Retreatment with Brentuximab Vedotin in Patients with Relapsed/Refractory CD30+ Malignancies: A Retrospective Medical Chart Review Study in Spain-The BELIEVE Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
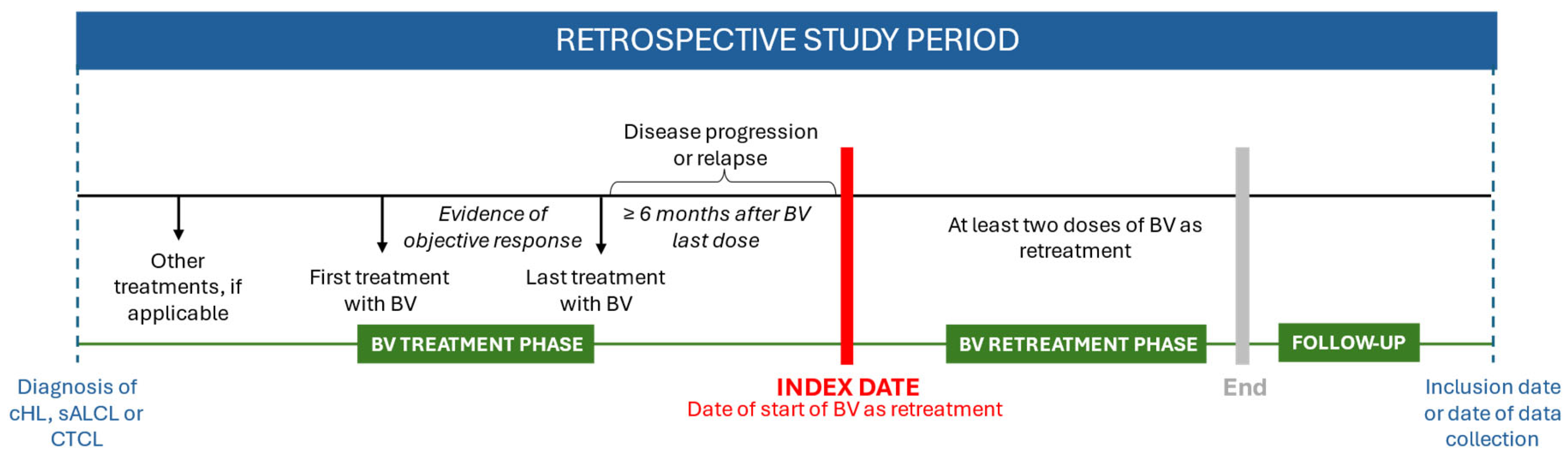
