Fluid-Suppressed Amide Proton Transfer-Weighted Imaging Outperforms Leakage-Corrected Dynamic Susceptibility Contrast Perfusion in Distinguishing Progression from Radionecrosis in Brain Metastases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. MRI Acquisition
2.3. MRI Data Post-Processing
- Compute leakage-corrected relative rCBV maps from DSC perfusion;
- Compute B0 and B1 maps from the WASAB1 sequence [15];
- Co-register fluid-suppressed APTw and leakage-corrected rCBV maps with structural sequences;
- Delineate regions of interest (ROIs) in the lesion and in the contralateral normal appearing white matter (cNAWM).
2.4. Statistical Analyses
- difference in the average fluid-suppressed APTw values calculated for the ROIs, defined as ΔAPTw = APTwlesion − APTwcNAWM;
- ratio of the average leakage-corrected rCBV values calculated for the ROIs, expressed as ΔrCBV = rCBVlesion/rCBVcNAWM.
3. Results
3.1. Characteristics of Subjects
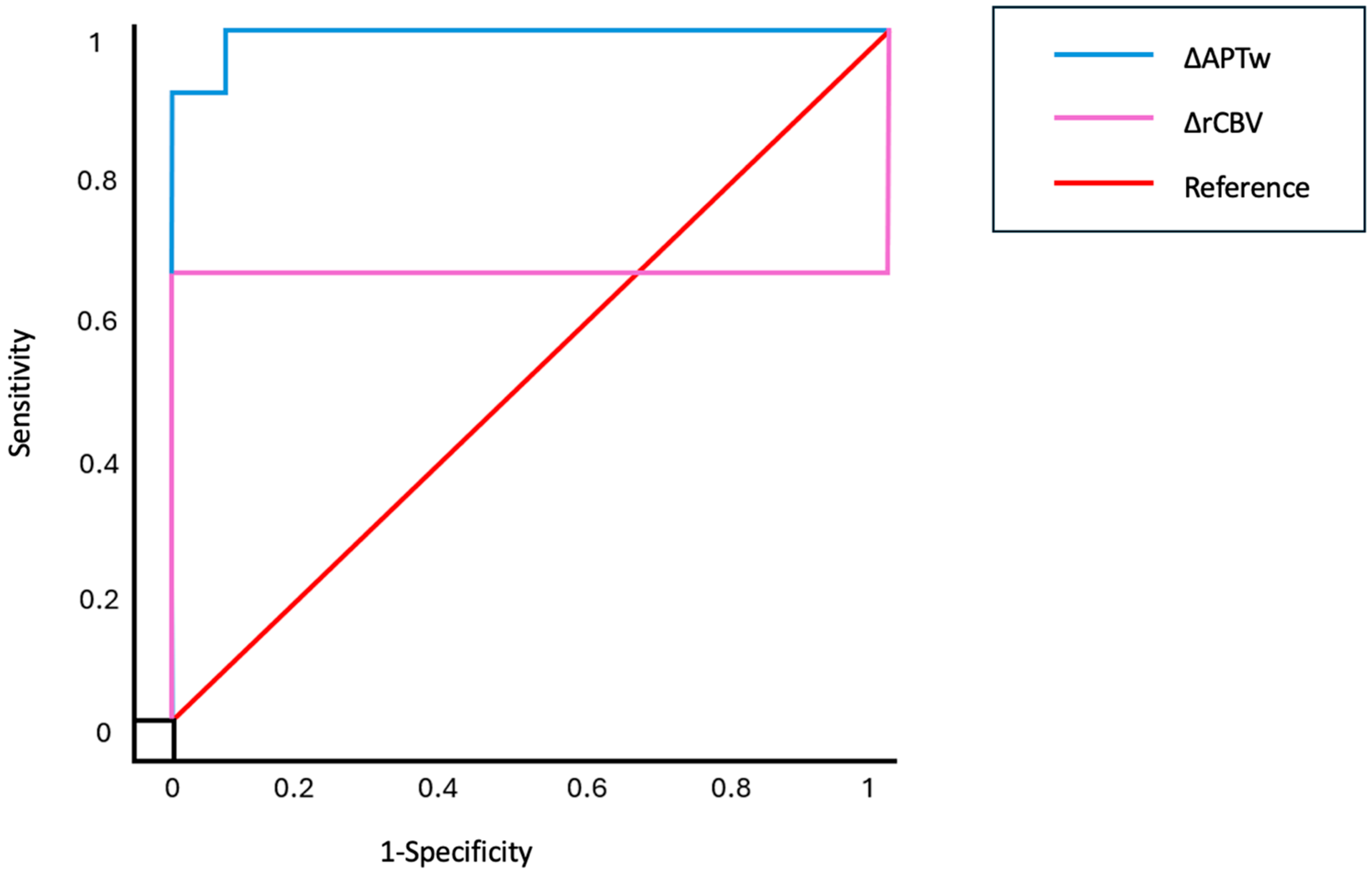
3.2. Ability of Fluid-Suppressed APTw and Leakage-Corrected rCBV to Distinguish Between RN and TP
3.3. Accuracy of Fluid-Suppressed APTw and Leakage-Corrected rCBV Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F.S.APT | Fluid-Suppressed Amide Proton Transfer |
| L.C.rCBV | Leakage-Corrected relative Cerebral Blood Volume |
| RN | Radionecrosis |
| TP | Tumor Progression |
References
- Nichelli, L.; Casagranda, S. Current emerging MRI tools for radionecrosis and pseudoprogression diagnosis. Curr. Opin. Oncol. 2021, 33, 597–607. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; La Rosa, A.; Brown, P.D.; Vogelbaum, M.A.; Navarria, P.; Bodensohn, R.; Niyazi, M.; Karschnia, P.; Minniti, G. Multidisciplinary management strategies for recurrent brain metastasis after prior radiotherapy: An overview. Neuro-Oncology 2025, 27, 597–615. [Google Scholar] [CrossRef]
- Bernhardt, D.; König, L.; Grosu, A.-L.; Rieken, S.; Krieg, S.M.; Wick, W.; Wiestler, B.; Schmidt-Graf, F.; Sahm, F.; Gempt, J.; et al. DEGRO practical guideline for central nervous system radiation necrosis part 2: Treatment. Strahlenther. Onkol. 2022, 198, 971–980. [Google Scholar] [CrossRef]
