Outcome of Debulking the Mesenteric Mass in Symptomatic Patients with Locally Advanced Small Intestine Neuroendocrine Tumors
Simple Summary
Abstract
1. Introduction
2. Methods
- (a)
- (b)
- The tumor was classified as locally advanced or unresectable because mesenteric disease encircled the mesenteric vascular root above the level of the horizontal part of the duodenum and/or extended to the retroperitoneum (Table 1);
- (c)
- Patients were symptomatic with episodes of abdominal pain and/or bowel obstruction.
2.1. Statistics
2.2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamarca, A.; Bartsch, D.K.; Caplin, M.; Kos-Kudla, B.; Kjaer, A.; Partelli, S.; Rinke, A.; Janson, E.T.; Thirlwell, C.; van Velthuysen, M.F.; et al. European Neuroendocrine Tumor Society (ENETS) 2024 guidance paper for the management of well-differentiated small intestine neuroendocrine tumours. J. Neuroendocrinol. 2024, 36, e13423. [Google Scholar] [CrossRef] [PubMed]
- Koea, J. Commonwealth Neuroendocrine Tumour Research Collaborative (CommNETs) Surgical Section. Management of Locally Advanced and Unresectable Small Bowel Neuroendocrine Tumours. World J. Surg. 2021, 45, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.J.; Pasieka, J.L.; Dixon, E.; Rorstad, O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery 2008, 144, 645–653, discussion 651–653. [Google Scholar] [CrossRef] [PubMed]
- Pasquer, A.; Walter, T.; Hervieu, V.; Forestier, J.; Scoazec, J.-Y.; Lombard-Bohas, C.; Poncet, G. Surgical management of small bowel neuroendocrine tumors: Specific requirements and their impact on staging and prognosis. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S742–S749. [Google Scholar] [CrossRef]
- Watzka, F.M.; Fottner, C.; Miederer, M.; Weber, M.M.; Schad, A.; Lang, H.; Musholt, T.J. Surgical treatment of NEN of small bowel: A retrospective analysis. World J. Surg. 2016, 40, 749–758. [Google Scholar] [CrossRef]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef]
- Ohrvall, U.; Eriksson, B.; Juhlin, C.; Karacagil, S.; Rastad, J.; Hellman, P.; Akerström, G. Method for dissection of mesenteric metastases in mid-gut carcinoid tumors. World J. Surg. 2000, 24, 1402–1408. [Google Scholar] [CrossRef]
- Deguelte, S.; Perrier, M.; Hammoutene, C.; Cadiot, G.; Kianmanesh, R. Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 2319. [Google Scholar] [CrossRef]
