The Role of HPV in the Development of Cutaneous Squamous Cell Carcinoma—Friend or Foe?
Simple Summary
Abstract
1. Introduction
2. The Epidemiology of cSCC
3. Human Papillomaviruses
4. Frequency and Prevalence of Beta HPV
5. Beta HPV and Association with cSCC
6. What Are the Oncogenic Functions of Beta HPV?
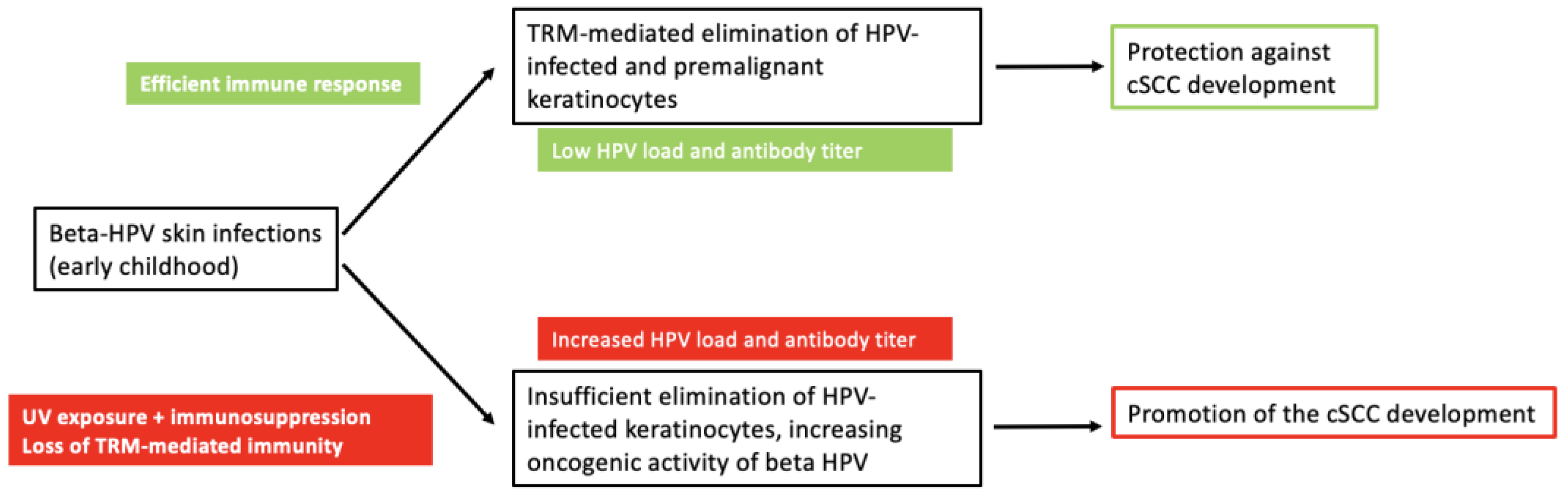
7. Protection and Benefits of Beta HPV
8. Beta HPV: The Oncogenic Co-Factor in Skin Tumourigenesis
9. Conclusions
Funding
Conflicts of Interest
References
- Schmitz, L.; Gambichler, T.; Gupta, G.; Stücker, M.; Dirschka, T. Actinic keratosis area and severity index (AKASI) is associated with the incidence of squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Eigentler, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Garbe, C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Investig. Dermatol. 2017, 137, 1860–1867. [Google Scholar] [CrossRef]
- Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef]
- Glogau, R.G. The risk of progression to invasive disease. J. Am. Acad. Dermatol. 2000, 42, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C. Epidemiology of actinic keratoses. Curr. Probl. Dermatol. 2015, 46, 1–7. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; van Akkooi, A.; Bataille, V.; Bastholt, L.; Dreno, B.; Dummer, R.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma. Part 1: Diagnostics and prevention-Update 2023. Eur. J. Cancer 2023, 193, 113251. [Google Scholar] [CrossRef] [PubMed]
- Traianou, A.; Ulrich, M.; Apalla, Z.; De Vries, E.; Bakirtzi, K.; Kalabalikis, D.; Ferrandiz, L.; Ruiz-de-Casas, A.; Moreno-Ramirez, D.; Sotiriadis, D.; et al. Risk factors for actinic keratosis in eight European centres: A case-control study. Br. J. Dermatol. 2012, 167 (Suppl. S2), 36–42. [Google Scholar] [CrossRef]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541. [Google Scholar] [CrossRef]
- Hariri, S.; Unger, E.R.; Sternberg, M.; Dunne, E.F.; Swan, D.; Patel, S.; Markowitz, L.E. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J. Infect. Dis. 2011, 204, 566–573. [Google Scholar] [CrossRef]
- Tiggelaar, S.M.; Lin, M.J.; Viscidi, R.P.; Ji, J.; Smith, J.S. Age-specific human papillomavirus antibody and deoxyribonucleic acid prevalence: A global review. J. Adolesc. Health 2012, 50, 110–131. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef]
- Gross, G.E.; Werner, R.N.; Avila Valle, G.L.; Bickel, M.; Brockmeyer, N.H.; Doubek, K.; Gallwas, J.; Gieseking, F.; Haase, H.; Hillemanns, P.; et al. Evidenz- und konsensbasierte (S3) Leitlinie: Impfprävention HPV-assoziierter Neoplasien. J. Dtsch. Dermatol. Ges. 2021, 19, 479–494. [Google Scholar] [CrossRef]
- Heppt, M.V.; Leiter, U.; Steeb, T.; Amaral, T.; Bauer, A.; Becker, J.C.; Breitbart, E.; Breuninger, H.; Diepgen, T.; Dirschka, T.; et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma—Short version, part 1: Diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J. Dtsch. Dermatol. Ges. 2020, 18, 275–294. [Google Scholar] [CrossRef]
