Fertility-Sparing Surgery for Non-Epithelial Ovarian Malignancies: Ten-Year Retrospective Study of Oncological and Reproductive Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
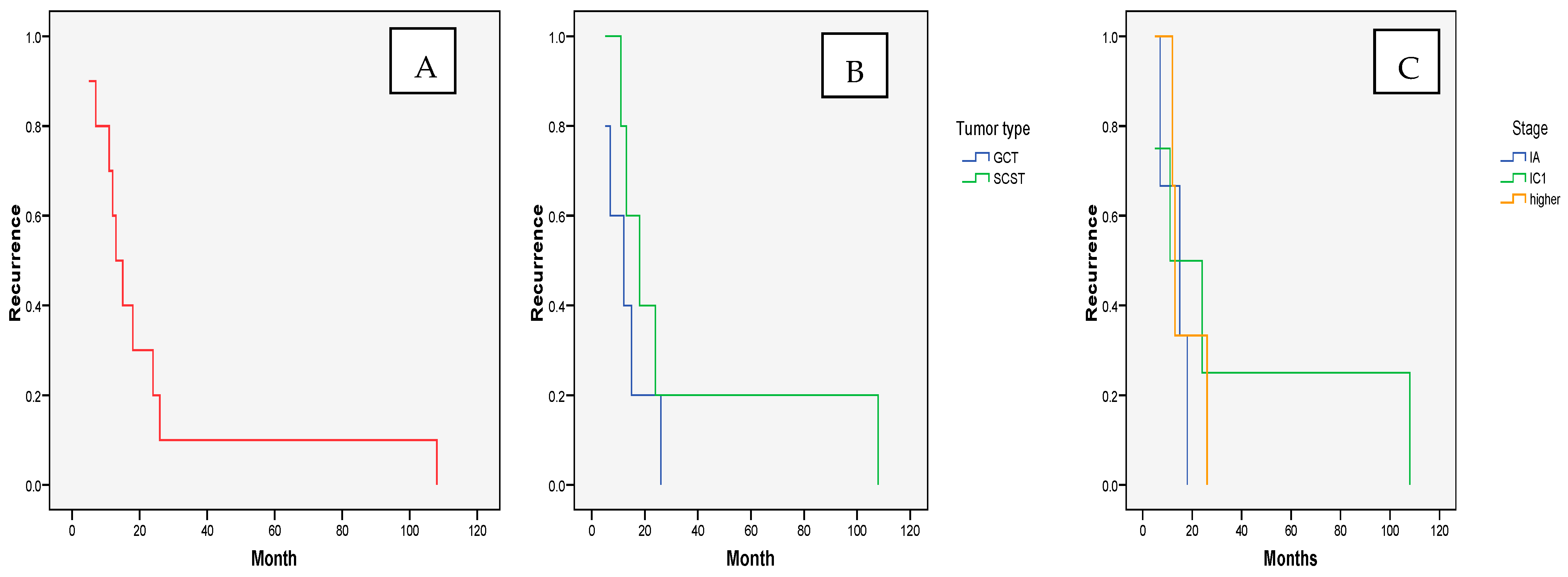
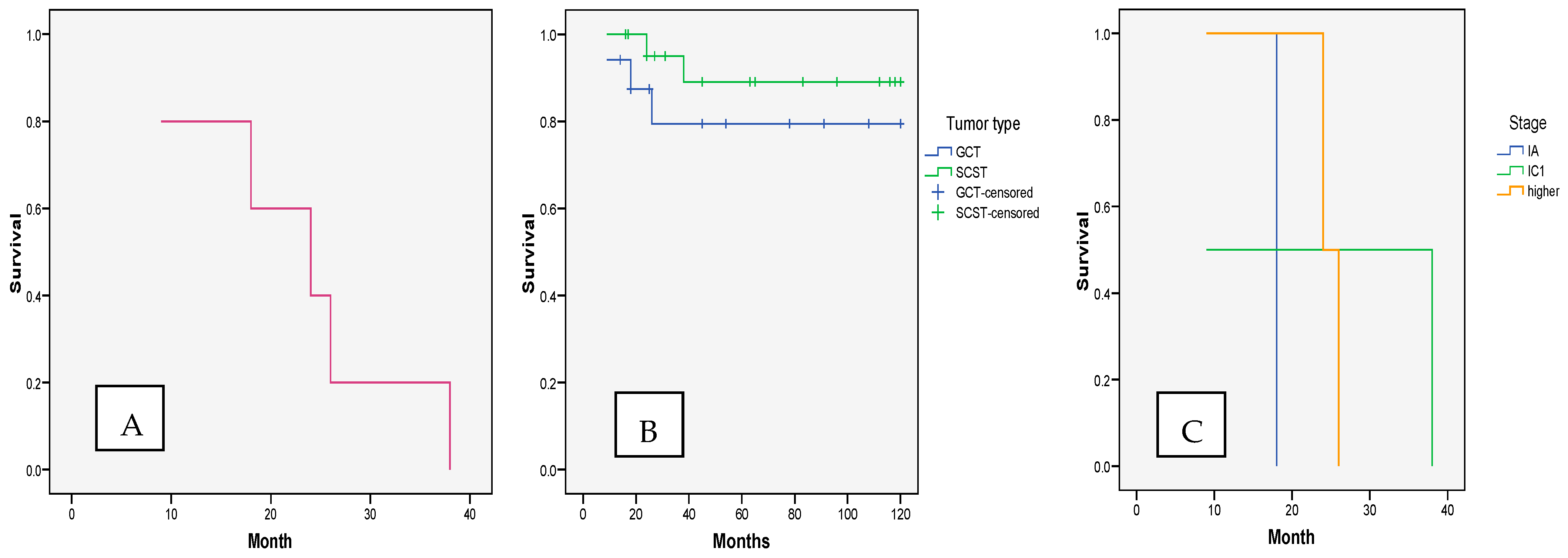
3.1. OncologicalOutcomes—Recurrence and Survival
3.2. Reproductive Outcomes
4. Discussion
5. Conclusions
6. Patients
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, A.; Shah, S.; Parker, J.; Soor, P.; Limbu, A.; Sheriff, M.; Boussios, S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int. J. Environ. Res. Public Health 2022, 19, 1106. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N.; ESMO Guidelines Committee. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef]
- Daviu, C.; Blaakaer, J.; Eriksson, A.G.Z.; Herrstedt, J.; Vandborg, M.P.; Rasmussen, A.M.O.; Fuglsang, K. Nonepithelial ovarian cancer–The current clinical practice in the Nordic countries. Survey from the surgical subcommittee of the Nordic society of gynecological oncology (NSGO). Acta Oncol. 2022, 61, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Schneider, D.T.; Planchamp, F.; Baust, K.; Braicu, E.I.; Concin, N.; Godzinski, J.; McCluggage, W.G.; Orbach, D.; Pautier, P.; et al. ESGO–SIOPE guidelines for the management of adolescents and young adults with non-epithelial ovarian cancers. Lancet Oncol. 2020, 21, e360–e368. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.P.; Tjota, M.Y.; Choi, D.N.B.; Chapel, D.B.; Kolin, D.L.; Euscher, E.D.; Barroeta, J.E.; Numan, T.A.; Xing, D.; Afkhami, M.; et al. Clinicopathologic and Molecular Characterization of Gynecologic Carcinosarcomas With a Mesonephric-Like Carcinomatous Component. Am. J. Surg. Pathol. 2025, 10, 1097. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, X.; Zhang, Z.; Xu, G.; Yu, X.; Li, S.; Zhang, Y.; Bian, L.; Zhang, B. Treatment Approach and Prognosis of Pediatric and Adolescent Nonepithelial Malignant Ovarian Tumors: A Retrospective Prognosis Analysis. J. Pediatr. Adolesc. Gynecol. 2018, 31, 304–310. [Google Scholar] [CrossRef]
- Bennetsen, A.; Baandrup, L.; Aalborg, G.; Kjaer, S. Non-epithelial ovarian cancer in Denmark – Incidence and survival over nearly 40 years. Gynecol. Oncol. 2020, 157, 693–699. [Google Scholar] [CrossRef]
