Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review
Simple Summary
Abstract
1. Introduction
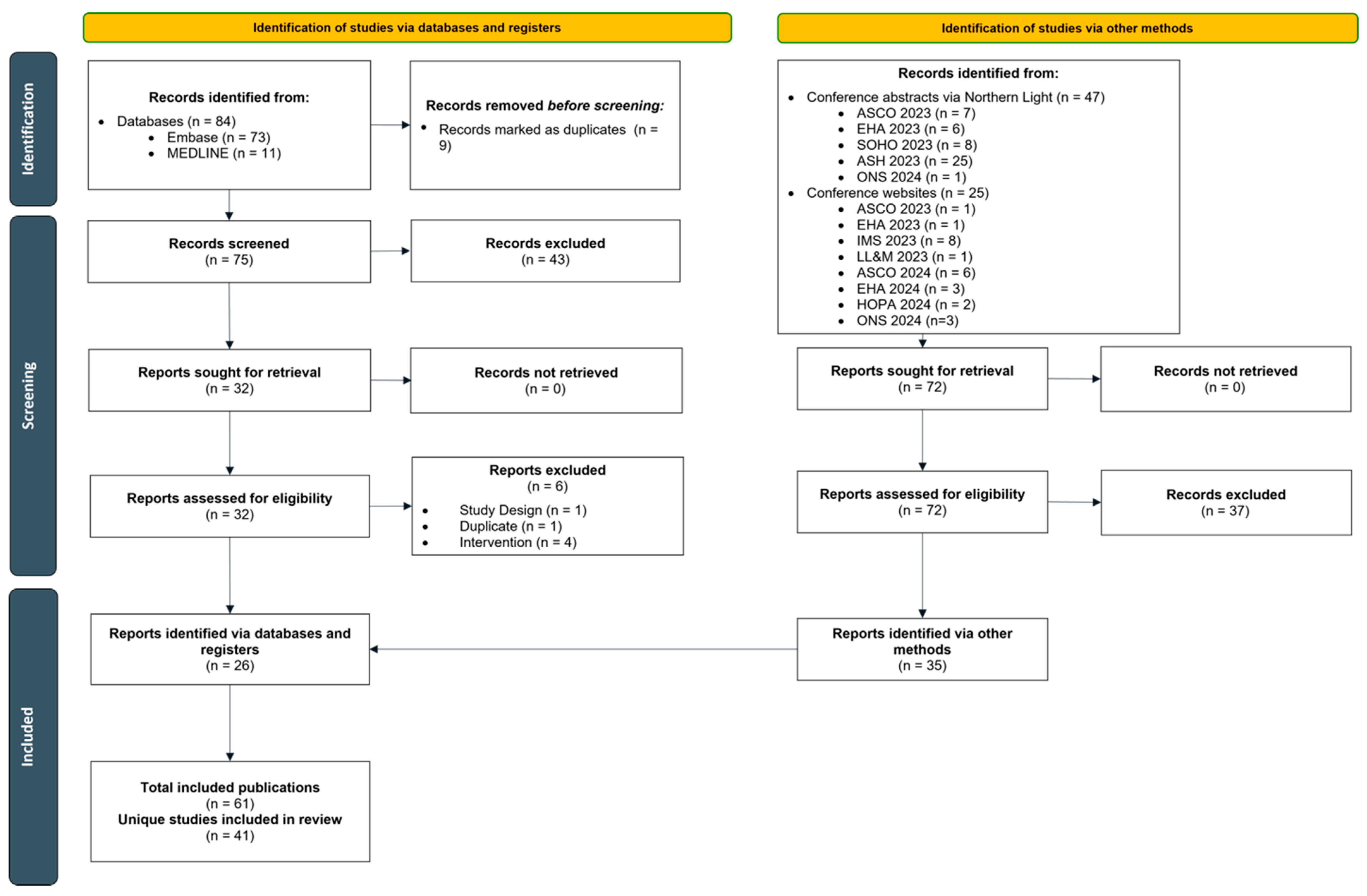
2. Materials and Methods
3. Results
3.1. Study Characteristics
3.2. Population Characteristics
3.3. Outcomes
3.3.1. Effectiveness Outcomes
3.3.2. Safety Outcomes
3.3.3. Healthcare Provider Practices and Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. PICOS Criteria
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Adult (≥18 years) patients with multiple myeloma | Patients aged < 18 years |
| Interventions | Teclistamab | Interventions not listed |
| Comparators | No restrictions | -- |
| Outcomes | No restrictions | -- |
| Study design | Real-world observational studies (prospective, retrospective) | Clinical trials Case reports or case series Pooled analyses of trials Systematic literature reviews a Non-systematic/narrative reviews |
| Publication type | Full-text publications Conference abstracts/posters b | Letters to editors Editorials Commentary Expert opinion Guidelines |
| Language | Only studies published in English | Studies published in a language other than English (even if the abstract is in English) |
| Time | Published between 1 January 2023 and 30 June 2024 | Published before 2023 |
Appendix B. Literature Search Strategies
| Database: Embase 1974 to 22 May 2024 Search Executed on 23 May 2024 | |||
|---|---|---|---|
| # | Criteria | Search Terms | Results |
| 1 | Population terms | exp multiple myeloma/ | 102,883 |
| 2 | exp plasmacytoma/ | 14,274 | |
| 3 | exp Paraproteinemias/ | 183,815 | |
| 4 | (myeloma or (multiple adj2 myeloma$) or plasmacytom$ or plasmocytom$ or mgus or (monoclonal adj2 gammopath$)).mp. | 149,247 | |
| 5 | or/1–4 | 221,071 | |
| 6 | Intervention terms | exp teclistamab/ | 406 |
| 7 | (teclistamab or Tecvayli or JNJ-64007957 or teclistamab-cqyv).mp. | 424 | |
| 8 | or/6–7 | 424 | |
| 9 | SIGN filters for observational studies | Clinical study/ | 166,793 |
| 10 | Case control study/ | 217,895 | |
| 11 | Family study/ | 25,824 | |
| 12 | Longitudinal study/ | 213,746 | |
| 13 | Retrospective study/ | 1,624,202 | |
| 14 | Prospective study/ | 919,580 | |
| 15 | Randomized controlled trials/ | 274,328 | |
| 16 | 14 not 15 | 908,367 | |
| 17 | Cohort analysis/ | 1,167,616 | |
| 18 | (Cohort adj (study or studies)).mp. | 517,217 | |
| 19 | (Case control adj (study or studies)).tw. | 176,157 | |
| 20 | (follow up adj (study or studies)).tw. | 76,223 | |
| 21 | (observational adj (study or studies)).tw. | 276,600 | |
| 22 | (epidemiologic$ adj (study or studies)).tw. | 125,861 | |
| 23 | (cross sectional adj (study or studies)).tw. | 373,627 | |
| 24 | (retrospective adj7 (study or studies or design or analysis or analyses or cohort or data or review)).ti,ab. | 1,254,000 | |
| 25 | or/9–13,16–24 | 4,466,453 | |
| 26 | Combined criteria | 5 and 8 and 25 | 96 |
| 27 | Language filter | limit 26 to english language | 96 |
| 28 | Date filter | limit 27 to yr=“2023-Current” | 72 |
| Database: Ovid MEDLINE(R) ALL <1946 to 22 May 2024> Search Executed on 23 May 2024 | |||
|---|---|---|---|
| # | Criteria | Search Terms | Results |
| 1 | Population terms | exp multiple myeloma/ | 49,135 |
| 2 | exp plasmacytoma/ | 8932 | |
| 3 | exp Paraproteinemias/ | 64,055 | |
| 4 | (myeloma or (multiple adj2 myeloma$) or plasmacytom$ or plasmocytom$ or mgus or (monoclonal adj2 gammopath$)).mp. | 84,636 | |
| 5 | or/1–4 | 96,987 | |
| 6 | Intervention terms | (teclistamab or Tecvayli or JNJ-64007957 or teclistamab-cqyv).mp. | 89 |
| 7 | SIGN filters for observational studies | Epidemiologic studies/ | 9543 |
| 8 | exp case control studies/ | 1,506,567 | |
| 9 | exp cohort studies/ | 2,607,741 | |
| 10 | Case control.tw. | 162,188 | |
| 11 | (cohort adj (study or studies)).tw. | 351,806 | |
| 12 | Cohort analy$.tw. | 13,035 | |
| 13 | (Follow up adj (study or studies)).tw. | 58,204 | |
| 14 | (observational adj (study or studies)).tw. | 178,369 | |
| 15 | Longitudinal.tw. | 345,820 | |
| 16 | Retrospective.tw. | 812,750 | |
| 17 | Cross sectional.tw. | 562,700 | |
| 18 | Cross-sectional studies/ | 502,656 | |
| 19 | or/7–18 | 4,003,471 | |
| 20 | Combined criteria | 5 and 6 and 19 | 11 |
| 21 | Language filter | limit 20 to english language | 11 |
| 22 | Date filter | limit 21 to yr=“2023-Current” | 11 |
| Database: Northern Light Life Sciences Conference Abstracts 2010–2024 Week 20 Search Executed on 23 May 2024 | |||
|---|---|---|---|
| # | Criteria | Search Terms | Results |
| 1 | Population terms | exp multiple myeloma/ | 27,388 |
| 2 | exp plasmacytoma/ | 1694 | |
| 3 | exp Paraproteinemias/ | 30,424 | |
| 4 | (myeloma or (multiple adj2 myeloma$) or plasmacytom$ or plasmocytom$ or mgus or (monoclonal adj2 gammopath$)).mp. | 30,055 | |
| 5 | or/1–4 | 32,556 | |
| 6 | Intervention terms | (teclistamab or Tecvayli or JNJ-64007957 or teclistamab-cqyv).mp. | 102 |
| 7 | Conference filter | Academy of Managed Care Pharmacy.cf. | 2749 |
| 8 | American Society of Clinical Oncology.cf. | 79,140 | |
| 9 | American Society of Hematology.cf. | 66,037 | |
| 10 | European Hematology Association.cf. | 31,727 | |
| 11 | European Society for Medical Oncology.cf. | 22,763 | |
| 12 | International Myeloma.cf. | 2497 | |
| 13 | Oncology Nursing Society.cf. | 1267 | |
| 14 | Society of Hematologic Oncology.cf. | 2052 | |
| 15 | or/7–14 | 208,232 | |
| 16 | Combined criteria | 5 and 6 and 15 | 82 |
| 17 | Date filter | limit 16 to yr=“2023-Current” | 47 |
Appendix C. List of Conferences Searched
| Conference | Dates Held | Search Method |
|---|---|---|
| Academy of Managed Care Pharmacy (AMCP) Meeting | 21–24 March 2023 15–18 April 2024 | Northern Light database via Ovid Hand search of website |
| AMCP Nexus | 16–19 October 2023 | Hand search of website |
| American Society for Transplantation and Cellular Therapy (ASTCT) and Center for International Blood and Marrow Transplant Research (CIBMTR) Tandem Meeting | 15–19 February 2023 21–24 February 2024 | Hand search of website |
| American Society of Clinical Oncology (ASCO) Annual Meeting | 2–6 June 2023 31 May–4 June 2024 | Northern Light database via Ovid Hand search of website |
| American Society of Hematology (ASH) Annual Meeting | 9–12 December 2023 | Hand search of website |
| European Hematology Association (EHA) Annual Congress | 8–11 June 2023 13–16 June 2024 | Northern Light database via Ovid Hand search of website |
| European Society for Medical Oncology (ESMO) | 20–24 October 2023 | Northern Light database via Ovid |
| European Myeloma Network (EMN) Meeting | 20–22 April 2023 18–20 April 2024 | Hand search of website |
| Hematology/Oncology Pharmacy Association (HOPA) Annual Conference | 29 March–1 April 2023 3–6 April 2024 | Hand search of website |
| International Conference on Oncology and Research Treatment (ORT) | 30 November–1 December 2023 | Hand search of website |
| International Myeloma Society (IMS) Annual Meeting | 27–30 September 2023 | Northern Light database via Ovid |
| Journal of the Advanced Practitioner in Oncology (JADPRO) Live | 9–12 November 2023 | Hand search of website |
| Lymphoma, Leukemia & Myeloma (LL&M) Congress | 18–21 October 2023 | Hand search of website |
| Oncology Nursing Society (ONS) Congress | 26–30 April 2023 24–28 April 2024 | Northern Light database via Ovid Hand search of website |
| Society of Hematologic Oncology (SOHO) Annual Meeting | 6–9 September 2023 | Northern Light database via Ovid |
Appendix D. Risk of Bias Assessment
| Domain | Response |
|---|---|
| Selection | |
| 1. Representativeness of the exposed cohort | Truly representative of the average _______________ (describe) in the community * Somewhat representative of the average ______________ in the community * Selected group of users (e.g., nurses, volunteers) No description of the derivation of the cohort |
| 2. Selection of the non-exposed cohort | Drawn from the same community as the exposed cohort * Drawn from a different source No description of the derivation of the non-exposed cohort |
| 3. Ascertainment of exposure | Secure record (e.g., surgical records) * Structured interview * Written self-report No description |
| 4. Demonstration that the outcome of interest was not present at the start of the study | Yes * No |
| Comparability | |
| 1. Comparability of cohorts on the basis of the design or analysis | Study controls for _____________ (select the most important factor) * Study controls for any additional factor (this criterion could be modified to indicate specific control for a second important factor) * |
| Outcomes | |
| 1. Assessment of outcome | Independent blind assessment * Record linkage * Self-report No description |
| 2. Was the follow-up long enough for the outcomes to occur? | Yes (select an adequate follow-up period for the outcome of interest) * No |
