Real-World Data on the Efficacy of Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma and Amplification 1q
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Objectives, Participants, and Oversights
2.2. Laboratory Analyses and Definition of Risk Criteria
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
| Number of Patients | Percentage | ||
|---|---|---|---|
| 74 (n) | 100% | ||
| Gender | Male | 46 | 62–2.1 |
| Female | 28 | 37.8 | |
| Median age | 62 yrs (53–71 IQR) | - | |
| Median age at daratumumab initialization | 66 yrs (56.75–75 IQR) | - | |
| Median follow-up | 5 yrs (3–7 IQR) | - | |
| Patient deaths | Total number | 29 | 39.4 |
| Male | 18 | 62.1 | |
| Female | 11 | 37.9 | |
| Cause of death | Disease related | 28 | 96.55 |
| Not disease related/unknown | 1 | 8.45 | |
| Revised International Staging System | I | 17 | 23.0 |
| II | 32 | 43.2 | |
| III | 25 | 33.8 | |
| Myeloma subtype | IgG | 34 | 45.9 |
| IgM | 1 | 1.4 | |
| IgA | 20 | 27 | |
| IgD | 2 | 2.7 | |
| Light-chain myeloma | 17 | 22.97 | |
| Kappa | 47 | 63.51 | |
| Lambda | 27 | 36.49 | |
| Cytogenetic aberration | del17p | 7 | 9.5 |
