Exploring the Relationship Between Perioperative Inflammatory Biomarkers and Oncological Recurrence in Patients Undergoing Pulmonary Cancer Surgery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patients Characteristics
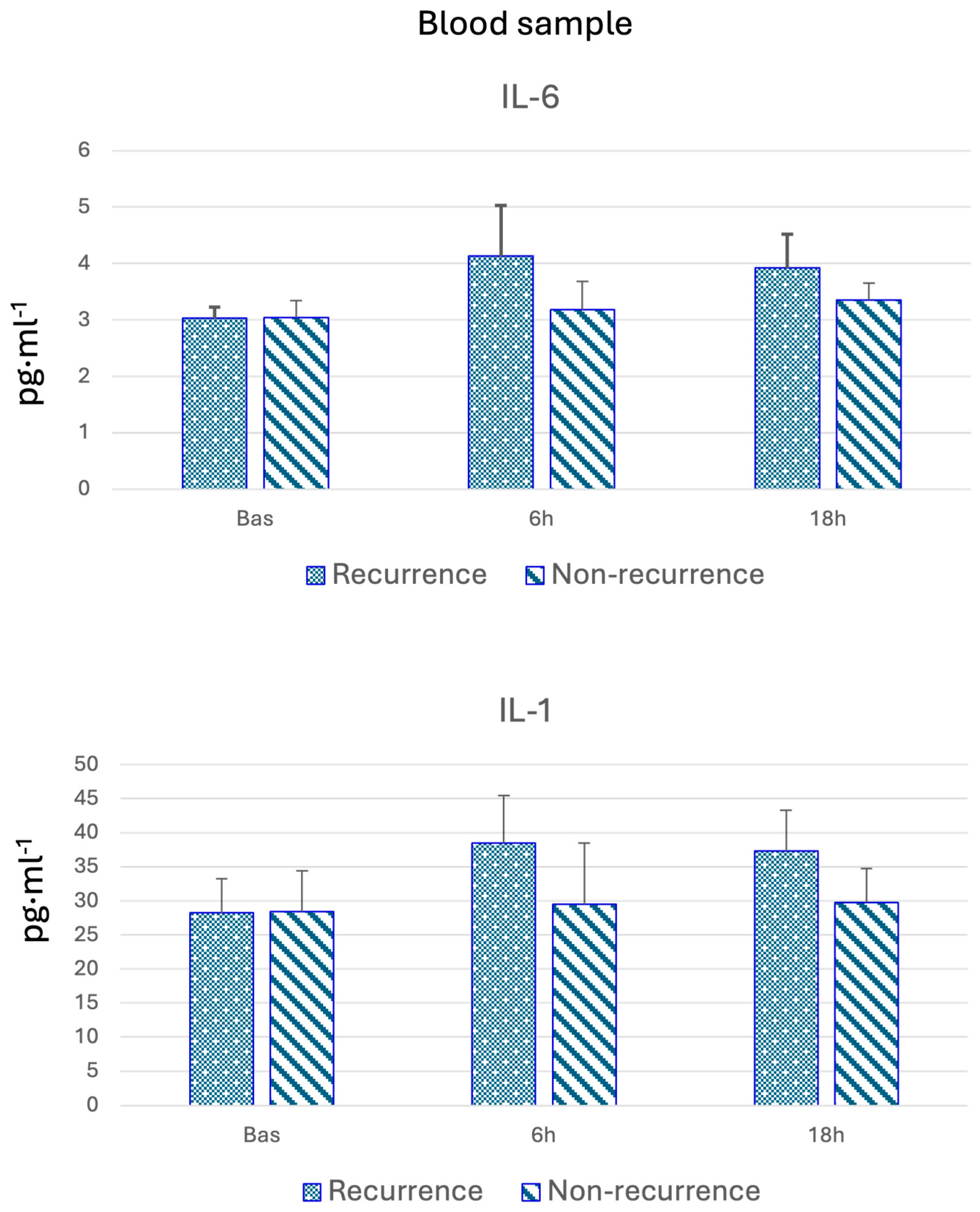
3.2. Blood Biomarkers
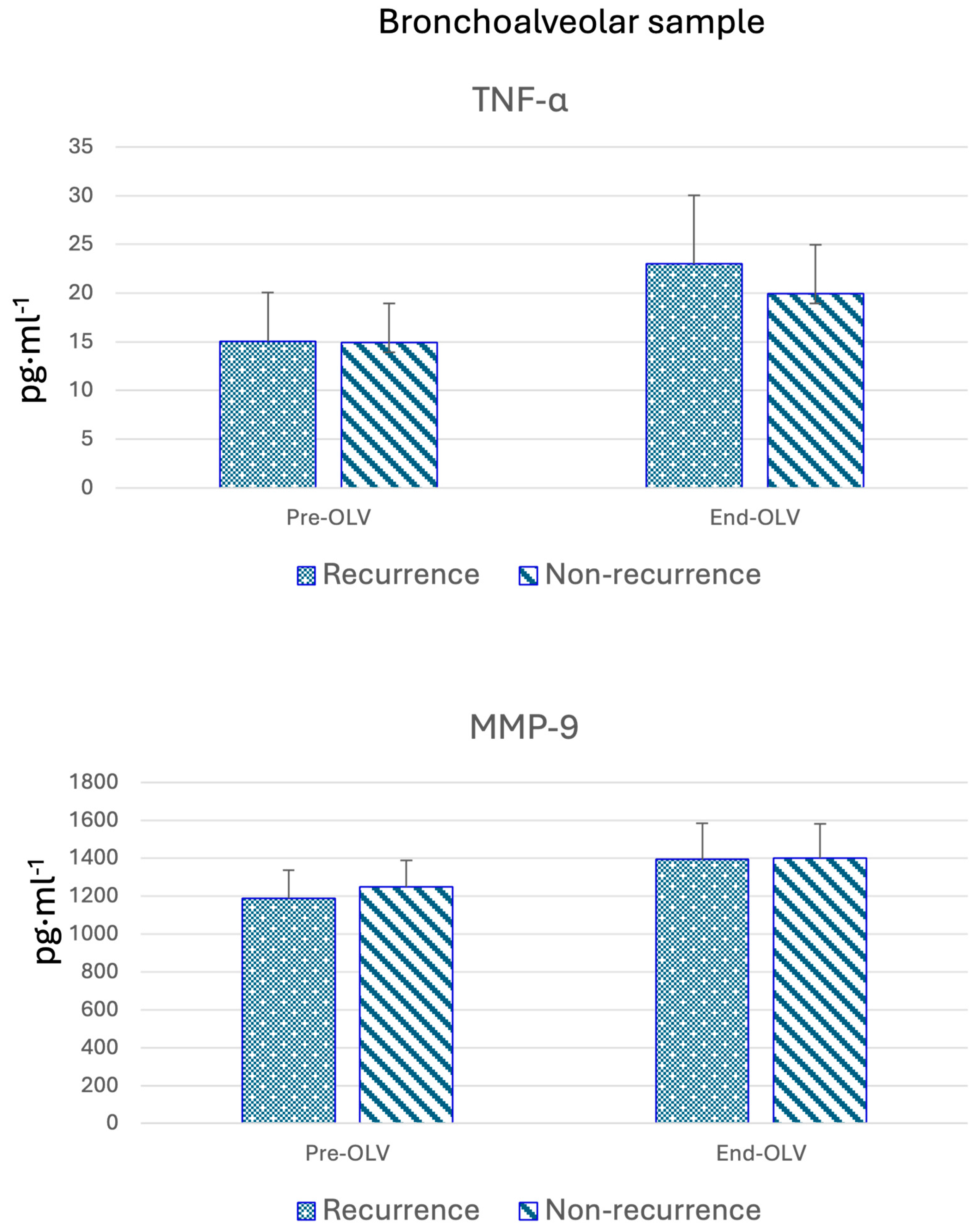
3.3. Bronchoalveolar Lavage Biomarkers
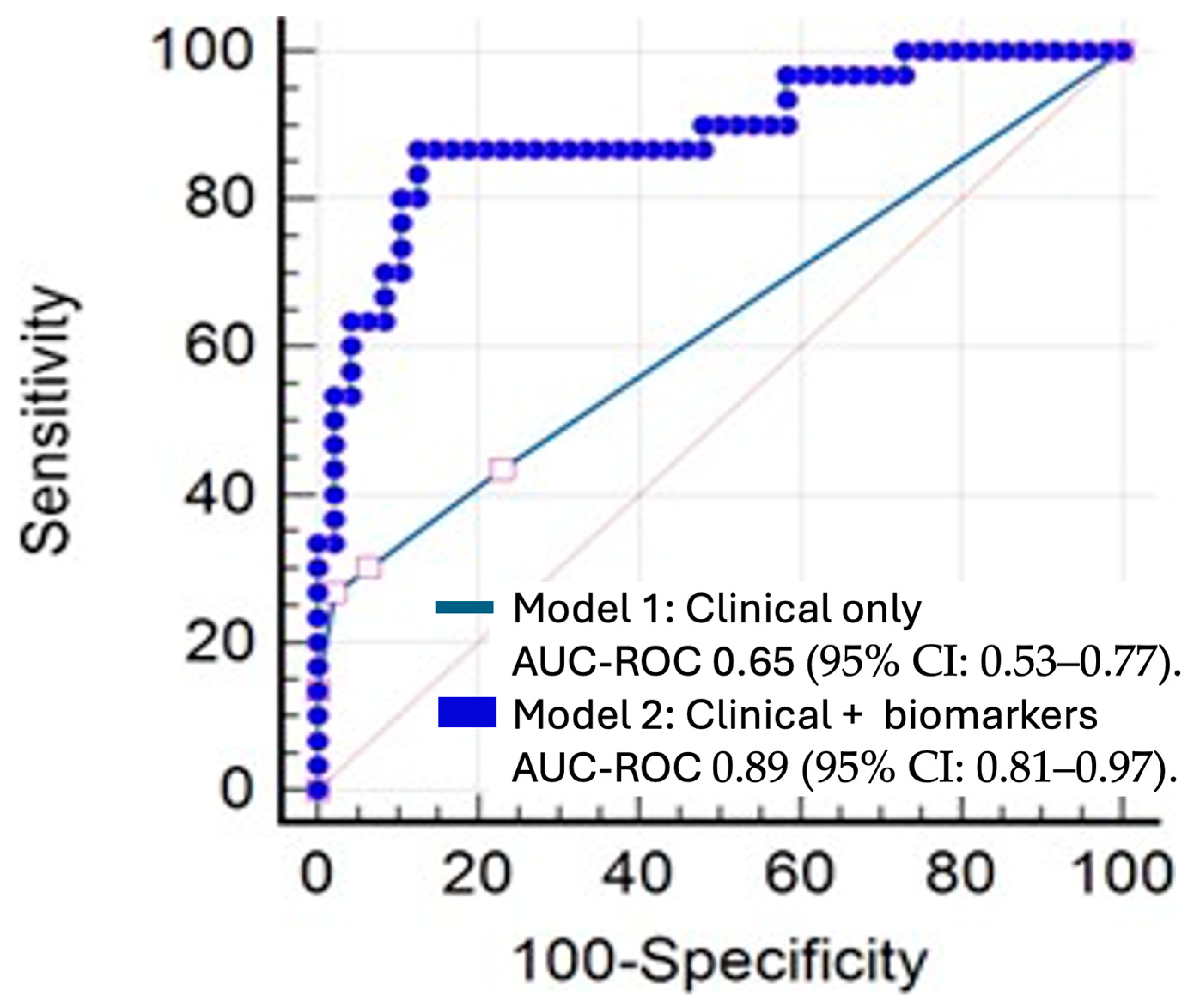
3.4. Predictive Factors for Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL | Interleukine |
| NSCLC | non-small-cell lung cancer |
| FEV1 | forced expired volume in the first second |
| FVC | forced vital capacity |
| BIS | bispectral index |
| PEEP | positive end-expiratory pressure |
| FiO2 | fraction-inspired oxygen |
| EtCO2 | end-tidal carbon dioxide |
| BAL | bronchoalveolar lavage |
| MMP | metalloproteinases |
| NO | nitric oxide |
| CO | carbon monoxide |
| IQR | interquartile range |
| ROC | Receiver Operating Characteristic |
| VATS | video-assisted thoracoscopy |
| ASA | American Society of Anesthesia |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France, 2024. Available online: https://gco.iarc.who.int/today (accessed on 13 March 2024).