| 3. Adequacy of the follow-up of cohorts | Complete follow up—all subjects accounted for * Subjects lost to follow up unlikely to introduce bias—small number lost—>____% (select an adequate %) follow up, or description provided of those lost) * Follow up rate <____% (select an adequate %) and no description of those lost No statement |
Appendix E. Summary Tables of All the Included Studies
| Study ID | Study Design | Data Source | Chart Review vs. Secondary Database | Setting | Study Timeframe | mFU (Months) | Sample Size | Relevant Outcomes Evaluated |
|---|---|---|---|---|---|---|---|---|
| Asoori 2023 [57] | Retrospective | Patients treated at UCSF | Chart review | Academic, single-center | NR—July 2023 | 3 | 46 | ORR, OS, PFS, infection AEs, IVIG use in AEs |
| Banerjee 2023a [44] | Retrospective | All-payer claims database (ARMMRD) | Secondary database | Multi-center | October 2022–July 2023 | -- | 182 | LOS, CRS |
| Banerjee 2023b [25,26] | Retrospective | Acentrus MM electronic medical records (EMRs) | Secondary database | Academic centers/community-based hospitals, multi-center | October 2022–November 2023 | 5.1 | 247 | LOS, CRS, ICANS, step-up dosing schedule, TTNT, or death |
| Bansal 2023 [45,58] | Retrospective | Patients treated at the Mayo Clinic, Rochester, MN | Chart review | Academic, single-center | October 2020–July 2023 | -- | 24 | LOS, CRS, remote monitoring, hospitalizations |
| Bansal 2024 [59] | Retrospective | Patients treated at the Mayo Clinic Rochester, MN | Chart review | Academic, single-center | December 2022–January 2024 | -- | 48 | ORR |
| Bolton 2024 [60] | Prospective | Patients treated at three Kaiser Permanente institutions | Chart review | Academic, multi-center | NR | -- | 9 | CRS, ICANS, discontinuations due to any cause |
| Catamero 2023 [61] | Retrospective | Patients treated at the Mount Sinai Hospital | Chart review | Academic, single-center | December 2022–May 2023 | -- | 26 | CRS, TCZ use in AE management |
| Charkviani 2024 [62] | Retrospective | Patients treated at the Mayo Clinic Healthcare System | Chart review | Academic, multi-center | December 2022–May 2023 | -- | 34 | Acute kidney injury (AKI) incidence and treatment |
| Dima 2023 [12,27,28,29,30] | Retrospective | Patients treated at USMIRC centers | Chart review | Academic, multi-center | August 2022–August 2023 | 3.8 | 106 | CRS, ICANS, infections, ORR, DOR, OS, PFS, TCZ use in AE management, hospitalizations |
| Faiman 2023 [63] | Retrospective | Patients treated at Cleveland Clinic | Chart review | Academic, single-center | December 2022–May 2023 | 2.5 | 26 | CRS, TCZ use in AE management, ICANS, infections, best response |
| Firestone 2023 [17,31,32,33] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | November 2022–July 2023 | 3.1 | 52 | Survival, PFS, ORR, safety |
| Ghamsari 2024 [64] | Retrospective | Patients treated at the University of California San Diego | Chart review | Academic, single-center | January 2023–June 2023 | -- | 18 | CRS, ICANS, infections, ORR, DOR, OS, PFS, TCZ use in AE management |
| Glenn 2024 [65] | Prospective | Patients treated at the Smilow Cancer Hospital at Yale New Haven Hospital | Chart review | Academic, single-center | NR | -- | 18 | CRS |
| Gong 2023 [21] | Retrospective | FDA Adverse Event Reporting System database | Secondary database | Multi-center | 2019–2023 | -- | 719 a | Reporting OR, CRS, ICANS, infection, non-ICANS neurotoxicity, mortality, hospitalizations |
| Gordon 2023 [66] | Retrospective | Patients treated at four New York City metro area centers | Chart review | Academic, multi-center | NR | -- | 58 | ORR, CRS, ICANS, tocilizumab use in AE management, discontinuations |
| Graf 2024 [67] | Retrospective | Patients treated at the Medical University of South Carolina | Chart review | Academic, single-center | November 2022–August 2023 | -- | 25 | Hospitalization, LOS, proportion of fever at each dose of teclistamab, incidence, severity, and onset of CRS |
| Grajales-Cruz 2023 [68,69] | Retrospective | Patients treated at the Lee Moffitt Cancer Center; chart review | Chart review | Academic, single-center | December 2022–October 2023 | 4.2 | 36 | ORR, PFS, OS, CRS, TCZ use in AE management, infection, LOS, ICU admission |
| Hamadeh 2024 [18,70,71] | Retrospective | Memorial Sloan Kettering Cancer Center’s institutional plasma cell disorders database | Chart review | Academic, single-center | November 2022–July 2023 | -- | 69 | CRS |
| Hebraud 2023 [51] | Retrospective | Patients treated at Institut Universitaire du Cancer de Toulouse | Chart review | Academic, single-center | January 2021–July 2023 | -- | 8 | CRS, ORR, TCZ, and IVIG use in AE management |
| Howard 2023 [47] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | NR | -- | 23 | LOS, unscheduled physician communications, hospitalizations |
| Kawasaki 2024 [72] | Retrospective | Patients treated at the University of California Davis (UCD) medical center | Chart review | Academic, single-center | December 2022–December 2023 | -- | 27 | CRS, ICANS, LOS, hematological toxicities, infections, hepatotoxicity, diarrhea, and IVIG in AE management |
| Kowalski 2023 [42] | Prospective | Patients treated at the University of Miami Hospital & Clinics, Sylvester Comprehensive Cancer Center | Chart review | Academic, single-center | October 2022–July 2023 | 3.4 b | 31 | Best response, ORR, CRS, ICANS, PFS |
| Kumar 2023 [73] | Prospective | Patients treated at the University of Connecticut Health Center | Chart review | Academic, single-center | November 2022–February 2023 | -- | 9 | Best response, ORR, CRS, ICANS |
| Lachenal 2023 [74] | Retrospective | Patients treated in the French AP program | Secondary database c | Multi-center | November 2022–October 2023 | 5.2 | 15 | CRS, TCZ use in AE management, ICANS, ORR, IVIG in AE management |
| Marin 2023 [43] | Prospective | Patients treated at the Emory University hospital | Chart review | Academic, single-center | December 2022–August 2023 | -- | 53 | CRS, TCZ use in AE management, TCZ dosing, ICANS, readmissions, |
| Midha 2023 [22] | Retrospective | Patients treated at the Dana–Farber Cancer Institute/Brigham and Women’s Hospital | Chart review | Academic, single-center | NR—August 2023 | 2.3 b | 56 | CRS, TCZ use in AE management, infections, ORR, PFS |
| Mohan 2024 [19,34] | Retrospective | Patients treated at five US academic centers | Chart review | Academic, multi-center | NR | 3.5 | 110 | CRS, ICANS, infections, ORR, best response, LOS, IVIG use in AE management, TCZ use in AE management |
| Mooney 2024 [46] | Retrospective | Patients treated at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital | Chart review | Academic, single-center | January 2023—NR | -- | 19 | Hospital admission, LOS, CRS, and ICANS |
| Nader 2023 [23] | Retrospective | Patients treated at the Indiana University School of Medicine d | Chart review | Academic, single-center d | 2000–2021 e | 33.6 f | 49 | PFS, OS, treatment response |
| Perrot 2023 [75] | Retrospective | Patients treated in the French AP program | Secondary database c | Multi-center | October 2022–April 2023 | 2.9 | 572 | Discontinuations, AE-related mortality |
| Pianko 2024 [35] | Retrospective | Komodo Healthcare MapTM | Secondary database | Multi-center | October 2022–December 2023 | 4.2 | 419 | Dosing schedule of teclistamab, time to less frequent dosing, TTNT |
| Rees 2024 [76] | Retrospective | Patients treated at the Mayo Clinic centers | Chart review | Academic, multi-center | April 2018–June 2023 | 9 | 41 | PFS, DOR, OS |
| Riedhammer 2024 [20,36] | Retrospective | Patients treated at 18 German centers | Chart review | Multi-center | July 2022–October 2023 | 5.5 | 123 | Time to response, best response, ORR, PFS, infections |
| Sandahl 2023 [7,77] | Retrospective | Patients treated at three Mayo Clinic centers | Chart review | Academic, multi-center | October 2022–September 2023 | -- | 49 | CRS, ICANS, TCZ use in AE management, admissions, LOS, time between TEC administration and check-out |
| Schaefers 2023 [78] | Prospective | Patients treated at the University Medical Center Hamburg-Eppendorf tertiary center | Chart review | Academic, single-center | July 2022–May 2023 | 3.4 b | 16 | CRS, ICANS, infections, neutropenia, ORR, OS, PFS, DOR |
| Tabbara 2024 [79] | Retrospective | Patients treated at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center; Inpatient/Outpatient unit | Chart review | Academic, single-center | January 2023–December 2023 | -- | 25 | Neutropenia, infection, CRS, ICANS, patients receiving dexamethasone, hospital admission, death |
| Tan-Asoori 2023 [37,38,39] | Retrospective | Patients treated at IMF-associated centers | Chart review | Academic, multi-center | NR—October 2023 | 5 | 204 | ORR, OS, PFS, CRS, ICANS, TCZ use in AE management, IVIG use in AE management, LOS |