- Zhuang, H.; Shi, S.; Yuan, Z.; Chang, J.Y. Bevacizumab treatment for radiation brain necrosis: Mechanism, efficacy and issues. Mol. Cancer 2019, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Welker, K.; Boxerman, J.; Kalnin, A.; Kaufmann, T.; Shiroishi, M.; Wintermark, M.; American Society of Functional Neuroradiology MR Perfusion Standards and Practice Subcommittee of the ASFNR Clinical Practice Committee. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am. J. Neuroradiol. 2015, 36, E41–E51. [Google Scholar] [CrossRef]
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reson. Med. 2022, 88, 546–574. [Google Scholar] [CrossRef] [PubMed]
- Nichelli, L.; Zaiss, M.; Casagranda, S. APT weighted imaging in diffuse gliomas. BJR|Open 2023, 5, 20230025. [Google Scholar] [CrossRef]
- Essed, R.A.; Prysiazhniuk, Y.; Wamelink, I.J.; Azizova, A.; Keil, V.C. Performance of amide proton transfer imaging to differentiate true progression from therapy-related changes in gliomas and metastases. Eur. Radiol. 2024, 35, 580–591. [Google Scholar] [CrossRef]
- Constanzo, J.; Masson-Côté, L.; Tremblay, L.; Fouquet, J.P.; Sarret, P.; Geha, S.; Whittingstall, K.; Paquette, B.; Lepage, M. Understanding the continuum of radionecrosis and vascular disorders in the brain following gamma knife irradiation: An MRI study. Magn. Reson. Med. 2017, 78, 1420–1431. [Google Scholar] [CrossRef]
- Zaiss, M.; Xu, J.; Goerke, S.; Khan, I.S.; Singer, R.J.; Gore, J.C.; Gochberg, D.F.; Bachert, P. Inverse Z-spectrum analysis for spillover-, MT-, and T1-corrected steady-state pulsed CEST-MRI-application to pH-weighted MRI of acute stroke: SIMPLE SPILLOVER-, MT-, AND T1-CORRECTED CEST-MRI. NMR Biomed. 2014, 27, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Schüre, J.; Casagranda, S.; Sedykh, M.; Liebig, P.; Papageorgakis, C.; Mancini, L.; Bisdas, S.; Nichelli, L.; Pinter, N.; Mechtler, L.; et al. Fluid suppression in amide proton transfer-weighted (APTw) CEST imaging: New theoretical insights and clinical benefits. Magn. Reson. Med. 2024, 91, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Schuenke, P.; Windschuh, J.; Roeloffs, V.; Ladd, M.E.; Bachert, P.; Zaiss, M. Simultaneous mapping of water shift and B1 (WASABI)—Application to field-Inhomogeneity correction of CEST MRI data. Magn. Reson. Med. 2017, 77, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Papageorgakis, C.; Firippi, E.; Gy, B.; Boutelier, T.; Khormi, I.; Al-iedani, O.; Lechner-Scott, J.; Ramadan, S.; Liebig, P.; Schuenke, P.; et al. Fast WASABI post-processing: Access to rapid B0 and B1 correction in clinical routine for CEST MRI. Magn. Reson. Imaging 2023, 102, 203–211. [Google Scholar] [CrossRef]