2.1. Study Design and Population
2.2. Data Sources and Measurements
2.3. Statistics
2.4. Treatment
3. Results
3.1. Patient Characteristics
3.2. Treatment Patterns
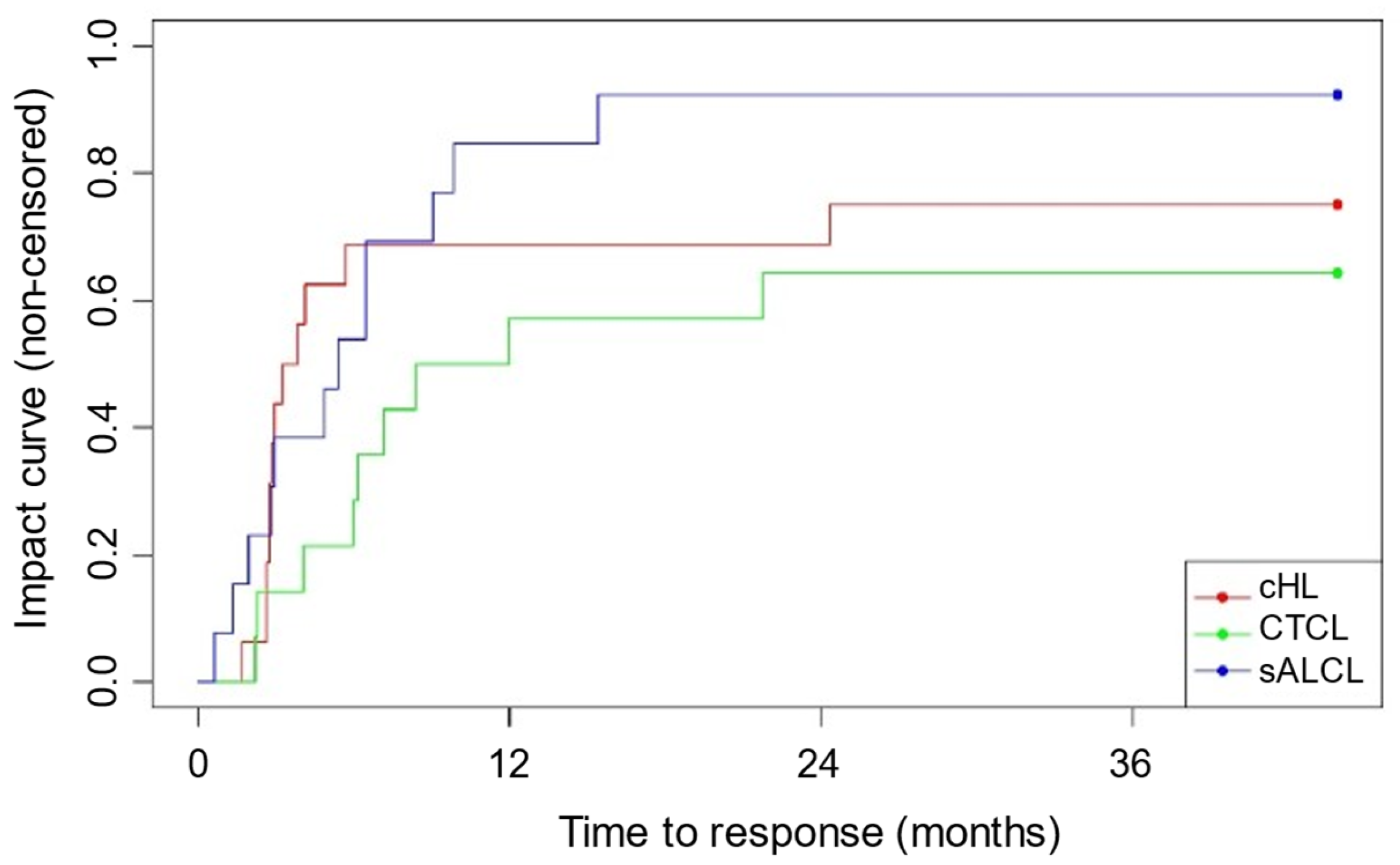
3.3. Overall Response Rate (ORR)
