HPV-16-Induced Squamous Cell Carcinoma in Hidradenitis Suppurativa: HPV Vaccination May Be Useful
Simple Summary
Abstract
1. Introduction
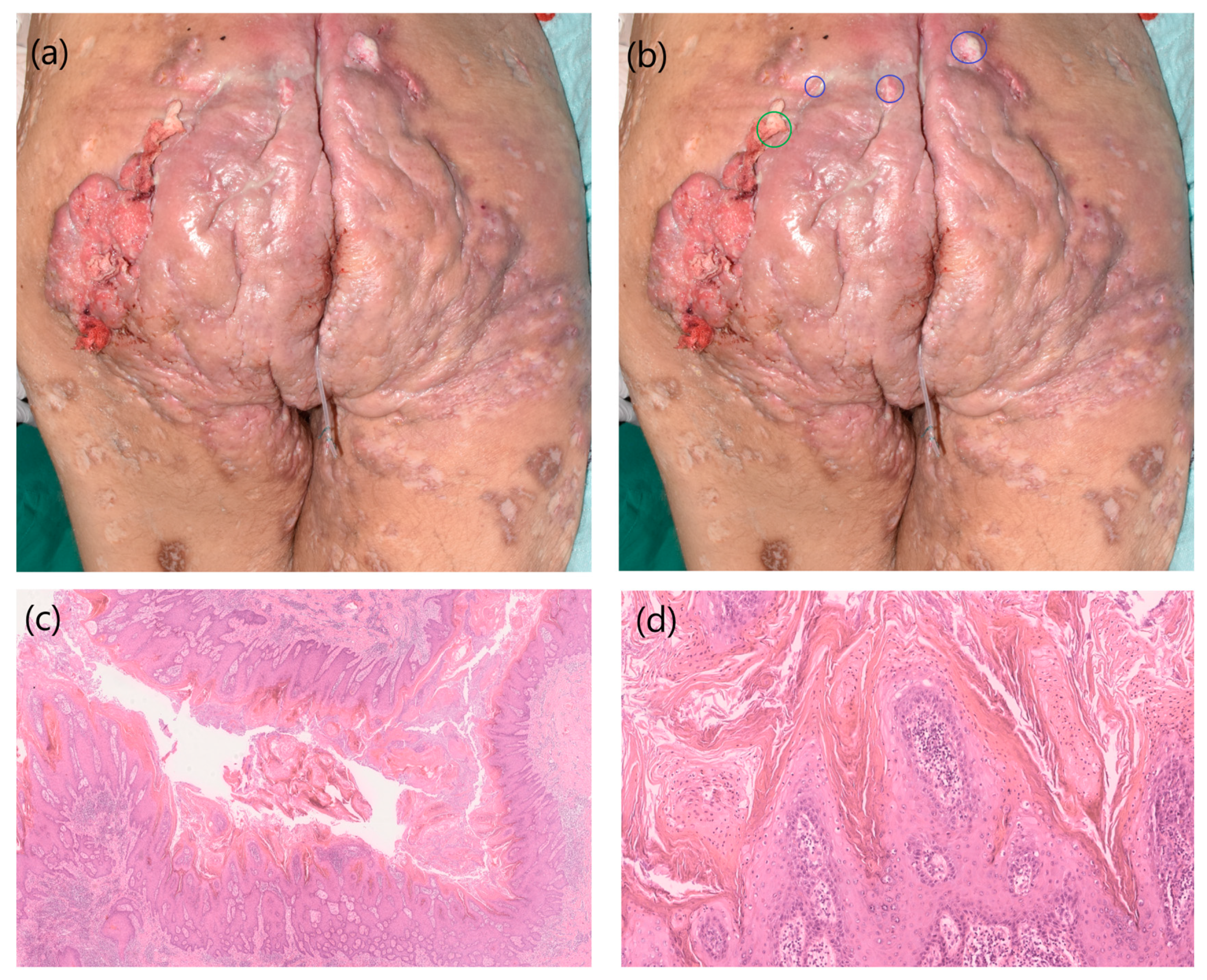
2. Case 1
3. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jourabchi, N.; Fischer, A.H.; Cimino-Mathews, A.; Waters, K.M.; Okoye, G.A. Squamous cell carcinoma complicating a chronic lesion of hidradenitis suppurativa: A case report and review of the literature. Int. Wound J. 2017, 14, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lai, Z.; He, M.; Zhai, B.; Zhou, L.; Long, X. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: A case report and literature review. Medicine 2017, 96, e5857. [Google Scholar] [CrossRef]
- Losanoff, J.E.; Sochaki, P.; Khoury, N.; Levi, E.; Salwen, W.A.; Basson, M.D. Squamous cell carcinoma complicating chronic suppurative hydradenitis. Am. Surg. 2011, 77, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Proby, C.M.; Harwood, C.A.; Neale, R.E.; Green, A.C.; Euvrard, S.; Naldi, L.; Tessari, G.; Feltkamp, M.C.W.; de Koning, M.N.C.; Quint, W.G.V.; et al. A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am. J. Transpl. 2011, 11, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Nindl, I.; Rösl, F. Molecular concepts of virus infections causing skin cancer in organ transplant recipients. Am. J. Transpl. 2008, 8, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Hasche, D.; Vinzón, S.E.; Rösl, F. Cutaneous Papillomaviruses and Non-melanoma Skin Cancer: Causal Agents or Innocent Bystanders? Front. Microbiol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef]
- Skelin, J.; Tomaić, V. Comparative Analysis of Alpha and Beta HPV E6 Oncoproteins: Insights into Functional Distinctions and Divergent Mechanisms of Pathogenesis. Viruses 2023, 15, 2253. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Cozma, E.-C.; Banciu, L.M.; Celarel, A.M.; Soare, E.; Srichawla, B.S.; Kipkorir, V.; Găman, M.-A. Molecular mechanisms of human papilloma virus related skin cancers: A review. Medicine 2024, 103, e38202. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, S.E.; Giuliano, A.R.; Palefsky, J.M.; Lazcano-Ponce, E.; Penny, M.E.; Cabello, R.E.; Moreira, E.D.; Baraldi, E.; Jessen, H.; Ferenczy, A.; et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: Results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2022, 22, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Lowy, D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012, 10, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, A.