- Ceppi, L.; Galli, F.; Lamanna, M.; Magni, S.; Dell’Orto, F.; Verri, D.; Marchette, M.D.; Lissoni, A.A.; Sina, F.; Giuliani, D.; et al. Ovarian function, fertility, and menopause occurrence after fertility-sparing surgery and chemotherapy for ovarian neoplasms. Gynecol. Oncol. 2019, 152, 346–352. [Google Scholar] [CrossRef]
- Johansen, G.; Dahm-Kähler, P.; Staf, C.; Rådestad, A.F.; Rodriguez-Wallberg, K.A. Fertility-sparing surgery for treatment of non-epithelial ovarian cancer: Oncological and reproductive outcomes in a prospective nationwide population-based cohort study. Gynecol. Oncol. 2019, 155, 287–293. [Google Scholar] [CrossRef]
- Thomakos, N.; Malakasis, A.; Machairiotis, N.; Zarogoulidis, P.; Rodolakis, A. Fertility Sparing Management in Non-Epithelial Ovarian Cancer. Which Patients, What Procedure and What Outcome? J. Cancer 2018, 9, 4659–4664. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, R.; Hua, K.; Zhang, Y. A retrospective study of reproductive outcomes after fertility-sparing surgery and postoperative adjuvant chemotherapy in malignant ovarian germ cell tumors and sex cord-stromal tumors. J. Ovarian Res. 2017, 10, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, B.; Yu, Y.; Chen, J.; Zhang, Y.; Yin, Y.; Yu, N.; Chen, G.; Zhu, S.; Huang, H.; Yuan, Y.; et al. Possibility of women treated with fertility-sparing surgery for non-epithelial ovarian tumors to safely and successfully become pregnant—a Chinese retrospective cohort study among 148 cases. Front. Med. 2018, 12, 509–517. [Google Scholar] [CrossRef]
- Zamani, N.; Poor, M.R.; Dizajmehr, S.G.; Alizadeh, S.; Gilani, M.M. Fertility sparing surgery in malignant ovarian Germ cell tumor (MOGCT): 15 years experiences. BMC Women’s Health 2021, 21, 282. [Google Scholar] [CrossRef]
- Gomes, T.A.; Campos, E.A.; Yoshida, A.; Sarian, L.O.; Andrade, L.A.L.d.A.; Derchain, S.F. Preoperative Differentiation of Benign and Malignant Non-epithelial Ovarian Tumors: Clinical Features and Tumor Markers. Rev. Bras. Hematol. Hemoter. 2020, 42, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Scambia, G.; Abu-Rustum, N.R.; Acien, M.; Arena, A.; Brucker, S.; Cheong, Y.; Collinet, P.; Fanfani, F.; Filippi, F.; et al. Fertility-sparing treatment and follow-up in patients with cervical cancer, ovarian cancer, and borderline ovarian tumours: Guidelines from ESGO, ESHRE, and ESGE. Lancet Oncol. 2024, 25, e602–e610. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Robertson, J.A.; Sanday, K.; Nicklin, J. Malignant ovarian germ cell tumours in the post-menopausal population. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 285–287. [Google Scholar] [CrossRef]
