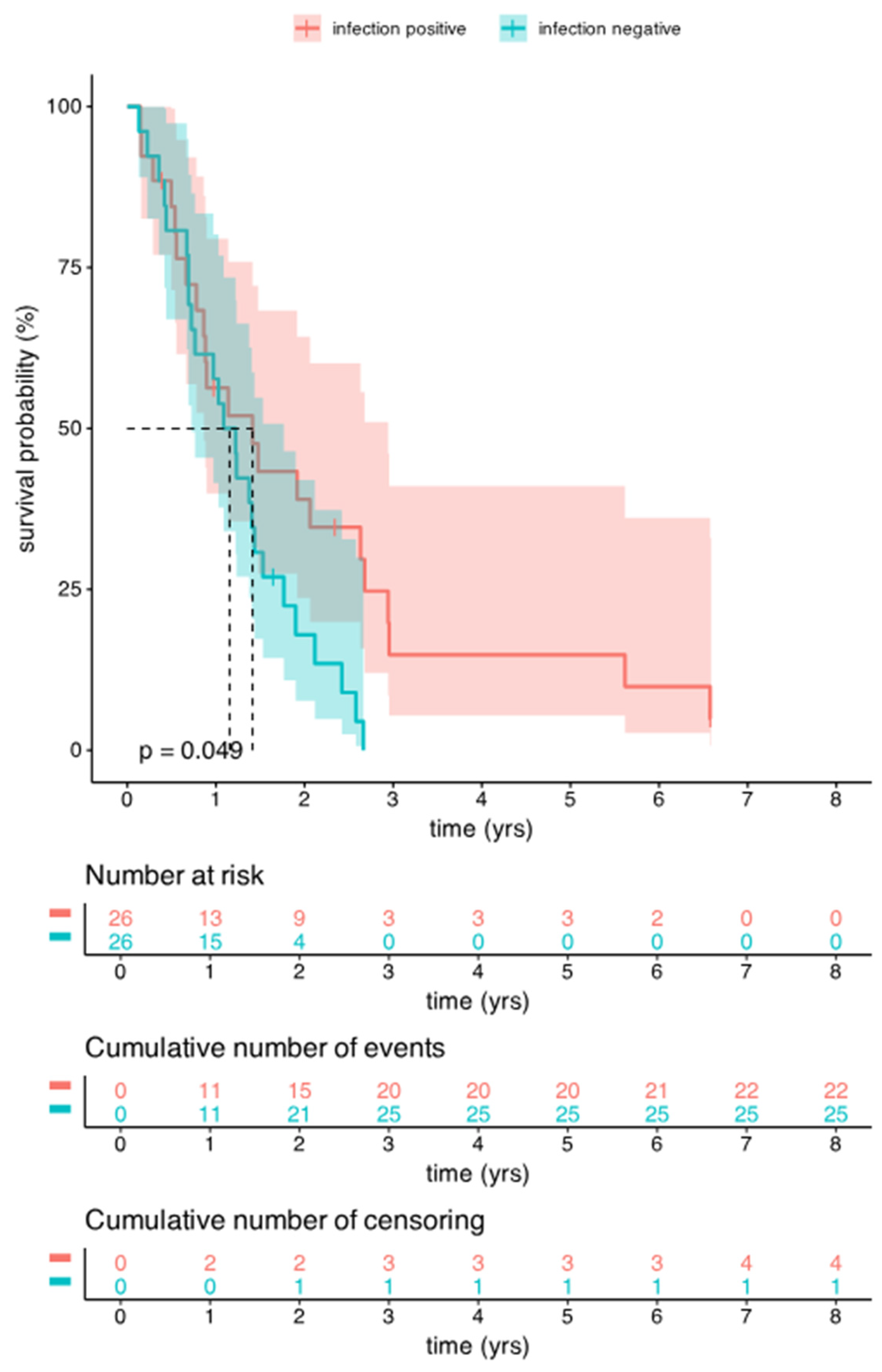
3.3. Comparison of Survival Data Between the Infected and Uninfected Groups of HGG Patients
3.3.1. OS Analysis Between the Infected and Uninfected Groups of HGG Patients
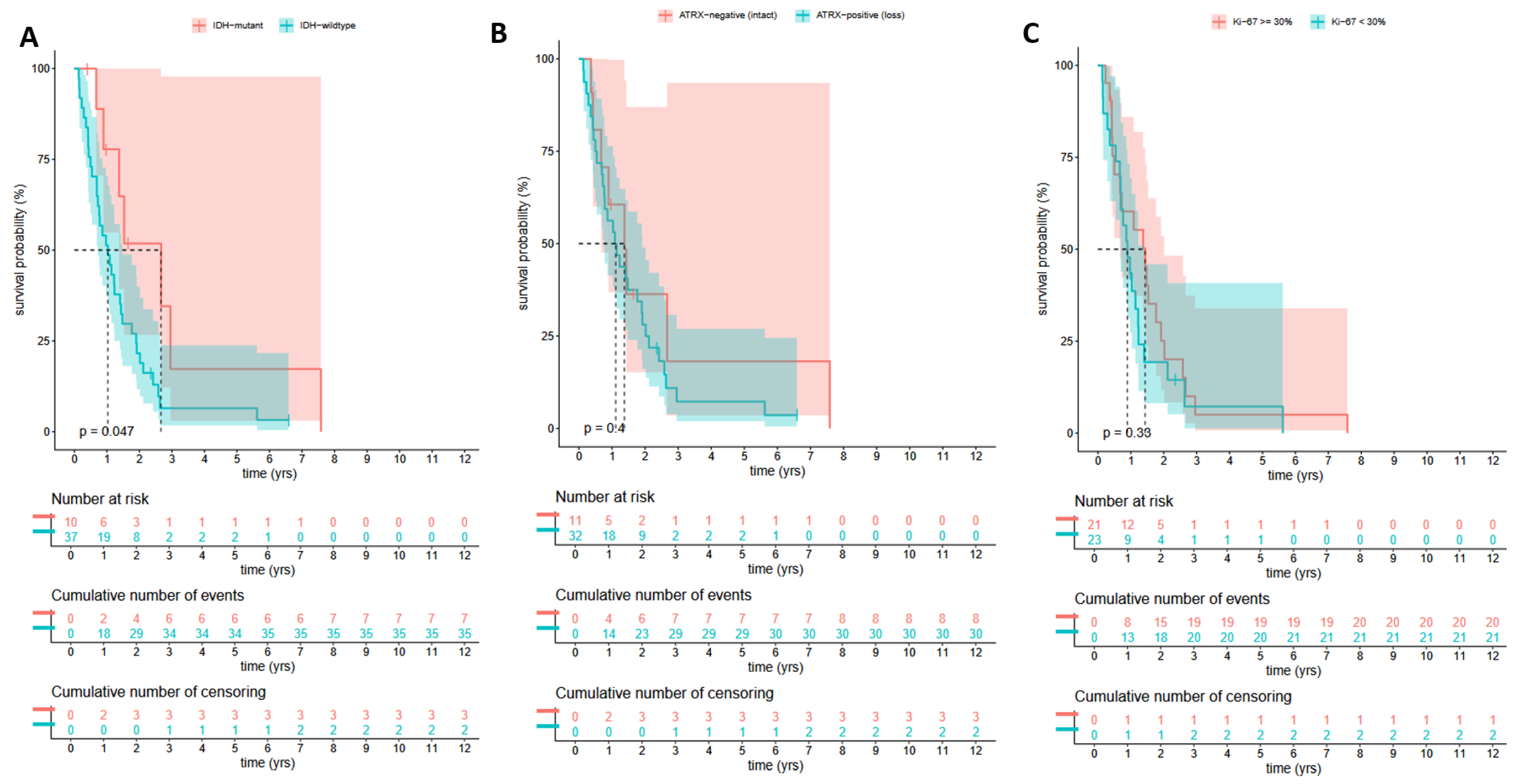
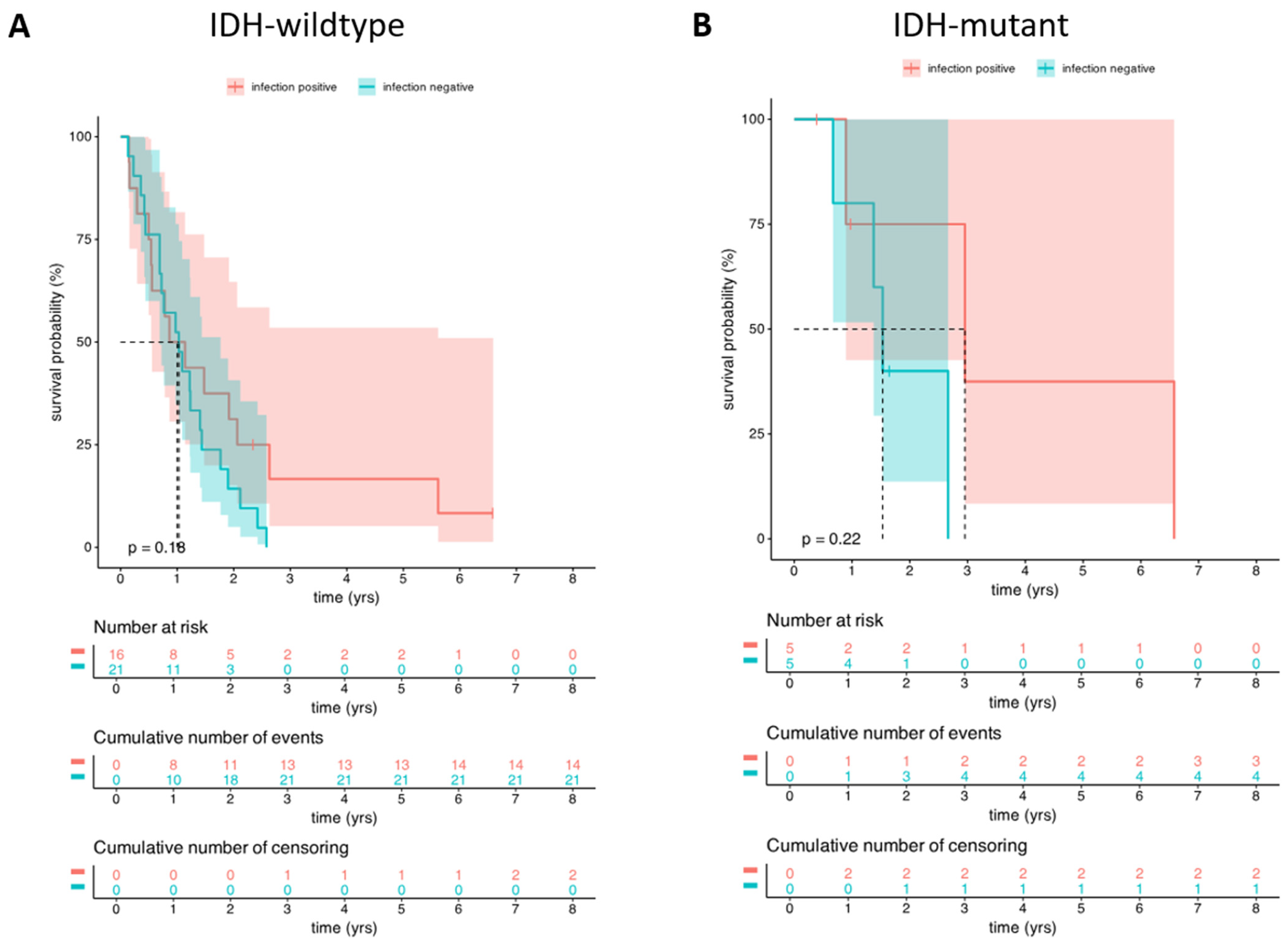
3.3.2. OS Analysis Between the Infected and Uninfected HGG Groups Based on Their IDH Mutations Status
3.4. The Microbiological Results of HGG Patients with Postoperative Infection
3.4.1. Case Reports of Three HGG Patients with Long Survival (>3-Years)