- Hoy, H.; Lynch, T.; Beck, M. Surgical treatment of lung cancer. Crit. Care Nurs. Clin. 2019, 31, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2015, 1856, 189–210. [Google Scholar]
- Choi, H.; Hwang, W. Perioperative inflammatory response and cancer recurrence in lung cancer surgery: A narrative review. Front. Surg. 2022, 9, 888630. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Nagji, A.S.; Bhamidipati, C.M.; Theodosakis, N.; Kozower, B.D.; Lau, C.L.; Jones, D.R. Tumor Recurrence After Complete Resection for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2012, 93, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, W.; Wang, Q.; Ni, Y.; Niu, Y.; Jiang, L. Assessment of systemic immune- inflammation index in predicting postoperative pulmonary complications in patients undergoing lung cancer resection. Surgery 2022, 172, 365–370. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028662. [Google Scholar] [CrossRef]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araujo, A.; Medeiros, R. The role of inflammation in lung cancer. In Inflammation and Cancer; Springer: Basel, Switzerland, 2014; pp. 1–23. [Google Scholar]
- Kumari, N.; Agrawal, U.; Mishra, A.K.; Kumar, A.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumor Biol. 2017, 39, 101042831769755. [Google Scholar] [CrossRef]
- Lim, J.U.; Yoon, H.K. Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine 2021, 138, 155363. [Google Scholar] [CrossRef]
- Marrugal, Á.; Ojeda, L.; Paz-Ares, L.; Molina-Pinelo, S.; Ferrer, I. Proteomic-Based Approaches for the Study of Cytokines in Lung Cancer. Dis. Markers 2016, 2016, 2138627. [Google Scholar] [CrossRef]
- Azevedo, A.; Cunha, V.; Teixeira, A.L.; Medeiros, R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J. Clin. Oncol. 2011, 2, 384. [Google Scholar] [CrossRef]
- Yamaji, H.; Iizasa, T.; Koh, E.; Suzuki, M.; Otsuji, M.; Chang, H.; Motohashi, S.; Yokoi, S.; Hiroshima, K.; Tagawa, M.; et al. Correlation between interleukin 6 production and tumor proliferation in non-small cell lung cancer. Cancer Immunol. Immunother. 2004, 53, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Webster, S.; Flower, D.; Woll, P. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br. J. Cancer 2004, 91, 1970–1976. [Google Scholar] [CrossRef]
- Lind, H.; Zienolddiny, S.; Ryberg, D.; Skaug, V.; Phillips, D.H.; Haugen, A. Interleukin 1 receptor antagonist gene polymorphism and risk of lung cancer: A possible interaction with polymorphisms in the interleukin 1 beta gene. Lung Cancer 2005, 50, 285–290. [Google Scholar] [CrossRef]
- Airoldi, I.; Di Carlo, E.; Cocco, C.; Caci, E.; Cilli, M.; Sorrentino, C.; Sozzi, G.; Ferrini, S.; Rosini, S.; Bertolini, G.; et al. IL-12 can target human lung adenocarcinoma cells and normal bronchial epithelial cells surrounding tumor lesions. PLoS ONE 2009, 4, e6119. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniak, K.; Czarnik-Kwaśniak, J.; Maziarz, A.; Aebisher, D.; Zielińska, K.; Karczmarek-Borowska, B.; Tabarkiewicz, J. Scientific reports concerning the impact of interleukin 4, interleukin 10 and transforming growth factor β on cancer cells. Cent. Eur. J. Immunol. 2019, 44, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.S.; Corrigan, A.E.; Dadah, H.; Stanislovas, J.; Zamani, P.; Makker, S.; Szabados, B.; Sideris, M. IL-10 in cancer: An essential thermostatic regulator between homeostatic immunity and inflammation—A comprehensive review. Future Oncol. 2022, 18, 3349–3365. [Google Scholar] [CrossRef]
- Alonso, A.; de la Gala, F.; Vara, E.; Hortal, J.; Piñeiro, P.; Reyes, A.; Simón, C.; Garutti, I. Lung and blood perioperative metalloproteinases in patients undergoing oncologic lung surgery: Prognostic implications. Thorac. Cancer 2024, 15, 307–315. [Google Scholar] [CrossRef]
- de la Gala, F.; Piñeiro, P.; Reyes, A.; Vara, E.; Olmedilla, L.; Cruz, P.; Garutti, I. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. BJA Br. J. Anaesth. 2017, 119, 655–663. [Google Scholar] [CrossRef]
- Sun, J.; Su, J.; Xie, Y.; Yin, M.T.; Huang, Y.; Xu, L.; Zhou, Q.; Zhu, B. Plasma IL-6/IL-10 Ratio and IL-8, LDH, and HBDH Level Predict the Severity and the Risk of Death in AIDS Patients with Pneumocystis Pneumonia. J. Immunol. Res. 2016, 2016, 1583951. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- MedCalc Software Ltd. MedCalc Version 22.023; MedCalc Software Ltd.: Ostend, Belgium, 2024; Available online: https://www.medcalc.org/ (accessed on 15 May 2023).