| Tan 2023 [53,80] | Retrospective | Premier healthcare database | Secondary database | Multi-center | November 2022–March 2023 | -- | 113 | LOS, step-up dosing schedule, hospitalizations, CRS, TCZ use in AEs |
| Tan 2024 [40,41] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | November 2022–March 2024 | Overall population: 9.5 Patients switching to less-frequent dosing: 6.4 | 86 | ORR, DOR, PFS, time to response |
| Varshavsky-Yanovsky 2023 [9,81] | Retrospective | Patients treated at Fox Chase Cancer Center | Chart review | Academic, single-center | December 2022–May 2023 | -- | 18 | CRS, ICANS |
| Venkatesh 2023 [82] | Retrospective | Patients treated at the University of Kansas | Chart review | Academic, single-center | NR—February 2023 | 3.1 | 22 | CRS, ICANS, ORR, mortality, best response |
| Study ID | Overall/Subgroup Details | Sample Size | Median Age (Range), Years | Female, n (%) | Race, n (%) | High Risk Cytogenetics, n (%) | Median Prior Lines of Therapy | Extramedullary Disease, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | Asian | ||||||||
| Asoori 2023 [57] | Overall | 46 | 71 (50–91) | 23 (50.0) a | 23 (50.0) | 2 (4.3) | 14 (30.4) | 13 (38.2) | 7 | -- |
| Banerjee 2023a [44] | Overall | 182 | 73 (39–85) | 91 (50.0) | 123 (67.6) | 20 (11.0) | 5 (2.7) | -- | -- | -- |
| Banerjee 2023b [25,26] | Overall | 247 | 69 (41–89) | 111 (44.9) | 138 (75.8) b | 23 (12.6) b | 21 (11.5) b | -- | -- | 14 (5.7) |
| Bansal 2023 [45,58] | Overall | 24 | 66 | 9 (39) a | -- | -- | -- | 9 (38) | 5 | -- |
| Bansal 2024 [59] | TEC treatment group c | 48 | -- | -- | -- | -- | -- | -- | -- | -- |
| Bolton 2024 [60] | Overall | 9 | -- | -- | -- | -- | -- | -- | -- | -- |
| Catamero 2023 [61] | Overall | 26 | -- | -- | -- | -- | -- | -- | -- | -- |
| Charkviani 2024 [62] | TEC treatment group | 34 | 66.4 (55.4–70.8) d | 11 (32.4) | 31 (91.2) | 1 (2.9) | 1 (2.9) | -- | -- | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | 66.5 (35–87) | 55 (54) a | 72 (68) | 28 (26) | 2 (2) | 56 (59) | 6 | 45 (42) |
| >70 years old | 33 | 75 (71–87) | -- | -- | -- | -- | 19 (58) | 6 | 13 (39) | |
| Patients with EMD | 45 | 68 (43–83) | 10 (22.0) a | 35 (78.0) | -- | -- | 25 (55.5) | 6 | 45 (100.0) | |
| Faiman 2023 [63] | Overall | 26 | 64 (45–75) | 14 e (54) | 19 e (73) | -- | -- | -- | 7 | -- |
| Firestone 2023 [17,31,32,33] | Overall | 52 | 70 (30–80) | -- | -- | -- | -- | 17 (33) | 7 | 18 (35) |
| Ghamsari 2024 [64] | Overall | 18 | 67 (50–83) | -- | -- | -- | -- | 11 (84.6) f,g | 6.5 | 8 (44) |
| Glenn 2024 [65] | Overall | 18 | -- | -- | -- | -- | -- | -- | -- | -- |
| Gong 2023 [21] | Overall | 719 cases h | 65 (57–72) e | 229 (31.8) | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [66] | Overall | 58 | 67 (45–88) | 31 (53.4) | 26 (45.6) | 15 (26.3) | 3 (5.2) | 21 (36.2) | 5 | 28 (48.3) |
| Graf 2024 [67] | Overall | 25 | 66 (37–78) | 12 (48.0) | 14 (56.0) | -- | -- | 9 (36.0) | 5 | 13 (52.0) |
| Grajales-Cruz 2023 [68,69] | Overall | 36 | 67 (48–88) | 16 (44.4) a | -- | -- | -- | 15 (44.1) | 7 | 16 (57.0) |
| Hamadeh 2024 [18,70,71] | Overall | 72 | (39–88) | 30 (41.7) i | 56 (77.8) i | 12 (16.7) i | -- | 36 (50.0) i | -- | 21 (29.2) i |
| Prior T-cell redirection therapies (TCRT) | 27 | 70 (51–88) | 10 (37) | 23 (85) | 2 (7) | -- | 19 (70) | 8 | 9 (33) | |
| No prior TCRT | 45 | 69 (39–88) | 20 (44) | 33 (73) | 10 (22) | -- | 17 (38) | 5 | 12 (27) | |
| Hebraud 2023 [51] | Overall | 8 | 69 (43–81) | -- | -- | -- | -- | -- | 4 | -- |
| Howard 2023 [47] | Overall | 23 | 67 (51–88) | -- | -- | -- | -- | 5 (22) | 6 | 6 (26) |
| Kawasaki 2024 [72] | Overall | 27 | -- | 12 (44.4) i | 18 (66.7) i | -- | -- | -- | -- | 8 (29.6) i |
| Dosing schedule 1, 3, 5 (days) | 23 | 69 | 9 (39) f | 16 (69) | -- | -- | -- | -- | 6 (26) | |
| Dosing schedule 1, 4, 7 (days) | 4 | 64 | 3 (75) f | 2 (50) | -- | -- | -- | -- | 2 (50) | |
| Kowalski 2023 [42] | Overall j | 31 | 71 (50–84) | 21 (68) | 22 (71) | -- | -- | 9 (29) | 5 | 24 (77) |
| Kumar 2023 [73] | Overall | 9 | 75 (41–81) | -- | -- | -- | -- | -- | 6 | -- |
| Lachenal 2023 [74] | Overall | 15 | 68 (58–83) | -- | -- | -- | -- | 7 (70.0) k | 4 | -- |
| Marin 2023 [43] | Overall | 53 | 69 (43–83) | 15 (28.3) a,i | 25 (47.2) i | 24 (45.3) i | 1 (1.9) i | 21 (39.6) i | -- | -- |
| No prophylactic tocilizumab (TCZ) | 15 | 58 (47–73) | 2 (13.3) a | 7 (46.7) | 8 (53.3) | -- | 4 (26.7) | 6 | -- | |
| Prophylactic TCZ | 38 | 69 (43–83) | 13 (34.2) a | 18 (47.4) | 16 (42.1) | 1 (2.6) | 17 (44.7) | 5 | -- | |
| Midha 2023 [22] | Overall | 56 | 69 (45–83) | 21 (37.5) a | -- | -- | -- | -- | 6 | 24 (42.9) |
| Mohan 2024 [19,34] | Overall | 110 | 68 (37–89) | 54 (49) | 67 (61) | 32 (29) | 2 (1.8) | 59 l (62) | 6 | 48 (44) |
| Mooney 2024 [46] | Overall | 19 | -- | -- | -- | -- | -- | -- | -- | -- |
| Nader 2023 [23] | Overall | 49 | 70 (59–75) d | 27 (55.0) | 41 (84.0) | 5 (10.0) | -- | -- | >4 | -- |
| Perrot 2023 [75] | Overall | 572 | 71 (64–76) d | 241 (42.1) | -- | -- | -- | 124 (21.7) | 4 | 121 (21.2) |
| Pianko 2024 [35] | Overall | 419 | 65 (58–73) d | 183 (43.7) | 185 (63.4) m | 91 (31.2) m | 16 (5.4) m | -- | 5 | -- |
| Rees 2024 [76] | TEC treatment group c | 41 | -- | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | 67 (35–87) | 53 e (43.1) | -- | -- | -- | 39 (36.8) n | 6 | 43 (36.1) |
| Sandahl 2023 [7,77] | Overall | 49 | 67.2 (38.7–84.2) | 18 (36.7) | 43 (87.8) | 3 (6.1) | 1 (2.0) | 31 (63.3) | -- | 3 (6.1) |
| Schaefers 2023 [78] | Overall | 16 | 65.5 (51–86) | 5 (31) | -- | -- | -- | 8 (80) | 6 | -- |
| Tabbara 2024 [79] | Overall | 25 | 70 (59–89) | 14 (56.0) | 19 (76.0) | 5 (20.0) | 0 (0.0) | -- | 6 | -- |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | 66 (33–91) | 91 (45) | 143 (70) | 15 (7) | 19 (9) | 90 (44) | 6 | 38 (19) |
| Tan 2023 [53,80] | Overall | 113 | 65 (58–74) d | 44 (38.9) | 74 (65.5) | 24 (21.2) | 3 (2.7) | -- | -- | -- |
| Tan 2024 [40,41] | Overall | 86 | 71 (64–78) d | 44 (51) | 65 (76) | 14 (16) | -- | 56 (71) o | 6 | 30 (38) o |
| Patients switched to a less frequent dosing schedule | 32 | 70 (65–78) d | 20 (62) a | -- | 3 e (9) | -- | 17 (59) | 6 | 10 (34) p | |
| Varshavsky-Yanovsky 2023 [9,81] | Overall | 18 | 66 (46–81) | -- | -- | -- | -- | -- | -- | -- |
| Venkatesh 2023 [82] | Overall | 22 | 70 (43–85) | 9 (41) g | -- | -- | -- | 9 (41) | 6 | 14 (64) |
| Study ID | Overall/Subgroup Details | Sample Size | MajesTEC-1 Ineligibility, n (%) | Prior BCMA Therapy, n (%) | ECOG PS ≥2, n (%) | Cytopenia, n (%) | Renal Impairment/Failure, n (%) | CrCl < 30 mL/min or 40 mL/min, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Anemia | Neutropenia | Thrombocytopenia | ||||||||
| Asoori 2023 [57] | Overall | 46 | 31 (67.3) | 16 (34.8) | -- | -- | -- | -- | -- | -- | 15 (32.6) |
| Banerjee 2023a [44] | Overall | 182 | -- | 15 (16.3) a | -- | -- | 108 (59.3) | 36 (19.8) | -- | 77 (42.3) | -- |
| Banerjee 2023b [25,26] | Overall | 247 | -- | 48 (19.4) | -- | -- | 126 (51.0) | 55 (22.3) | -- | 100 (40.5) | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | 88 (83) | 56 (53) | 35 (33) | 33 (31) | 27 (25) | 2 (2) | 21 (20) | 14 (13) | 14 (13) |
| >70 years old | 33 | -- | -- | 15 (45.0) | -- | -- | -- | -- | -- | -- | |
| Patients with EMD | 45 | -- | 25 (55.5) | 16 (35.5) | -- | -- | -- | -- | -- | -- | |
| Faiman 2023 [63] | Overall | 26 | -- | 9 (38) | -- | -- | -- | -- | -- | -- | -- |
| Firestone 2023 [17,31,32,33] | Overall | 52 | -- | 27 (52) | -- | -- | -- | -- | -- | -- | -- |
| Ghamsari 2024 [64] | Overall | 18 | -- | 7 (39) | -- | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [66] | Overall | 58 | 51 (87.9) | 23 (39.7) | 17 (29.8) | (48.3) | -- | -- | -- | 13 (22.4) | -- |
| Graf 2024 [67] | Overall | 25 | -- | 11 (44) | -- | -- | -- | -- | -- | -- | -- |
| Grajales-Cruz 2023 [68,69] | Overall | 36 | 36 (100.0) | 36 (100.0) | -- | -- | 7 (22.6) | 2 (6.45) | 10 (32.3) | -- | 6 (18.8) |
| Howard 2023 [47] | Overall | 23 | -- | 8 (35) | -- | -- | -- | -- | -- | -- | -- |
| Kowalski 2023 [42] | Overall c | 31 | 26 (84) | 4 (13) | 16 (52) d | 15 (48) e | -- | -- | -- | -- | -- |
| Lachenal 2023 [74] | Overall | 15 | -- | -- | -- | -- | -- | -- | -- | 15 (100) f | -- |
| Midha 2023 [22] | Overall | 56 | 45 (80.0) | 20 (35.7) | 17 (30.4) | -- | -- | -- | -- | -- | 11 (19.6) |
| Mohan 2024 [19,34] | Overall | 110 | -- | 38 (35) | -- | -- | -- | -- | -- | -- | -- |
| Perrot 2023 [75] | Overall | 572 | -- | 49 g (8.6) | 108 g (18.9) d | -- | -- | -- | -- | 102 g (17.8) | -- |
| Pianko 2024 [35] | Overall | 419 | -- | 102 (24.3) | -- | -- | 164 (39.1) | 50 (11.9) | -- | 206 (49.2) | -- |
| Rees 2024 [76] | TEC treatment group b | 41 | -- | 25 (61.0) | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | 48 g (39) | 45 (37.4) | -- | -- | -- | -- | -- | -- | -- |
| Sandahl 2023 [7,77] | Overall | 49 | -- | 17 (34.7) | -- | -- | 27 (55.1) | 14 (28.6) | -- | 15 (30.6) | -- |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | 122 (70) h | 91 (45.0) | -- | -- | -- | -- | -- | -- | 25 (12) |