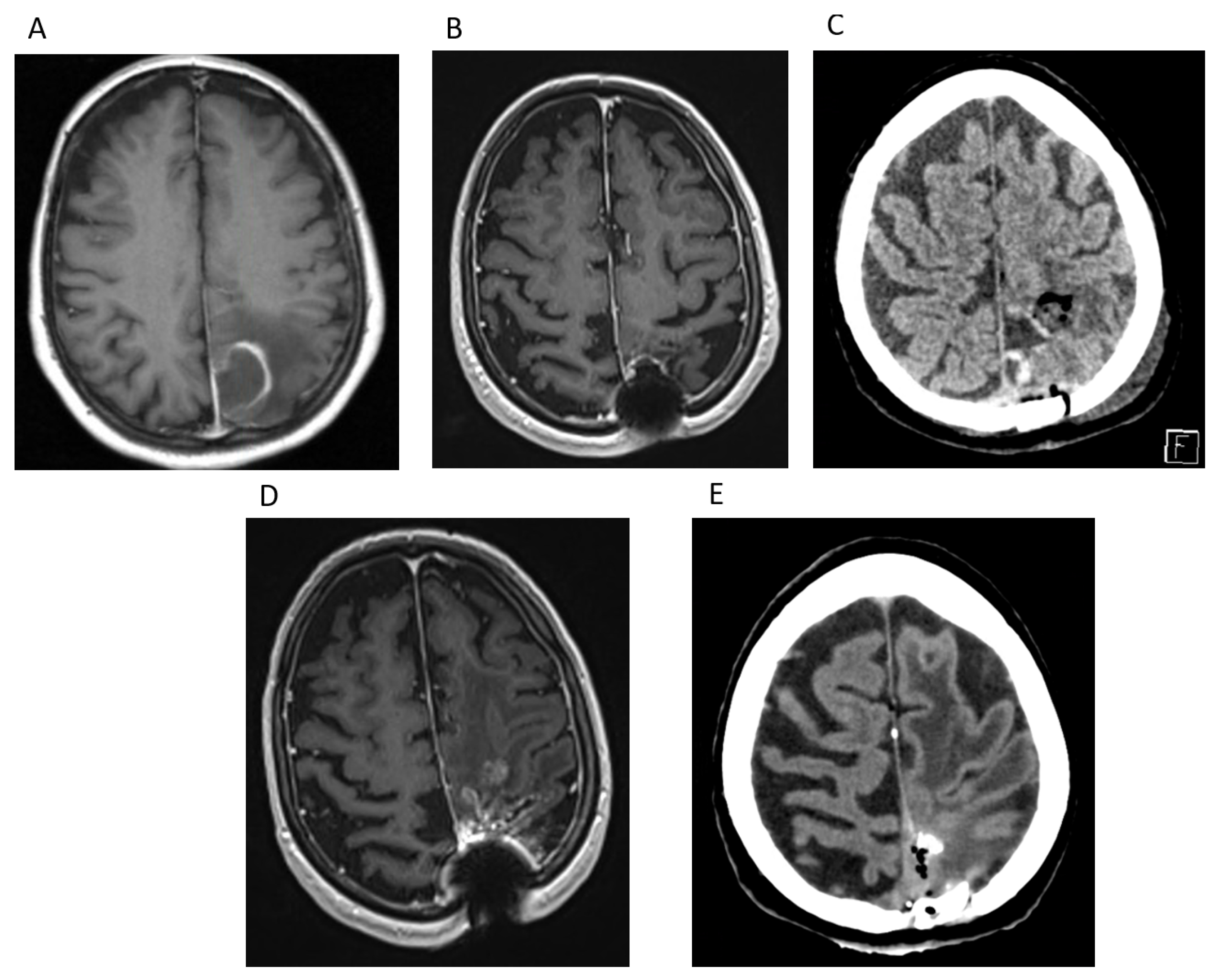
Case Report 1
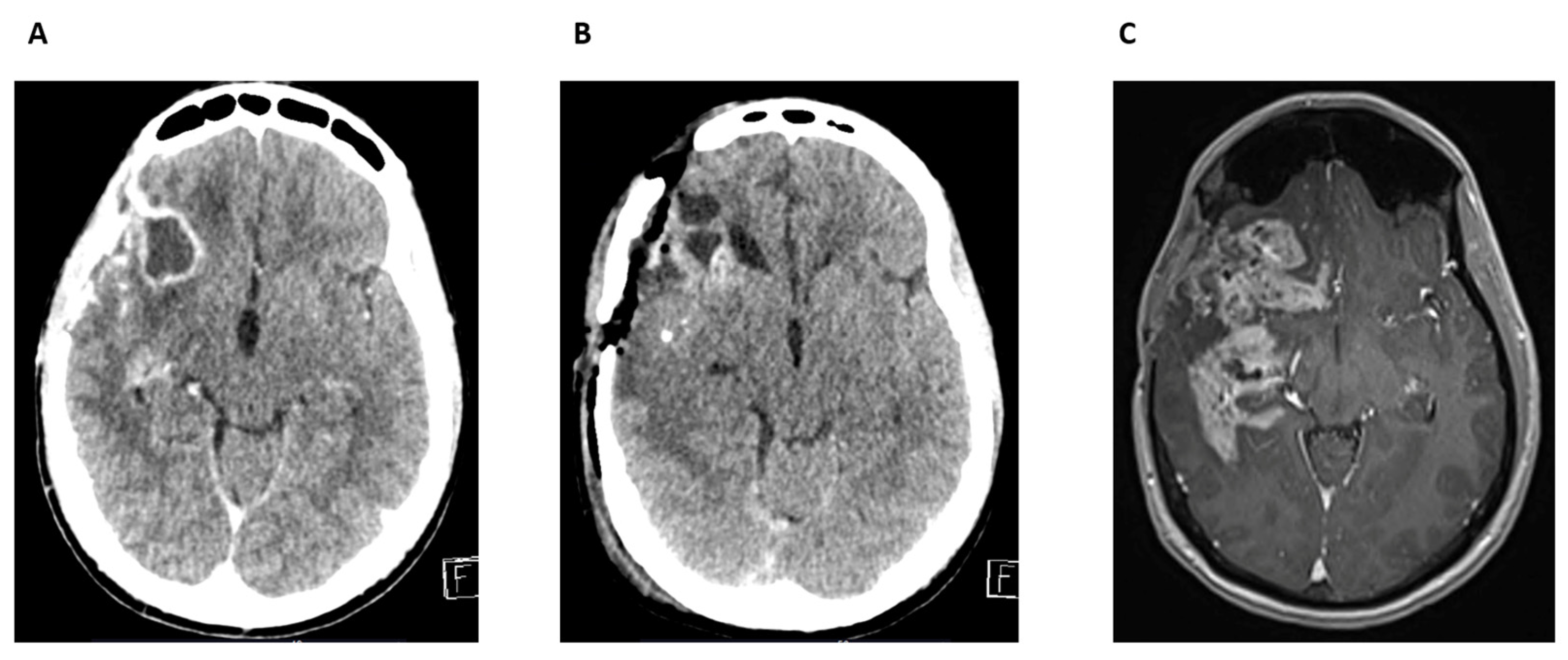
Case Report 2
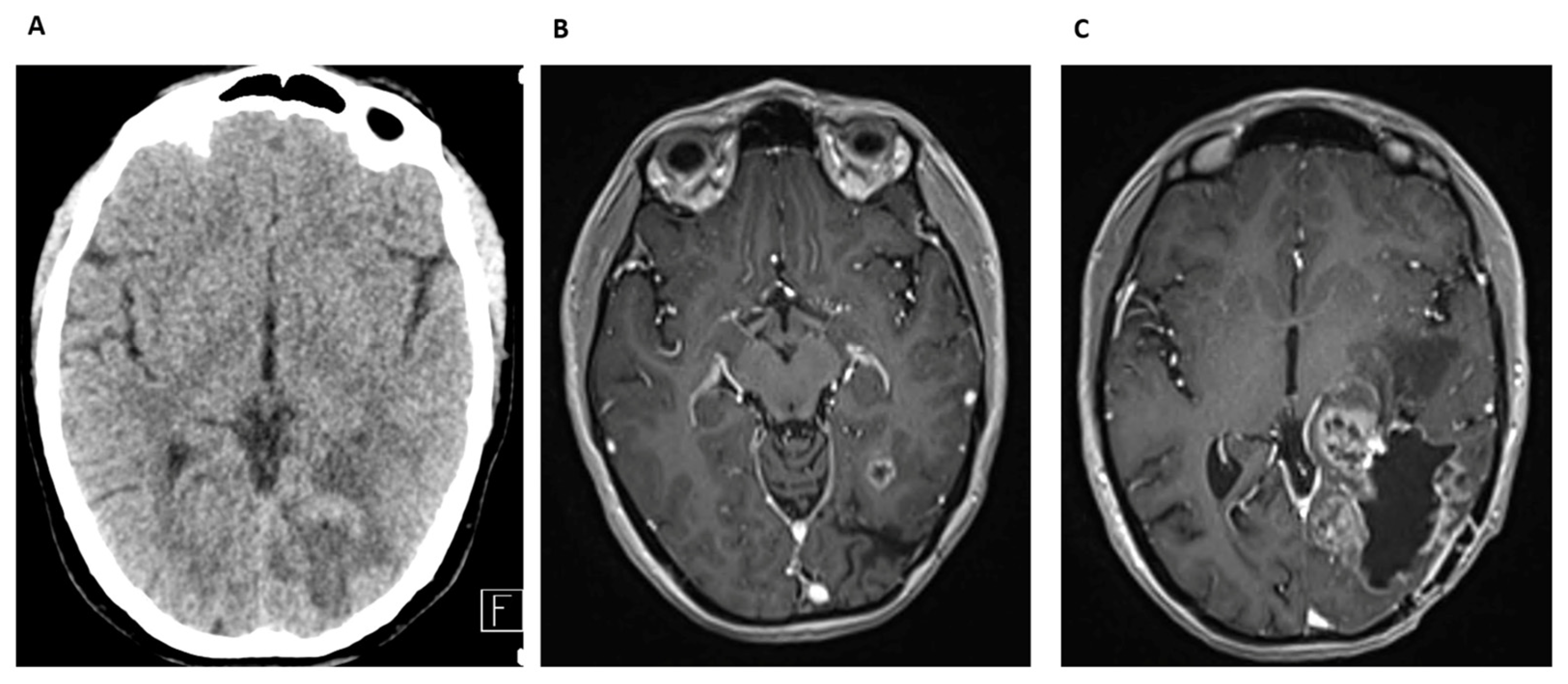
Case Report 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBM | GBM |
| KPS | Karnofsky Performance Score |
| WHO | Word Health Organization |
| IDH | isocitrate dehydrogenase |
| OS | overall survival |
| PFS | progression free survival |
| SSI | surgical site infection |
| SSI-MEN | surgical site infection—meningitis |
| SSI-IC | surgical site infection—intracranial abscess |
| SSI-CRAN | surgical site infection—craniotomy |