- IBM Corporation. IBM SPSS Statistics Base 27; IBM Corporation: Armonk, NY, USA, 2024. [Google Scholar]
- Lee, H.J.; Jo, J.; Son, D.S.; Lee, J.; Choi, Y.S.; Kim, K.; Shim, Y.M.; Kim, J. Predicting recurrence using the clinical factors of patients with non-small cell lung cancer after curative resection. J. Korean Med. Sci. 2009, 24, 824–830. [Google Scholar] [CrossRef][Green Version]
- Watanabe, K.; Masuda, H.; Noma, D. Anesthetic and analgesic techniques and perio- perative inflammation may affect the timing of recurrence after complete resection for non-small-cell lung cancer. Front. Surg. 2022, 9, 886241. [Google Scholar]
- Garutti, I.; Rancan, L.; Abubakra, S.; Simón, C.; Paredes, S.D.; Ortega, J.; Huerta, L.; Ramos, S.; Vara, E. Effects of Intraoperative Infusion of Esmolol on Systemic and Pulmonary Inflammation in a Porcine Experimental Model of Lung Resection Surgery. Anesth. Analg. 2019, 128, 168–175. [Google Scholar] [PubMed]
- Garutti, I.; De la Gala, F.; Piñeiro, P.; Rancan, L.; Vara, E.; Reyes, A.; Puente-Maestu, L.; Bellón, J.M.; Simón, C. Usefulness of combining clinical and biochemical parameters for prediction of postoperative pulmonary complications after lung resection surgery. J. Clin. Monit. Comput. 2019, 33, 1043–1054. [Google Scholar] [PubMed]
- Baudouin, S.V. Lung injury after thoracotomy. Br. J. Anaesth. 2003, 91, 132–142. [Google Scholar]
| Variable | No Recurrence (n = 54) | Recurrence (n = 39) | p-Value |
|---|---|---|---|
| Anesthetic Group | 0.336 | ||
| Propofol | 25 | 22 | |
| Inhalational | 29 | 17 | |
| Status | <0.01 | ||
| Deceased | 18 | 37 | |
| Alive | 36 | 2 | |
| Type of Associated Complications | 0.364 | ||
| No complications | 27 | 15 | |
| Minor complications | 21 | 16 | |
| Major complications | 6 | 8 | |
| Staging (T) | 0.022 | ||
| T1–T2 | 52 | 32 | |
| T3–T4 | 2 | 7 | |
| Medical Complications | 0.238 | ||
| Yes | 20 | 20 | |
| No | 30 | 18 | |
| Surgical Complications | 0.754 | ||
| Yes | 15 | 12 | |
| No | 39 | 27 | |
| PPC | 0.653 | ||
| Yes | 13 | 11 | |
| No | 41 | 28 | |
| Sex | 0.13 | ||
| Female | 22 | 10 | |
| Male | 32 | 29 | |
| Tobacco Use | 0.252 | ||
| Never | 20 | 11 | |
| Former smoker > 6 months | 21 | 11 | |
| Former smoker < 6 months | 7 | 8 | |
| Smoker | 6 | 9 | |
| Anesthetic Risk | 0.328 | ||
| ASA I | 2 | 2 | |
| ASA II | 32 | 17 | |
| ASA III | 20 | 20 | |
| VATS | 0.659 | ||
| Open | 4 | 2 | |
| Closed | 50 | 37 | |
| Surgery | 0.021 | ||
| Pneumonectomy/Bilobectomy | 2 | 8 | |
| Lobectomy | 42 | 22 | |
| Segmentectomy | 10 | 9 | |
| Age (years) | 65.5 (60–73.75) | 65 (59–72.5) | 0.427 |