| Tan 2023 [53,80] | Overall | 113 | -- | -- | -- | -- | 65 (57.5) | -- | -- | 32 (28.3) | -- |
| Tan 2024 [40,41] | Overall | 86 | -- | 32 (37) | 5 (10) i | -- | -- | -- | -- | -- | 9 (10) |
| Patients switched to less frequent dosing schedule | 32 | -- | 10 (31) | -- | -- | -- | -- | -- | -- | -- | |
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | Overall Response Rate, n (%) | ||
|---|---|---|---|---|---|---|
| PR or Better | VGPR or Better | CR or Better | ||||
| Asoori 2023 [57] | Overall | 46 | Median of 3 months | 32 b (70.0) | 25 (54.3) c,d | 6 (13.0) c,d |
| Bansal 2024 [59] | TEC treatment group a | 48 | January 2024 cut-off | 29 b (61.0) | -- | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 104 | Median of 3.8 months | 70 (66) b | 49 (46)c | 31 (29) c |
| >70 years old | 34 | Median of 3.8 months e | 24 (71) | -- | 10 (30) f | |
| Faiman 2023 [63] | Overall | 26 | Median of 2.5 months | 15 (60) c | 9 (36) c | 4 (16) c |
| Firestone 2023 [17,31,32,33] | Overall | 47 | Median of 3.1 months | 30 (64) | 18 b (38) | -- |
| Prior anti-BCMA exposure | 26 | Median of 3.1 months e | 13 (50.0) | -- | -- | |
| Faiman 2023 [63] | Overall | 18 | June 2023 cut-off | 9 b (50) | 9 b (50) | -- |
| Gordon 2023 [66] | Overall | 58 | -- | 29 b (50.0) | 14 b (24.1) | -- |
| Grajales-Cruz 2023 [68,69] | Overall | 36 | Median of 4.2 months | 19 b (52.8) | 16 b (44.5) c | 16 b (44.5) c |
| Hebraud 2023 [51] | Overall | 8 | July 2023 cut-off | -- | 6 (75) d | -- |
| Kowalski 2023 [42] | Patients with secretory disease g | 30 | Median of 3.4 months h | 15 (50) | -- | 9 (30) c |
| Kumar 2023 [73] | Overall | 9 | 3-month evaluation | 6 (66.7) | 4 (44.4) c | 3 (33.3) c |
| Lachenal 2023 [74] | Overall | 15 | Median of 5.2 months | 13 (86.7) d | 11 (73.3) c,e | 4 (26.7) c,d |
| Midha 2023 [22] | Overall | 56 | Median of 2.3 months h | 30 b (53.6) | -- | -- |
| Mohan 2024 [19,34] | Overall | 98 | Median of 3.5 months | 61 (62) | 50 b (51) | 20 b (20) |
| Nader 2023 [23] | Overall | 27 | Median of 33.6 months i | 19 (70) | -- | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | Median of 5.5 months | 73 b (59.3) | 59 b (48.0) c | 27 b (22.0) c,j |
| Schaefers 2023 [78] | Overall | 16 | Median of 3.4 months h | 7 b (44) | 5 b (31) | -- |
| Tan-Asoori 2023 [37,38,39] | Overall | 180 | Median of 5 months | 115 b (64) | 90 b (50) c | 34 b (19) c |
| Tan 2024 [40,41] | Overall | 77 | Median of 9.5 months | 47 (61) | 33 (43) | -- |
| Prior BCMA-directed therapy | 32 | Median of 9.5 months e | 14 (43) | -- | -- | |
| Venkatesh 2023 [82] | Overall | 22 | Median of 3.1 months | 11 b (50) | -- | -- |
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | CRS, n (%) | ICANS, n (%) | Infections, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 1 | Grade 2 | Grade 3+ | Any Grade | Grade 1 | Grade 2 | Grade 3+ | Any Grade | Grade 3+ | ||||
| Chart review—mixed inpatients and outpatient or not reported | |||||||||||||
| Asoori 2023 [57] | Overall | 46 | Median of 3 months | -- | -- | -- | -- | -- | -- | -- | -- | 39 (84.8) | 14 (35.8) a |
| Bansal 2023 [45,58] | Overall b | 24 | July 2023 cut-off | 13 (54.2) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Catamero 2023 [61] | Overall | 26 | May 2023 cut-off | 20 c (78) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| CRS patients | 20 | May 2023 cut-off | -- | 17 c (85) | 3 c (15) | 0 (0) | -- | -- | -- | -- | -- | -- | |
| Glenn 2024 [65] | Overall | 18 | -- | 1 (5.6) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [66] | Overall | 58 | -- | 30 c (52) | -- | -- | -- | 6 c (11) | -- | -- | -- | -- | -- |
| Grajales-Cruz 2023 [68,69] | Overall | 36 | Median of 4.2 months | 21 (58.3) | 15 (45.5) | 4 (12.1) | 0 (0) | -- | -- | -- | -- | 21 (58.3) | -- |
| Hamadeh 2024 [18,70,71] | Overall | 72 | July 2023 cut-off | 46 c (63.9) d | 36 c (50.0) d | 10 c (13.9) d | -- | -- | -- | -- | -- | -- | -- |
| Prior TCRT | 27 | July 2023 cut-off | 10 c (37) | 8 c (30) | 2 c (7) | -- | -- | -- | -- | -- | -- | -- | |
| No prior TCRT | 45 | July 2023 cut-off | 36 c (80) | 28 c (62) | 8 c (18) | -- | -- | -- | -- | -- | -- | -- | |
| Kawasaki 2024 [72] | Overall | 27 | -- | -- | 13 c (48.1) d,e | -- | 4 c (14.8) d | -- | -- | -- | -- | -- | |
| Dosing schedule 1, 3, 5 (days) | 23 | -- | -- | 11 (48) e | -- | 4 (17) | -- | -- | -- | -- | -- | ||
| Dosing schedule 1, 4, 7 (days) | 4 | -- | -- | 2 (50) e | -- | 0 (0) | -- | -- | -- | -- | -- | ||
| Kowalski 2023 [42] | Prophylactic tocilizumab | 31 | Median of 3.4 months f | 4 c (13) | -- | -- | -- | 3 c (10) | -- | -- | -- | 8 (26) | -- |
| Kumar 2023 [73] | Overall | 9 | 3-month evaluation | 7 (77.8) d | -- | -- | -- | 1 (11.1) d | -- | -- | -- | -- | -- |
| Midha 2023 [22] | Overall | 56 | Median of 2.3 months f | 29 (51.8) | -- | -- | 1 (1.8) | -- | -- | -- | -- | 32 (57.1) | -- |
| Mohan 2024 [19,34] | Overall | 110 | Median of 3.5 months | 62 c (56) | 57 c (51.8) d,e | 5 (4.5) d | 12 (11) | -- | -- | 5 (4.5) | 44 (40) | 29 (26) | |
| Riedhammer 2024 [20,36] | Overall | 123 | Median of 5.5 months | 72 (58.5) | -- | -- | 2 (1.6) | -- | -- | -- | -- | 67 (54.5) | 33 (26.8) |
| Tabbara 2024 [79] | Overall | 25 | December 2023 cut-off | -- | 15 (60) e | 0 (0) | 4 (16) | -- | 2 (8) | 0 (0) | 6 (24) | 3 (12) | |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | Median of 5 months | 110 (53.9) c,d | 84 (41.2) c,d | 25 (12.3) c,d | 1 (0.5) c,d | -- | -- | -- | -- | 115 (60.0) | -- |
| Venkatesh 2023 [82] | Overall | 22 | Median of 3.1 months | -- | 9 (41.0) d,e | -- | 5 (23) | -- | -- | 2 (9) | -- | -- | |
| Chart review—inpatient monitoring | |||||||||||||
| Bolton 2024 [60] | Overall | 9 | -- | -- | 3 (33.3) | -- | -- | -- | 2 (22.2) | -- | -- | -- | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | Median of 3.8 months | 68 (64) | 57 (54) | 10 (9) | 1 (1) | 15 (14) | 5 (5) | 7 (6) | 3 (3) | 33 (31) | -- |
| >70 years old | 33 | July 2023 cut-off | 22 (67) | -- | -- | 1 (3) | 7 (21) | -- | -- | 0 (0) | 11 (33) | -- | |
| Faiman 2023 [63] | Overall | 26 | Median of 2.5 months | 22 (85) | -- | -- | -- | 5 (19) | -- | -- | -- | 7 (26.9) e | -- |
| Firestone 2023 [17,31,32,33] | Overall | 52 | Median of 3.1 months | 27 (52) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Ghamsari 2024 [64] | Overall | 18 | June 2023 cut-off | 12 (67) | -- | 4 (22.2) d | 0 (0) | 2 (11) | -- | 1 (5.6) d | -- | 12 c (67) | -- |
| Graf 2024 [67] | Overall | 25 | August 2023 cut-off | 13 (52) | 12 (48) | 1 (4) | -- | 1 (4) | -- | -- | -- | -- | -- |
| Marin 2023 [43] | Overall | 53 | August 2023 cut-off | 21 c (39.6) g | -- | -- | -- | 5 (9.4) c | -- | -- | -- | -- | -- |
| Prophylactic TCZ | 38 | August 2023 cut-off | 10 c (26.3) | -- | -- | -- | 2 c (5.3) | -- | -- | -- | -- | -- | |
| No prophylactic TCZ | 15 | August 2023 cut-off | 11 c (73.3) | -- | -- | -- | 3 c (20.0) | -- | -- | -- | -- | -- | |
| Mooney 2024 [46] | Patients admitted to hospital | 15 | -- | 13 (86.7) d,h | -- | -- | -- | 13 (86.7) d,h | -- | -- | -- | -- | -- |
| Schaefers 2023 [78] | Overall | 16 | Median of 3.4 months f | 4 (25) | -- | -- | 0 (0) | 0 (0) | -- | -- | 0 (0) | 13 (81) | 9 (56) |
| Tan-Asoori 2023 [37,38,39] | Inpatients | 160 | Median of 5 months | 94 (59) | 72 (45) | 22 (14) | 0 (0) | -- | -- | -- | -- | -- | -- |
| Chart review—outpatient monitoring | |||||||||||||
| Hebraud 2023 [51] | Overall | 8 | July 2023 cut-off | 3 (37.5) d | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Sandahl 2023 [7,77] | Overall | 45 | September 2023 cut-off | 13 (28.9) | 10 (22.2) | 2 (4.4) | 1 (2.2) | 2 (4.4) | -- | -- | -- | -- | -- |
| Tan-Asoori 2023 [37,38,39] | Outpatients | 44 | Median of 5 months | 16 (36) | 12 (27) | 3 (7) | 1 (2) | -- | -- | -- | -- | -- | -- |
| Varshavsky-Yanovsky 2023 [9,81] | Overall | 18 | 6-month evaluation | -- | 5 (27.8) | 1 (5.6) | -- | -- | -- | -- | -- | -- | -- |
| Secondary databases | |||||||||||||
| Banerjee 2023a [44] | Overall | 131 | July 2023 cut-off | 54 (41.2) | 39 (29.8) | 9 (6.9) | 2 (1.5) | -- | -- | -- | -- | -- | -- |
| Banerjee 2023b [25,26] | Overall | 76 | Median of 5.1 months | 14 (18.4) | 8 (10.5) | 3 (3.9) | 1 (1.3) | 3 (3.9) | 2 (2.6) | 1 (1.3) | 0 (0) | -- | -- |
| Gong 2023 [21] | Total count of AEs | 1317 events i | -- | 130 (9.5) events | -- | -- | -- | 34 (2.5) events | -- | -- | -- | 214 (15.7) events | -- |
| Lachenal 2023 [74] | Overall | 15 | Median of 5.2 months | -- | 11 (73.3) d,e | -- | 1 (6.7) d | -- | -- | -- | 9 (60.0) d | -- | |
| Tan 2023 [53,80] | Overall | 58 | April 2023 cut-off | 15 (25.9) | 11 (19.0) | 4 (6.9) | -- | -- | -- | -- | -- | -- | -- |
Appendix F. Additional Patient Characteristics and Effectiveness Outcomes
| Study ID | Overall/Subgroup Details | n | Prior BCMA CAR T, n (%) | Prior BCMA ADC, n (%) | Prior BCMA BsAb, n (%) | Prior Non-BCMA, n (%) | Prior AlloSCT, n (%) | Prior AutoSCT, n (%) | Triple-Class, n (%) | Penta-Class, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAR-T | BsAb | Exposed | Refractory | Exposed | Refractory | ||||||||
