Adjuvant Therapy in “Intermediate-Risk” Early-Stage Cervical Cancer: To Treat or Not to Treat? Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
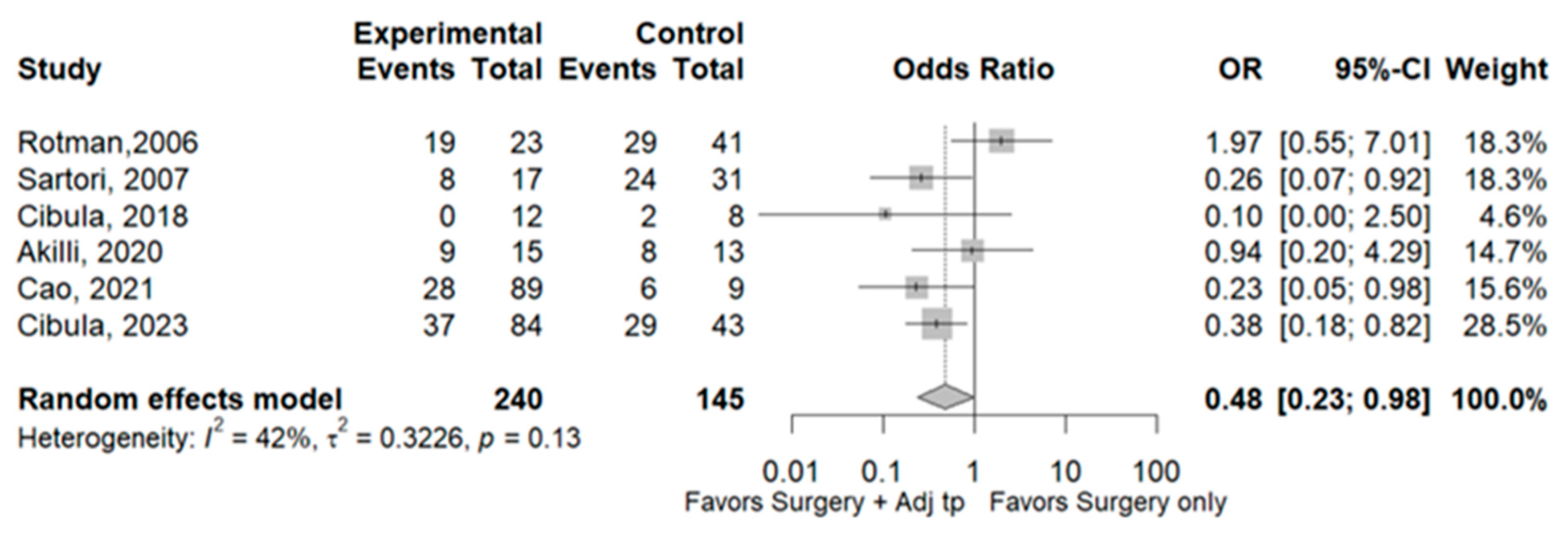
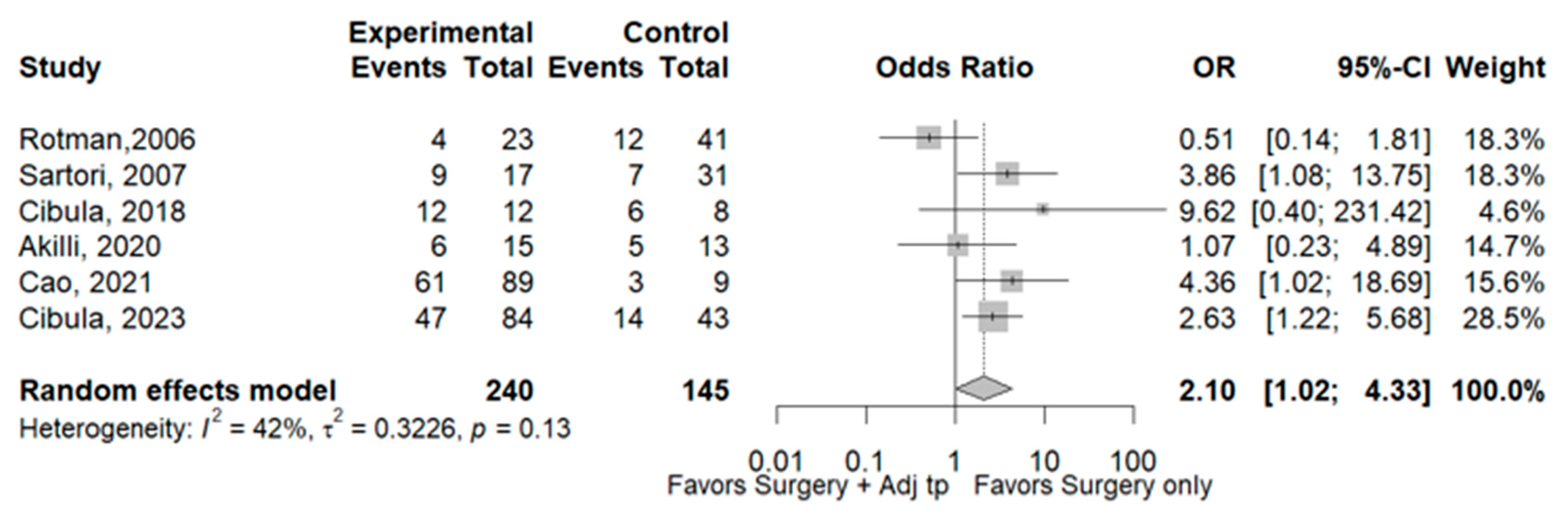
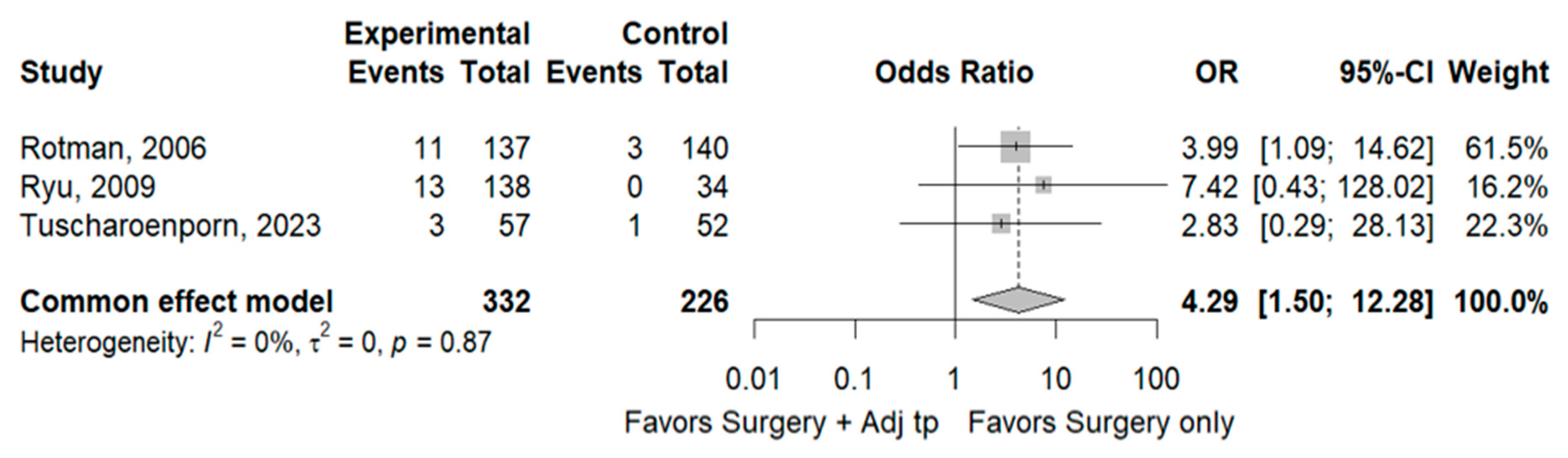
3.1. Recurrence
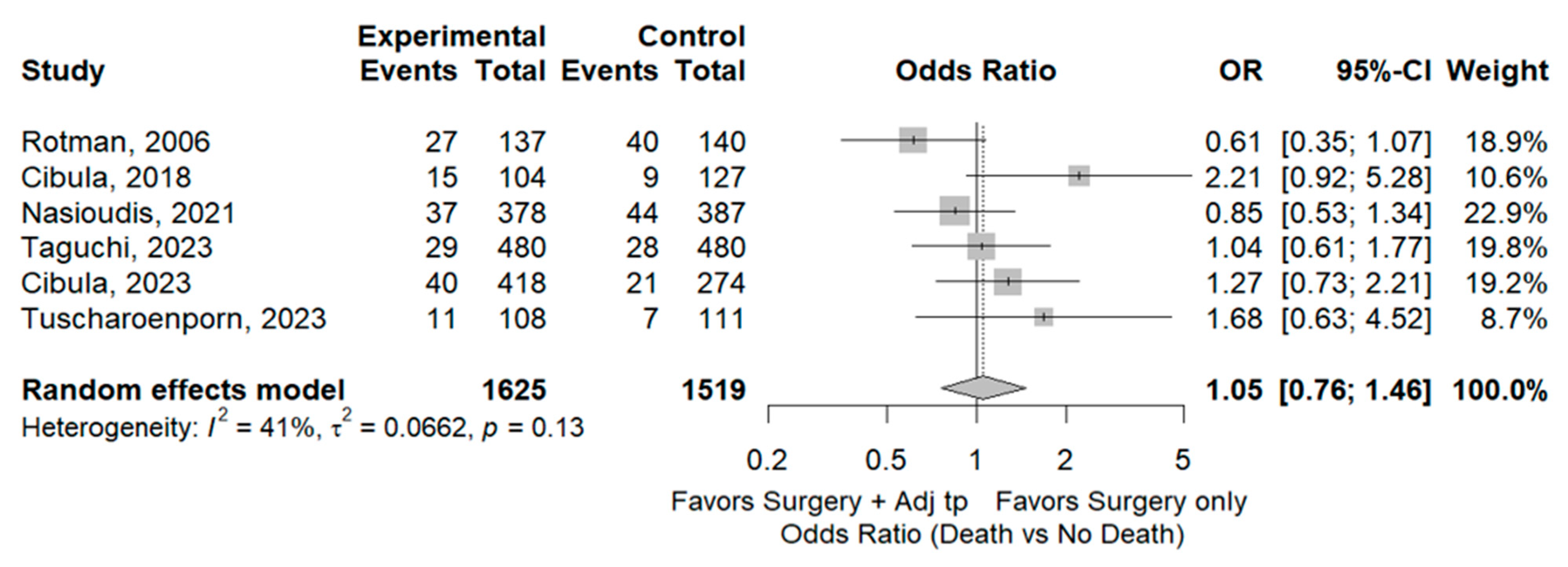
3.2. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023*. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Wagar, M.K.; Hsu, H.-C.; Hoegl, J.; Valzacchi, G.M.R.; Fernandes, A.; Cucinella, G.; Aker, S.S.; Jayraj, A.S.; Mauro, J.; et al. Cervical cancer: A new era. Int. J. Gynecol. Cancer 2024, 34, 1946–1970. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Akilli, H.; Jarkovsky, J.; van Lonkhuijzen, L.; Scambia, G.; Meydanli, M.M.; Ortiz, D.I.; Falconer, H.; Abu-Rustum, N.R.; Odetto, D.; et al. Role of adjuvant therapy in intermediate-risk cervical cancer patients—Subanalyses of the SCCAN study. Gynecol. Oncol. 2023, 170, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A Randomized Trial of Pelvic Radiation Therapy versus No Further Therapy in Selected Patients with Stage IB Carcinoma of the Cervix after Radical Hysterectomy and Pelvic Lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104, Erratum in Int. J. Gynaecol. Obstet. 2010, 108, 176. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. 2006, 65, 169–176. [Google Scholar] [CrossRef]
- Sartori, E.; Tisi, G.; Chiudinelli, F.; La Face, B.; Franzini, R.; Pecorelli, S. Early stage cervical cancer: Adjuvant treatment in negative lymph node cases. Gynecol. Oncol. 2007, 107, S170–S174. [Google Scholar] [CrossRef]