- Casagranda, S.; Papageorgakis, C.; Romdhane, F.; Firippi, E.; Boutelier, T.; Mancini, L.; Zaiss, M.; Bisdas, S.; Longo, D. Principal Component Selections and Filtering by Spatial Information Criteria for Multi-Acquisition CEST MRI Denoising. In Proceedings of the ISMRM 31st Annual Meeting, London, UK, 7–12 May 2022; p. 2080. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef]
- Papageorgakis, C.; Zucchelli, M.; Dipasquale, O.; Mancini, L.; Bisdas, S.; Liebig, P.; Zaiss, M.; Casagranda, S. Decorrelation Algorithm for Correcting B1 Artifacts in APTw Imaging at 3 Tesla. In Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM), Toronto, ON, Canada, 4–9 May 2024; p. 4469. [Google Scholar] [CrossRef]
- Zhou, J.; Tryggestad, E.; Wen, Z.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.-X.; Ford, E.; et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef]
- Hong, X.; Liu, L.; Wang, M.; Ding, K.; Fan, Y.; Ma, B.; Lal, B.; Tyler, B.; Mangraviti, A.; Wang, S.; et al. Quantitative multiparametric MRI assessment of glioma response to radiotherapy in a rat model. Neuro-Oncology 2014, 16, 856–867. [Google Scholar] [CrossRef]
- Jiang, S.; Eberhart, C.G.; Lim, M.; Heo, H.-Y.; Zhang, Y.; Blair, L.; Wen, Z.; Holdhoff, M.; Lin, D.; Huang, P.; et al. Identifying Recurrent Malignant Glioma after Treatment Using Amide Proton Transfer-Weighted MR Imaging: A Validation Study with Image-Guided Stereotactic Biopsy. Clin. Cancer Res. 2019, 25, 552–561. [Google Scholar] [CrossRef]
- Hou, H.; Diao, Y.; Yu, J.; Xu, M.; Wang, L.; Li, Z.; Song, T.; Liu, Y.; Yuan, Z. Differentiation of true progression from treatment response in high-grade glioma treated with chemoradiation: A comparison study of 3D-APTW and 3D-PcASL imaging and DWI. NMR Biomed. 2023, 36, e4821. [Google Scholar] [CrossRef]
- Park, Y.W.; Ahn, S.S.; Kim, E.H.; Kang, S.-G.; Chang, J.H.; Kim, S.H.; Zhou, J.; Lee, S.-K. Differentiation of recurrent diffuse glioma from treatment-induced change using amide proton transfer imaging: Incremental value to diffusion and perfusion parameters. Neuroradiology 2021, 63, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Park, K.J.; Kim, S.J.; Kim, J.H.; Smith, S.A. Pre- and Posttreatment Glioma: Comparison of Amide Proton Transfer Imaging with MR Spectroscopy for Biomarkers of Tumor Proliferation. Radiology 2016, 278, 514–523. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, J.Y.; Kim, H.S.; Oh, J.-Y.; Jung, S.C.; Kim, S.J.; Keupp, J.; Oh, M.; Kim, J.S. Amide proton transfer imaging seems to provide higher diagnostic performance in post-treatment high-grade gliomas than methionine positron emission tomography. Eur. Radiol. 2018, 28, 3285–3295. [Google Scholar] [CrossRef] [PubMed]
- Mehrabian, H.; Desmond, K.L.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Differentiation between Radiation Necrosis and Tumor Progression Using Chemical Exchange Saturation Transfer. Clin. Cancer Res. 2017, 23, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Mehrabian, H.; Chan, R.W.; Sahgal, A.; Chen, H.; Theriault, A.; Lam, W.W.; Myrehaug, S.; Tseng, C.; Husain, Z.; Detsky, J.; et al. Chemical Exchange Saturation Transfer MRI for Differentiating Radiation Necrosis From Tumor Progression in Brain Metastasis—Application in a Clinical Setting. J. Magn. Reson. Imaging 2023, 57, 1713–1725. [Google Scholar] [CrossRef]
- Chan, R.W.; Lam, W.W.; Chen, H.; Murray, L.; Zhang, B.; Theriault, A.; Endre, R.; Moon, S.; Liebig, P.; Maralani, P.J.; et al. Is pulsed saturation transfer sufficient for differentiating radiation necrosis from tumor progression in brain metastases? Neuro-Oncol. Adv. 2024, 6, vdae132. [Google Scholar] [CrossRef]
- Schultheiss, T.E.; Kun, L.E.; Ang, K.K.; Stephens, L.C. Radiation response of the central nervous system. Int. J. Radiat. Oncol. 1995, 31, 1093–1112. [Google Scholar] [CrossRef]
- Valk, P.E.; Dillon, W.P. Radiation injury of the brain. AJNR Am. J. Neuroradiol. 1991, 12, 45–62. [Google Scholar]
- Furuse, M.; Nonoguchi, N.; Kawabata, S.; Miyatake, S.-I.; Kuroiwa, T. Delayed brain radiation necrosis: Pathological review and new molecular targets for treatment. Med. Mol. Morphol. 2015, 48, 183–190. [Google Scholar] [CrossRef]
- Hamon, G.; Lecler, A.; Ferré, J.-C.; Bourdillon, P.; Duron, L.; Savatovsky, J. 3-Tesla amide proton transfer-weighted imaging (APT-WI): Elevated signal also in tumor mimics. Eur. Radiol. 2024. [Google Scholar] [CrossRef]