| ATRX | Alpha-thalassemia X-linked mutant retardation syndrome |
| TMZ | temozolomide |
| CT | computed tomography |
| MRI | Magnetic Resonance Imaging |
| ASA | acetyl salicylic acid |
| HGG | high-grade glioma |
| AA | anaplastic astrocytoma |
| MHC | major histocompatibility complex |
References
- World Health Organization. Central Nervous System Tumours, 6th ed.; WHO classification of tumours series; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Heilinger, T.; Lavelle, E.C.; Prehn, J.H. Advances in immunotherapy for the treatment of glioblastoma. J. Neurooncol. 2017, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Qin, Y.; You, S.H.; Min, J.J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Kazim, S.F.; Martinez, E.; Hough, T.J.; Spangler, B.Q.; Bowers, C.A.; Chohan, M.O. The Survival Benefit of Postoperative Bacterial Infections in Patients with Glioblastoma Multiforme: Myth or Reality? Front. Neurol. 2021, 12, 615593. [Google Scholar] [CrossRef]
- Solar, P.; Mackerle, Z.; Hendrych, M.; Pospisil, P.; Lakomy, R.; Valekova, H.; Hermanova, M.; Jancalek, R. Prolonged survival in patients with local chronic infection after high-grade glioma treatment: Two case reports. Front. Oncol. 2022, 12, 1073036. [Google Scholar] [CrossRef]
- Bohman, L.E.; Gallardo, J.; Hankinson, T.C.; Waziri, A.E.; Mandigo, C.E.; McKhann, G.M., 2nd; Sisti, M.B.; Canoll, P.; Bruce, J.N. The survival impact of postoperative infection in patients with glioblastoma multiforme. Neurosurgery 2009, 64, 828–834, discussion 825–834. [Google Scholar] [CrossRef]
- R_core_team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 5 April 2025).