- Ryu, S.-Y.; Park, S.-I.; Nam, B.-H.; Cho, C.-K.; Kim, K.; Kim, B.-J.; Kim, M.-H.; Choi, S.-C.; Lee, E.-D.; Lee, K.-H. Is Adjuvant Chemoradiotherapy Overtreatment in Cervical Cancer Patients with Intermediate Risk Factors? Int. J. Radiat. Oncol. 2011, 79, 794–799. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R.; Fischerova, D.; Pather, S.; Lavigne, K.; Slama, J.; Alektiar, K.; Ming-Yin, L.; Kocian, R.; Germanova, A.; et al. Surgical treatment of “intermediate risk” lymph node negative cervical cancer patients without adjuvant radiotherapy—A retrospective cohort study and review of the literature. Gynecol. Oncol. 2018, 151, 438–443. [Google Scholar] [CrossRef]
- Akilli, H.; Tohma, Y.A.; Bulut, A.N.; Karakas, L.A.; Haberal, A.N.; Kuscu, U.E.; Ayhan, A. Comparison of no adjuvant treatment and radiotherapy in early-stage cervical carcinoma with intermediate risk factors. Int. J. Gynecol. Obstet. 2020, 149, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wen, H.; Feng, Z.; Han, X.; Zhu, J.; Wu, X. Role of adjuvant therapy after radical hysterectomy in intermediate-risk, early-stage cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; A Latif, N.; Ii, R.L.G.; Haggerty, A.F.; Cory, L.; Kim, S.H.; A Morgan, M.; Ko, E.M. Role of adjuvant radiation therapy after radical hysterectomy in patients with stage IB cervical carcinoma and intermediate risk factors. Int. J. Gynecol. Cancer 2021, 31, 829–834. [Google Scholar] [CrossRef]
- Turkmen, O.; Kilic, F.; Tokalioglu, A.A.; Cakir, C.; Yuksel, D.; Kilic, C.; Boran, N.; Comert, G.K.; Turan, T. The effect of adjuvant radiotherapy on oncological outcomes in patients with early-stage cervical carcinoma with only intermediate-risk factors: A propensity score matching analysis. J. Obstet. Gynaecol. 2022, 42, 3204–3211. [Google Scholar] [CrossRef]
- Taguchi, A.; Kato, K.; Hara, K.; Furusawa, A.; Nakajima, Y.; Ishizawa, C.; Tanikawa, M.; Sone, K.; Mori, M.; Shimada, M.; et al. Heterogeneous treatment effects of adjuvant therapy for patients with cervical cancer in the intermediate-risk group. Cancer Med. 2023, 12, 18557–18567. [Google Scholar] [CrossRef]