- Sağnıç, S.; Karadağ, C.; Tuncer, H.A.; Doğan, S.; Şimşek, T. Reproductive and oncologic outcomes in women with non-epithelial ovarian cancer: Single center experience over 25 years. J. Turk. Soc. Obstet. Gynecol. 2023, 20, 97–104. [Google Scholar] [CrossRef]
- Hemmingsen, C.H.; Kjaer, S.K.; Bennetsen, A.K.K.; Dehlendorff, C.; Baandrup, L. The association of reproductive factors with risk of non-epithelial ovarian cancer and comparison with serous ovarian cancer. Gynecol. Oncol. 2021, 162, 469–474. [Google Scholar] [CrossRef]
- Kempf, E.; Desamericq, G.; Vieites, B.; Diaz-Padilla, I.; Calvo, E.; Estevez, P.; Garcia-Arreza, A.; Martinez-Maestre, M.; Duran, I. Clinical and pathologic features of patients with non-epithelial ovarian cancer: Retrospective analysis of a single institution 15-year experience. Clin. Transl. Oncol. 2017, 19, 173–179. [Google Scholar] [CrossRef]
- Mikuš, M.; Benco, N.; Matak, L.; Planinić, P.; Ćorić, M.; Lovrić, H.; Radošević, V.; Puževski, T.; Bajt, M.; Vujić, G. Fertility-sparing surgery for patients with malignant ovarian germ cell tumors: 10 years of clinical experience from a tertiary referral center. Arch. Gynecol. Obstet. 2020, 301, 1227–1233. [Google Scholar] [CrossRef]
- Piątek, S.; Szymusik, I.; Sobiczewski, P.; Michalski, W.; Kowalska, M.; Ołtarzewski, M.; Bidziński, M. Obstetric Results after Fertility-Sparing Management of Non-Epithelial Ovarian Cancer. Cancers 2023, 15, 4170. [Google Scholar] [CrossRef]
- Klar, M.; Plett, H.; Harter, P.; Heitz, F.; Kommoss, S.; Hartkopf, A.D.; Grube, M.; Roser, E.; Sehouli, J.; Braicu, I.; et al. Treatment and survival of patients with malignant ovarian sex cord-stromal cell tumours: An analysis of the Arbeitsgemeinschaft für Gynäkologische Onkologie (AGO) study group CORSETT database. J. Surg. Oncol. 2023, 128, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Analysis of outcomes and prognostic factors after fertility-sparing surgery in malignant ovarian germ cell tumors. Gynecol. Oncol. 2017, 145, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, H.; Colonna-Márquez, L.E. Non-epithelial ovarian carcinoma: What is the optimal staging surgery? Chin. Clin. Oncol. 2020, 9, 50. [Google Scholar] [CrossRef]