- Therneau, T.M. A Package for Survival Analysis in R. Available online: https://CRAN.R-project.org/package=survival (accessed on 5 April 2025).
- Kassambara, A.K.M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. Available online: https://CRAN.R-project.org/package=survminer (accessed on 5 April 2025).
- Miller, J.T.; Rahimi, S.Y.; Lee, M. History of infection control and its contributions to the development and success of brain tumor operations. Neurosurg. Focus 2005, 18, e4. [Google Scholar] [CrossRef]
- Dashti, S.R.; Baharvahdat, H.; Spetzler, R.F.; Sauvageau, E.; Chang, S.W.; Stiefel, M.F.; Park, M.S.; Bambakidis, N.C. Operative intracranial infection following craniotomy. Neurosurg. Focus 2008, 24, E10. [Google Scholar] [CrossRef]
- McClelland, S., 3rd; Hall, W.A. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin. Infect. Dis. 2007, 45, 55–59. [Google Scholar] [CrossRef]
- Turkalp, Z.; Karamchandani, J.; Das, S. IDH mutation in glioma: New insights and promises for the future. JAMA Neurol. 2014, 71, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Solomou, G.; Finch, A.; Asghar, A.; Bardella, C. Mutant IDH in Gliomas: Role in Cancer and Treatment Options. Cancers 2023, 15, 2883. [Google Scholar] [CrossRef]
- De Bonis, P.; Albanese, A.; Lofrese, G.; de Waure, C.; Mangiola, A.; Pettorini, B.L.; Pompucci, A.; Balducci, M.; Fiorentino, A.; Lauriola, L.; et al. Postoperative infection may influence survival in patients with glioblastoma: Simply a myth? Neurosurgery 2011, 69, 864–868, discussion 868–869. [Google Scholar] [CrossRef] [PubMed]
- Salle, H.; Deluche, E.; Couve-Deacon, E.; Beaujeux, A.C.; Pallud, J.; Roux, A.; Dagain, A.; de Barros, A.; Voirin, J.; Seizeur, R.; et al. Surgical Site Infections after glioblastoma surgery: Results of a multicentric retrospective study. Infection 2021, 49, 267–275. [Google Scholar] [CrossRef]
- Shen, H.; Lin, Y.; Chu, Z.; Wang, G.; Chu, W. Case report: Diagnosis and treatment of delayed epidural pyogenic abscess after brain tumor operation: A report of 5 cases and review of the literature. Front. Surg. 2023, 10, 1202387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Ugiliweneza, B.; Burton, E.; Woo, S.Y.; Boakye, M.; Skirboll, S. The effect of postoperative infection on survival in patients with glioblastoma. J. Neurosurg. 2017, 127, 807–811. [Google Scholar] [CrossRef]
- Walker, D.G.; Pamphlett, R. Prolonged survival and pulmonary metastasis after local cure of glioblastoma multiforme. J. Clin. Neurosci. 1999, 6, 67–68. [Google Scholar] [CrossRef]
- Park, D.M.; DeAngelis, L.M. Delayed infection of the Ommaya reservoir. Neurology 2002, 59, 956–957. [Google Scholar] [CrossRef]
- Bowles, A.P., Jr.; Perkins, E. Long-term remission of malignant brain tumors after intracranial infection: A report of four cases. Neurosurgery 1999, 44, 636–642, discussion 633–642. [Google Scholar] [CrossRef]
- Yamamura, M.; Amano, Y.; Sakagami, H.; Yamanaka, Y.; Nishimoto, Y.; Yoshida, H.; Yamaguchi, M.; Ohata, H.; Momose, K.; Takeda, M. Calcium mobilization during nicotine-induced cell death in human glioma and glioblastoma cell lines. Anticancer. Res. 1998, 18, 2499–2502. [Google Scholar]