- Tuscharoenporn, T.; Muangmool, T.; Charoenkwan, K. Adjuvant pelvic radiation versus observation in intermediate-risk early-stage cervical cancer patients following primary radical surgery: A propensity score-adjusted analysis. J. Gynecol. Oncol. 2023, 34, e42. [Google Scholar] [CrossRef]
- Huang, H.; Feng, Y.-L.; Wan, T.; Zhang, Y.-N.; Cao, X.-P.; Huang, Y.-W.; Xiong, Y.; Huang, X.; Zheng, M.; Li, Y.-F.; et al. Effectiveness of Sequential Chemoradiation vs Concurrent Chemoradiation or Radiation Alone in Adjuvant Treatment After Hysterectomy for Cervical Cancer. JAMA Oncol. 2021, 7, 361–369. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Cibula, D.; Köhler, C.; Bajsová, S.; Sebestova, S.; Zapardiel, I.; Di Martino, G.; van Lonkhuijzen, L.; Sehnal, B.; Sánchez, O.A.; Gil Ibañez, B.; et al. 526MO Pathological assessment of sentinel lymph node in early-stage cervical cancer: Results from the prospective SENTIX trial (CEEGOG-CX01; ENGOT-CX2). Ann. Oncol. 2022, 33, S786. [Google Scholar] [CrossRef]
- Pan, T.L.; Pareja, R.; Chiva, L.; Rodriguez, J.; Munsell, M.F.; Iniesta, M.D.; Manzour, N.; Frumovitz, M.; Ramirez, P.T. Accuracy of pre-operative tumor size assessment compared to final pathology and frequency of adjuvant treatment in patients with FIGO 2018 stage IB2 cervical cancer. Int. J. Gynecol. Cancer 2024, 34, 1861–1866. [Google Scholar] [CrossRef]
- Bianchi, T.; Grassi, T.; Di Martino, G.; Negri, S.; Trezzi, G.; Fruscio, R.; Landoni, F. Low-Volume Metastases in Cervical Cancer: Does Size Matter? Cancers 2024, 16, 1107. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines® Insights: Cervical Cancer, Version 1.2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1224–1233. [Google Scholar] [CrossRef]