- Bartsch, D.K.; Windel, S.; Kanngießer, V.; Jesinghaus, M.; Holzer, K.; Rinke, A.; Maurer, E. Vessel-Sparing Lymphadenectomy Should Be Performed in Small Intestine Neuroendocrine Neoplasms. Cancers 2022, 14, 3610. [Google Scholar] [CrossRef]
- Van Den Heede, K.; van Beek, D.J.; Van Slycke, S.; Borel Rinkes, I.; Norlén, O.; Stålberg, P.; Nordenström, E. Surgery for advanced neuroendocrine tumours of the small bowel: Recommendations based on a consensus meeting of the European Society of Endocrine Surgeons (ESES). Br. J. Surg. 2024, 111, znae082. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C. North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Niederle, B.; Pape, U.F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Comp. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Maurer, E.; Bartsch, D.K. Lokale Resektion von neuroendokrinen Tumoren des Dünndarms (SI-NEN): Aktuelle Prinzipien [Local resection of small intestine neuroendocrine neoplasms (SI-NEN): Current principles]. Chirurgie 2024, 95, 818–824. [Google Scholar] [CrossRef]
- Hounschell, C.A.; Higginbotham, S.; Al-Kasspooles, M.; Selby, L.V. Gastroenteropancreatic Neuroendocrine Tumor with Peritoneal Metastasis: A Review of Current Management. Cancers 2024, 16, 3472. [Google Scholar] [CrossRef]
- Pantongrag-Brown, L.; Buetow, P.C.; Carr, N.J.; Lichtenstein, J.E.; Buck, J.L. Calcification and fibrosis in mesenteric carcinoid tumor: CT findings and pathologic correlation. AJR Am. J. Roentgenol. 1995, 164, 387–391. [Google Scholar] [CrossRef]
- Graf, S.D.; Keber, C.U.; Hattesohl, A.; Teply-Szymanski, J.; Hattesohl, S.; Guder, M.; Gercke, N.; Di Fazio, P.; Slater, E.P.; Jesinghaus, M.; et al. Mesenteric fibrosis in patients with small intestinal neuroendocrine tumors is associated with enrichment of alpha-smooth muscle actin-positive fibrosis and COMP-expressing stromal cells. J. Neuroendocrinol. 2024, 36, e13364. [Google Scholar] [CrossRef]
- Cattell, R.B.; Braasch, J.W. A technique for the exposure of the third and fourth portions of the duodenum. Surg. Gynecol. Obstet. 1960, 111, 378–379. [Google Scholar]
- Oderich, G.S.; Erben, Y.; Debus, E.S. Klinische Anatomie und Physiologie des viszeralen Arteriensystems. In Operative und interventionelle Gefäßmedizin. Springer Reference Medizin; Debus, E., Gross-Fengels, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Shaikh, H.; Wehrle, C.J.; Khorasani-Zadeh, A. Anatomy, Abdomen and Pelvis: Superior Mesenteric Artery. [Updated 2023 July 24]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519560/ (accessed on 4 December 2024).