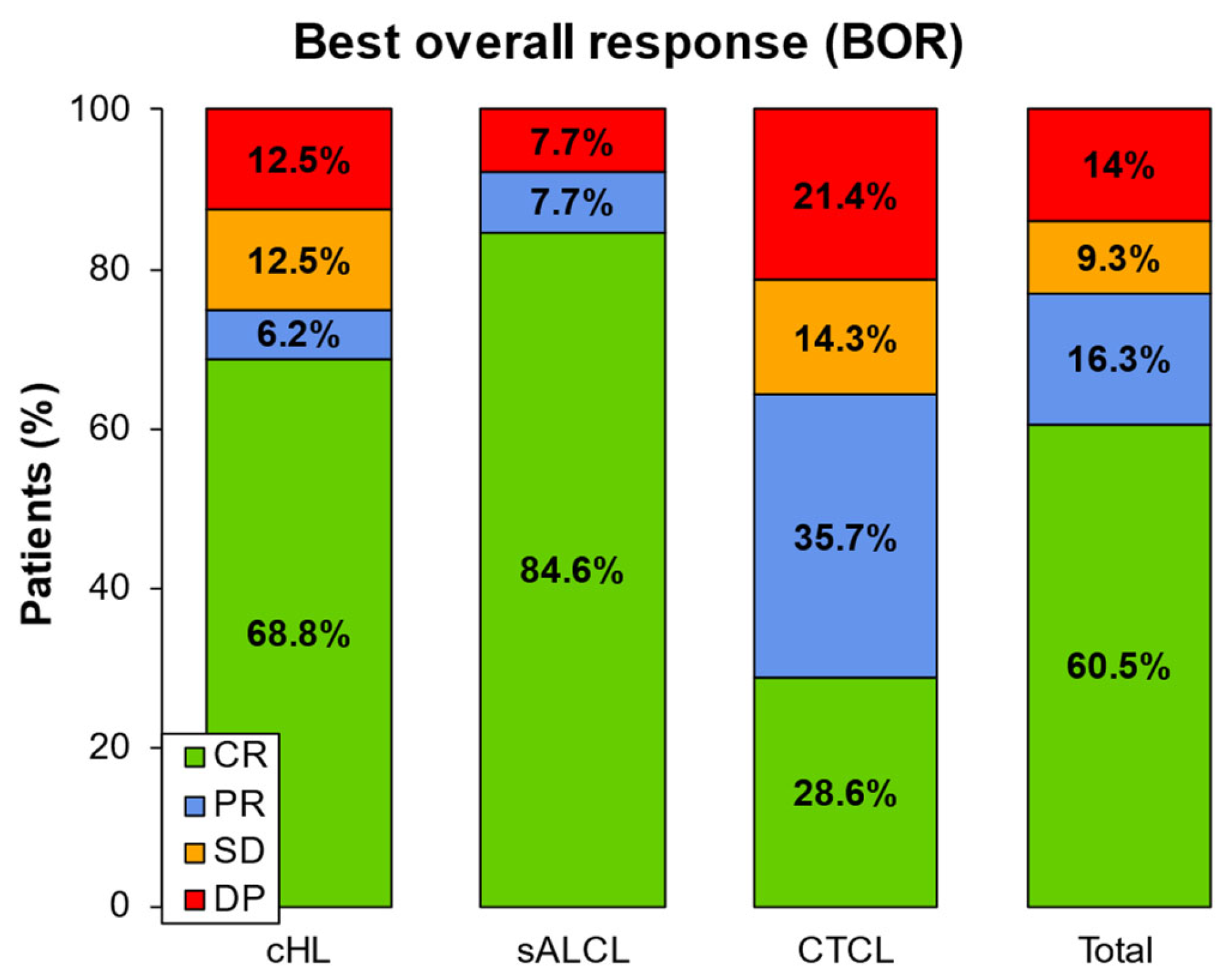
3.4. Best Overall Response (BOR)
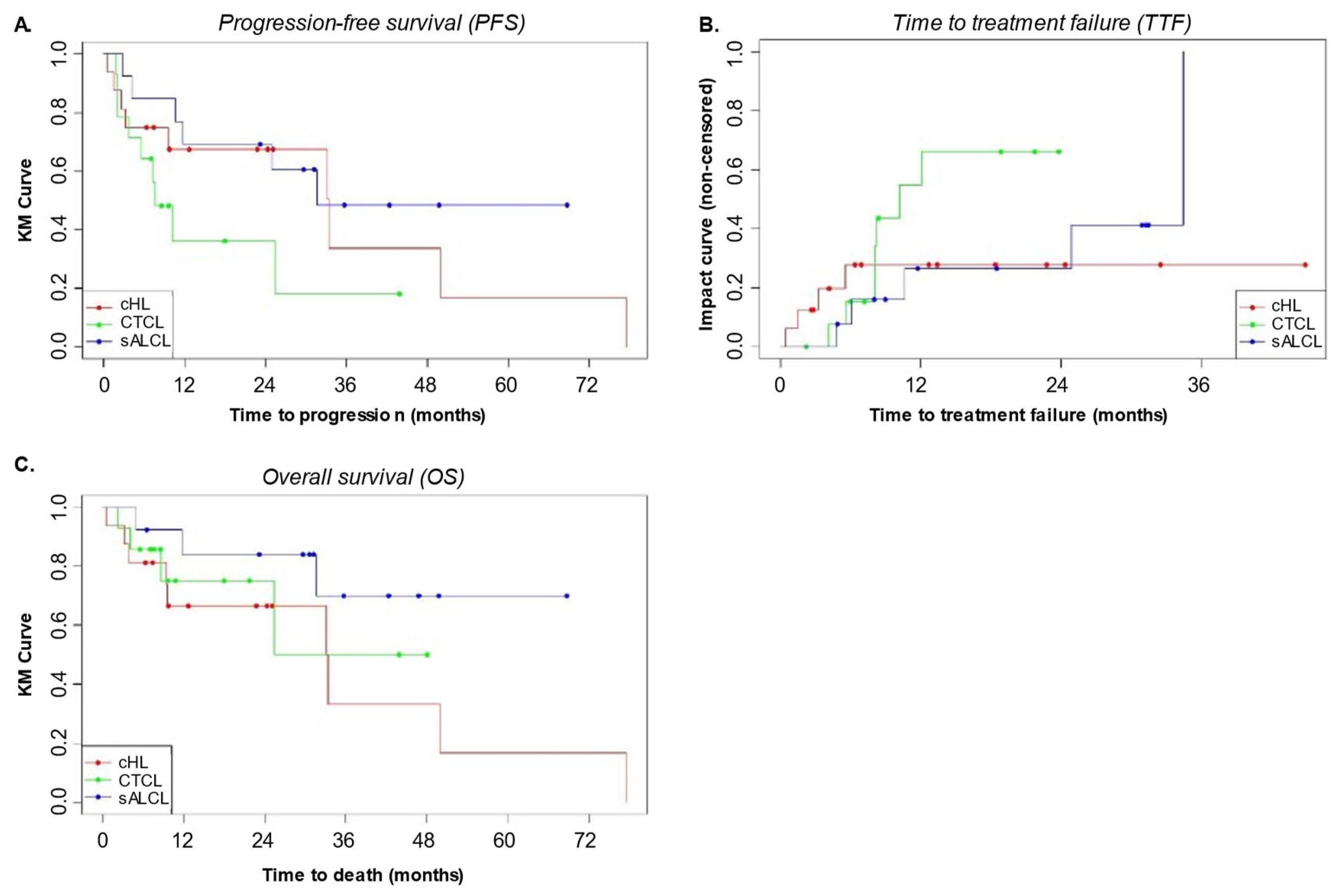
3.5. Progression Free Survival (PFS), Time to Treatment Failure (TTF), and Overall Survival (OS)
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takeda. Adcetris(R) (Brentuximab Vedotin) SmPc; CIMA: Navarra, Spain, 2025. Available online: https://cima.aemps.es/cima/dochtml/ft/112794001/FT_112794001.html (accessed on 18 February 2025).