| Weight (kg) | 70 (64.5–80.75) | 70 (62–80.5) | 0.604 |
| Height (cm) | 166 (160–172) | 167 (160–173) | 0.679 |
| BMI | 26 (23.9–28.6) | 24.57 (22.69–28.4) | 0.167 |
| Variable | RECURRENCE | Basal | p-Value | Postoperative 6 h | p-Value | Postoperative 18 h | p-Value |
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |||||
| TNFα (pg·mL−1) | Yes | 7.11 (6.38–7.36) | 0.90 | 9.28 (8.02–11.54) | 0.14 | 8.68 (7.60–9.59) | 0.18 |
| No | 7.01 (6.59–7.53) | 8.44 (7.58–10.92) | 8.22 (7.45–9.45) | ||||
| IL-1 (pg·mL−1) | Yes | 28.23 (24.55–31.10) | 0.45 | 38.48 (24.73–44.13) | 0.55 | 37.30 (25.17–42.35) | 0.57 |
| No | 28.44 (24.59–32.35) | 29.51 (24.22–45.47) | 29.74 (26.18–42.89) | ||||
| IL-2 (pg·mL−1) | Yes | 0.86 (0.81–0.88) | 0.93 | 1.17 (0.97–1.43) | 0.14 | 1.24 (0.94–1.43) | 0.34 |
| No | 0.85 (0.80–0.90) | 0.98 (0.92–1.39) | 0.98 (0.93–1.38) | ||||
| IL-4 (pg·mL−1) | Yes | 0.33 (0.30–0.38) | 0.57 | 0.37 (0.35–0.40) | 0.76 | 0.39 (0.37–0.47) | 0.39 |
| No | 0.33 (0.30–0.38) | 0.37 (0.35–0.40) | 0.39 (0.37–0.47) | ||||
| IL-6 (pg·mL−1) | Yes | 3.03 (2.83–3.14) | 0.41 | 4.13 (3.06–5.09) | 0.03 | 3.92 (3.09–4.43) | 0.23 |
| No | 3.04 (2.90–3.20) | 3.18 (2.86–4.89) | 3.35 (2.90–4.35) | ||||
| IL-7 (pg·mL−1) | Yes | 2.80 (2.49–3.12) | 0.42 | 6.03 (4.01–7.84) | 0.09 | 4.26 (3.47–4.65) | 0.42 |
| No | 2.75 (2.40–3.01) | 4.21 (3.90–7.03) | 3.88 (3.05–4.66) | ||||
| IL-8 (pg·mL−1) | Yes | 0.93 (0.63–1.23) | 0.74 | 16.01 (7.20–23.93) | 0.60 | 2.48 (1.48–3.33) | 0.31 |
| No | 0.97 (0.70–1.08) | 9.85 (7.24–22.79) | 2.07 (1.15–3.19) | ||||
| IL-10 (pg·mL−1) | Yes | 0.09 (0.08–0.10) | 0.53 | 0.09 (0.08–0.10) | 0.82 | 0.09 (0.08–0.10) | 0.73 |
| No | 0.09 (0.08–0.10) | 0.09 (0.08–0.10) | 0.09 (0.08–0.10) | ||||
| MCP1 (pg·mL−1) | Yes | 248.62 (211.12–268.19) | 0.19 | 373.14 (347.59–395.09) | 0.16 | 377.31 (356.61–396.45) | 0.18 |
| No | 234.04 (202.54–253.31) | 356.15 (328.99–381.76) | 364.62 (322.93–394.79) | ||||
| NO (mmHg) | Yes | 31.29 (29.35–33.34) | 0.33 | 27.48 (25.42–30.96) | 0.64 | 28.45 (25.98–31.21) | 0.70 |
| No | 30.82 (28.41–33.13) | 28.25 (25.86–30.63) | 29.10 (25.70–30.86) | ||||
| CO (mmHg) | Yes | 2.61 (2.37–2.88) | 0.41 | 2.77 (2.69–3.04) | 0.59 | 2.86 (2.75–2.98) | 0.20 |
| No | 2.66 (2.48–2.86) | 2.81 (2.70–3.02) | 2.92 (2.81–3.03) | ||||
| IL-6/IL-10 | Yes | 34.12 (30.70–42.04) | 0.39 | 48.34 (32.55–55.24) | 0.10 | 42.63 (34.98–46.98) | 0.26 |
| No | 34.58 (30.44–37.18) | 41.08 (29.44–50.69) | 40.36 (30.54–47.45) | ||||
| IL-8/IL-10 | Yes | 10.25 (6.60–14.57) | 1.00 | 118.56 (76.68–231.73) | 0.48 | 24.90 (11.97–36.62) | 0.34 |
| No | 10.67 (8.15–12.49) | 166.61 (83.65–253.82) | 26.51 (16.44–35.38) | ||||