- Broussard, A.; Wehrle, C.J.; Samra, N.S. Anatomy, Abdomen and Pelvis: Superior Mesenteric Vein. [Updated 2023 August 8]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545162/ (accessed on 4 December 2024).
- Bufacchi, P.; Gomes-Jorge, M.; Walter, T.; Poncet, G.; Pasquer, A. Mesenteric sparing approach for advanced nodal extent in small intestinal neuroendocrine tumors. Is there a limit to the vascular resection in order to avoid creating a short small bowel syndrome? An anatomic research study. Surg. Radiol. Anat. 2024, 46, 811–823. [Google Scholar] [CrossRef]
- Pasquer, A.; Walter, T.; Milot, L.; Hervieu, V.; Poncet, G. Principles of Surgical Management of Small Intestinal NET. Cancers 2021, 13, 5473. [Google Scholar] [CrossRef]
- Neddermeyer, M.; Kanngießer, V.; Maurer, E.; Bartsch, D.K. Indocyanine Green Near-Infrared Fluoroangiography Is a Useful Tool in Reducing the Risk of Anastomotic Leakage Following Left Colectomy. Front. Surg. 2022, 29, 850256. [Google Scholar] [CrossRef] [PubMed]
- Publication of WHO Classification of Tumours, 5th Edition, Volume 1: Digestive System Tumours—IARC. Available online: https://www.iarc.who.int/fr/news-events/publication-of-who-classification-of-tumours-5th-edition-volume-1-digestive-system-tumours/ (accessed on 4 December 2024).
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS); Netzwerk Neuroendokrine Tumoren (NeT) e.V. (Patientenvertretung); Bundesorganisation Selbsthilfe NeuroEndokrine Tumoren e.V. (NET-sgh) (Patientenvertretung); Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e.V. (DGHO), und Arbeitsgemeinschaft Internistische Onkologie (AIO) der Deutschen Krebsgesellschaft e.V.; Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie e.V. (DGAV); Deutsche Gesellschaft für Chirurgie (DGCH); Deutsche Gesellschaft für Endoskopie und Bildgebende Verfahren (DGEBV); Deutsche Gesellschaft für Nuklearmedizin e.V. (DGNM); Deutsche Gesellschaft für Innere Medizin (DGIM); Deutsche Gesellschaft für Endokrinologie (DGE); et al. S2k-Leitlinie Neuroendokrine Tumore [Practice guideline neuroendocrine tumors—AWMF-Reg. 021-27]. Z. Gastroenterol. 2018, 56, 583–681. [Google Scholar] [CrossRef]
- Knigge, U.; Capdevila, J.; Bartsch, D.K.; Baudin, E.; Falkerby, J.; Kianmanesh, R.; Kos-Kudla, B.; Niederle, B.; van Dijkum, E.N.; O’Toole, D.; et al. ENETS consensus recommendations for the standards of care in neuroendocrine tumors: Follow-up and documentation. Neuroendocrinology 2009, 105, 310–319. [Google Scholar] [CrossRef]