- Levinson, K.; Beavis, A.L.; Purdy, C.; Rositch, A.F.; Viswanathan, A.; Wolfson, A.H.; Kelly, M.G.; Tewari, K.S.; McNally, L.; Guntupalli, S.R.; et al. Beyond Sedlis—A novel histology-specific nomogram for predicting cervical cancer recurrence risk: An NRG/GOG ancillary analysis. Gynecol. Oncol. 2021, 162, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kitahara, Y.; Satoh, T.; Takei, Y.; Takano, M.; Nagao, S.; Sekiguchi, I.; Suzuki, M. Analysis of the effect of adjuvant radiotherapy on outcomes and complications after radical hysterectomy in FIGO stage IB1 cervical cancer patients with intermediate risk factors (GOTIC Study). World J. Surg. Oncol. 2016, 14, 173. [Google Scholar] [CrossRef]
- Kim, H.; Park, W.; Kim, Y.S.; Kim, Y.J. Chemoradiotherapy is not superior to radiotherapy alone after radical surgery for cervical cancer patients with intermediate-risk factor. J. Gynecol. Oncol. 2020, 31, e35. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W.; Tang, J.; Hu, K.; Zhang, F. Comparison of outcomes between early-stage cervical cancer patients without high-risk factors undergoing adjuvant concurrent chemoradiotherapy and radiotherapy alone after radical surgery. BMC Cancer 2024, 24, 548. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, R.; Jin, D.; Yin, Z.; Hu, B.; Li, R.; Wu, D. Comparison of adjuvant chemoradiotherapy versus radiotherapy in early-stage cervical cancer patients with intermediate-risk factors: A systematic review and meta-analysis. Taiwan J. Obstet. Gynecol. 2022, 61, 15–23. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Fan, Y.; Zhang, P.; Ruan, J.-Y.; Mu, Y.; Li, J.-K. Cervical Cancer Recurrence and Patient Survival After Radical Hysterectomy Followed by Either Adjuvant Chemotherapy or Adjuvant Radiotherapy with Optional Concurrent Chemotherapy: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 823064. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Eriksson, A.G.; Planchamp, F.; Morice, P.; Taylor, A.; Sturdza, A.; Coza, O.F.; Halaska, M.J.; Martinelli, F.; Armbrust, R.; et al. European Society of Gynaecological Oncology expanded quality indicators and accreditation for cervical cancer management. Int. J. Gynecol. Cancer 2024, 34, 480–489. [Google Scholar] [CrossRef]
- Cibula, D.; Borčinová, M.; Kocian, R.; Feltl, D.; Argalacsova, S.; Dvorak, P.; Fischerová, D.; Dundr, P.; Jarkovsky, J.; Höschlová, E.; et al. CERVANTES: An international randomized trial of radical surgery followed by adjuvant (chemo) radiation versus no further treatment in patients with early-stage, intermediate-risk cervical cancer (CEEGOG-CX-05; ENGOT-CX16). Int. J. Gynecol. Cancer 2022, 32, 1327–1331. [Google Scholar] [CrossRef]

| Study | Design | Pts (n) | Adjuvant Therapy | FIGO Stage (2009) | Histology | Tumor Size | Intermediate-Risk Criteria |
|---|---|---|---|---|---|---|---|
| Sedlis, 1999 [4] | RCT | 277 | No 140 (50.5) RT 137 (49.5) | IB | SCC: No 115 (82.1); RT 103 (75.2) Non-SCC: No 25 (17.9); RT 34 (24.8) | ≤3 cm: No 58 (42.6); RT 39 (29.3) >3 cm: No 78 (57.4); RT 94 (70.7) | LVSI+, deep third, any: No 69 (49.3); RT 60 (43.8) LVSI+, middle third, ≥2 cm: No 37 (26.4); RT 28 (20.4) LVSI+, superficial third, ≥5 cm: No 0 (0.0); RT 1 (0.7) LVSI−, middle/deep third, ≥4 cm: No 34 (24.3); RT 48 (35.1) |
| Rotman, 2006 [7] | RCT | 277 | No 140 (50.5) RT 137 (49.5) | IB | SCC: No 115 (82.1); RT 103 (75.2) Non-SCC: No 25 (17.9); RT 34 (24.8) | ≤3 cm: No 58 (42.6); RT 39 (29.3) >3 cm: No 78 (57.4); RT 94 (70.7) | LVSI+, deep third, any: No 69 (49.3); RT 60 (43.8) LVSI+, middle third, ≥2 cm: No 37 (26.4); RT 28 (20.4) LVSI+, superficial third, ≥5 cm: No 0 (0.0); RT 1 (0.7) LVSI−, middle/deep third, ≥4 cm: No 34 (24.3); RT 48 (35.1) |