| MMP2 (pg·mL−1) | Yes | 2.22 (1.94–2.48) | 0.71 | 4.06 (3.77–6.19) | 0.93 | 5.00 (4.42–6.21) | 0.72 |
| No | 2.21 (1.96–2.48) | 4.99 (3.69–6.24) | 5.44 (4.33–6.20) | ||||
| MMP3 (pg·mL−1) | Yes | 1.40 (1.30–1.60) | 0.10 | 2.95 (2.46–3.93) | 0.03 | 1.89 (1.50–4.16) | 0.68 |
| No | 1.52 (1.33–1.84) | 3.86 (2.82–4.12) | 3.95 (1.51–4.14) | ||||
| MMP7 (pg·mL−1) | Yes | 0.51 (0.45–0.63) | 0.81 | 0.65 (0.55–0.82) | 0.79 | 0.65 (0.50–0.86) | 0.77 |
| No | 0.52 (0.47–0.63) | 0.66 (0.52–0.88) | 0.65 (0.50–0.86) | ||||
| MMP9 (pg·mL−1) | Yes | 853.49 (752.04–944.03) | 0.44 | 961.28 (900.34–1082.41) | 0.07 | 993.09 (881.85–1299.96) | 0.28 |
| No | 811.19 (713.42–927.06) | 1112.75 (931.7–1363.64) | 1211.37 (877.75–1362.5) |
| Variable | Recurrence | Baseline Median (IQR) | p-Value | End of Surgery Median (IQR) | p-Value |
|---|---|---|---|---|---|
| IL-1 (pg·mL−1) | Yes | 129.52 (121.10–149.74) | 0.510 | 183.40 (169.34–206.84) | 0.606 |
| No | 128.81 (113.68–147.44) | 188.72 (167.80–219.65) | |||
| TNFα (pg·mL−1) | Yes | 15.05 (13.81–15.52) | 0.643 | 23.01 (20.48–24.97) | 0.073 |
| No | 14.94 (13.92–16.63) | 20.94 (19.98–23.38) | |||
| IL-2 (pg·mL−1) | Yes | 2.19 (1.99–2.60) | 0.321 | 3.22 (2.96–3.92) | 0.315 |
| No | 2.14 (1.95–2.30) | 3.16 (2.90–3.79) | |||
| IL-6 (pg·mL−1) | Yes | 6.33 (5.76–6.67) | 0.975 | 7.60 (7.18–8.06) | 0.264 |
| No | 6.25 (5.80–6.92) | 7.42 (6.86–7.94) | |||
| IL-10 (pg·mL−1) | Yes | 40.75 (39.14–42.38) | 0.901 | 42.06 (39.96–44.27) | 0.753 |
| No | 40.92 (39.98–42.08) | 41.37 (39.67–44.35) | |||
| MCP1 (pg·mL−1) | Yes | 374.70 (366.26–390.77) | 0.750 | 545.96 (529.67–574.70) | 0.232 |
| No | 382.59 (349.05–399.56) | 541.09 (517.49–555.15) | |||
| IL-4 (pg·mL−1) | Yes | 0.41 (0.38–0.43) | 0.978 | 0.86 (0.73–0.93) | 0.300 |
| No | 0.41 (0.38–0.43) | 0.80 (0.71–0.91) | |||
| IL-7 (pg·mL−1) | Yes | 3.13 (2.88–3.28) | 0.060 | 5.21 (4.86–5.76) | 0.630 |
| No | 3.20 (2.98–3.62) | 5.08 (4.96–5.60) | |||
| IL-8 (pg·mL−1) | Yes | 2.76 (2.51–3.06) | 0.436 | 50.88 (25.79–57.44) | 0.503 |
| No | 2.71 (2.05–2.97) | 32.55 (26.52–51.49) | |||
| IL-12 (pg·mL−1) | Yes | 0.07 (0.06–0.07) | 0.191 | 0.13 (0.11–0.15) | 0.350 |
| No | 0.07 (0.06–0.08) | 0.13 (0.10–0.14) | |||
| VEGF (pg·mL−1) | Yes | 106.32 (93.31–115.82) | 0.150 | 118.84 (103.85–130.48) | 0.085 |
| No | 98.82 (87.22–110.87) | 108.63 (98.79–124.53) | |||
| NO (mmHg) | Yes | 12.50 (6.15–15.85) | 0.394 | 11.05 (8.01–20.69) | 0.358 |
| No | 8.15 (6.02–14.44) | 9.49 (7.43–17.41) | |||
| CO (mmHg) | Yes | 6.39 (5.71–6.90) | 0.767 | 8.17 (7.82–9.18) | 0.506 |
| No | 6.20 (5.77–7.22) | 8.30 (7.43–9.10) | |||