| t(4;14) | 16 | 21.6 | |
| amp1q21 | 26 | 35.1 | |
| gain1q | 17 | 23 | |
| t(14;20) | 0 | 0 | |
| t(14;16) | 0 | 0 | |
| Del1p32 | 3 | 4.1 | |
| Cytogenetic risk | High-Risk R2-ISS | 29 | 39.2 |
| Double-Hit R2-ISS | 10 | 13.5 | |
| High-Risk IMS2024 | 24 | 32.4 | |
| Previous lines of therapy | 0 | 9 | 12.2 |
| 1 | 17 | 23 | |
| 2 | 28 | 37.8 | |
| 3 or more | 20 | 27 | |
| Previous stem cell transplant | Autologous median age | 44 | 59.7 |
| Allogeneic | 1 | 2.2 | |
| No stem cell transplant | 25 | 33.8 | |
| Not available | 1 | 2.2 | |
| Plasma cell infiltration | ≥60% | 20 | 27 |
| <60% | 50 | 67.6 | |
| Not available | 4 | 5.4 | |
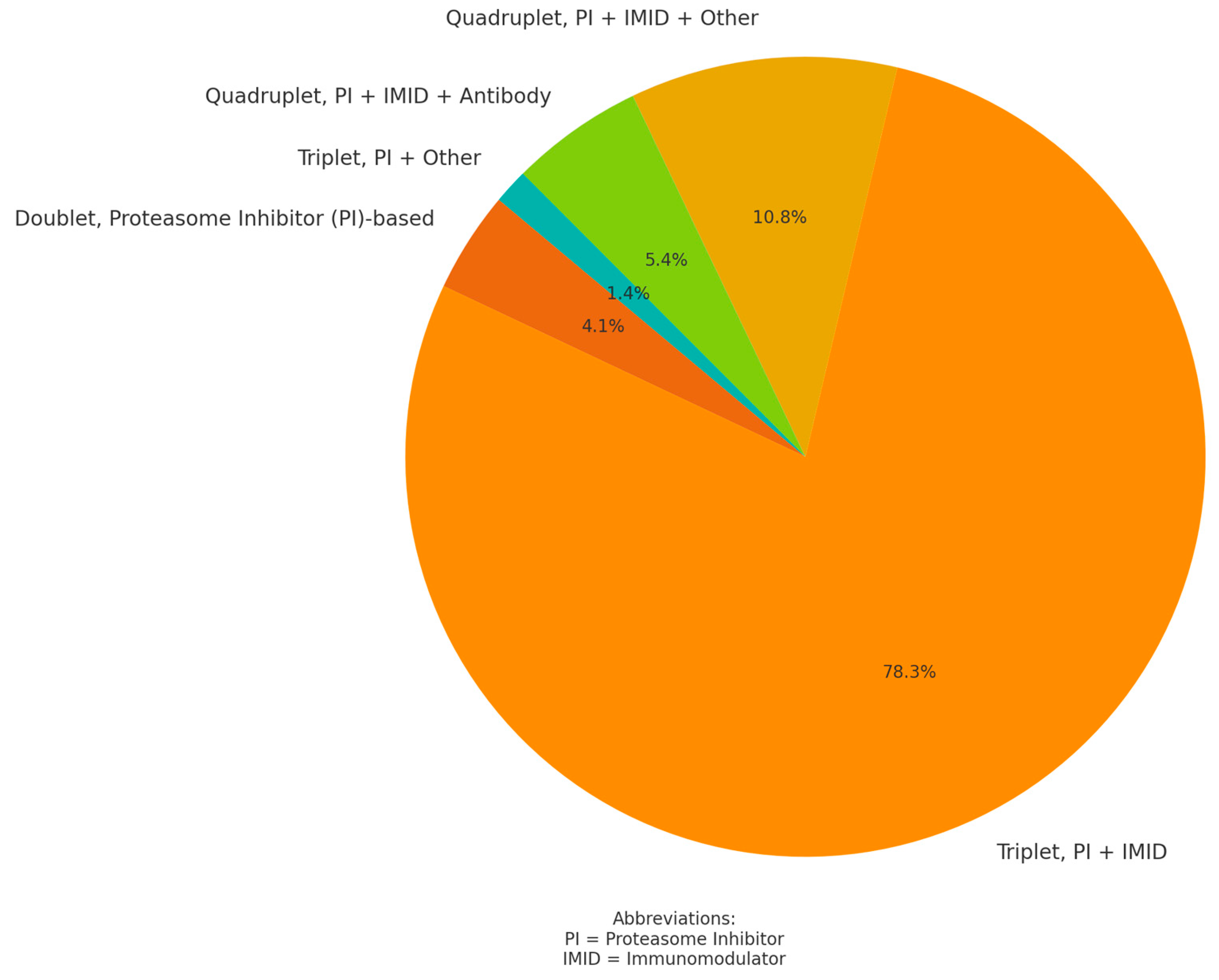
3.2. Daratumumab Treatment
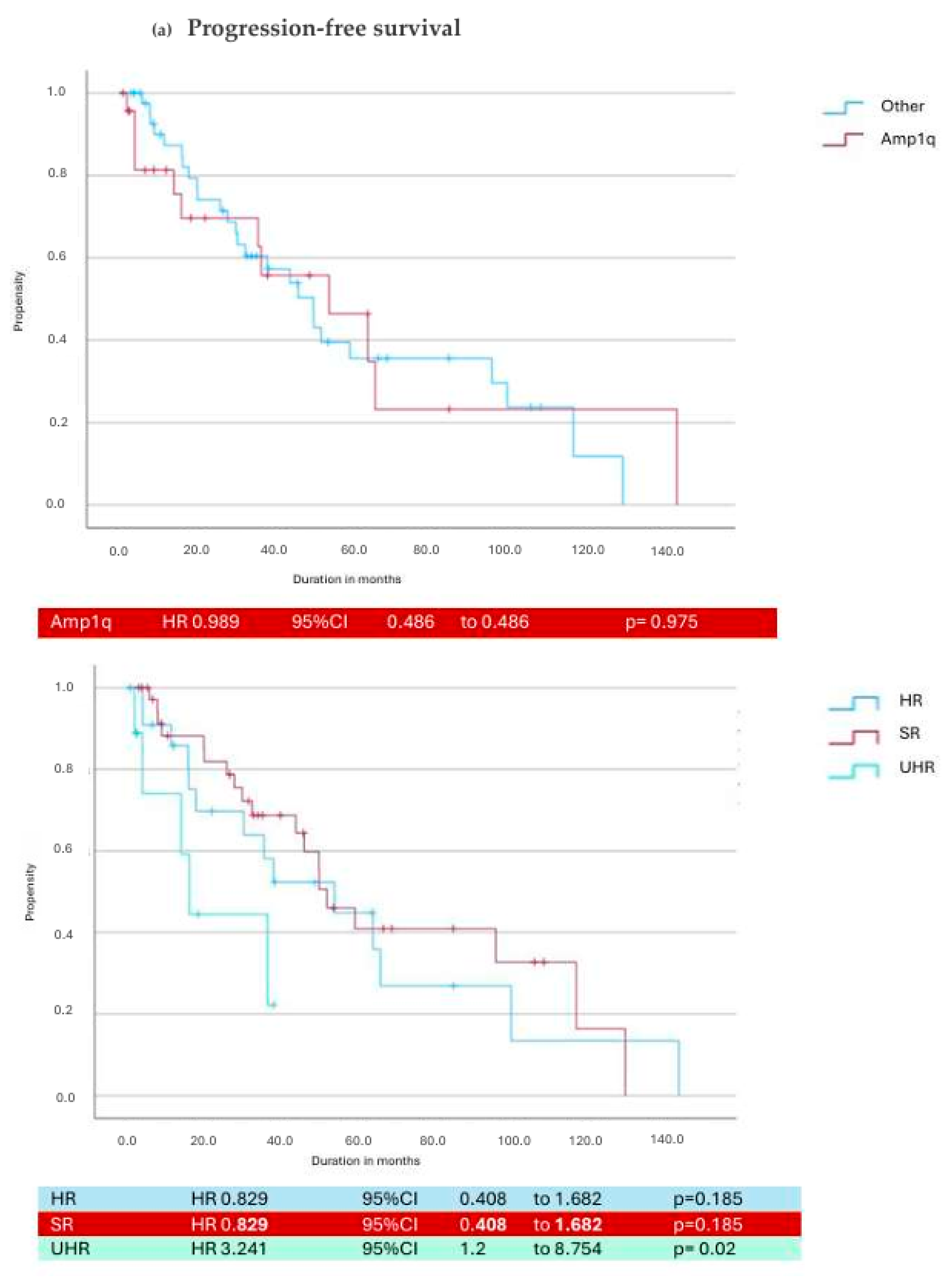
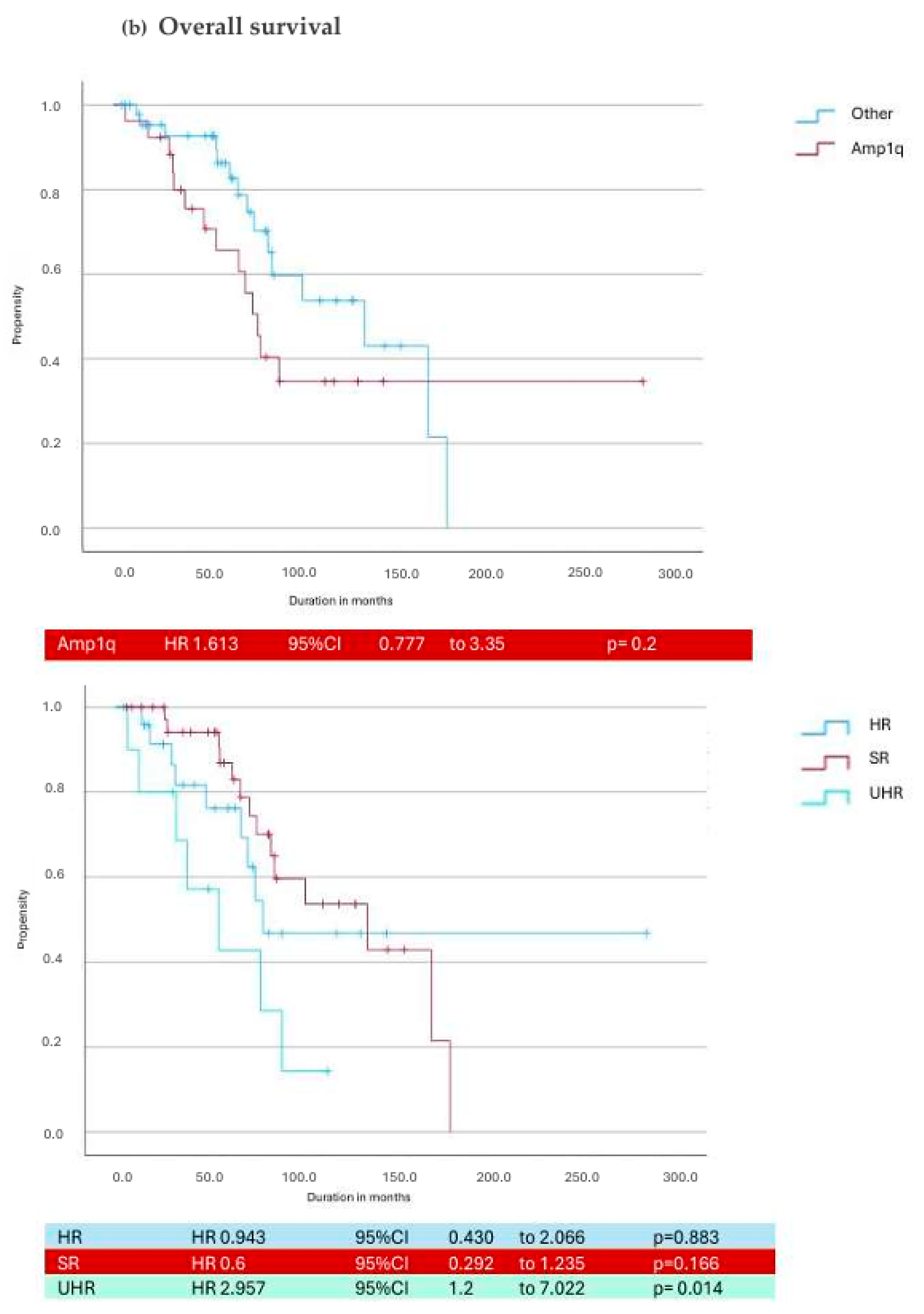
3.3. Daratumumab in Cytogenetic Subgroups
| Cytogenetic Risk | Median PFS (Months) | Median OS (Months) | Median Duration of Treatment (Months) |
|---|---|---|---|
| Standard Risk | 17.3 (8.6–27.6) | 67 (49.5–101) | 10.28 (3.5–22) |
| High-Risk R2ISS | 13.1 (3.3–26.4) | 55.3 (27.6–85.6) | 10.48 (3.17–25.8) |