- Jiang, M.; Bennani, N.N.; Feldman, A.L. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev. Hematol. 2017, 10, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Barta, S.K.; Liu, N.; DerSarkissian, M.; Chang, R.; Ye, M.; Duh, M.S.; Surinach, A.; Fanale, M.; Yu, K.S. Real-World Treatment Patterns and Clinical Outcomes With Brentuximab Vedotin or Other Standard Therapies in Patients With Previously Treated Cutaneous T-Cell Lymphoma in the United States. Clin. Lymphoma Myeloma Leuk. 2023, 24, e21–e32.e4. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Chen, R.; Fanale, M.A.; Brice, P.; Gopal, A.; Smith, S.E.; Advani, R.; Matous, J.V.; Ramchandren, R.; Rosenblatt, J.D.; et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J. Hematol. Oncol. 2014, 7, 24. [Google Scholar] [CrossRef]
- Fukuhara, N.; Yamamoto, G.; Tsujimura, H.; Chou, T.; Shibayama, H.; Yanai, T.; Shibuya, K.; Izutsu, K. Retreatment with brentuximab vedotin in patients with relapsed/refractory classical Hodgkin lymphoma or systemic anaplastic large-cell lymphoma: A multicenter retrospective study. Leuk. Lymphoma 2019, 61, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Sano, D.; Ahmed, S.; Liu, J.; Knowles, S.; Lee, S.T. Efficacy and Safety of Retreatment with Brentuximab Vedotin in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma or CD30-Expressing Peripheral T Cell Lymphoma. Blood 2022, 140, 12003–12004. [Google Scholar] [CrossRef]
- Sano, D.; Liu, N.; Knowles, S.; MacEwan, J.P.; Wang, S.; Wogen, J.; Yu, K.S.; Lee, S.T. Brentuximab Vedotin Retreatment in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma or Peripheral T-Cell Lymphoma: A Retrospective United States Claims Analysis. Curr. Oncol. 2024, 31, 2598–2609. [Google Scholar] [CrossRef]
- Munir, F.; Hardit, V.; Sheikh, I.N.; AlQahtani, S.; He, J.; Cuglievan, B.; Hosing, C.; Tewari, P.; Khazal, S. Classical Hodgkin Lymphoma: From Past to Future—A Comprehensive Review of Pathophysiology and Therapeutic Advances. Int. J. Mol. Sci. 2023, 24, 10095. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Dulery, R.; Bazarbachi, A.H.; Savani, M.; Hamed, R.A.; Bazarbachi, A.; Mohty, M. Latest advances in the management of classical Hodgkin lymphoma: The era of novel therapies. Blood Cancer J. 2021, 11, 126. [Google Scholar] [CrossRef]
- Stein, H.; Foss, H.D.; Durkop, H.; Marafioti, T.; Delsol, G.; Pulford, K.; Pileri, S.; Falini, B. CD30(+) anaplastic large cell lymphoma: A review of its histopathologic, genetic, and clinical features. Blood 2000, 96, 3681–3695. [Google Scholar]
- Pierce, J.M.R.; Mehta, A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev. Hematol. 2016, 10, 29–37. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Bonthapally, V.; Huebner, D.; Lutes, R.; Chi, A.; Pileri, S. Panoptic clinical review of the current and future treatment of relapsed/refractory T-cell lymphomas: Cutaneous T-cell lymphomas. Crit. Rev. Oncol. 2016, 99, 228–240. [Google Scholar] [CrossRef]