| MMP2 (pg·mL−1) | Yes | 5.03 (4.42–12.58) | 0.575 | 9.77 (8.60–16.50) | 0.761 |
| No | 4.89 (4.31–12.00) | 9.72 (8.36–15.38) | |||
| MMP3 (pg·mL−1) | Yes | 3.37 (3.00–3.77) | 0.047 | 9.30 (7.12–9.86) | 0.582 |
| No | 3.78 (2.97–4.07) | 8.44 (6.96–9.84) | |||
| MMP7 (pg·mL−1) | Yes | 0.50 (0.47–0.52) | 0.368 | 0.53 (0.51–0.55) | 0.755 |
| No | 0.51 (0.47–0.53) | 0.53 (0.51–0.56) | |||
| MMP9 (pg·mL−1) | Yes | 1188.68 (1007.70–1244.50) | 0.020 | 1394.42 (1359.35–1500.73) | 0.346 |
| No | 1250.98 (1158.85–1291.16) | 1402.43 (1341.95–1433.73) |
| Variables | Coefficient (B) | p-Value |
|---|---|---|
| Model 1 | ||
| Type of surgery | 0.051 | |
| Lobectomy | −2.045 | 0.015 |
| Segmentectomy | −1.727 | 0.066 |
| T3/T4 staging | 1.739 | 0.043 |
| Model 2 | ||
| Type of surgery | 0.021 | |
| Lobectomy | −4.16 | 0.006 |
| Segmentectomy | −3.493 | 0.028 |
| IL6 plasma at 6 h | −1.718 | 0.02 |
| MCP1 plasma at 18 h | 0.021 | 0.032 |
| IL6/IL10 plasma at 6 h | 0.111 | 0.014 |
| MMP3 plasma at baseline | 2.93 | 0.023 |
| TNFα BAL at surgery end | 0.428 | 0.016 |
| MMP9 BAL at surgery start | −0.006 | 0.017 |
| MMP9 BAL at surgery end | 0.009 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente, E.; Morgado, O.; de la Gala, F.; Vara, E.; Zuluaga, P.; Reyes, A.; Simón, C.M.; Hortal, J.; Piñeiro, P.; Garutti, I. Exploring the Relationship Between Perioperative Inflammatory Biomarkers and Oncological Recurrence in Patients Undergoing Pulmonary Cancer Surgery. Cancers 2025, 17, 1159. https://doi.org/10.3390/cancers17071159
de la Fuente E, Morgado O, de la Gala F, Vara E, Zuluaga P, Reyes A, Simón CM, Hortal J, Piñeiro P, Garutti I. Exploring the Relationship Between Perioperative Inflammatory Biomarkers and Oncological Recurrence in Patients Undergoing Pulmonary Cancer Surgery. Cancers. 2025; 17(7):1159. https://doi.org/10.3390/cancers17071159
Chicago/Turabian Stylede la Fuente, Elena, Oscar Morgado, Francisco de la Gala, Elena Vara, Pilar Zuluaga, Almudena Reyes, Carlos M. Simón, Javier Hortal, Patricia Piñeiro, and Ignacio Garutti. 2025. "Exploring the Relationship Between Perioperative Inflammatory Biomarkers and Oncological Recurrence in Patients Undergoing Pulmonary Cancer Surgery" Cancers 17, no. 7: 1159. https://doi.org/10.3390/cancers17071159
APA Stylede la Fuente, E., Morgado, O., de la Gala, F., Vara, E., Zuluaga, P., Reyes, A., Simón, C. M., Hortal, J., Piñeiro, P., & Garutti, I. (2025). Exploring the Relationship Between Perioperative Inflammatory Biomarkers and Oncological Recurrence in Patients Undergoing Pulmonary Cancer Surgery. Cancers, 17(7), 1159. https://doi.org/10.3390/cancers17071159