| Double Hit | 6.2 (1.4–16.4) | 42.8 (25.9–74.6) * | 6.1 (1.1–16.5) |
| High Risk IMS 2024 | 7 (2.7–18.1) * | 40.12 (21.1–74.5) * | 5.2 (2–15) |
| Amp 1q | 7.03 (1.95–22.4) | 61.2 (32–83.8) | 8 (2.5–24) |
| Gain 1q | 14 (1.8–21.4) | 53 (38.7–69.2) | 11.5 (1.9–21.8) |
| T(4;14) | 8.1 (4.5–19.1) | 44 (22.4–63) | 6.1 (1.7–19.1) |
| Del17p | 5.7 (1.1–16) | 17.18 (6.2–77.5) | 5.03 (0.41–40.7) |
| Del(1p32) | 13.4 (5.7–14.6) | 40.7 (15–77.6) | 10 (5.03–13) |
3.4. Next Line of Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Sonneveld, P.; Hungria, V.; Nooka, A.K.; Estell, J.A.; Barreto, W.; Corradini, P.; Min, C.-K.; Medvedova, E.; Weisel, K.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin. Lymphoma Myeloma Leuk. 2020, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Suzuki, K.; Plesner, T.; et al. Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef]
- Martin, T.; Richardson, P.G.; Facon, T.; Moreau, P.; Perrot, A.; Spicka, I.; Bisht, K.; Inchauspé, M.; Casca, F.; Macé, S.; et al. Primary outcomes by 1q21+ status for isatuximab-treated patients with relapsed/refractory multiple myeloma: Subgroup analyses from ICARIA-MM and IKEMA. Haematologica 2022, 107, 2485–2491. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Derman, B.A.; Cooperrider, J.; Rosenblatt, J.; Avigan, D.E.; Rampurwala, M.; Barnidge, D.; Major, A.; Karrison, T.; Jiang, K.; Ramsland, A.; et al. Final analysis of a phase II trial of daratumumab, carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma without transplant. Blood Cancer J. 2024, 14, 87. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Costa, L.J.; Chhabra, S.; Medvedova, E.; Dholaria, B.R.; Schmidt, T.M.; Godby, K.N.; Silbermann, R.; Dhakal, B.; Bal, S.; Giri, S.; et al. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone with Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2022, 40, 2901–2912. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.-A.; Leleu, X.P.; Beksac, M.; Pour, L.; Hájek, R.; Liu, Z.; Minarik, J.; Moreau, P.; Romejko-Jarosinska, J.; et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 391, 1597–1609. [Google Scholar] [CrossRef]
- Goudarzi, Z.; Shahtaheri, R.S.; Najafpour, Z.; Hamedifar, H.; Ebrahimi, H. Cost-effectiveness and budget impact analysis of Daratumumab, Lenalidomide and dexamethasone for relapsed-refractory multiple myeloma. Cost Eff. Resour. Alloc. 2024, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Walker, B.; Kumar, S.K.; Spicka, I.; Moreau, P.; Martin, T.; Costa, L.J.; Richter, J.; Fukao, T.; Macé, S.; et al. Chromosomal 1q21 abnormalities in multiple myeloma: A review of translational, clinical research, and therapeutic strategies. Expert Rev. Hematol. 2021, 14, 1099–1114. [Google Scholar] [CrossRef]
- D’Agostino, M.; Cairns, D.A.; Lahuerta, J.J.; Wester, R.; Bertsch, U.; Waage, A.; Zamagni, E.; Mateos, M.-V.; Dall’Olio, D.; van de Donk, N.W.C.J.; et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J. Clin. Oncol. 2022, 40, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Kortüm, M.; Auner, H.W.; Bassermann, F.; Driessen, C.; Einsele, H.; Engelhardt, M.; Goldschmidt, H.; Gunsilius, E.; Krauth, M.T.; Kraywinkel, K.; et al. Multiple Myeloma. Onkopedia. 2024. Available online: https://www.onkopedia.com/de/onkopedia/guidelines/multiples-myelom/@@guideline/html/index.html#ID0E5OAI (accessed on 26 March 2025).