- Lambert, E.A.; Holmes, S. Differentiating Radiation-Induced Necrosis from Tumor Progression After Stereotactic Radiosurgery for Brain Metastases, Using Evaluation of Blood Flow with Arterial Spin Labeling (ASL): The Importance of Setting a Baseline. In Gamma Knife Neurosurgery in the Management of Intracranial Disorders II; Chernov, M.F., Hayashi, M., Chen, C.C., McCutcheon, I.E., Eds.; Acta Neurochirurgica Supplement; Springer International Publishing: Cham, Switzerland, 2021; Volume 128, pp. 113–119. [Google Scholar] [CrossRef]

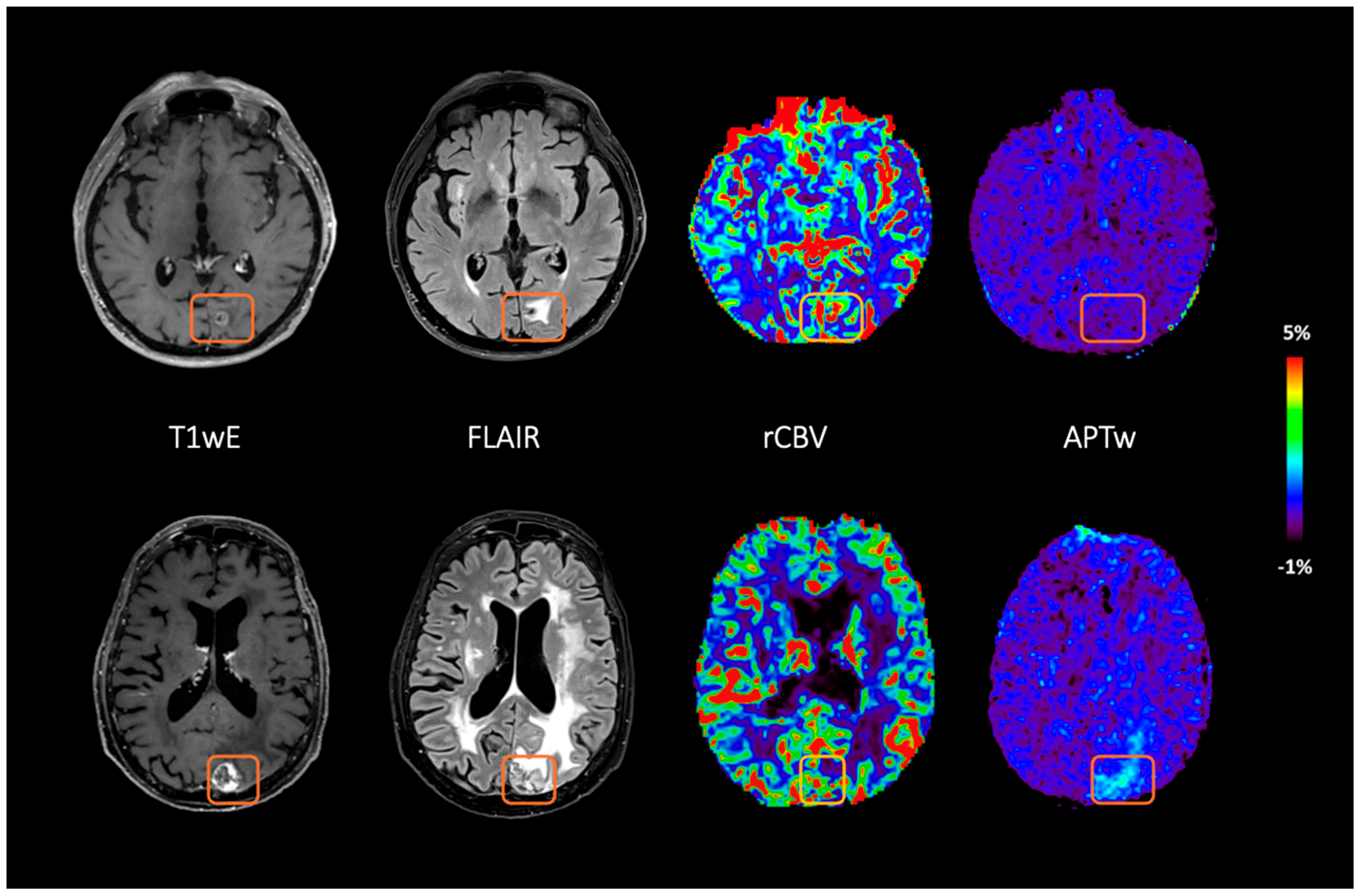
| Subject | Sex | Age (Years) | Primary Tumor | SRS (Median Dose/ Number of Fraction) | Previous Radiation | SRS to MRI (Years) | Average F.S.APT | Average L.C.rCBV | Subsequent Patient Care | Diagnosis | Evaluation Method | Follow-Up Time (Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S01 | M | 47 | Lung | 27 Gy/3 | No | 0.93 | 0.34 | 1.12 | Follow-up | RN | Follow-up | 2.30 |
| S02 | F | 44 | Germinal tumor | 27 Gy/1 | No | 3.15 | 0.53 | 0.74 | Re-irradiation | TP | Follow-up | 3.22 |
| S03 | F | 55 | Breast | 20 Gy/1 | No | 0.24 | 0.61 | 0.48 | Re-irradiation | TP | Histology | 2.85 |
| S04 | F | 44 | Breast | 20 Gy/1 | No | 3.29 | 0.47 | 2.93 | Re-irradiation | TP | Follow-up | 3.64 |
| S05 | M | 69 | Lung | N.A. | N.A. | 0.49 | 0.09 | 1.26 | Follow-up | RN | MDT | 1.00 |
| S06 | M | 63 | Melanoma | N.A. | SRS, 1 fraction | 0.51 | 0.85 | 2.23 | Re-irradiation | TP | Histology | 2.23 |
| S07 | F | 49 | Lung | 20 Gy/1 | No | 0.71 | 0.57 | 3.36 | Re-irradiation | TP | Histology | 3.73 |
| S08 | F | 64 | Lung | N.A. | SRS, 2 fractions | 2.84 | 0.93 | 2.19 | Follow-up | TP | Follow-up | 1.13 |
| S09 | F | 59 | Breast | 27 Gy/3 | No | 2.82 | 0.23 | 0.87 | Follow-up | RN | Follow-up | 3.49 |
| S10 | M | 54 | Lung | 20 Gy/1 | No | 1.81 | 0.35 | 1.30 | Follow-up | RN | Follow-up | 2.45 |
| S11 | F | 86 | Neuroendocrine | 20 Gy/1 | No | 0.47 | 0.26 | 1.54 | Follow-up | RN | MDT | 0.19 |
| S12 | F | 72 | Lung | 20 Gy/2 | No | 1.48 | 0.62 | 0.69 | Follow-up | TP | Histology | N.A. |
| S13 | M | 80 | Melanoma | 27 Gy/3 | No | 1.86 | 0.06 | 2.01 | Follow-up | RN | Follow-up | 1.65 |
| S14 | F | 68 | Lung | 20 Gy/1 | No | 1.29 | 0.76 | 2.12 | Immunotherapy | TP | Histology | 3.38 |
| S15 | F | 75 | Breast | 20 Gy/1 | No | 3.39 | 0.65 | 4.36 | Chemotherapy | TP | Follow-up | 1.83 |
| S16 | F | 51 | Melanoma | 24 Gy/1 | No | 0.47 | 0.33 | 1.10 | Follow-up | RN | Follow-up | 0.19 |