- Laskaratos, F.M.; Walker, M.; Wilkins, D.; Tuck, A.; Ramakrishnan, S.; Phillips, E.; Gertner, J.; Megapanou, M.; Papantoniou, D.; Shah, R.; et al. Evaluation of Clinical Prognostic Factors and Further Delineation of the Effect of Mesenteric Fibrosis on Survival in Advanced Midgut Neuroendocrine Tumours. Neuroendocrinology 2018, 107, 292–304. [Google Scholar] [CrossRef]
- Lardière-Deguelte, S.; de Mestier, L.; Appéré, F.; Vullierme, M.P.; Zappa, M.; Hoeffel, C.; Noaves, M.; Brixi, H.; Hentic, O.; Ruszniewski, P.; et al. Toward a Preoperative Classification of Lymph Node Metastases in Patients with Small Intestinal Neuroendocrine Tumors in the Era of Intestinal-Sparing Surgery. Neuroendocrinology 2016, 103, 552–559. [Google Scholar] [CrossRef]
- Daskalakis, K.; Karakatsanis, A.; Hessman, O.; Stuart, H.C.; Welin, S.; Tiensuu Janson, E.; Öberg, K.; Hellman, P.; Norlén, O.; Stålberg, P. Association of a Prophylactic Surgical Approach to Stage IV Small Intestinal Neuroendocrine Tumors with Survival. JAMA Oncol. 2017, 4, 183–189. [Google Scholar] [CrossRef]
- Hellman, P.; Hessman, O.; Akerström, G.; Stålberg, P.; Hennings, J.; Björck, M.; Eriksson, L.G. Stenting of the superior mesenteric vein in midgut carcinoid disease with large mesenteric masses. World J. Surg. 2010, 34, 1373–1379. [Google Scholar] [CrossRef]
- Søreide, K.; Stättner, S.; Hallet, J. Surgery as a Principle and Technical Consideration for Primary Tumor Resection of Small Bowel Neuroendocrine Tumors. Ann. Surg. Oncol. 2024, 31, 1125–1137. [Google Scholar] [CrossRef]
- Conley, D.; Hurst, P.R.; Stringer, M.D. An investigation of human jejunal and ileal arteries. Anat. Sci. Int. 2010, 85, 23–30. [Google Scholar] [CrossRef]
- Kitchens, W.H.; Elias, N.; Blaszkowsky, L.S.; Cosimi, A.B.; Hertl, M. Partial abdominal evisceration and intestinal autotransplantation to resect a mesenteric carcinoid tumor. World J. Surg. Oncol. 2011, 31, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kaçmaz, E.; Chen, J.W.; Tanis, P.J.; Nieveen van Dijkum, E.J.M.; Engelsman, A.F. Postoperative morbidity and mortality after surgical resection of small bowel neuroendocrine neoplasms: A systematic review and meta-analysis. J. Neuroendocrinol. 2021, 33, e13008. [Google Scholar] [CrossRef] [PubMed]
- Blaževic, A.; Zandee, W.T.; Franssen, G.J.H.; Hofland, J.; van Velthuysen, M.-L.F.; Hofland, L.J.; Feelders, R.A.; de Herder, W.W. Mesenteric fibrosis and palliative surgery in small intestinal neuroendocrine tumours. Endocr. Relat. Cancer 2018, 25, 245–254. [Google Scholar]