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Solomon, D.; Bratti, M.C.; Schiller, J.T.; Gonzalez, P.; Dubin, G.; Porras, C.; et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: A randomized trial. JAMA 2007, 298, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Gay, J.; Johnson, N.; Kavuru, V.; Phillips, M. Utility of the Human Papillomavirus Vaccination in Management of HPV-associated Cutaneous Lesions. Skin Ther. Lett. 2021, 26, 6–8. [Google Scholar]
- Șandru, F.; Radu, A.-M.; Petca, A.; Dumitrașcu, M.C.; Petca, R.-C.; Roman, A.-M. Unveiling the Therapeutic Horizon: HPV Vaccines and Their Impact on Cutaneous Diseases-A Comprehensive Review. Vaccines 2024, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, G.; Herzum, A.; Serviddio, G.; Occella, C.; Parodi, A.; Drago, F. Efficacy of Human Papillomavirus Vaccines for Recalcitrant Anogenital and Oral Warts. J. Clin. Med. 2023, 12, 7317. [Google Scholar] [CrossRef]
- Nofal, E.; Emam, S.; Aldesoky, F.; Ghonemy, S.; Adelshafy, A. Intralesional bivalent human papilloma virus vaccine as a treatment for anogenital warts versus topical podophyllin resin 25%: A pilot study. Dermatol. Ther. 2022, 35, e15384. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Nofal, E.; Abdelkhalek, N.; Ehab, R. Intralesional bivalent and quadrivalent human papillomavirus vaccines didn't significantly enhance the response of multiple anogenital warts when co-administered with intralesional Candida antigen immunotherapy. A randomized controlled trial. Arch. Dermatol. Res. 2023, 315, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.K.; Kim, D.H.; Yoon, M.S. Condyloma accuminatum treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, 18). J. Am. Acad. Dermatol. 2011, 64, e130–e132. [Google Scholar] [CrossRef] [PubMed]
- Bossart, S.; Gabutti, M.P.; Seyed Jafari, S.M.; Hunger, R.E. Nonavalent human papillomavirus vaccination as alternative treatment for genital warts. Dermatol. Ther. 2020, 33, e13771. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; zur Hausen, H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Miazga, W.; Tatara, T.; Gujski, M.; Ostrowski, J.; Pinkas, J.; Religioni, U. Analysis of Implementation Strategies for Nationwide HPV Vaccination Programs Across European Union Countries. Vaccines 2024, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and Immunisation. JCVI Statement on HPV Vaccination of Men Who Have Sex with Men; JCVI, 2015.