| S17 | M | 69 | Melanoma | N.A. | N.A. | 0.23 | 0.69 | 2.08 | N.A. | RN | MDT | 0.18 |
| S18 | F | 60 | Lung | 20 Gy/1 | WBRT, SRS, 10 fractions | 7.15 | 0.68 | 0.82 | Re-irradiation | TP | Histology | 1.43 |
| S19 | F | 77 | Breast | 33 Gy/3 | No | 2.09 | 0.31 | 0.90 | Anti-angiogenic | RN | Follow-up | 1.17 |
| S20_L1 | F | 54 | Breast | 20 Gy/1 | No | 2.10 | 0.69 | 2.96 | Re-irradiation | TP | MDT | 1.11 |
| S20_L2 | F | 55 | Breast | 27 Gy/1 | No | 0.37 | 0.05 | 0.99 | Follow-up | RN | Follow-up | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichelli, L.; Casagranda, S.; Dipasquale, O.; Bensemain, M.; Papageorgakis, C.; Zucchelli, M.; Jacob, J.; Valery, C.; Mathon, B.; Liebig, P.; et al. Fluid-Suppressed Amide Proton Transfer-Weighted Imaging Outperforms Leakage-Corrected Dynamic Susceptibility Contrast Perfusion in Distinguishing Progression from Radionecrosis in Brain Metastases. Cancers 2025, 17, 1175. https://doi.org/10.3390/cancers17071175
Nichelli L, Casagranda S, Dipasquale O, Bensemain M, Papageorgakis C, Zucchelli M, Jacob J, Valery C, Mathon B, Liebig P, et al. Fluid-Suppressed Amide Proton Transfer-Weighted Imaging Outperforms Leakage-Corrected Dynamic Susceptibility Contrast Perfusion in Distinguishing Progression from Radionecrosis in Brain Metastases. Cancers. 2025; 17(7):1175. https://doi.org/10.3390/cancers17071175
Chicago/Turabian StyleNichelli, Lucia, Stefano Casagranda, Ottavia Dipasquale, Mehdi Bensemain, Christos Papageorgakis, Mauro Zucchelli, Julian Jacob, Charles Valery, Bertrand Mathon, Patrick Liebig, and et al. 2025. "Fluid-Suppressed Amide Proton Transfer-Weighted Imaging Outperforms Leakage-Corrected Dynamic Susceptibility Contrast Perfusion in Distinguishing Progression from Radionecrosis in Brain Metastases" Cancers 17, no. 7: 1175. https://doi.org/10.3390/cancers17071175
APA StyleNichelli, L., Casagranda, S., Dipasquale, O., Bensemain, M., Papageorgakis, C., Zucchelli, M., Jacob, J., Valery, C., Mathon, B., Liebig, P., Zaiss, M., & Lehéricy, S. (2025). Fluid-Suppressed Amide Proton Transfer-Weighted Imaging Outperforms Leakage-Corrected Dynamic Susceptibility Contrast Perfusion in Distinguishing Progression from Radionecrosis in Brain Metastases. Cancers, 17(7), 1175. https://doi.org/10.3390/cancers17071175