- de Mestier, L.; Lardière-Deguelte, S.; Brixi, H.; O’Toole, D.; Ruszniewski, P.; Cadiot, G.; Kianmanesh, R. Updating the surgical management of peritoneal carcinomatosis in patients with neuroendocrine tumors. Neuroendocrinology 2015, 101, 105–111. [Google Scholar] [CrossRef]
- Calomino, N.; Poto, G.E.; Carbone, L.; Bagnacci, G.; Piccioni, S.; Andreucci, E.; Nenci, L.; Marano, L.; Verre, L.; Petrioli, R.; et al. Neuroendocrine tumors’ patients treated with somatostatin analogue could complicate with emergency cholecystectomy. Ann. Ital. Chir. 2023, 94, 518–522. [Google Scholar]

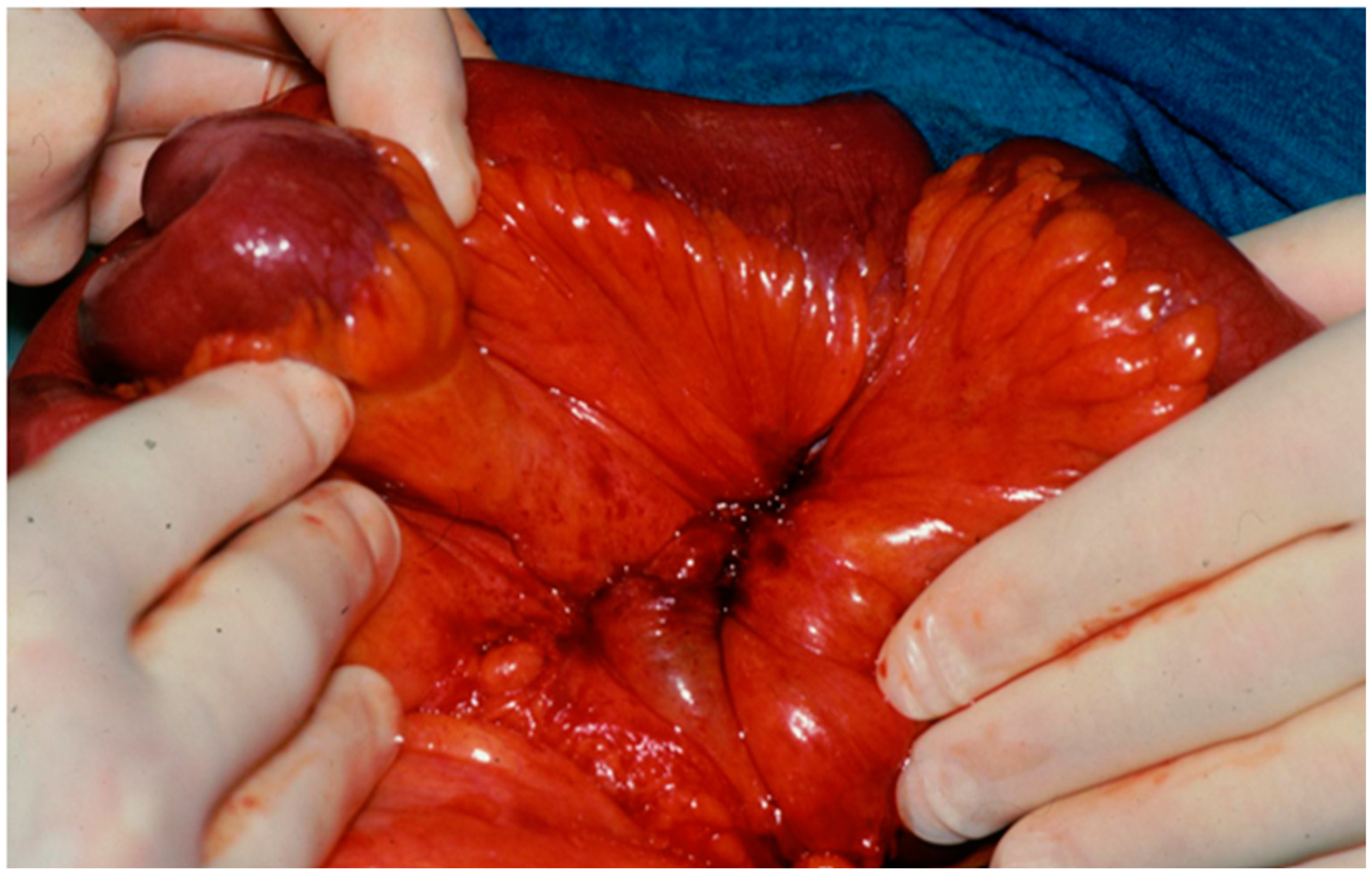
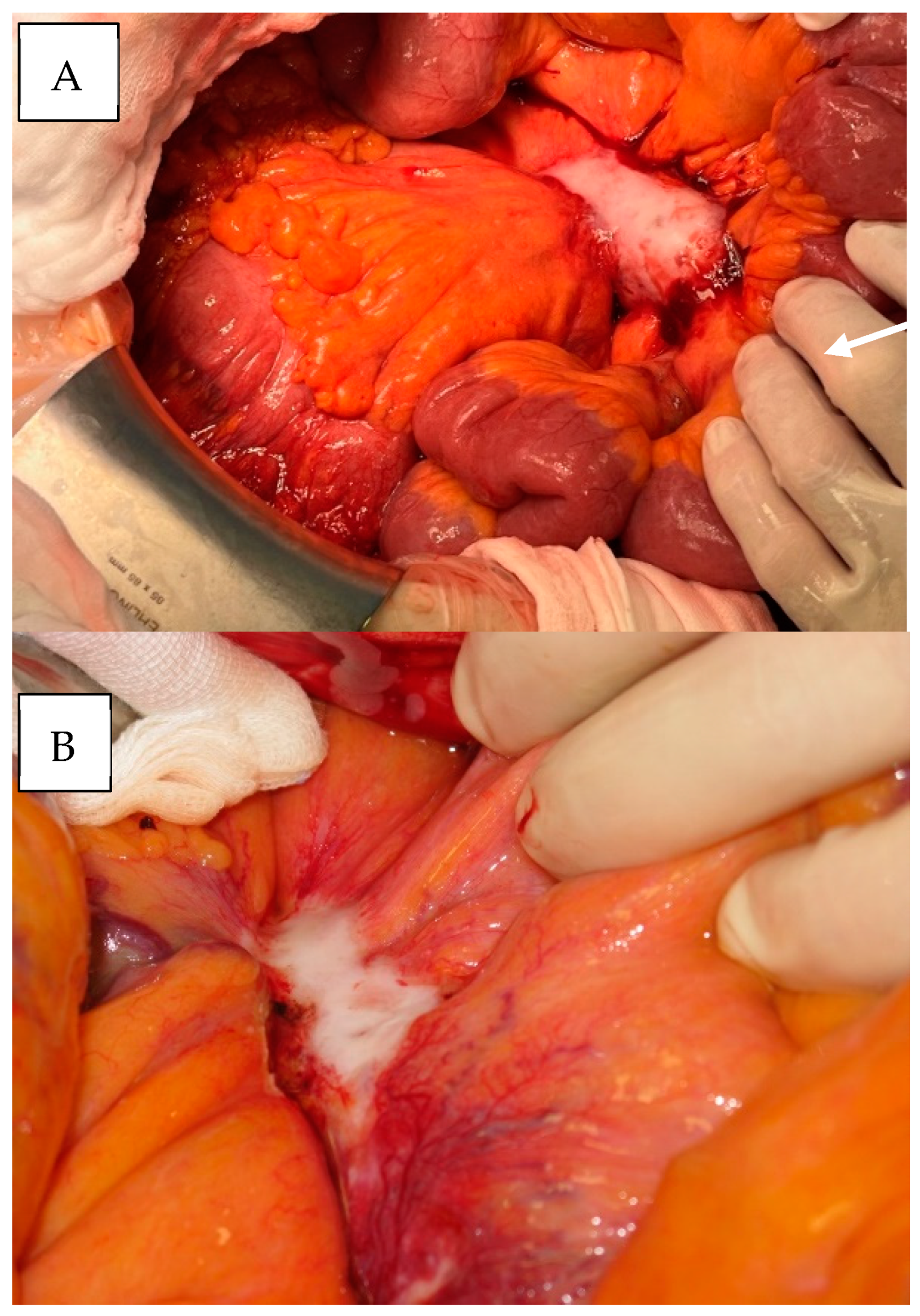
| Resectable | Mesenteric disease (nodal metastases and desmoplasia) up to the of the outlet of the ileocolic artery from the mesenteric superior artery |
| Borderline resectable | Mesenteric disease up to the level of the inferior pancreas body without encircling the mesenteric vessel root, nor the first two jejunal arteries, and without extension to the retroperitoneum |
| Locally advanced or unresectable | Mesenteric disease that encircles the mesenteric vessel root, including the first two jejunal arteries, above the level of the inferior wall of the horizontal part of the duodenum and/or extension to the retroperitoneum |
| Proportion of locally advanced cases of all SI-NENs (2012–2024) | 29/202 (14%) |
| Age, years median (range) at Dx | 63 (46–78) |
| Gender (male/female) | 23/6 |
| Abdominal symptoms | 29/29 (100%) |
| Abdominal pain | 22/29 (76%) |
| Symptoms of bowel obstruction | 11/29 (38%) |
| Weight loss | 22/29 (76%) |
| Patients with stage III/stage IV disease | 2 (7%)/27 (93%) |
Previous SI-NEN surgery
| 14/29 (48%) 10/14 (71%) 4/14 (29%) |
| Occlusion or compression of PV/SMV on imaging | 18/29 (62%) |
| Mesenteric mass > 10 mm at level 3 on imaging | 16/29 (55%) |
| Advanced mesenteric desmoplasia on imaging * | 21/29 (72%) |
| Previous SSA treatment | 22/29 (76%) |
| Previous PRRT treatment | 7/29 (24%) |
| Carcinoid syndrome | 12/29 (41%) |
| Hedinger syndrome | 1/29 (3%) |
Distant metastases
| 27/29 (93%) 23/27 (85%) 11/27 (41%) 20/27 (74%) 4/27 (15%) |