- Werner, R.N.; Stockfleth, E.; Connolly, S.M.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.K.; Jacobs, A.; Kerl, H.; Lim, H.W.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Kessels, J.; Merks, I.; Nelemans, P.J.; Kelleners-Smeets, N.W.J.; Mosterd, K.; Essers, B.A.B. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br. J. Dermatol. 2020, 183, 738–744. [Google Scholar] [CrossRef]
- Lai, V.; Cranwell, W.; Sinclair, R. Epidemiology of skin cancer in the mature patient. Clin. Dermatol. 2018, 36, 167–176. [Google Scholar] [CrossRef]
- Stang, A.; Khil, L.; Kajüter, H.; Pandeya, N.; Schmults, C.D.; Ruiz, E.S.; Karia, P.S.; Green, A.C. Incidence and mortality for cutaneous squamous cell carcinoma: Comparison across three continents. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S8), 6–10. [Google Scholar] [CrossRef]
- Lounas, M.; Ylitalo, L.; Salmi, T.; Jernman, J.; Palve, J.; Luukkaala, T.; Korhonen, N. Recent Changes in the Incidence and Characteristics of Cutaneous Squamous Cell Carcinomas in Finland from 2006 to 2020: A Retrospective Cohort Study. Acta Derm. Venereol. 2024, 104, adv39891. [Google Scholar] [CrossRef]
- Keim, U.; Katalinic, A.; Holleczek, B.; Wakkee, M.; Garbe, C.; Leiter, U. Incidence, mortality and trends of cutaneous squamous cell carcinoma in Germany, the Netherlands, and Scotland. Eur. J. Cancer 2023, 183, 60–68. [Google Scholar] [CrossRef]
- Ibrahim, N.; Ali, S.R.; Dobbs, T.D.; Gibson, J.A.G.; Hutchings, H.A.; Whitaker, I.S. The incidence of non-melanoma skin cancer in the UK and the Republic of Ireland: A systematic review. Eur. J. Dermatol. 2023, 33, 218–229. [Google Scholar] [CrossRef]
- Adalsteinsson, J.A.; Olafsdottir, E.; Ratner, D.; Waldman, R.; Feng, H.; Ungar, J.; Silverberg, J.I.; Kristjansson, A.K.; Jonasson, J.G.; Tryggvadottir, L. Invasive and in situ squamous cell carcinoma of the skin: A nationwide study in Iceland. Br. J. Dermatol. 2021, 185, 537–547. [Google Scholar] [CrossRef]
- Yang, D.D.; Borsky, K.; Jani, C.; Crowley, C.; Rodrigues, J.N.; Matin, R.N.; Marshall, D.C.; Salciccioli, J.D.; Shalhoub, J.; Goodall, R. Trends in keratinocyte skin cancer incidence, mortality and burden of disease in 33 countries between 1990 and 2017. Br. J. Dermatol. 2023, 188, 237–246. [Google Scholar] [CrossRef]
- Wehner, M.R.; Cidre Serrano, W.; Nosrati, A.; Schoen, P.M.; Chren, M.M.; Boscardin, J.; Linos, E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 78, 663–672.e3. [Google Scholar] [CrossRef]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; DeSalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Clifford, G.; Franceschi, S.; Diaz, M.; Muñoz, N.; Villa, L.L. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006, 24 (Suppl. S3), S26–S34. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef]
- Hasche, D.; Vinzón, S.E.; Rösl, F. Cutaneous Papillomaviruses and Non-melanoma Skin Cancer: Causal Agents or Innocent Bystanders? Front. Microbiol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Antonsson, A.; Forslund, O.; Ekberg, H.; Sterner, G.; Hansson, B.G. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 2000, 74, 11636–11641. [Google Scholar] [CrossRef]