- Mohan, M.; Weinhold, N.; Schinke, C.; Thanedrarajan, S.; Rasche, L.; Sawyer, J.R.; Tian, E.; van Rhee, F.; Zangari, M. Daratumumab in high-risk relapsed/refractory multiple myeloma patients: Adverse effect of chromosome 1q21 gain/amplification and GEP70 status on outcome. Br. J. Haematol. 2020, 189, 67–71. [Google Scholar] [CrossRef]
- Harrison, S.J.; Perrot, A.; Alegre, A.; Simpson, D.; Wang, M.C.; Spencer, A.; Delimpasi, S.; Hulin, C.; Sunami, K.; Facon, T.; et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br. J. Haematol. 2021, 194, 120–131. [Google Scholar] [CrossRef]
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.-Y.; et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front. Immunol. 2020, 11, 1771. [Google Scholar] [CrossRef]
- Nijhof, I.S.; Casneuf, T.; van Velzen, J.; van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.W.J.; van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [Google Scholar] [CrossRef]
- Chung, D.J.; Pronschinske, K.B.; Shyer, J.A.; Sharma, S.; Leung, S.; Curran, S.A.; Lesokhin, A.M.; Devlin, S.M.; Giralt, S.A.; Young, J.W. T-cell Exhaustion in Multiple Myeloma Relapse after Autotransplant: Optimal Timing of Immunotherapy. Cancer Immunol. Res. 2016, 4, 61–71. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.C.J.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Lagrue, K.; Carisey, A.; Morgan, D.J.; Chopra, R.; Davis, D.M. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood 2015, 126, 50–60. [Google Scholar] [CrossRef]
- Parrondo, R.D.; Gardner, L.B.; Alhaj Moustafa, M.; Roy, V.; Sher, T.; Rasheed, A.; Warsame, R.M.; Larsen, J.T.; Gonsalves, W.I.; Kourelis, T.; et al. Therapeutic Outcomes of Relapsed-Refractory Multiple Myeloma Patients with 1q21+Treated with Daratumumab-Based Regimens: A Retrospective Analysis. Blood 2022, 140, 7237–7238. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.C.; Wellard, C.; Moore, E.; Ninkovic, S.; Chng, W.-J.; Spencer, A.; Mollee, P.; Hocking, J.; Ho, P.J.; Janowski, W.; et al. Impact of 1q21 Gain and Amplification on Daratumumab-Treated Multiple Myeloma Patients: Real-World Data from the Australia-New Zealand and Asia-Pacific Myeloma and Related Diseases Registries. Blood 2023, 142, 3325. [Google Scholar] [CrossRef]
- Leypoldt, L.B.; Tichy, D.; Besemer, B.; Hänel, M.; Raab, M.S.; Mann, C.; Munder, M.; Reinhardt, H.C.; Nogai, A.; Görner, M.; et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2024, 42, 26–37. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]

| sOR | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|
| Lower Value | Upper Value | |||
| Sex | 1.1 | 0.39 | 2.88 | 0.905 |
| Age | 0.38 | 0.92 | 1.56 | 0.379 |
| SCT | 1.25 | 0.35 | 4.47 | 0.727 |
| Double hit myeloma | 5.24 | 1.01 | 27.24 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benda, M.; Reimann, P.; Bletzacher, E.; Muendlein, A.; Bernhard, B.; Hartmann, B.; Huynh, M.; Gasser, K.; Zojer, N.; Lang, T.; et al. Real-World Data on the Efficacy of Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma and Amplification 1q. Cancers 2025, 17, 1261. https://doi.org/10.3390/cancers17081261
Benda M, Reimann P, Bletzacher E, Muendlein A, Bernhard B, Hartmann B, Huynh M, Gasser K, Zojer N, Lang T, et al. Real-World Data on the Efficacy of Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma and Amplification 1q. Cancers. 2025; 17(8):1261. https://doi.org/10.3390/cancers17081261
Chicago/Turabian StyleBenda, Magdalena, Patrick Reimann, Elena Bletzacher, Axel Muendlein, Benda Bernhard, Bernd Hartmann, Minh Huynh, Klaus Gasser, Niklas Zojer, Theresia Lang, and et al. 2025. "Real-World Data on the Efficacy of Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma and Amplification 1q" Cancers 17, no. 8: 1261. https://doi.org/10.3390/cancers17081261
APA StyleBenda, M., Reimann, P., Bletzacher, E., Muendlein, A., Bernhard, B., Hartmann, B., Huynh, M., Gasser, K., Zojer, N., Lang, T., Göbel, G., Bohn, J.-P., Schmidt, S., Gunsilius, E., Nachbaur, D., Jukic, E., Locher, M., Willenbacher, E., Willenbacher, W., ... Steiner, N. (2025). Real-World Data on the Efficacy of Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma and Amplification 1q. Cancers, 17(8), 1261. https://doi.org/10.3390/cancers17081261