| Sartori, 2007 [8] | Retrospective | 331 | No 215 (65.0) RT 116 (35.0) | IB | NA | NA | NA |
| Ryu, 2009 [9] | Retrospective | 172 | No 34 (19.8) RT 49 (28.5) CCRT 89 (51.7) | IB1 107 (62.2) IB2 18 (10.4) IIA 47 (27.3) | SCC 130 (75.5) Non-SCC 42 (24.5) | NA | LVSI+, deep third, any: 38 (54.3) LVSI+, middle third, ≥2 cm: 8 (11.4) LVSI+, superficial third, ≥5 cm: 1 (1.4) LVSI−, middle/deep third, ≥4 cm 23 (32.9) |
| Cibula, 2018 [10] | Retrospective | 231 | No 127 (55.0) RT 6 (2.6) CCRT 98 (42.4) | IB | SCC: No 96 (75.6); RT: 67 (64.4) Non-SCC: No 31 (24.4); RT: 37 (35.6) | ≤2 cm: No 34 (26.8); 21 (20.4) 2–4 cm: No 78 (61.4); 61 (59.2) >4 cm: No 15 (11.8); 21 (20.4) | DSI: No 13.0 ± 6.3 mm; RT 13.4 ± 6.0 mm LVSI+: No 67 (52.8); RT 78 (75.0) |
| Akilli, 2020 [11] | Retrospective | 134 | No 66 (49.3) RT 68 (50.7) | IB | SCC: No 55 (83.3); RT 53 (77.9) Non-SCC: No 10 (15.2); RT 14 (20.6) | <4 cm: No 49 (74.2); RT 40 (58.8) ≥4 cm: No 17 (25.8); RT 28 (41.2) | DSI > 1/3: No 58 (87.9); RT 62 (91.2) LVSI+: No 42 (63.6); RT 57 (83.8) |
| Cao, 2021 [12] | Retrospective | 861 | No 85 (9.9) RT 283 (32.9) CCRT 493 (57.2) | IB 414 (48.1) IIA 447 (51.9) | NA | 2–4 cm: No 19 (22.6); RT 56 (19.8); CCRT 135 (27.4) ≥4 cm: No 65 (77.4); RT 227 (80.2); CCRT 354 (72.0) | LVSI+, deep third, any: No 14 (16.5); RT 55 (19.4); CCRT 124 (25.2) LVSI+, middle third, ≥2 cm: No 14 (16.5); RT 58 (20.5); CCRT 127 (25.8) LVSI+, superficial third, ≥5 cm: No 2 (2.4); RT 3 (1.1); CCRT 3 (0.6) LVSI−, middle/deep third, ≥4 cm: No 55 (64.7); RT 167 (59.0); CCRT 239 (48.5) |
| Nasioudis, 2021 [13] | Retrospective | 765 | No 387 (50.6) RT 378 (49.4) | IB | SCC: No 271 (70.0); RT 253 (67.0) Non-SCC: No 116 (30.0); RT 125 (33.0) | 2–4 cm: No 201 (51.9); RT 180 (47.6) ≥4 cm: No 186 (48.1); RT 198 (52.4) | LVSI+: No 239 (62.9); RT 263 (72.9) |