- de Koning, M.N.C.; Weissenborn, S.J.; Abeni, D.; Bouwes Bavinck, J.N.; Euvrard, S.; Green, A.C.; Harwood, C.A.; Naldi, L.; Neale, R.; Nindl, I.; et al. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J. Gen. Virol. 2009, 90, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G. Epithelial stem cells: A folliculocentric view. J. Investig. Dermatol. 2006, 126, 1459–1468. [Google Scholar] [CrossRef]
- Antonsson, A.; Karanfilovska, S.; Lindqvist, P.G.; Hansson, B.G. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 2003, 41, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Ma, Y.; Madupu, R.; Karaoz, U.; Nossa, C.W.; Yang, L.; Yooseph, S.; Yachimski, P.S.; Brodie, E.L.; Nelson, K.E.; Pei, Z. Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J. Virol. 2014, 88, 4786–4797. [Google Scholar] [CrossRef]
- de Jong, S.J.; Créquer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef]
- Béziat, V. Human genetic dissection of papillomavirus-driven diseases: New insight into their pathogenesis. Hum. Genet. 2020, 139, 919–939. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.J.; Imahorn, E.; Itin, P.; Uitto, J.; Orth, G.; Jouanguy, E.; Casanova, J.L.; Burger, B. Epidermodysplasia Verruciformis: Inborn Errors of Immunity to Human Beta-Papillomaviruses. Front. Microbiol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Zaravinos, A.; Kanellou, P.; Spandidos, D.A. Viral DNA detection and RAS mutations in actinic keratosis and nonmelanoma skin cancers. Br. J. Dermatol. 2010, 162, 325–331. [Google Scholar] [CrossRef]
- Neagu, N.; Dianzani, C.; Venuti, A.; Bonin, S.; Voidăzan, S.; Zalaudek, I.; Conforti, C. The role of HPV in keratinocyte skin cancer development: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 40–46. [Google Scholar] [CrossRef]
- Forslund, O.; Iftner, T.; Andersson, K.; Lindelof, B.; Hradil, E.; Nordin, P.; Stenquist, B.; Kirnbauer, R.; Dillner, J.; de Villiers, E.M. Cutaneous human papillomaviruses found in sun-exposed skin: Beta-papillomavirus species 2 predominates in squamous cell carcinoma. J. Infect. Dis. 2007, 196, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Arron, S.T.; Ruby, J.G.; Dybbro, E.; Ganem, D.; Derisi, J.L. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2011, 131, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Accardi, R.; Gheit, T. Cutaneous HPV and skin cancer. Presse Med. 2014, 43, e435–e443. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, S.J.; Nindl, I.; Purdie, K.; Harwood, C.; Proby, C.; Breuer, J.; Majewski, S.; Pfister, H.; Wieland, U. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J. Investig. Dermatol. 2005, 125, 93–97. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30 (Suppl. S5), F55–F70. [Google Scholar] [CrossRef]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; zur Hausen, H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef]