| Asoori 2023 [57] | Overall | 46 | 9 (19.6) a | 4 (8.7) a | 3 (6.5) a | -- | -- | -- | -- | -- | 41 (89.1) | -- | -- |
| Banerjee 2023a [44] | Overall | 92 | 8 (8.7) | 9 (9.8) b | -- | -- | -- | 43 (46.7) | -- | -- | -- | -- | |
| Banerjee 2023b [25,26] | Overall | 247 | 27 (10.9) | 25 (10.1) | 1 (<1) | -- | -- | -- | -- | -- | -- | -- | -- |
| Bansal 2024 [59] | TEC treatment group | 48 | 20 (71.4) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | -- | -- | -- | -- | -- | 3 (3) | 61 (58) | -- | 97 (92) | -- | 68 (64) |
| >70 years old | 33 | -- | -- | -- | -- | -- | -- | 19 (58) | -- | 32 (97) | -- | 19 (58) | |
| Patients with EMD | 45 | -- | -- | -- | -- | -- | -- | 29 (64) | -- | 42 (93) | -- | 26 (58) | |
| Faiman 2023 [63] | Overall | 26 | -- | 1 (3.8) a | -- | -- | -- | 10 (38) c | -- | 26 (100) | -- | (92) | |
| Firestone 2023 [17,31,32,33] | Overall | 52 | 19 (37) | 16 (31) | 2 (4) | 3 (6) | 5 (10) | 3 (6) | 40 (77) | 50 (96) | -- | -- | 35 (67) |
| Ghamsari 2024 [64] | Overall | 18 | 5 (27.8) a | 2 (11.1) a | -- | -- | -- | -- | -- | -- | 18 (100.0) | -- | 17 (94.4) |
| Gordon 2023 [66] | Overall | 58 | 12 (20.7) | 15 (25.9) b | -- | -- | -- | 2 (3.4) | 40 (69) | 58 (100.0) | -- | 42 (72.4) | -- |
| Graf 2024 [67] | Overall | 25 | -- | -- | -- | --- | -- | -- | -- | -- | 20 (80) | -- | 12 (48) |
| Grajales-Cruz 2023 [68,69] | Overall | 36 | 26 (72.2) | 4 (11.1) | -- | -- | -- | -- | 25/33 (75.8) c | -- | 35 (97.2) | -- | 22 (61.1) |
| Hamadeh 2024 [18,70,71] | Overall | 72 | 19 (27.5) d | -- | 3 (4.2) d | 2 (2.8) d,e | 7 (9.7) d,f | 5 (6.9) d | 48 (66.7) d | -- | -- | -- | -- |
| Prior TCRT | 27 | 19/21 (90) | -- | 3/10 (30) | 2/21 (10) e | 7/10 (70) f | 3 (11) | 19 (70) | -- | -- | -- | -- | |
| No prior TCRT | 45 | -- | -- | -- | -- | -- | 2 (1) | 29 (64) | -- | -- | -- | -- | |
| Howard 2023 [47] | Overall | 23 | -- | -- | -- | -- | -- | 1 (4) | 16 (70) | -- | 20 (87) | -- | 10 (43) |
| Kawasaki 2024 [72] | Overall | 27 | -- | -- | -- | -- | -- | -- | 18 (66.7) | -- | 27 (100.0) d | -- | -- |
| Dosing schedule 1, 3, 5 (days) | 23 | -- | -- | -- | -- | -- | -- | 15 (65) | -- | 23 (100) | -- | -- | |
| Dosing schedule 1, 4, 7 (days) | 4 | -- | -- | -- | -- | -- | -- | 3 (75) | -- | 4 (100) | -- | -- | |
| Kowalski 2023 [42] | Overall g | 31 | -- | -- | -- | -- | -- | 12 (39) h | 31 (100) | 26 (84) | 21 (68) | 6 (19) | |
| Marin 2023 [43] | Overall | 53 | -- | -- | -- | -- | -- | -- | -- | -- | 53 (100.0) d,i | -- | -- |
| No prophylactic TCZ | 15 | -- | -- | -- | -- | -- | -- | -- | -- | 15 (100.0) i | -- | -- | |
| Prophylactic TCZ | 38 | -- | -- | -- | -- | -- | -- | -- | -- | 38 (100.0) i | -- | -- | |
| Midha 2023 [22] | Overall | 56 | 13 (23.2) | 12 (23.2) | -- | -- | -- | 33 (58.9) j | -- | -- | -- | -- | |
| Mohan 2024 [19,34] | Overall | 110 | -- | -- | -- | -- | -- | -- | 96 (87) | -- | 95 (86) | -- | 84 (76) |
| Perrot 2023 [75] | Overall | 572 | -- | -- | -- | -- | -- | -- | -- | -- | 398 k (69.6) | -- | 183 k (32.0) |
| Pianko 2024 [35] | Overall | 419 | 51 (12.2) | 71 (16.9) | 0 (0.0) | -- | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | 21 (17.1) a,l | 23 (18.7) a | -- | -- | -- | -- | -- | -- | 114 k (92.6) | -- | 74 k (60.2) |
| Sandahl 2023 [7,77] | Overall | 49 | 10 (20.4) | 5 (10.2) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Schaefers 2023 [78] | Overall | 16 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 16 (100) |
| Tabbara 2024 [79] | Overall | 25 | 1 (4) | -- | -- | -- | -- | 13 (52) j | -- | -- | -- | -- | |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | 40 (19.6) a | 25 (12.3) a | 5 (2.5) a | -- | -- | -- | -- | -- | 123/156 (79.0) | -- | 56/150 (37.0) |
| Tan 2024 [40,41] | Overall | 86 | 21/32 (66) | 19/32 (60) | 3/32 (9) | -- | -- | 3 (3) | 53 (62) | -- | -- | -- | -- |
| Venkatesh 2023 [82] | Overall | 22 | -- | -- | -- | -- | -- | 17 (77) j | -- | 20 (91) | -- | 13 (59) | |
| Study ID | Overall/Subgroup Details | Sample Size | Hemodialysis/dialysis, n (%) | Peripheral Neuropathy, n (%) | Hypogammaglobulinemia, n (%) | CNS Involvement, n (%) |
|---|---|---|---|---|---|---|
| Asoori 2023 [57] | Overall | 46 | 2 (5.4) | -- | 32 (69.6) | -- |
| Banerjee 2023a [44] | Overall | 124 | 9 (4.9) | 57 (31.3) | 52 (28.6) | -- |
| Banerjee 2023b [25,26] | Overall | 247 | -- | 89 (36) | 39 (15.8) | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | -- | -- | -- | 4 (4) |
| Grajales-Cruz 2023 [68,69] | Overall | 33 | -- | -- | 17 (53.1) | -- |
| Kowalski 2023 [42] | Overall b | 31 | -- | -- | -- | 2 (7) |
| Lachenal 2023 [74] | Overall | 15 | 15 (100) c | -- | -- | -- |
| Midha 2023 [22] | Overall | 56 | -- | -- | -- | 7 (12.5) |
| Pianko 2024 [35] | Overall | 419 | -- | -- | 125 (29.8) | -- |
| Sandahl 2023 [7,78] | Overall | 49 | -- | 28 (57.1) | 20 (40.8) | -- |
| Tabbara 2024 [79] | Overall | 25 | 1 (4.0) | -- | -- | -- |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | 2 (1) a | -- | 105/153 (69) | -- |
| Tan 2023 [53,80] | Overall | 113 | -- | 41 (36.3) | -- | -- |
| Tan 2024 [40,41] | Overall | 86 | 2 (2) | 35 (41) | -- | -- |
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | Overall Response Rate, n (%) | Stratified Best Response, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR or Better | VGPR or Better | CR or Better | PD | SD | PR | VGPR | CR | sCR | ||||
| Asoori 2023 [57] | Overall | 46 | Median of 3 months | 32 a (70.0) | -- | -- | -- | -- | 7 (15.2) b | 19 (41.3) b | 6 (13.0) b | |
| Dima 2023 [12,27,28,29,30] | Overall | 104 | Median of 3.8 months | 70 (66) | -- | -- | 26 (24) | 10 (9.5) | 21 (20) | 18 (17) | 31 (29) | |
| >70 years old | 34 | Median of 3.8 months | 24 (71) | -- | 10 (30) c | -- | -- | -- | -- | -- | -- | |
| Faiman 2023 [63] | Overall | 26 | Median of 2.5 months | -- | -- | -- | 6 (24) | 4 (16) | 6 (24) | 5 (20) | 4 (16) | -- |
| Firestone 2023 [17,31,32,33] | Overall | 47 | Median of 3.1 months | 30 (64) | 18 a (38) | -- | -- | -- | -- | -- | -- | -- |
| Ghamsari 2024 [64] | Overall | 18 | June 2023 cut-off | 9 a (50) | 9 a (50) | -- | -- | -- | -- | -- | -- | -- |
| Gordon 2023 [66] | Overall | 58 | -- | 29 a (50.0) | 14 a (24.1) | -- | -- | 8 a (13.1) b | 15 a (25.9) | 14 a (24.1) | -- | -- |
| Grajales-Cruz 2023 [65,66] | Overall | 36 | Median of 4.2 months | 19 a (52.8) | -- | -- | -- | -- | 3 a (8.3) | 0 (0.0) | 16 a (44.5) | |
| Hebraud 2023 [51] | Overall | 8 | July 2023 cut-off | -- | 6 (75) b | -- | -- | -- | -- | -- | -- | -- |
| Kowalski 2023 [42] | Patients with secretory disease d | 30 | Median of 3.4 months e | 15 (50) | -- | -- | 4 (13) | 11 (37) | 4 (13) | -- | 9 (30) | -- |
| Kumar 2023 [73] | Overall | 9 | 3 months | 6 (66.7) | -- | -- | 3 (33.3) b | -- | 2 (22.2) b | 1 (11.1) b | 1 (11.1) b | 2 (22.2) b |
| Lachenal 2023 [74] | Overall | 15 | Median of 5.2 months | 13 (86.7) b | -- | -- | -- | -- | 2 (13.3) b | 7 (46.7) b | 2 (13.3) b | 2 (13.3) b |
| Midha 2023 [22] | Overall | 56 | Median of 2.3 months e | 30 a (53.6) | -- | -- | -- | -- | -- | -- | -- | -- |
| Mohan 2024 [19,34] | Overall | 98 | Median of 3.5 months | 61 (62) | 50 a (51) | 20 a (20) | -- | -- | -- | -- | -- | -- |
| Nader 2023 [23] | Overall | 27 | 33.6 months f | 19 (70) | -- | -- | -- | -- | -- | -- | -- | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | Median of 5.5 months | 73 a (59.3) | -- | -- | -- | -- | 14 a (11.4) | 32 a (26.0) | 27 a (22.0) g | |
| Schaefers 2023 [78] | Overall | 16 | 3.4 months e | 7 a (44) | 5 a (31) | -- | 7 a (44) | 2 a (13) | 2 a (13) | -- | -- | -- |
| Tan-Asoori 2023 [37,38,39] | Overall | 180 | Median of 5 months | 115 a (64) | -- | -- | 40 a (22) | 25 a (14) | 25 a (14) | 56 a (31) | 34 a (19) | |
| Tan 2024 [40,41] | Overall | 86 | Median of 9.5 months | 47 (61.0) | 33 (43.0) | -- | -- | -- | -- | -- | -- | -- |
| Prior BCMA-directed therapy | 32 | Median of 9.5 months | 14 (43) | -- | -- | -- | -- | -- | -- | -- | -- | |
| Venkatesh 2023 [82] | Overall | 22 | Median of 3.1 months | 11 a (50) | -- | -- | -- | -- | -- | -- | -- | |
Appendix G. Summary of MajesTEC-1 Trial Characteristics and Outcomes
| Characteristic | MajesTEC-1 (n = 165) |
|---|---|
| Study description | |
| Study design | Clinical trial |
| MajesTEC-1 ineligibility, % | 0 |
| Median follow-up, months | 23 |
| Median prior LOTs | 5 |
| Select efficacy outcomes | |
| ORR, % (overall) | 63 |
| CR or better, % | 45.5 |
| VGPR or better, % | 59.4 |
| Median PFS, months (95% CI) | 11.3 (8.8–16.4) |
| Median OS, months (95% CI) | 21.9 (15.1—NE) |
| Select safety outcomes | |
| CRS (any grade), % | 72 |
| ICANS (any grade), % | 3 |
| Infections (any grade), % | 80 |
References
- National Cancer Institute. SEER Cancer Stat Facts: Myeloma. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 2 August 2024).