- Kelly, K.A.; Edenfield, L.; Seegars, M.B.; Vaidya, R.; Feldman, S.R.; Strowd, L.C. A Case Series on the Use of Brentuximab Vedotin for the Treatment of Mycosis Fungoides. J. Drugs Dermatol. 2023, 22, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Muniesa, C.; Gallardo, F.; García-Doval, I.; Estrach, M.; Combalia, A.; Morillo-Andújar, M.; Vicente, F.D.l.C.; Machan, S.; Moya-Martínez, C.; Rovira, R.; et al. Brentuximab vedotin in the treatment of cutaneous T-cell lymphomas: Data from the Spanish Primary Cutaneous Lymphoma Registry. Eur. J. Cancer 2022, 173, S40–S41. [Google Scholar] [CrossRef]
- NCI. Common Terminology Criteria for Adverse Events (CTCAE); U.S. Department of Health and Human Services: Washington, DC, USA, 2017. [Google Scholar]
- Chen, R.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Huebner, D.; et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016, 128, 1562–1566. [Google Scholar] [CrossRef]
- Chen, R.; Palmer, J.M.; Thomas, S.H.; Tsai, N.-C.; Farol, L.; Nademanee, A.; Forman, S.J.; Gopal, A.K. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood 2012, 119, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.; Christensen, J.H.; Morschhauser, F.; Domenech, E.D.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2018, 393, 229–240. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Scarisbrick, J.J.; Dummer, R.; Whittaker, S.; Duvic, M.; Kim, Y.H.; Quaglino, P.; Zinzani, P.L.; Bechter, O.; Eradat, H.; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician’s choice in cutaneous T-cell lymphoma: Final data. Blood Adv. 2021, 5, 5098–5106. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Brentuximab Vedotin (SGN-35) in Patients with Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J. Clin. Oncol. 2012, 30, 2190–2196. [Google Scholar] [CrossRef]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; De Vos, S.; et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012, 30, 2183–2189. [Google Scholar]
- Horwitz, S.M.; Advani, R.H.; Bartlett, N.L.; Jacobsen, E.D.; Sharman, J.P.; O’Connor, O.A.; Siddiqi, T.; Kennedy, D.A.; Oki, Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014, 123, 3095–3100. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef]
- Randall, M.P.; Spinner, M.A. Optimizing Treatment for Relapsed/Refractory Classic Hodgkin Lymphoma in the Era of Immunotherapy. Cancers 2023, 15, 4509. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Walewski, J.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Viviani, S.; et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood 2018, 132, 2639–2642. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chem-otherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Gianni, A.M.; Carella, A.; et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 385, 1853–1862. [Google Scholar] [CrossRef]
- Gopal, A.K.; Ramchandren, R.; O’Connor, O.A.; Berryman, R.B.; Advani, R.H.; Chen, R.; Smith, S.E.; Cooper, M.; Rothe, A.; Matous, J.V.; et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood 2012, 120, 560–568. [Google Scholar] [CrossRef]
| cHL (n = 16) | sALCL (n = 13) | CTCL (n = 14) | Total (n = 43) | ||
|---|---|---|---|---|---|
| Age at the time of visit (years) | Mean (SD) | 36.2 (13.3) | 51.3 (10.8) | 52.9 (13.5) | 46.2 (14.6) |
| Min–Max | 18.0–62.0 | 31.0–70.0 | 34.0–76.0 | 18.0–76.0 | |
| Gender | Male | 9 (56.2%) | 8 (61.5%) | 8 (57.1%) | 25 (58.1%) |