- Deshmukh, A.A.; Cantor, S.B.; Fenwick, E.; Chiao, E.Y.; Nyitray, A.G.; Stier, E.A.; Goldstone, S.E.; Wilkin, T.; Chhatwal, J. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: The time is now. Vaccine 2017, 35, 5102–5109. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, V.; Petráš, M.; Lomozová, D.; Dlouhý, P.; Králová Lesná, I.; Pilka, R. Reduced risk of CIN2+ recurrence in women immunized with a 9-valent HPV vaccine post-excision: Retrospective cohort study. Hum. Vaccin. Immunother. 2024, 20, 2343552. [Google Scholar] [CrossRef] [PubMed]
- Rykkelid, M.; Wennberg, H.M.; Richardsen, E.; Sørbye, S.W. Post-Conization HPV Vaccination and Its Impact on Viral Status: A Retrospective Cohort Study in Troms and Finnmark, 2022. Pathogens 2024, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Swedish, K.A.; Factor, S.H.; Goldstone, S.E. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: A nonconcurrent cohort study. Clin. Infect. Dis. 2012, 54, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Frazer, I.H. Interaction of human papillomaviruses with the host immune system: A well evolved relationship. Virology 2009, 384, 410–414. [Google Scholar] [CrossRef]
- Nakagawa, M.; Stites, D.P.; Patel, S.; Farhat, S.; Scott, M.; Hills, N.K.; Palefsky, J.M.; Moscicki, A.B. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J. Infect. Dis. 2000, 182, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Lavogiez, C.; Delaporte, E.; Darras-Vercambre, S.; Martin De Lassalle, E.; Castillo, C.; Mirabel, X.; Laurent, F.; Patenotre, P.; Gheit, T.; Talmant, J.C.; et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology 2010, 220, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, J.J.; Shah, K.K.; Hallemeier, C.L.; Baum, C.L.; Davis, M.D.P. Squamous Cell Carcinoma in Perineal, Perianal, and Gluteal Hidradenitis Suppurativa: Experience in 12 Patients. Dermatol. Surg. 2019, 45, 519–526. [Google Scholar] [CrossRef]
- Kim, S.; Arduino, J.M.; Roberts, C.C.; Marsico, M.; Liaw, K.-L.; Skjeldestad, F.E. Incidence and predictors of human papillomavirus-6,-11,-16, and-18 infection in young Norwegian women. Sex. Transm. Dis. 2011, 38, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.P.; Tortolero-Luna, G.; Romaguera, J.; Pérez, C.M.; González, D.; Muñoz, C.; González, L.; Marrero, E.; Suárez, E.; Palefsky, J.M.; et al. Seroprevalence of HPV 6, 11, 16 and 18 and correlates of exposure in unvaccinated women aged 16–64 years in Puerto Rico. Papillomavirus Res. 2018, 5, 109–113. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Rached, N.; Käpynen, R.; Doerler, M.; Ocker, L.; Frost, C.; Haven, Y.; Bechara, F.G. HPV-16-Induced Squamous Cell Carcinoma in Hidradenitis Suppurativa: HPV Vaccination May Be Useful. Cancers 2025, 17, 702. https://doi.org/10.3390/cancers17040702
Abu Rached N, Käpynen R, Doerler M, Ocker L, Frost C, Haven Y, Bechara FG. HPV-16-Induced Squamous Cell Carcinoma in Hidradenitis Suppurativa: HPV Vaccination May Be Useful. Cancers. 2025; 17(4):702. https://doi.org/10.3390/cancers17040702
Chicago/Turabian StyleAbu Rached, Nessr, Riina Käpynen, Martin Doerler, Lennart Ocker, Carolin Frost, Yannik Haven, and Falk G. Bechara. 2025. "HPV-16-Induced Squamous Cell Carcinoma in Hidradenitis Suppurativa: HPV Vaccination May Be Useful" Cancers 17, no. 4: 702. https://doi.org/10.3390/cancers17040702
APA StyleAbu Rached, N., Käpynen, R., Doerler, M., Ocker, L., Frost, C., Haven, Y., & Bechara, F. G. (2025). HPV-16-Induced Squamous Cell Carcinoma in Hidradenitis Suppurativa: HPV Vaccination May Be Useful. Cancers, 17(4), 702. https://doi.org/10.3390/cancers17040702