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Hari, P.; Romanus, D.; Palumbo, A.; Luptakova, K.; Rifkin, R.M.; Tran, L.M.; Raju, A.; Farrelly, E.; Noga, S.J.; Blazer, M.; et al. Prolonged Duration of Therapy Is Associated with Improved Survival in Patients Treated for Relapsed/Refractory Multiple Myeloma in Routine Clinical Care in the United States. Clin. Lymphoma Myeloma Leuk 2018, 18, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Joseph, N.; He, J.; Crivera, C.; Fu, A.Z.; Garrett, A.; Shah, N. Healthcare Costs of Multiple Myeloma Patients with Four or More Prior Lines of Therapy, Including Triple-Class Exposure in the United States. Oncol. Ther. 2022, 10, 411–420. [Google Scholar] [CrossRef]
- Bazarbachi, A.H.; Al Hamed, R.; Malard, F.; Harousseau, J.L.; Mohty, M. Relapsed refractory multiple myeloma: A comprehensive overview. Leukemia 2019, 33, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rivas, J.A.; Rios-Tamayo, R.; Encinas, C.; Alonso, R.; Lahuerta, J.J. The changing landscape of relapsed and/or refractory multiple myeloma (MM): Fundamentals and controversies. Biomark. Res. 2022, 10, 1–23. [Google Scholar] [CrossRef]
- Sandahl, T.B.; Soefje, S.A.; Calay, E.S.; Lin, Y.; Fonseca, R.; Ailawadhi, S.; Parrondo, R.; Lin, D.; Wu, B.; Silvert, E.; et al. Real-World Treatment Outcomes of Teclistamab Under an Outpatient Model for Step-up Dosing Administration. Blood 2023, 142, 5154. [Google Scholar] [CrossRef]
- Derman, B.A.; Roach, M.; Lin, D.; Wu, B.; Murphy, R.; Kim, N.; Doyle, M.; Prood, N.; Fowler, J.; Marshall, A.; et al. Panel Interview of Oncology Practices with Emergent Experience of Teclistamab in the Real World: The Tec-Pioneer Study. Blood 2023, 142, 7249. [Google Scholar] [CrossRef]
- Varshavsky-Yanovsky, N.A.; Styler, M.; Khanal, R.; Abdelmessieh, P.; Fung, H. P940: An outpatient model for teclistamab step-up dosing administration—Initial experiences at fox chase cancer center BMT program. HemaSphere 2023, 7, e605007f. [Google Scholar]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Garfall, A.L.; Nooka, A.K.; van de Donk, N.W.C.J.; Moreau, P.; Bhutani, M.; Oriol, A.; Martin, T.G.; Rosiñol, L.; Mateos, M.-V.; Bahlis, N.J.; et al. Long-term follow-up from the phase 1/2 MajesTEC-1 trial of teclistamab in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, 7540. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.A.; Ahmed, N.; Jia, X.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; et al. Safety and Efficacy of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Real-World Experience. Transplant. Cell. Ther. 2023, 30, 308.e1–308.e13. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Kumar, A.; Loughran, T.; Alsina, M.; Durie, B.G.; Djulbegovic, B. Management of multiple myeloma: A systematic review and critical appraisal of published studies. Lancet Oncol. 2003, 4, 293–304. [Google Scholar] [CrossRef]
- Maiese, E.M.; Ainsworth, C.; Le Moine, J.G.; Ahdesmaki, O.; Bell, J.; Hawe, E. Comparative Efficacy of Treatments for Previously Treated Multiple Myeloma: A Systematic Literature Review and Network Meta-analysis. Clin. Ther. 2018, 40, 480–494.e423. [Google Scholar] [CrossRef] [PubMed]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Firestone, R.S.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.; Wang, A.; Zhu, M.; Patel, D.; Tan, C.R.; Hultcrantz, M.; et al. CD8 effector T cells enhance response in BCMA-exposed and -naïve multiple myeloma. Blood Adv. 2023, 8, 1600–1611. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.; Firestone, R.S.; Wang, A.X.; Korde, N.; Hultcrantz, M.L.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; et al. Patterns of CRS with teclistamab in relapsed/refractory multiple myeloma with or without prior T-cell redirection therapy. Blood Adv. 2024, 8, 3038–3044. [Google Scholar] [CrossRef]
- Mohan, M.; Monge, J.; Shah, N.; Luan, D.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in relapsed refractory multiple myeloma: Multi-institutional real-world study. Blood Cancer J. 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Riedhammer, C.; Bassermann, F.; Besemer, B.; Bewarder, M.; Brunner, F.; Carpinteiro, A.; Einsele, H.; Faltin, J.; Frenking, J.; Gezer, D.; et al. Real-world analysis of teclistamab in 123 RRMM patients from Germany. Leukemia 2024, 38, 365–371. [Google Scholar] [CrossRef]
- Gong, Z.; Umoru, G.; Monge, J.; Shah, N.; Mohyuddin, G.R.; Radhakrishnan, S.; Chakraborty, R.; Schinke, C.; D’Souza, A.; Mohan, M. Adverse Effects and Non-Relapse Mortality of BCMA-Directed Immunotherapies: An FDA Adverse Event Reporting System (FAERS) Database Study. Blood 2023, 142, 358. [Google Scholar] [CrossRef]
- Midha, S.; Laubach, J.; Mo, C.; Sperling, A.; Nadeem, O.; Bianchi, G.; Hartley-Brown, M.; Ramsdell, L.; Reyes, K.; Costello, P.; et al. Real world experience of patients treated with teclistamab: aBCMA-directed bispecific t-cell engaging therapy for multiple myeloma. In Proceedings of the Internation Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Nader, S.M.; Jines, S.T.; Collier, C.; Zimmers, T.; Abonour, R.; Farag, S.; Zaid, M.A.; Lee, K.P.; Suvannasankha, A. Characterization of Anthropometric Changes in Patients with Relapsed/Refractory Myeloma Treated with BCMA Bispecific Antibody Teclistamab. Blood 2023, 142, 6683. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Moreau, P.; Garfall, A.L.; Bhutani, M.; Oriol, A.; Nooka, A.K.; Martin, T.G.; Rosiñol, L.; Mateos, M.-V.; Bahlis, N.J.; et al. Long-term follow-up from MajesTEC-1 of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2023, 41, 8011. [Google Scholar] [CrossRef]
- Banerjee, R.; Chang, H.-Y.; Lin, D.; Fu, A.Z.; Hester, L.; Kim, N.; Fowler, J.; Walker, S.; Gifkins, D.; Hearty, C.; et al. Real-World Patterns of Step-Up Dosing Period and Early Safety Outcomes in US Patients Treated with Teclistamab for Multiple Myeloma. In Proceedings of the Lymphoma, Leukemia & Myeloma Congress, New York, NY, USA, 18–21 October 2023; pp. S3–S57. [Google Scholar]
- Banerjee, R.; Chang, H.-Y.; Lin, D.; Harper, J.S.; Fu, A.Z.; Kim, N.; Fowler, J.; Fernandez, M.; Doyle, M.; Min, E.E.; et al. Teclistamab (TEC) step-up dosing (SUD) and treatment dose schedule de-escalation in the real-world (RW) setting—An analysis of multicenter electronic medical records. In Proceedings of the European Hematology Association 2024, Barcelona, Spain, 15 August 2024. [Google Scholar]
- Dima, D.; Davis, J.A.; Ahmed, N.; Sannareddy, A.; Shaikh, H.; Mahmoudjafari, Z.; Khouri, J.; Kaur, G.; Strouse, C.; Valent, J.; et al. Real-World Safety and Efficacy of Teclistamab for Patients with Heavily Pretreated Relapsed-Refractory Multiple Myeloma. Blood 2023, 142, 91. [Google Scholar] [CrossRef]
- Dima, D.; Sannareddy, A.; Ahmed, N.; Davis, J.A.; Shaikh, H.; Mahmoudjafari, Z.; Duco, M.; Khouri, J.; Kaur, G.; Lochner, J.; et al. Toxicity and Efficacy Outcomes of Teclistamab in Patients with Relapsed-Refractory Multiple Myeloma (RRMM) Above the Age of 70 Years: A Multicenter Study. Blood 2023, 142, 3330. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.; Ahmed, N.; Jia, X.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; et al. Safety and Efficacy of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Real-World Experience from the US Myeloma Innovations Research Collaborative (USMIRC). Transplant. Cell. Ther. 2024, 30, S384. [Google Scholar] [CrossRef]
- Dima, D.; Davis, J.; Ahmed, N.; Sannareddy, A.; Shaikh, H.; Shune, L.; Kaur, G.; Khouri, J.; Afrough, A.; Strouse, C.S.; et al. Outcomes of BCMA-Directed Chimeric Antigen Receptor T-Cell (CART) Therapy and Teclistamab in Patients with Relapse-Refractory Multiple Myeloma with Extramedullary Disease: A Real-World Experience of the US Myeloma Innovations Research Collaborative (USMIRC). Transplant. Cell. Ther. 2024, 30, S384–S385. [Google Scholar] [CrossRef]
- Firestone, R.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.S.; Wang, A.; Zhu, M.; Patel, D.; Tan, C.; Hultcrantz, M.; et al. Evaluating Tumor-Intrinsic and Patient-Specific Mechanisms of Resistance to Teclistamab in Anti-BCMA Exposed and Naïve Multiple Myeloma. Blood 2023, 142, 333. [Google Scholar] [CrossRef]
- Firestone, R.; Shekarkhand, T.; Patel, D.; Hultcrantz, M.; Lesokhin, A.; Mailankody, S.; Hassoun, H.; Tan, C.; Shah, U.; Korde, N.; et al. P962: Commercial Teclistamab in anti-BCMA therapy exposed relapsed refractory multiple myeloma patients: The MSKCC experience. HemaSphere 2023, 7, e81198bb. [Google Scholar]
- Firestone, R.; Shekarkhand, T.; Patel, D.; Tan, C.R.C.; Hultcrantz, M.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; Shah, U.A.; Korde, N.; et al. Evaluating the efficacy of commercial teclistamab in relapsed refractory multiple myeloma patients with prior exposure to anti-BCMA therapies. J. Clin. Oncol. 2023, 41, 8049. [Google Scholar] [CrossRef]
- Mohan, M.; Shah, N.; Luan, D.; Monge, J.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in Relapsed Refractory Multiple Myeloma: Multi-Institutional Real-World Study. Blood 2023, 142, 545. [Google Scholar] [CrossRef]
- Matthew, J.; Pianko, J.H.; Lin, D.; Chang, H.-Y.; Nina, K.; Harper, J.S.; Fowler, J.; Fernandez, M.; Doyle, M.; Hester, L.; et al. Real-world schedule de-escalation of teclistamab in patients with relapsed or refractory multiple myeloma—A US national healthcare claims analysis. In Proceedings of the European Hematology Association 2024, Barcelona, Spain, 15 August 2024. [Google Scholar]
- Riedhammer, C.; Bassermann, F.; Besemer, B.; Bewarder, M.; Brunner, F.; Carpinteiro, A.; Einsele, H.; Faltin, J.; Frenking, J.; Gezer, D.; et al. Real-World Analysis of Teclistamab in 115 RRMM Patients from Germany. Blood 2023, 142, 3329. [Google Scholar] [CrossRef]
- Maringanti, S.A.; Lin, Y.; Estritis, S.; Martinez-Lopez, J.; Bansal, R.; Fotiou, D.; Corona, M.; Chhabra, S.; Brunaldi, L.; Corraes, A.D.M.S.; et al. MM-527 Real World Evaluation of Teclistamab for the Treatment of Relapsed or Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2023, 23, S506–S507. [Google Scholar] [CrossRef]
- Yi, L.S.A.; Brunaldi, L.; Bansal, R.; De Menezes Silva Corraes, A.; Chhabra, S.; Parrondo, R.; Ailawadhi, S.; Kastritis, E.; Fotiou, D.; Popat, R.; et al. Real world evaluation of Teclistamab in patients with RRMM: Results from the IMF Immunotherapy Database Project. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Asoori, S.; Popat, R.; Martínez-Lopez, J.; Kastritis, E.; Brunaldi, L.; Bansal, R.; De Menezes Silva Corraes, A.; Yong, K.; Mactier, C.; Corona, M.; et al. Real-World Evaluation of Teclistamab for the Treatment of Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2023, 142, 3347. [Google Scholar] [CrossRef]