| Female | 7 (43.8%) | 5 (38.5%) | 6 (42.9%) | 18 (41.9%) | |
| Ethnicity | Caucasian | 13 (86.7%) | 12 (92.3%) | 12 (85.7%) | 37 (88.1%) |
| Hispanic | 2 (13.3%) | 1 (7.7%) | 1 (7.1%) | 4 (9.5%) | |
| Other | 0 (0%) | 0 (0%) | 1 (7.1%) | 1 (2.4%) | |
| N missing | 1 | 0 | 0 | 1 | |
| ECOG performance status at BV retreatment initiation | Grade 0 | 6 (50%) | 7 (53.8%) | 5 (41.7%) | 18 (48.6%) |
| Grade I | 5 (41.7%) | 5 (38.5%) | 6 (50%) | 16 (43.2%) | |
| Grade II | 1 (8.3%) | 0 (0%) | 1 (8.3%) | 2 (5.4%) | |
| Grade III | 0 (0%) | 1 (7.7%) | 0 (0%) | 1 (2.7%) | |
| Grade IV | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade V | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| N missing | 4 | 0 | 2 | 6 | |
| Clinical stage (Ann Arbor classification) at BV retreatment initiation | Stage I | 1 (8.3%) | 3 (23.1%) | - | 4 (16%) |
| Stage II | 6 (50%) | 2 (15.4%) | - | 8 (32%) | |
| Stage III | 2 (16.7%) | 2 (15.4%) | - | 4 (16%) | |
| Stage IVa | 2 (16.7%) | 2 (15.4%) | - | 4 (16%) | |
| Stage IVb | 1 (8.3%) | 4 (30.8%) | - | 5 (20%) | |
| N missing | 4 | 0 | - | 18 | |
| Clinical stage (EORTC) at BV retreatment initiation | Stage IA | - | - | 1 (9.1%) | 1 (9.1%) |
| Stage IB | - | - | 1 (9.1%) | 1 (9.1%) | |
| Stage IIA | - | - | 0 (0%) | 0 (0%) | |
| Stage IIB | - | - | 3 (27.3%) | 3 (27.3%) | |
| Stage III | - | - | 0 (0%) | 0 (0%) | |
| Stage IVA | - | - | 2 (18.2%) | 2 (18.2%) | |
| Stage IVB | - | - | 4 (36.4%) | 4 (36.4%) | |
| N missing | - | - | 3 | 32 | |
| cHL (n = 16) | sALCL (n = 13) | CTCL (n = 14) | Total (n = 43) | ||
|---|---|---|---|---|---|
| No. of treatments before BV retreatment | Mean (SD) | 5.8 (3.1) | 3.9 (2.1) | 8.8 (6.9) | 6.2 (4.8) |
| Median | 5.5 | 4.0 | 6.5 | 5.0 | |
| (Min–Max) | (2.0–12.0) | (1.0–7.0) | (2.0–30.0) | (1.0–30.0) | |
| Treatments between first BV and BV retreatment | No | 3 (18.8%) | 2 (15.4%) | 2 (14.3%) | 7 (16.3%) |
| Yes | 13 (81.2%) | 11 (84.6%) | 12 (85.7%) | 36 (83.7%) | |
| Mean (SD) | 1.5 (1.2) | 1.5 (0.8) | 1.6 (0.9) | 1.6 (1.0) | |
| Median | 1.0 | 1.0 | 1.0 | 1.0 | |
| (Min–Max) | (1.0–5.0) | (1.0–3.0) | (1.0–3.0) | (1.0–5.0) | |
| No. of treatments after BV retreatment | Mean (SD) | 1.2 (2.4) | 0.9 (0.9) | 1.1 (1.8) | 1.1 (1.8) |
| Median | 0.0 | 1.0 | 1.0 | 1.0 | |
| (Min–Max) | (0.0–7.0) | (0.0–2.0) | (0.0–7.0) | (0.0–7.0) | |
| No. of BV treatment cycles before BV retreatment | Mean (SD) | 5.9 (4.4) | 8.5 (4.6) | 9.2 (4.0) | 7.8 (4.5) |
| Median | 4.0 | 6.0 | 8.5 | 6.0 | |
| (Min–Max) | (2.0;16.0) | (4.0;16.0) | (3.0;16.0) | (2.0;16.0) | |
| No. of BV retreatment cycles | Mean (SD) | 7.0 (5.4) | 8.3 (5.5) | 7.6 (3.7) | 7.6 (4.8) |
| Median | 4.5 | 6.0 | 7.0 | 6.0 | |
| (Min–Max) | (2.0–18.0) | (3.0–20.0) | (3.0–14.0) | (2.0–20.0) | |
| cHL (n = 16) | sALCL (n = 14) | CTCL (n = 13) | Total (n = 43) | ||
|---|---|---|---|---|---|
| No. of autologous transplants before first BV treatment | 0 | 10 (62.5%) | 11 (84.6%) | 13 (92.9%) | 34 (79.1%) |
| 1 | 6 (37.5%) | 2 (15.4%) | 1 (7.1%) | 9 (20.9%) | |
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| No. of autologous transplants after first BV treatment | 0 | 11 (68.8%) | 7 (53.8%) | 14 (100%) | 32 (74.4%) |
| 1 | 4 (25%) | 6 (46.2%) | 0 (0%) | 10 (23.3%) | |