- El Helali, A.; Kwok, G.S.T.; Tse, K.Y. Adjuvant and post-surgical treatment in non-epithelial ovarian cancer. Best Pr. Res. Clin. Obstet. Gynaecol. 2022, 78, 74–85. [Google Scholar] [CrossRef]
- Saani, I.; Raj, N.; Sood, R.; Ansari, S.; Mandviwala, H.A.; Sanchez, E.; Boussios, S. Clinical Challenges in the Management of Malignant Ovarian Germ Cell Tumours. Int. J. Environ. Res. Public. Heal. 2023, 20, 6089. [Google Scholar] [CrossRef] [PubMed]
- Uccello, M.; Boussios, S.; Samartzis, E.P.; Moschetta, M. Systemic anti-cancer treatment in malignant ovarian germ cell tumours (MOGCTs): Current management and promising approaches. Ann. Transl. Med. 2020, 8, 1713. [Google Scholar] [CrossRef]
- Fumagalli, D.; Jayraj, A.; Olearo, E.; Capasso, I.; Hsu, H.-C.; Tzur, Y.; Piedimonte, S.; Jugeli, B.; Santana, B.N.; De Vitis, L.A.; et al. Primary versus interval cytoreductive surgery in patients with rare epithelial or non-epithelial ovarian cancer. Int. J. Gynecol. Cancer 2025, 35, 101664. [Google Scholar] [CrossRef]
- Ronsini, C.; Restaino, S.; Budani, M.C.; Porcelli, G.; Tiboni, G.M.; Fanfani, F. Fertility sparing treatment for bilateral borderline ovarian tumor: A case report and management strategy explication. Minerva Obstet. Gynecol. 2023, 75, 583–587. [Google Scholar] [CrossRef]
| Parameters | Whole Sample | GCT | SCST | Between Groups p | |||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | ||
| Patient’s age | 26.87 | 6.95 | 23.88 | 4.59 | 29.18 | 7.65 | 0.016 |
| Parity before cancer | 0.46 | 0.72 | 0.35 | 0.61 | 0.55 | 0.81 | 0.415 |
| Follow-up in months | 62.92 | 45.63 | 64.00 | 45.43 | 77.59 | 42.31 | 0.342 |
| Recurrence in months | 23.91 | 30.29 | 13.01 | 8.28 | 34.80 | 41.23 | 0.281 |
| Adverse outcome in months | 23.01 | 10.68 | 17.66 | 8.50 | 31.01 | 9.89 | 0.203 |
| Parameters | Whole Sample | GCT | SCST | Between Groups p | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |||
| Ovarian tumor histology | dysgerminoma | 8 | 20.5 | 8 | 47.1 | / | / | / |
| teratoma | 5 | 12.8 | 5 | 29.4 | / | / | ||
| yolk sack | 4 | 10.3 | 4 | 23.5 | / | / | ||
| granulosa—adult | 17 | 43.6 | / | / | 17 | 43.6 | ||
| granulosa—juvenile | 4 | 10.3 | / | / | 4 | 10.3 | ||
| Sertoli–Leidig | 1 | 2.6 | / | / | 1 | 2.6 | ||
| Cancer stage | IA | 21 | 53.8 | 10 | 58.8 | 11 | 50.0 | 0.636 |
| IC1 | 13 | 33.3 | 5 | 29.4 | 8 | 36.4 | ||
| IC2 | 3 | 7.7 | 1 | 5.9 | 2 | 9.1 | ||
| IC3 | 1 | 2.6 | 0 | 0 | 1 | 4.5 | ||