- Tan, C.R.C.; Derkach, A.; Maclachlan, K.; Hultcrantz, M.; Hassoun, H.; Mailankody, S.; Shah, U.A.; Rajeeve, S.; Shah, G.L.; Scordo, M.; et al. Real-world schedule de-escalation of teclistamab in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, 7536. [Google Scholar] [CrossRef]
- Tan, C.R.C.; Derkach, A.; Maclachlan, K.; Hultcrantz, M.; Hassoun, H.; Mailankody, S.; Shah, U.A.; Rajeeve, S.; Shah, G.L.; Scordo, M.; et al. Real-world schedule de-escalation of teclistamab in patients with relapsed/refractory multiple myeloma. In Proceedings of the European Hematology Association 2024, Barcelona, Spain, 15 August 2024. [Google Scholar]
- Kowalski, A.; Lykon, J.L.; Diamond, B.; Coffey, D.; Kaddoura, M.; Maura, F.; Hoffman, J.E.; Kazandjian, D.; Landgren, O. Tocilizumab Prophylaxis for Patients Treated with Teclistamab: A Single-Center Experience. Blood 2023, 142, 4709. [Google Scholar] [CrossRef]
- Marin, E.; Scott, S.; Maples, K.; Joseph, N.S.; Hofmeister, C.C.; Gupta, V.A.; Dhodapkar, M.V.; Kaufman, J.L.; Lonial, S.; Nooka, A.K. Prophylactic Tocilizumab to Prevent Cytokine Release Syndrome (CRS) with Teclistamab Administration. Blood 2023, 142, 2008. [Google Scholar] [CrossRef]
- Banerjee, R.; Kim, N.; Kohli, M.; Hester, L.; Achter, E.; Fowler, J.; Umeh, E.; Lin, D.; Aweh, G.; Gifkins, D.; et al. Evolving Real-World Characteristics and Step-up Dosing Among Early Initiators of Teclistamab for Multiple Myeloma—A National All-Payer Claims Database Study. Blood 2023, 142, 5087. [Google Scholar] [CrossRef]
- Bansal, R.; Paludo, J.; de Menezes Silva Corraes, A.; Megan, S.; Khurana, A.; Hampel, P.; Durani, U.; Dingli, D.; Hayman, S.; Kapoor, P.; et al. Outpatient practice utilization for CAR-T and T cell engager in patients with lymphoma and multiple myeloma. J. Clin. Oncol. 2023, 41, 1533. [Google Scholar] [CrossRef]
- Kathy Mooney, N.A.; Anderson, K.; Zukas, A. Taking a BiTE out of Hospital Admission Days Using a Team Approach to Managing Patients at Risk for Treatment Related Toxicities. In Proceedings of the Oncology Nursing Society 2024, Washington DC, USA, 24–28 April 2024. [Google Scholar]
- Howard, A.J.; Shekarkhand, T.; Hamadeh, I.S.; Wang, A.; Patel, D.; Tan, C.; Hultcrantz, M.; Mailankody, S.; Hassoun, H.; Shah, U.A.; et al. Identifying Causes of Unscheduled Healthcare Interactions and Changes to Patient Disposition in Individuals Receiving Outpatient Commercial Bispecific Antibody Therapy in Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2023, 142, 3707. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Raje, N.; Anderson, K.; Einsele, H.; Efebera, Y.; Gay, F.; Hammond, S.P.; Lesokhin, A.M.; Lonial, S.; Ludwig, H.; Moreau, P.; et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: Consensus recommendations from an expert panel. Blood Cancer J. 2023, 13, 116. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Usmani, S.; Cohen, A.D.; van de Donk, N.W.C.J.; Leleu, X.; Gállego Pérez-Larraya, J.; Manier, S.; Nooka, A.K.; Mateos, M.V.; Einsele, H.; et al. International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of T-cell-engaging bispecific antibodies in multiple myeloma. Lancet Oncol. 2024, 25, e205–e216. [Google Scholar] [CrossRef] [PubMed]
- Hebraud, B.; Granell, M.; Lapierre, L.; Mouchel, P.L.; Beziat, G.; Sicard, N.; Bonneau, M.; Higue, J.; Sougy, F.; Perriat, S.; et al. French Monocentric Experience of Outpatient Step-up Dosing of Teclistamab in Relapsed Refractory Multiple Myeloma. Blood 2023, 142, 4736. [Google Scholar] [CrossRef]
- Derman, B.A.; Roach, M.; Lin, D.; Wu, B.; Murphy, R.; Kim, N.; Doyle, M.; Prood, N.; Fowler, J.; Marshall, A.; et al. Panel Interview of ONcology practices with Emergent Experience of teclistamab in the Real world: The TecPIONEER Study. Curr. Med. Res. Opin. 2024, 40, 1053–1058. [Google Scholar] [CrossRef]
- Tan, C.R.; Chinaeke, E.; Kim, N.; Hester, L.; Fowler, J.; Gifkins, D.; Lin, D.; Walker, S.; Fu, A.Z.; Wu, B. MM-344 Real-World Patient Profile and Step-Up Dosing Process of Early Initiators of Teclistamab for Multiple Myeloma (MM) in a Hospital Setting in the US: Premier Healthcare Database Study. Clin. Lymphoma Myeloma Leuk. 2023, 23, S491. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network; Health Improvement Scotland. SIGN Search Filters. 2021. Available online: https://www.sign.ac.uk/what-we-do/methodology/search-filters/ (accessed on 23 May 2024).
- Wells, G.S.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 October 2016).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Asoori, S.; Martin, T.; Wolf, J.; Chung, A.; Arora, S. CT-630 Real World Evaluation of Teclistamab: A Focus on Infections in Patients with Relapsed Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2023, 23, S536–S537. [Google Scholar] [CrossRef]
- Bansal, R.; Paludo, J.; Corraes, A.; Spychalla, M.; Haugen, K.; Khurana, A.; Hampel, P.J.; Durani, U.; Dingli, D.; Hayman, S.R.; et al. Outpatient Management of CAR-T and Teclistamab for Patients with Lymphoma and Multiple Myeloma. Blood 2023, 142, 253. [Google Scholar] [CrossRef]
- Bansal, R.; De Menezes Silva Corraes, A.; Brunaldi, L.; Sandahl, T.B.; Rees, M.J.; Hayman, S.R.; Binder, M.; Abdallah, N.; Dingli, D.; Cook, J.; et al. Real world outcome of patients with multiple myeloma who received bispecific antibodies after CAR-T therapy. J. Clin. Oncol. 2024, 42, 7520. [Google Scholar] [CrossRef]
- Deborah Bolton, A.B.; Martin, J.; Monsale, S. Administration of Teclistamab Shortly after Food and Drug Administration Approval in a Community Hospital System. In Proceedings of the Oncology Nursing Society Congress 2024, Washington DC, USA, 24–28 April 2024. [Google Scholar]
- Catamero, D.D. Real world experience of teclistamab using a promptmanagement strategy for cytokine release syndrome. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Charkviani, M.; Vaughan, L.E.; Sandahl, T.B.; Lin, Y.; Leung, N.; Herrmann, S.M. Incidence of acute kidney injury in patients with relapsed and refractory multiple myeloma treated with teclistamab vs chimeric antigen receptor T-cell therapy. J. Clin. Oncol. 2024, 42, 7542. [Google Scholar] [CrossRef]
- Beth Faiman, D.D.; Duco, M.; Rice, M.; Rudoni, J.; Anwer, F.; Khouri, J.; Mazzoni, S.; Raza, S.; Samaras, C.; Williams, L.; et al. Initial Report of a Single Institution Experience with Teclistamabfor Relapsed or Refractory Multiple Myeloma Including Prior BCMA. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Ghamsari, F.; Trando, A.; Medley, K.; Martino, J.; Block, S.; Doan, T.; Wells, K.; Quach, A.C.; Cheng, R.G.; Costello, C.; et al. Real-world outcomes of teclistamab for the treatment of relapsed/refractory multiple myeloma at UC San Diego Health: A single-institution experience. J. Clin. Oncol. 2024, 42, e19504. [Google Scholar] [CrossRef]
- Melanie, R.; Glenn, K.G.; Bucknall, J.; Ricca, M. Teclistamab Early Discharge Program. In Proceedings of the Oncology Nursing Society Congress 2024, Washington DC, USA, 24–28 April 2024. [Google Scholar]
- Gordon, B.; Fogel, L.; Varma, G.; Mejia Saldarriaga, M.; Ahn, J.; Aleman, A.; Caro, J.; Chen, X.; Monge, J.; Parmar, H.; et al. Teclistamab Demonstrates Clinical Activity in Real-World Patients Ineligible for the Pivotal Majestec-1 Trial. Blood 2023, 142, 4741. [Google Scholar] [CrossRef]
- Graf, K.C.; Davis, J.A.; Cendagorta, A.M.; Granger, K.; Gaffney, K.; Green, K.; Hess, B.T.; Hashmi, H. ‘Fast but Not so Furious’: A Condensed but Safe and Cost-Effective Step-up Dosing Regimen of Teclistamab for Relapsed Refractory Multiple Myeloma. Transplant. Cell. Ther. 2024, 30, S381. [Google Scholar] [CrossRef]
- Grajales-Cruz, A.S.K.; Blue, B.; Hansen, D.; Puglianini, O.C.; Freeman, C.; De Avila, G.; Ochoa-Bayona, L.; Liu, H.; Nishihori, T.; Frederick; et al. Safety and Efficacy of Standard of Care (SOC) Teclistamab (TEC) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM), a single center experience. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Grajales-Cruz, A.F.; Castaneda, O.; Hansen, D.K.; Vazquez-Martinez, M.A.; Blue, B.; Khadka, S.; Liu, H.; Ochoa-Bayona, J.L.; Freeman, C.L.L.; Locke, F.L.; et al. Teclistamab Induces Favorable Responses in Patients with Relapsed and Refractory Multiple Myeloma after Prior BCMA-Directed Therapy. Blood 2023, 142, 3351. [Google Scholar] [CrossRef]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.; Firestone, R.; Wang, A.; Korde, N.; Hulcrantz, M.; Lesokhin, A.; Mailankody, S.; Hassoun, H.; et al. Patterns of Cytokine Release Syndrome with Teclistamab in Relapsed/Refractory Multiple Myeloma with or without Prior T-Cell Redirection Therapy. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Hamadeh, I.S.; Shekarkhand, T.; Rueda, C.; Firestone, R.; Wang, A.; Korde, N.; Hultcrantz, M.; Lesokhin, A.; Mailankody, S.; Hassoun, H.; et al. Patterns of Cytokine Release Syndrome with Teclistamab in Relapsed/Refractory Multiple Myeloma with or without Prior T-Cell Redirection Therapy. Blood 2023, 142, 1961. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Steele, A.P.; Rosenberg, A.; Guglielmo, J. Safety Outcomes of Teclistamab Accelerated Dose Escalation. In Proceedings of the Hematology/Oncology Pharmacy Association 2024, Tampa, FL, USA, 3–6 April 2024. [Google Scholar]
- Swarup Kumar, K.H.; Clark, J.; Soto, A.A.; Rounds, A. Early outcomes and therapy modification strategies in Multiple Myeloma patients treated with teclistamab, CD3XBCMA BITE: A singlecenter experience. In Proceedings of the International Myeloma Society Annual Meeting and Exposition, Athens, Greece, 27–3 September 2023. [Google Scholar]
- Lachenal, F.; Lebreton, P.; Bouillie, S.; Pica, G.M.; Aftisse, H.; Pascal, L.; Montes, L.; Macro, M.; Vignon, M.; Harel, S.; et al. Teclistamab in Relapsed Refractory Multiple Myeloma Patients on Dialysis: A French Experience. Blood 2023, 142, 4739. [Google Scholar]
- Harrell, S.; de Coella, J.-M.S.; Sonntag, C.; Cazaubiel, T. P-014 teclistamab in relapsed and refractory multiple myeloma: Patients characteristics from post marketing acces (acces precoce) in France. In Proceedings of the Internation Myeloma Society Annual Meeting and Exposition, Athens, Greece, 17–30 September 2023. [Google Scholar]
- Rees, M.J.; Mammadzadeh, A.; Bolarinwa, A.; Elhaj, M.E.; Bohra, A.; Ailawadhi, S.; Parrondo, R.; Chhabra, S.; Lin, Y.; Binder, M.; et al. Class comparison of BCMA-directed therapies in relapsed multiple myeloma. J. Clin. Oncol. 2024, 42, 7515. [Google Scholar] [CrossRef]
- Sandahl, T.B.; Soefje, S.A.; Calay, E.S.; Lin, Y.; Fonseca, R.; Ailawadhi, S.; Parrondo, R.; Lin, D.; Wu, B.; Silvert, E.; et al. Real-World Treatment Outcomes of Teclistamab Under an Outpatient Model for Step-Up Dosing Administration. In Proceedings of the Hematology/Oncology Pharmacy Association 2024, Tampa, FL, USA, 3–6 April 2024. [Google Scholar]