- Collins, S.I.; Constandinou-Williams, C.; Wen, K.; Young, L.S.; Roberts, S.; Murray, P.G.; Woodman, C.B. Disruption of the E2 gene is a common and early event in the natural history of cervical human papillomavirus infection: A longitudinal cohort study. Cancer Res. 2009, 69, 3828–3832. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Skelin, J.; Tomaić, V. Comparative Analysis of Alpha and Beta HPV E6 Oncoproteins: Insights into Functional Distinctions and Divergent Mechanisms of Pathogenesis. Viruses 2023, 15, 2253. [Google Scholar] [CrossRef] [PubMed]
- Iftner, T.; Elbel, M.; Schopp, B.; Hiller, T.; Loizou, J.I.; Caldecott, K.W.; Stubenrauch, F. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. EMBO J. 2002, 21, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Wallace, N.A.; Robinson, K.; Howie, H.L.; Galloway, D.A. β-HPV 5 and 8 E6 disrupt homology dependent double strand break repair by attenuating BRCA1 and BRCA2 expression and foci formation. PLoS Pathog. 2015, 11, e1004687. [Google Scholar] [CrossRef]
- Muschik, D.; Braspenning-Wesch, I.; Stockfleth, E.; Rösl, F.; Hofmann, T.G.; Nindl, I. Cutaneous HPV23 E6 prevents p53 phosphorylation through interaction with HIPK2. PLoS ONE 2011, 6, e27655. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Kramer, R.E.; Tan, M.J.; Hayes, S.D.; Harper, J.W.; Howley, P.M. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J. Virol. 2012, 86, 13174–13186. [Google Scholar] [CrossRef]
- McElhinny, A.S.; Li, J.L.; Wu, L. Mastermind-like transcriptional co-activators: Emerging roles in regulating cross talk among multiple signaling pathways. Oncogene 2008, 27, 5138–5147. [Google Scholar] [CrossRef]
- Wallace, N.A.; Robinson, K.; Galloway, D.A. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J. Virol. 2014, 88, 6112–6127. [Google Scholar] [CrossRef]
- Makielski, K.R.; Lee, D.; Lorenz, L.D.; Nawandar, D.M.; Chiu, Y.F.; Kenney, S.C.; Lambert, P.F. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology 2016, 495, 52–62. [Google Scholar] [CrossRef]
- Ciccarese, G.; Drago, F.; Broccolo, F.; Pastorino, A.; Pizzatti, L.; Atzori, L.; Pilloni, L.; Santinelli, D.; Urbani, A.; Parodi, A.; et al. Oncoviruses and melanomas: A retrospective study and literature review. J. Med. Virol. 2023, 95, e27924. [Google Scholar] [CrossRef]
- Feng, M.; Duan, R.; Gao, Y.; Zhang, H.; Qiao, Y.; Li, Q.; Zhao, F. Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Intraepithelial Neoplasia in Chinese Women Living With HIV. Front. Cell Infect. Microbiol. 2021, 11, 703259. [Google Scholar] [CrossRef]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Iwasaki, A. Tissue instruction for migration and retention of TRM cells. Trends Immunol. 2015, 36, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.M.; Bonneau, R.H.; Kinchington, P.R.; Hendricks, R.L. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 2003, 18, 593–603. [Google Scholar] [CrossRef]
- Djenidi, F.; Adam, J.; Goubar, A.; Durgeau, A.; Meurice, G.; de Montpréville, V.; Validire, P.; Besse, B.; Mami-Chouaib, F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015, 194, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
- Corgnac, S.; Malenica, I.; Mezquita, L.; Auclin, E.; Voilin, E.; Kacher, J.; Halse, H.; Grynszpan, L.; Signolle, N.; Dayris, T.; et al. CD103(+)CD8(+) T(RM) Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep. Med. 2020, 1, 100127. [Google Scholar] [CrossRef]