| Turkmen, 2022 [14] | Retrospective | 134 | No 67 (50.0) RT 67 (50.0) | IB | NA | NA | NA |
| Cibula, 2023 [3] | Retrospective | 692 | No 274 (39.6) RT 418 (60.4) | IB | SCC: No 192 (70.3); RT 299 (71.7) Non-SCC: No 82 (29.7); RT 119 (28.3) | 2–3.99 cm: No 156 (56.9); RT 241 (57.7) ≥4 cm: No 118 (43.1); RT 177 (42.3) | DSI > 1/3: NA LVSI+: No 194 (77.3); RT 332 (81.2) |
| Taguchi, 2023 [15] | Retrospective | 960 | No 480 (50.0) CT or RT 480 (50.0) | IB | NA | NA | NA |
| Tuscharoenporn, 2023 [16] | Retrospective | 219 | No 111 (50.7) CCRT or RT 108 (49.3) | IB 194 (88.6) IIA 25 (11.4) | SCC: No 81 (73.0); Adj 76 (70.4) Non-SCC: No 30 (27.0); Adj 32 (29.6) | No 3.12 ± 1.37 Adj 3.16 ± 1.33 | DSI: Middle1/3 No 14 (12.6); Adj 21 (19.4); Outer1/3 No 97 (87.4); Adj 87 (80.6) LVSI+: No 100 (90.1); Adj 99 (91.7) |
| Study | Median FUP (Months) | Recurrence (n) | Site of Recurrence | 5 y OS (%) |
|---|---|---|---|---|
| Sedlis, 1999 [4] | 120.0 | No 39 (27.9) RT 21 (15.3) | Local: No 27 (69.2); RT 18 (85.7) Distant: No 10 (25.6); RT 3 (14.3) | No 78.6 RT 86.9 |
| Rotman, 2006 [7] | 240.0 | No 43 (30.7) RT 24 (17.5) | Local: No 29 (67.4); RT 19 (79.2) Distant: No 12 (27.9); RT 4 (16.7) | No 71.4 RT 80.3 |
| Sartori, 2007 [8] | 79.0 | No 31 (14.4) RT 17 (14.7) | Local: No 24 (77.4); RT 8 (47.1) Distant: No 7 (22.6); RT 9 (52.9) | NA |
| Ryu, 2009 [9] | 44.6 | No 6 (17.6) RT 4 (8.1) CRT 89 2 (2.2) | NA | No 94.1 RT 97.6 CRT 98.6 |
| Cibula, 2018 [10] | 89.8 | No 8 (6.3) RT/CRT 12 (12.2) | Local: No 2 (25.0); RT 0 (0.0) Distant: No 6 (75.0); RT 12 (100) | No 92.9 RT/CRT 85.6 |
| Akilli, 2020 [11] | 61.1 | No 13 (19.7) RT 15 (22.1) | Local: No 8 (61.5); RT 9 (60.0) Distant: No 5 (38.5); RT 6 (40.0) | NA |
| Cao, 2021 [12] | 63.0 | No 6 (6.8) RT 15 (5.3) CRT 13 (2.6) | Local: No 6 (60.0); RT 15 (36.6); CCRT 13 (24.5) Distant: No 3 (30.0); RT 25 (61.0); CCRT 36 (67.9) | NA |