| IIIC | 1 | 2.6 | 1 | 5.9 | 0 | 0 | ||
| I Surgery type | UCE | 5 | 12.8 | 1 | 5.9 | 4 | 18.2 | 0.828 |
| USOE | 14 | 35.9 | 6 | 35.3 | 8 | 36.4 | ||
| staging | 20 | 51.3 | 10 | 58.8 | 10 | 45.5 | ||
| II Surgery | no | 28 | 71.8 | 13 | 76.5 | 15 | 68.2 | 0.573 |
| staging | 11 | 28.2 | 4 | 23.5 | 7 | 31.8 | ||
| I Chemo- therapy | no | 20 | 51.3 | 7 | 41.2 | 13 | 59.1 | 0.273 |
| yes | 19 | 48.7 | 10 | 58.8 | 9 | 40.9 | ||
| Recurrence | no | 29 | 74.4 | 12 | 70.6 | 17 | 77.3 | 0.640 |
| yes | 10 | 25.6 | 5 | 29.4 | 5 | 22.7 | ||
| Recurrence localization | peritoneum | 4 | 40.0 | 1 | 20.0 | 3 | 60.0 | 0.154 |
| lymph nodes | 2 | 20.0 | 1 | 20.0 | 1 | 20.0 | ||
| ovaries | 3 | 30.0 | 2 | 40.0 | 1 | 20.0 | ||
| multiple | 1 | 10.0 | 1 | 20.0 | 0 | 0 | ||
| II Chemo- therapy | no | 30 | 76.9 | 13 | 76.5 | 17 | 77.3 | 0.954 |
| yes | 9 | 23.1 | 4 | 23.5 | 5 | 22.7 | ||
| Final radicalization | no | 35 | 89.7 | 16 | 94.1 | 19 | 86.4 | 0.734 |
| yes | 4 | 10.3 | 1 | 5.9 | 3 | 13.6 | ||
| Surviving follow-up | no | 5 | 12.8 | 3 | 17.6 | 2 | 9.1 | 0.434 |
| yes | 34 | 87.2 | 14 | 82.4 | 20 | 90.9 | ||
| Parameters | B Coefficient | Wald Coefficient | p | Odds Ratio | Lower 95% Confidence Interval | Higher 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Age | −0.207 | 1.708 | 0.191 | 0.813 | 0.596 | 1.109 |
| Parity | 0.586 | 0.179 | 0.672 | 1.797 | 0.119 | 7.080 |
| Histology | −0.051 | 0.016 | 0.899 | 0.951 | 0.436 | 2.071 |
| Stage | 2.339 | 5.714 | 0.017 | 1.374 | 1.524 | 7.631 |
| I Surgery type | 0.053 | 0.002 | 0.967 | 1.055 | 0.083 | 3.373 |
| II Surgery type | −1.689 | 0.036 | 0.689 | 0.019 | 0.002 | 3.681 |
| Staging complete | −2.022 | 1.159 | 0.282 | 0.132 | 0.003 | 5.255 |
| Chemotherapy I | −1.600 | 1.595 | 0.207 | 0.202 | 0.017 | 2.419 |
| Constant | 2.601 | 0.210 | 0.047 | 4.958 |
| Parameters | B Coefficient | Wald Coefficient | p | Odds Ratio | Lower 95% Confidence Interval | Higher 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Age | 0.376 | 1.365 | 0.243 | 1.457 | 0.775 | 2.740 |
| Parity | 2.501 | 1.812 | 0.114 | 0.355 | 0.792 | 5.331 |
| Histology | −0.537 | 0.339 | 0.560 | 0.584 | 0.096 | 3.564 |
| Stage | −0.107 | 0.009 | 0.925 | 0.899 | 0.097 | 8.324 |
| I Surgery type | −1.717 | 0.383 | 0.536 | 0.180 | 0.001 | 4.157 |
| II Surgery type | −4.231 | 0.044 | 0.981 | 0.015 | 0.166 | 8.661 |
| Staging complete | 3.449 | 1.247 | 0.491 | 1.476 | 0.223 | 5.672 |