- Schaefers, C.T.; Alsdorf, W.; Schaefers, C.; Kosch, R.; Leypoldt, L.; Bokemeyer, C.; Weisel, K.K.A. BCMA x CD3 bispecifc antibody treatment with teclistamab in relapsed and/or refractory multiple myeloma: A real-world monocentric analysis. Oncol. Res. Treat. 2023, 46, 1–354. [Google Scholar] [CrossRef]
- Tabbara, N.; Singel, M.; Allen, N.; Mooney, K.; Shedeck, A.; Zukas, A.; Campion, K.; Sollenberger, C.; Gocke, C.B.; Ali, S.A.; et al. Ambulatory teclistamab administration in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, 11146. [Google Scholar] [CrossRef]
- Tan, C.R.; Kim, N.; Chinaeke, E.; Hester, L.; Fowler, J.; Gifkins, D.; Lin, D.; Walker, S.; Fu, A.Z.; Wu, B. Real-World Patient Profile and Step-up Dosing Process of Early Initiators of Teclistamab for Multiple Myeloma in US Hospitals—An Updated Analysis Using Premier Healthcare Database. Blood 2023, 142, 3792. [Google Scholar] [CrossRef]
- Yanovsky, A.V.; Styler, M.; Khanal, R.; Abdelmessieh, P.; Fung, H. MM-595 Feasibility and Safety of Outpatient Model for Teclistamab Step-Up Dosing Administration: A Single Center Experience. Clin. Lymphoma Myeloma Leuk. 2023, 23, S509. [Google Scholar] [CrossRef]
- Venkatesh, P.; Atrash, S.; Paul, B.; Alkharabsheh, O.; Afrough, A.; Mahmoudjafari, Z.; Mushtaq, M.U.; Hashmi, H.; Davis, J.A.; Abdallah, A.-O.A. Efficacy of teclistamab in patients (pts) with heavily pretreated, relapsed/refractory multiple myeloma (RRMM), including those refractory to penta RRMM and BCMA (B-cell maturation antigen) directed therapy (BDT). J. Clin. Oncol. 2023, 41, e20044. [Google Scholar] [CrossRef]
| Study ID | Study Design | Data Source | Chart Review vs. Secondary Database | Setting | Study Timeframe | mFU (Months) | Sample Size | Relevant Outcomes Evaluated |
|---|---|---|---|---|---|---|---|---|
| Banerjee 2023b [25,26] | Retrospective | Acentrus MM electronic medical records (EMRs) | Secondary database | Academic centers/community-based hospitals, multi-center | October 2022–November 2023 | 5.1 | 247 | LOS, CRS, ICANS, step-up dosing schedule, time to next treatment (TTNT), or death |
| Dima 2023 [12,27,28,29,30] | Retrospective | Patients treated at USMIRC centers | Chart review | Academic, multi-center | August 2022–August 2023 | 3.8 | 106 | CRS, ICANS, infections, ORR, DOR, OS, PFS, TCZ use in AE management, hospitalizations |
| Firestone 2023 [17,31,32,33] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | November 2022–July 2023 | 3.1 | 52 | Survival, PFS, ORR, safety |
| Mohan 2024 [19,34] | Retrospective | Patients treated at five US academic centers | Chart review | Academic, multi-center | NR | 3.5 | 110 | CRS, ICANS, infections, ORR, best response, LOS, IVIG use in AE management, TCZ use in AE management |
| Pianko 2024 [35] | Retrospective | Komodo Healthcare MapTM | Secondary database | Multi-center | October 2022–December 2023 | 4.2 | 419 | Dosing schedule of teclistamab, time to less frequent dosing, TTNT |
| Riedhammer 2024 [20,36] | Retrospective | Patients treated at 18 German centers | Chart review | Multi-center | July 2022–October 2023 | 5.5 | 123 | Time to response, best response, ORR, PFS, infections |
| Tan-Asoori 2023 [37,38,39] | Retrospective | Patients treated at IMF-associated centers | Chart review | Academic, multi-center | NR—October 2023 | 5 | 204 | ORR, OS, PFS, CRS, ICANS, TCZ use in AE management, IVIG use in AE management, LOS |
| Tan 2024 [40,41] | Retrospective | Patients treated at the Memorial Sloan Kettering Cancer Center | Chart review | Academic, single-center | November 2022–March 2024 | Overall population: 9.5 Patients switching to less-frequent dosing: 6.4 | 86 | ORR, DOR, PFS, time to response |
| Study ID | Overall/Subgroup Details | Sample Size | Median Age (Range), Years | Female, n (%) | Race, n (%) | High - Risk Cytogenetics, n (%) | Median Prior Lines of Therapy | Extramedullary Disease, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | Asian | ||||||||
| Banerjee 2023b [25,26] | Overall | 247 | 69 (41–89) | 111 (44.9) | 138 (75.8) a | 23 (12.6) a | 21 (11.5) a | -- | -- | 14 (5.7) |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | 66.5 (35–87) | 55 (54) b | 72 (68) | 28 (26) | 2 (2) | 56 (59) | 6 | 45 (42) |
| Firestone 2023 [17,31,32,33] | Overall | 52 | 70 (30–80) | -- | -- | -- | -- | 17 (33) | 7 | 18 (35) |
| Mohan 2024 [19,34] | Overall | 110 | 68 (37–89) | 54 (49) | 67 (61) | 32 (29) | 2 (1.8) | 59 c (62) | 6 | 48 (44) |
| Pianko 2024 [35] | Overall | 419 | 65 (58–73) d | 183 (43.7) | 185 (63.4) e | 91 (31.2) e | 16 (5.4) e | -- | 5 | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | 67 (35–87) | 53 f (43.1) | -- | -- | -- | 39 (36.8) g | 6 | 43 (36.1) |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | 66 (33–91) | 91 (45) | 143 (70) | 15 (7) | 19 (9) | 90 (44) | 6 | 38 (19) |
| Tan 2024 [40,41] | Overall | 86 | 71 (64–78) d | 44 (51) | 65 (76) | 14 (16) | -- | 56 (71) h | 6 | 30 (38) h |
| Study ID | Overall/Subgroup Details | Sample Size | MajesTEC-1 Ineligible Patients, n (%) | Prior BCMA Therapy, n (%) | ECOG PS ≥2, n (%) | Cytopenia, n (%) | Renal Impairment/Failure, n (%) | CrCl <30 mL/min or 40 mL/min, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Anemia | Neutropenia | Thrombocytopenia | ||||||||
| Banerjee 2023b [25,26] | Overall | 247 | -- | 48 (19.4) | -- | -- | 126 (51.0) | 55 (22.3) | -- | 100 (40.5) | -- |
| Dima 2023 [12,27,28,29,30] | Overall | 106 | 88 (83) | 56 (53) | 35 (33) | 33 (31) | 27 (25) | 2 (2) | 21 (20) | 14 (13) | 14 (13) |
| Firestone 2023 [17,31,32,33] | Overall | 52 | -- | 27 (52) | -- | -- | -- | -- | -- | -- | -- |
| Mohan 2024 [19,34] | Overall | 110 | -- | 38 (35) | -- | -- | -- | -- | -- | -- | -- |
| Pianko 2024 [35] | Overall | 419 | -- | 102 (24.3) | -- | -- | 164 (39.1) | 50 (11.9) | -- | 206 (49.2) | -- |
| Riedhammer 2024 [20,36] | Overall | 123 | 48 a (39) | 45 (37.4) | -- | -- | -- | -- | -- | -- | |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | 122 (70) b | 91 (45.0) | -- | -- | -- | -- | -- | -- | 25 (12) |
| Tan 2024 [40,41] | Overall | 86 | -- | 32 (37) | 5 (10) c | -- | -- | -- | -- | -- | 9 (10) |
| Study ID | Overall/Subgroup Details | Timepoint/mFU | Response Evaluable Population Size | Overall Response Rate, n (%) | Survival Evaluable Population Size | PFS | OS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PR or Better | VGPR or Better | CR or Better | Median, Months (95% CI) | 6-Month Rate, % (95% CI) | Median, Months (95% CI) | 6-Month Rate, % (95% CI) | |||||
| Dima 2023 [12,27,28,29,30] | Overall | Median of 3.8 months | 104 | 70 a (66) | 49 (46) b | 31 (29) b | 106 | 5.4 (3.4, NR) | -- | NR | 70 (61, 80) |
| >70 years old | Median of 3.8 months c | 34 | 24 (71) | -- | 10 (30) d | 33 | 5.4 (2.8, NR) | -- | -- | -- | |
| Firestone 2023 [17,31,32,33] | Overall | Median of 3.1 months | 47 | 30 (64) | 18 a (38) | -- | 52 | NR | -- | -- | -- |
| Prior anti-BCMA exposure | Median of 3.1 months c | 26 | 13 (50.0) | -- | -- | 27 | 3.4 | -- | -- | -- | |
| Mohan 2024 [19,34] | Overall | Median of 3.5 months | 98 | 61 (62) | 50 a (51) | 20 a (20) | 110 | NR | 52 (42, 64) | NR | 80 (72, 89) |
| Riedhammer 2024 [20,36] | Overall | Median of 5.5 months | 123 | 73 a (59.3) | 59 a (48.0) b | 27 a (22.0) b,e | 123 | 8.7 | -- | NR | -- |
| Tan-Asoori 2023 [37,38,39] | Overall | Median of 5 months | 180 | 115 a (64) | 90 a (50) b | 34 a (19) b | 204 | 13 | 57.7 | 15 | 76.2 |
| Tan 2024 [40,41] | Overall | Median of 9.5 months | 77 | 47 (61) | 33 (43) | -- | 86 | -- | 52.4 (42.4, 64.7) | -- | -- |
| Prior BCMA-directed therapy | Median of 6.4 months | 32 | 14 (43) | -- | -- | 32 | -- | 90.0 (79.1, 100.0) | -- | -- | |
| Study ID | Overall/Subgroup Details | Sample Size | Timepoint/mFU | CRS, n (%) | ICANS, n (%) | Infections, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 1 | Grade 2 | Grade 3+ | Any Grade | Grade 1 | Grade 2 | Grade 3+ | Any Grade | Grade 3+ | ||||
| Chart review—mixed inpatients and outpatient, or not reported | |||||||||||||
| Mohan 2024 [19,34] | Overall | 110 | Median of 3.5 months | 62 a (56) | 57 a (51.8) b,c | 5 (4.5) b | 12 (11) | -- | -- | 5 (4.5) | 44 (40) | 29 (26) | |
| Riedhammer 2024 [20,36] | Overall | 123 | Median of 5.5 months | 72 (58.5) | -- | -- | 2 (1.6) | -- | -- | -- | -- | 67 (54.5) | 33 (26.8) |
| Tan-Asoori 2023 [37,38,39] | Overall | 204 | Median of 5 months | 110 (53.9) a,b | 84 (41.2) a,b | 25 (12.3) a,b | 1 (0.5) a,b | -- | -- | -- | -- | 115 (60.0) | -- |
| Chart Review—inpatient monitoring | |||||||||||||
| Dima 2023 [12,27,28,29,30] | Overall | 106 | Median of 3.8 months | 68 (64) | 57 (54) | 10 (9) | 1 (1) | 15 (14) | 5 (5) | 7 (6) | 3 (3) | 33 (31) | -- |
| >70 years old | 33 | July 2023 cut-off | 22 (67) | -- | -- | 1 (3) | 7 (21) | -- | -- | 0 (0) | 11 (33) | -- | |
| Firestone 2023 [17,31,32,33] | Overall | 52 | Median of 3.1 months | 27 (52) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tan-Asoori 2023 [37,38,39] | Inpatients | 160 | Median of 5 months | 94 (59) | 72 (45) | 22 (14) | 0 (0) | -- | -- | -- | -- | -- | -- |
| Chart review—outpatient monitoring | |||||||||||||
| Tan-Asoori 2023 [37,38,39] | Outpatients | 44 | Median of 5 months | 16 (36) | 12 (27) | 3 (7) | 1 (2) | -- | -- | -- | -- | -- | -- |
| Secondary databases | |||||||||||||
| Banerjee 2023b [25,26] | Overall | 76 | Median of 5.1 months | 14 (18.4) | 8 (10.5) | 3 (3.9) | 1 (1.3) | 3 (3.9) | 2 (2.6) | 1 (1.3) | 0 (0) | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derman, B.; Tan, C.; Steinfield, I.; Wilson, F.R.; Lin, D.; Wu, B.; Fernandez, M.; Fowler, J.; Paner-Straseviciute, A.; Kim, N.; et al. Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review. Cancers 2025, 17, 1235. https://doi.org/10.3390/cancers17071235
Derman B, Tan C, Steinfield I, Wilson FR, Lin D, Wu B, Fernandez M, Fowler J, Paner-Straseviciute A, Kim N, et al. Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review. Cancers. 2025; 17(7):1235. https://doi.org/10.3390/cancers17071235
Chicago/Turabian StyleDerman, Benjamin, Carlyn Tan, Ian Steinfield, Florence R. Wilson, Dee Lin, Bingcao Wu, Mariana Fernandez, Jessica Fowler, Agne Paner-Straseviciute, Nina Kim, and et al. 2025. "Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review" Cancers 17, no. 7: 1235. https://doi.org/10.3390/cancers17071235
APA StyleDerman, B., Tan, C., Steinfield, I., Wilson, F. R., Lin, D., Wu, B., Fernandez, M., Fowler, J., Paner-Straseviciute, A., Kim, N., Doyle, M., Marshall, A., Cheadle, J., Keeping, S., & Liu, J. J. (2025). Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review. Cancers, 17(7), 1235. https://doi.org/10.3390/cancers17071235