- Nusair, A.R.; El Nekidy, W.S.; Reynolds, L.; Evans, D.; El-Lababidi, R.; Alatoom, A. Comprehensive Approach to Reduce Surgical Site Infections in Patients Undergoing Neurosurgical Procedures. Surg. Infect. 2021, 22, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Troeman, D.P.R.; Hazard, D.; Timbermont, L.; Malhotra-Kumar, S.; van Werkhoven, C.H.; Wolkewitz, M.; Ruzin, A.; Goossens, H.; Bonten, M.J.M.; Harbarth, S.; et al. Postoperative Staphylococcus aureus Infections in Patients with and Without Preoperative Colonization. JAMA Netw. Open 2023, 6, e2339793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, L.; Fan, S. The characteristics of surgical site infection with class I incision in neurosurgery. BMC Surg. 2025, 25, 97. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Prozan, L.; Popovits, N.; Lidar, Z.; Ben-Ami, R. Risk Factors and Outcomes of Cutibacterium acnes Postoperative Central Nervous System Infection: A Case-Control Study. World Neurosurg. 2020, 137, e251–e256. [Google Scholar] [CrossRef]
- Kelly, M.E.; Fourney, D.R.; Guzman, R.; Sadanand, V.; Griebel, R.W.; Sanche, S.E. Propionibacterium acnes infections after cranial neurosurgery. Can. J. Neurol. Sci. 2006, 33, 292–295. [Google Scholar] [CrossRef]
- Nisbet, M.; Briggs, S.; Ellis-Pegler, R.; Thomas, M.; Holland, D. Propionibacterium acnes: An under-appreciated cause of post-neurosurgical infection. J. Antimicrob. Chemother. 2007, 60, 1097–1103. [Google Scholar] [CrossRef]
- Jimenez-Martinez, E.; Cuervo, G.; Hornero, A.; Ciercoles, P.; Gabarros, A.; Cabellos, C.; Pelegrin, I.; Garcia-Somoza, D.; Adamuz, J.; Carratala, J.; et al. Risk factors for surgical site infection after craniotomy: A prospective cohort study. Antimicrob. Resist. Infect. Control 2019, 8, 69. [Google Scholar] [CrossRef]
- Roe, J.M.; Seely, K.; Bussard, C.J.; Eischen Martin, E.; Mouw, E.G.; Bayles, K.W.; Hollingsworth, M.A.; Brooks, A.E.; Dailey, K.M. Hacking the Immune Response to Solid Tumors: Harnessing the Anti-Cancer Capacities of Oncolytic Bacteria. Pharmaceutics 2023, 15, 2004. [Google Scholar] [CrossRef]
- Huang, S.W.; Lim, S.K.; Yu, Y.A.; Pan, Y.C.; Lien, W.J.; Mou, C.Y.; Hu, C.J.; Mou, K.Y. Overcoming the nutritional immunity by engineering iron-scavenging bacteria for cancer therapy. Elife 2024, 12, RP90798. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of the Patients | Patients with Infections (26) | Age-Matched Controls (26) |
|---|---|---|
| Mean age (years) (min–max) | 56 (30–75) | 56 (33–77) |
| Male (%) | 17 (65%) | 16 (61.5%) |
| Localization (%) | ||
| frontal | 10 (38%) | 9 (35%) |
| occipital | 1 (4%) | 1 (4%) |
| temporal | 8 (31%) | 10 (38%) |
| parietal | 7 (27%) | 6 (23%) |
| Postoperative infection (%) | ||
| SSI | 7 (27%) | 0 |
| SSI-IC | 13 (50%) | 0 |
| SSI-MEN | 3 (11.5%) | 0 |
| SSI-CRAN | 3 (11.5%) | 0 |
| PFS (days) | ||
| mean | 343 | 309 |
| median | 179 | 259 |
| OS (days) | ||
| mean | 674 | 442 |
| median | 388 | 422 |
| Patients | Age at Diagnosis (Years) | Localization | Reoperation | IDH-Type | ATRX | Ki-67 % | PFS (Days) | OS (Days) | Infection Type | Time Elapsed After Surgery and Infection (Days) | Microbiological Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | temporal | yes | IDH-wild | neg | 45 | 80 | 202 | SSI-MEN | 5 | Escherichia coli, Enterococcus faecalis |
| 2 | 56 | frontal | no | IDH-wild | pos | 30 | 176 | 539 | SSI-IC | 170 | ND |
| 3 | 60 | frontal | yes | IDH-wild | pos | 22.5 | 54 | 54 | SSI-IC | 21 | negative |
| 4 | 49 | temporal | yes | ND | ND | ND | 378 | 1075 | SSI | 954 | Staphylococcus aureus |
| 5 | 73 | temporal | yes | IDH-wild | pos | 30 | 147 | 753 | SSI-IC | 202 | Staphylococcus epidermidis |