| Nasioudis, 2021 [13] | 45.0 | NA | NA | No 87.1 RT 88.4 |
| Turkmen, 2022 [14] | 60.0 | No 12 (17.9) RT 7 (10.4) | NA | NA |
| Cibula, 2023 [3] | 55.2 | No 44 (16.1) RT 84 (20.1) | Local: No 29 (65.9); RT 14 (16.7) Distant: No 37 (84.1); RT 47 (55.6) | No 92.4 RT 90.4 |
| Taguchi, 2023 [15] | NA | No 57 (11.9) CT or RT 47 (9.8) | NA | No 94.2 CT or RT 94.0 |
| Tuscharoenporn, 2023 [16] | 76.1 | No 13 (11.7) CRT or RT 9 (8.3) | NA | No 93.7 CRT or RT 89.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripepi, C.; Cracco, F.; Ricci, G.; Nappi, L.; Restaino, S.; Vizzielli, G.; Carlucci, S.; Stabile, G. Adjuvant Therapy in “Intermediate-Risk” Early-Stage Cervical Cancer: To Treat or Not to Treat? Systematic Review and Meta-Analysis. Cancers 2025, 17, 1320. https://doi.org/10.3390/cancers17081320
Ripepi C, Cracco F, Ricci G, Nappi L, Restaino S, Vizzielli G, Carlucci S, Stabile G. Adjuvant Therapy in “Intermediate-Risk” Early-Stage Cervical Cancer: To Treat or Not to Treat? Systematic Review and Meta-Analysis. Cancers. 2025; 17(8):1320. https://doi.org/10.3390/cancers17081320
Chicago/Turabian StyleRipepi, Chiara, Francesco Cracco, Giuseppe Ricci, Luigi Nappi, Stefano Restaino, Giuseppe Vizzielli, Stefania Carlucci, and Guglielmo Stabile. 2025. "Adjuvant Therapy in “Intermediate-Risk” Early-Stage Cervical Cancer: To Treat or Not to Treat? Systematic Review and Meta-Analysis" Cancers 17, no. 8: 1320. https://doi.org/10.3390/cancers17081320
APA StyleRipepi, C., Cracco, F., Ricci, G., Nappi, L., Restaino, S., Vizzielli, G., Carlucci, S., & Stabile, G. (2025). Adjuvant Therapy in “Intermediate-Risk” Early-Stage Cervical Cancer: To Treat or Not to Treat? Systematic Review and Meta-Analysis. Cancers, 17(8), 1320. https://doi.org/10.3390/cancers17081320