- Ganesan, A.P.; Clarke, J.; Wood, O.; Garrido-Martin, E.M.; Chee, S.J.; Mellows, T.; Samaniego-Castruita, D.; Singh, D.; Seumois, G.; Alzetani, A.; et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017, 18, 940–950. [Google Scholar] [CrossRef]
- Jouanneau, E.; Black, K.L.; Veiga, L.; Cordner, R.; Goverdhana, S.; Zhai, Y.; Zhang, X.X.; Panwar, A.; Mardiros, A.; Wang, H.; et al. Intrinsically de-sialylated CD103(+) CD8 T cells mediate beneficial anti-glioma immune responses. Cancer Immunol. Immunother. 2014, 63, 911–924. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Nelson, B.H. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 926–935. [Google Scholar] [CrossRef]
- Clarke, J.; Panwar, B.; Madrigal, A.; Singh, D.; Gujar, R.; Wood, O.; Chee, S.J.; Eschweiler, S.; King, E.V.; Awad, A.S.; et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J. Exp. Med. 2019, 216, 2128–2149. [Google Scholar] [CrossRef]
- Barrett, J.W.; Brownwright, A.J.; Primavera, M.J.; Palli, S.R. Studies of the nucleopolyhedrovirus infection process in insects by using the green fluorescence protein as a reporter. J. Virol. 1998, 72, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Son, H.G.; Ha, D.T.; Xia, Y.; Li, T.; Blandin, J.; Oka, T.; Azin, M.; Conrad, D.N.; Zhou, C.; Zeng, Y.; et al. Commensal papillomavirus immunity preserves the homeostasis of highly mutated normal skin. Cancer Cell 2025, 43, 36–48.e10. [Google Scholar] [CrossRef]
- Patel, J.J.; Levy, D.A.; Nguyen, S.A.; Knochelmann, H.M.; Day, T.A. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma-Systematic review and meta-analysis. Head Neck 2020, 42, 774–786. [Google Scholar] [CrossRef]
- Wang, B.C.; Cao, R.B.; Li, P.D.; Fu, C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: A systematic review and meta-analysis. Cancer Med. 2019, 8, 5969–5978. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.J.; Wang, H.; Liu, H.H.; Dong, Z.Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 13404. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.R.; Schran, S.; Persa, O.D.; Aguilar, C.; Thelen, M.; Lehmann, J.; Garcia-Marquez, M.A.; Wennhold, K.; Preugszat, E.; Zentis, P.; et al. Post-transplant Malignancies Show Reduced T-cell Abundance and Tertiary Lymphoid Structures as Correlates of Impaired Cancer Immunosurveillance. Clin. Cancer Res. 2022, 28, 1712–1723. [Google Scholar] [CrossRef]
- Hochheiser, K.; Gyorki, D.E.; Gebhardt, T. Skin colonization with beta papilloma virus drives tissue immunity and resistance to squamous cell cancer. Immunol. Cell Biol. 2020, 98, 9–11. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Nishisgori, C. Current concept of photocarcinogenesis. Photochem. Photobiol. Sci. 2015, 14, 1713–1721. [Google Scholar] [CrossRef]
- Valejo Coelho, M.M.; Matos, T.R.; Apetato, M. The dark side of the light: Mechanisms of photocarcinogenesis. Clin. Dermatol. 2016, 34, 563–570. [Google Scholar] [CrossRef]