| 6 | 53 | temporal | yes | IDH-wild | pos | 20 | 39 | 416 | SSI-IC | 15 | ND |
| 7 | 57 | parietal | yes | IDH-mutant | neg | 25 | 327 | 327 | SSI-IC | 217 | Klebsiella aerogenes |
| 8 | 60 | parietal | yes | IDH-wild | pos | ND | 234 | 285 | SSI-IC | 68 | negative |
| 9 | 51 | frontal | yes | IDH-wild | neg | 15 | 182 | 322 | SSI-IC | 217 | negative |
| 10 | 68 | parietal | yes | ND | pos | 55 | 162 | 181 | SSI-IC | 45 | Propionibacterium acnes |
| 11 | 65 | frontal | yes | IDH-wild | neg | 20 | 57 | 57 | SSI-IC | 25 | negative |
| 12 | 67 | frontal | yes | IDH-wild | pos | 27.5 | 27 | 197 | SSI-IC | 102 | Staphylococcus epidermidis, Cutibacterium acnes |
| 13 | 64 | parietal | yes | IDH-wild | pos | ND | 1096 | 2406 | SSI-IC | 2356 | Enterobacter cloacae, Peptoniphilus species, Peptococcus niger Actinomyces turicensis |
| 14 | 56 | parietal | yes | ND | ND | 20 | 173 | 243 | SSI | 229 | ND |
| 15 | 48 | parietal | yes | ND | ND | ND | 516 | 516 | SSI-CRAN | 487 | Methicillin resistant Staphylococcus aureus, Pseudomonas aeruginosa |
| 16 | 53 | parietal | yes | IDH-wild | pos | 15 | 60 | 315 | SSI-CRAN | 68 | negative |
| 17 | 58 | frontal | no | IDH-wild | pos | 25 | 289 | 961 | SSI | 14 | negative |
| 18 | 57 | temporal | yes | IDH-wild | pos | 40 | 462 | 700 | SSI-IC | 484 | Staphylococcus epidermidis |
| 19 | 30 | frontal | ND | IDH-mutant | neg | 40 | 2116 | 2402 | SSI-CRAN | 2175 | Staphylococcus aureus |
| 20 | 75 | temporal | yes | ND | ND | ND | 950 | 978 | SSI | 96 | Methicillin-resistant Staphylococcus aureus |
| 21 | 63 | temporal | no | IDH-wild | pos | 22.5 | 106 | 106 | SSI | 35 | negative |
| 22 | 35 | frontal | no | IDH-mutant | pos | 45 | 933 | 1079 | SSI | 6 | negative |
| 23 | 37 | occipital | no | IDH-wild | pos | 25 | 1818 | 2051 | SSI | 1943 | negative |
| 24 | 48 | frontal | yes | IDH-wild | pos | 22.5 | 702 | 858 | SSI-IC | 33 | negative |
| 25 | 52 | temporal | yes | IDH-mutant | neg | 17.5 | 359 | 359 | SSI-MEN | 10 | Enterococcus faecalis, Staphylococcus epidermidis |
| 26 | 37 | frontal | yes | IDH-mutant | neg | 55 | 145 | 145 | SSI-MEN | 20 | negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berényi, G.; Szabó, D.; Agócs, G.; Andrássy, B.; Fedorcsák, I.; Erőss, L.; Sipos, L. Impact of Infection on Survival Outcomes in High-Grade Gliomas: A Retrospective Analysis of 26 Cases in Our Fifteen-Year Experience—Janus Faced Phenomenon. Cancers 2025, 17, 1348. https://doi.org/10.3390/cancers17081348
Berényi G, Szabó D, Agócs G, Andrássy B, Fedorcsák I, Erőss L, Sipos L. Impact of Infection on Survival Outcomes in High-Grade Gliomas: A Retrospective Analysis of 26 Cases in Our Fifteen-Year Experience—Janus Faced Phenomenon. Cancers. 2025; 17(8):1348. https://doi.org/10.3390/cancers17081348
Chicago/Turabian StyleBerényi, György, Dóra Szabó, Gergely Agócs, Blanka Andrássy, Imre Fedorcsák, Loránd Erőss, and László Sipos. 2025. "Impact of Infection on Survival Outcomes in High-Grade Gliomas: A Retrospective Analysis of 26 Cases in Our Fifteen-Year Experience—Janus Faced Phenomenon" Cancers 17, no. 8: 1348. https://doi.org/10.3390/cancers17081348
APA StyleBerényi, G., Szabó, D., Agócs, G., Andrássy, B., Fedorcsák, I., Erőss, L., & Sipos, L. (2025). Impact of Infection on Survival Outcomes in High-Grade Gliomas: A Retrospective Analysis of 26 Cases in Our Fifteen-Year Experience—Janus Faced Phenomenon. Cancers, 17(8), 1348. https://doi.org/10.3390/cancers17081348