| Chemotherapy I | 1.200 | 0.165 | 0.684 | 3.321 | 0.010 | 6.245 |
| Recurrence | −4.892 | 0.361 | 0.042 | 0.055 | 3.417 | 8.981 |
| Chemotherapy II | 2.777 | 0.015 | 0.866 | 2.620 | 0.012 | 4.371 |
| Radicalization | 1.434 | 0.277 | 0.599 | 4.197 | 0.020 | 4.275 |
| Constant | 9.557 | 0.421 | 0.028 | 3.905 |
| Parameters | Whole Sample | GCT | SCST | Between-Group p | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |||
| Pregnancy attempted | no | 29 | 74.4 | 12 | 70.6 | 17 | 77.3 | 0.640 |
| yes | 10 | 25.6 | 5 | 29.4 | 5 | 22.7 | ||
| Pregnancy achieved | no | 3 | 30.0 | 2 | 40.0 | 1 | 20.0 | 0.513 |
| yes | 7 | 70.0 | 3 | 60.0 | 4 | 80.0 | ||
| Delivery mode | vaginal | 5 | 71.4 | 1 | 33.3 | 4 | 100 | 0.074 |
| caesarean section | 2 | 28.6 | 2 | 66.7 | 0 | 0 | ||
| Delivery time | term | 5 | 71.4 | 2 | 66.7 | 4 | 80.0 | 0.248 |
| preterm | 2 | 28.6 | 1 | 33.3 | 1 | 20.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likic Ladjevic, I.; Dotlic, J.; Stefanovic, K.; Milosevic, B.; Beleslin, A.; Mihaljevic, O.; Bila, J.; Vukovic, I.; Radojevic, M.; Vilendecic, Z. Fertility-Sparing Surgery for Non-Epithelial Ovarian Malignancies: Ten-Year Retrospective Study of Oncological and Reproductive Outcomes. Cancers 2025, 17, 1304. https://doi.org/10.3390/cancers17081304
Likic Ladjevic I, Dotlic J, Stefanovic K, Milosevic B, Beleslin A, Mihaljevic O, Bila J, Vukovic I, Radojevic M, Vilendecic Z. Fertility-Sparing Surgery for Non-Epithelial Ovarian Malignancies: Ten-Year Retrospective Study of Oncological and Reproductive Outcomes. Cancers. 2025; 17(8):1304. https://doi.org/10.3390/cancers17081304
Chicago/Turabian StyleLikic Ladjevic, Ivana, Jelena Dotlic, Katarina Stefanovic, Branislav Milosevic, Aleksandra Beleslin, Olga Mihaljevic, Jovan Bila, Ivana Vukovic, Milos Radojevic, and Zoran Vilendecic. 2025. "Fertility-Sparing Surgery for Non-Epithelial Ovarian Malignancies: Ten-Year Retrospective Study of Oncological and Reproductive Outcomes" Cancers 17, no. 8: 1304. https://doi.org/10.3390/cancers17081304
APA StyleLikic Ladjevic, I., Dotlic, J., Stefanovic, K., Milosevic, B., Beleslin, A., Mihaljevic, O., Bila, J., Vukovic, I., Radojevic, M., & Vilendecic, Z. (2025). Fertility-Sparing Surgery for Non-Epithelial Ovarian Malignancies: Ten-Year Retrospective Study of Oncological and Reproductive Outcomes. Cancers, 17(8), 1304. https://doi.org/10.3390/cancers17081304