- Bouwes Bavinck, J.N.; Feltkamp, M.C.W.; Green, A.C.; Fiocco, M.; Euvrard, S.; Harwood, C.A.; Nasir, S.; Thomson, J.; Proby, C.M.; Naldi, L.; et al. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am. J. Transplant. 2018, 18, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Weissenborn, S.; Abeni, D.; Bavinck, J.N.; Euvrard, S.; Feltkamp, M.C.; Green, A.C.; Harwood, C.; de Koning, M.; Naldi, L.; et al. Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2013, 22, 719–727. [Google Scholar] [CrossRef]
- Iannacone, M.R.; Gheit, T.; Waterboer, T.; Giuliano, A.R.; Messina, J.L.; Fenske, N.A.; Cherpelis, B.S.; Sondak, V.K.; Roetzheim, R.G.; Michael, K.M.; et al. Case-control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1303–1313. [Google Scholar] [CrossRef]
- Proby, C.M.; Harwood, C.A.; Neale, R.E.; Green, A.C.; Euvrard, S.; Naldi, L.; Tessari, G.; Feltkamp, M.C.; de Koning, M.N.; Quint, W.G.; et al. A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am. J. Transplant. 2011, 11, 1498–1508. [Google Scholar] [CrossRef]
- Genders, R.E.; Mazlom, H.; Michel, A.; Plasmeijer, E.I.; Quint, K.D.; Pawlita, M.; van der Meijden, E.; Waterboer, T.; de Fijter, H.; Claas, F.H.; et al. The presence of betapapillomavirus antibodies around transplantation predicts the development of keratinocyte carcinoma in organ transplant recipients: A cohort study. J. Investig. Dermatol. 2015, 135, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Hasche, D.; Stephan, S.; Braspenning-Wesch, I.; Mikulec, J.; Niebler, M.; Gröne, H.J.; Flechtenmacher, C.; Akgül, B.; Rösl, F.; Vinzón, S.E. The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLoS Pathog. 2017, 13, e1006723. [Google Scholar] [CrossRef]
- Schaper, I.D.; Marcuzzi, G.P.; Weissenborn, S.J.; Kasper, H.U.; Dries, V.; Smyth, N.; Fuchs, P.; Pfister, H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005, 65, 1394–1400. [Google Scholar] [CrossRef]
- Hufbauer, M.; Lazić, D.; Akgül, B.; Brandsma, J.L.; Pfister, H.; Weissenborn, S.J. Enhanced human papillomavirus type 8 oncogene expression levels are crucial for skin tumorigenesis in transgenic mice. Virology 2010, 403, 128–136. [Google Scholar] [CrossRef]
- Morgan, H.J.; Olivero, C.; Shorning, B.Y.; Gibbs, A.; Phillips, A.L.; Ananthan, L.; Lim, A.X.H.; Martuscelli, L.; Borgogna, C.; De Andrea, M.; et al. HPV8-induced STAT3 activation led keratinocyte stem cell expansion in human actinic keratoses. JCI Insight 2024, 9, e177898. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervenis, V. The Role of HPV in the Development of Cutaneous Squamous Cell Carcinoma—Friend or Foe? Cancers 2025, 17, 1195. https://doi.org/10.3390/cancers17071195
Dervenis V. The Role of HPV in the Development of Cutaneous Squamous Cell Carcinoma—Friend or Foe? Cancers. 2025; 17(7):1195. https://doi.org/10.3390/cancers17071195
Chicago/Turabian StyleDervenis, Vasileios. 2025. "The Role of HPV in the Development of Cutaneous Squamous Cell Carcinoma—Friend or Foe?" Cancers 17, no. 7: 1195. https://doi.org/10.3390/cancers17071195
APA StyleDervenis, V. (2025). The Role of HPV in the Development of Cutaneous Squamous Cell Carcinoma—Friend or Foe? Cancers, 17(7), 1195. https://doi.org/10.3390/cancers17071195