| Type of procedure - Debulking MM with small bowel resection - Debulking MM with right hemicolectomy/ileocecal resection - Debulking MM with resection of ileotransversostomy - Debulking MM without bowel resection - No debulking MM, but resection of ischemic bowel - Additional resection of liver metastases | Number n = 29 14/29 (48%) 10/29 (34%) 2/29 (7%) 2/29 (7%) 1/29 (3%) 14/29 (48%) |
| Operating time (min.), median (range) | 262 (156–411) |
| Patients with advanced mesenteric desmoplasia intraoperatively * | 29/29 (100%) |
| Patients with ≥200 cm small bowel length after debulking | 26/29 (90%) |
| Grading, G1/G2/G3 | 19 (66%)/9 (31%)/1 (3%) |
| LN resected in patients with debulking, median (range) | 20 (7–40) |
| Postoperative complications ≥CD 3 | 4/29 (14%) |
| Perioperative mortality | 1/29 (3%) |
| Improvement in bowel obstruction | 11/11 (100%) |
| Improvement in abdominal pain | 20/22 (91%) |
| Additional SSA treatment | 28/29 (97%) |
| Additional PRRT | 9/29 (31%) |
| Additional local treatments (e.g., TACE, stenting SMA/SMV, duodenal stent) | 9/29 (31%) |
| Additional abdominal surgery for SI-NENs | 2/29 (7%) |
| Median follow-up (range) after debulking surgery, months | 28 (1–142) |
| Patients alive with disease at study endpoint | 21/29 (72%) |
| 5-year survival after debulking surgery | 0.7 (95%CI 0.5–0.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartsch, D.K.; Krasser-Gercke, N.; Rinke, A.; Mahnken, A.; Jesinghaus, M.; Eilsberger, F.; Maurer, E. Outcome of Debulking the Mesenteric Mass in Symptomatic Patients with Locally Advanced Small Intestine Neuroendocrine Tumors. Cancers 2025, 17, 1318. https://doi.org/10.3390/cancers17081318
Bartsch DK, Krasser-Gercke N, Rinke A, Mahnken A, Jesinghaus M, Eilsberger F, Maurer E. Outcome of Debulking the Mesenteric Mass in Symptomatic Patients with Locally Advanced Small Intestine Neuroendocrine Tumors. Cancers. 2025; 17(8):1318. https://doi.org/10.3390/cancers17081318
Chicago/Turabian StyleBartsch, Detlef K., Norman Krasser-Gercke, Anja Rinke, Andreas Mahnken, Moritz Jesinghaus, Friederike Eilsberger, and Elisabeth Maurer. 2025. "Outcome of Debulking the Mesenteric Mass in Symptomatic Patients with Locally Advanced Small Intestine Neuroendocrine Tumors" Cancers 17, no. 8: 1318. https://doi.org/10.3390/cancers17081318
APA StyleBartsch, D. K., Krasser-Gercke, N., Rinke, A., Mahnken, A., Jesinghaus, M., Eilsberger, F., & Maurer, E. (2025). Outcome of Debulking the Mesenteric Mass in Symptomatic Patients with Locally Advanced Small Intestine Neuroendocrine Tumors. Cancers, 17(8), 1318. https://doi.org/10.3390/cancers17081318