| 2 | 1 (6.2%) | 0 (0%) | 0 (0%) | 1 (2.3%) | |
| No. of allogenic transplants before BV retreatment | 0 | 11 (68.8%) | 11 (84.6%) | 13 (92.9%) | 35 (81.4%) |
| 1 | 5 (31.2%) | 1 (7.7%) | 0 (0%) | 6 (14%) | |
| 2 | 0 (0%) | 1 (7.7%) | 1 (7.1%) | 2 (4.7%) | |
| No. of allogenic transplants after BV retreatment | 0 | 12 (75%) | 10 (76.9%) | 12 (85.7%) | 34 (79.1%) |
| 1 | 4 (25%) | 3 (23.1%) | 2 (14.3%) | 9 (20.9%) | |
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| cHL | sALCL | CTCL | Total | ||
|---|---|---|---|---|---|
| AEs related to BV retreatment | Yes | 7 (53.8%) | 7 (53.8%) | 4 (28.6%) | 18 (45%) |
| No | 6 (46.2%) | 6 (46.2%) | 10 (71.4%) | 22 (55%) | |
| N missing | 3 | 0 | 0 | 3 | |
| Type of AEs per patient * | Peripheral Motor Neuropathy | 2 (12.5%) | 0 (0%) | 0 (0%) | 2 (4.7%) |
| Peripheral Sensory Neuropathy | 1 (6.2%) | 5 (38.5%) | 3 (21.4%) | 9 (20.9%) | |
| Neutropenia | 2 (12.5%) | 2 (15.4%) | 1 (7.1%) | 5 (11.6%) | |
| Febrile Neutropenia | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Renal dysfunction | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Liver dysfunction | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Pulmonary disorder | 0 (0%) | 1 (7.7%) | 0 (0%) | 1 (2.3%) | |
| Others with clinical relevance | 4 (25%) | 5 (38.5%) | 1 (7.1%) | 10 (23.3%) | |
| Severity of the AE per patient * | Severe | 2 (12.5%) | 3 (23.1%) | 3 (21.4%) | 8 (18.6%) |
| Non-Severe | 6 (37.5%) | 7 (53.8%) | 2 (14.3%) | 15 (34.9%) | |
| N valid | 8 | 10 | 5 | 23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sureda, A.; García-Sanz, R.; Domingo-Domenech, E.; Capote, F.J.; Gutiérrez, A.; Rodriguez, A.; Aguiar, D.; Giraldo, P.; Infante, M.S.; López-Jiménez, J.; et al. Retreatment with Brentuximab Vedotin in Patients with Relapsed/Refractory CD30+ Malignancies: A Retrospective Medical Chart Review Study in Spain-The BELIEVE Study. Cancers 2025, 17, 1137. https://doi.org/10.3390/cancers17071137
Sureda A, García-Sanz R, Domingo-Domenech E, Capote FJ, Gutiérrez A, Rodriguez A, Aguiar D, Giraldo P, Infante MS, López-Jiménez J, et al. Retreatment with Brentuximab Vedotin in Patients with Relapsed/Refractory CD30+ Malignancies: A Retrospective Medical Chart Review Study in Spain-The BELIEVE Study. Cancers. 2025; 17(7):1137. https://doi.org/10.3390/cancers17071137
Chicago/Turabian StyleSureda, Anna, Ramón García-Sanz, Eva Domingo-Domenech, Francisco Javier Capote, Antonio Gutiérrez, Antonia Rodriguez, David Aguiar, Pilar Giraldo, María Stefanía Infante, Javier López-Jiménez, and et al. 2025. "Retreatment with Brentuximab Vedotin in Patients with Relapsed/Refractory CD30+ Malignancies: A Retrospective Medical Chart Review Study in Spain-The BELIEVE Study" Cancers 17, no. 7: 1137. https://doi.org/10.3390/cancers17071137
APA StyleSureda, A., García-Sanz, R., Domingo-Domenech, E., Capote, F. J., Gutiérrez, A., Rodriguez, A., Aguiar, D., Giraldo, P., Infante, M. S., López-Jiménez, J., Martínez, C., Sánchez-González, B., Ortiz-Romero, P. L., Grande, M., & Baeza-Montañez, L. (2025). Retreatment with Brentuximab Vedotin in Patients with Relapsed/Refractory CD30+ Malignancies: A Retrospective Medical Chart Review Study in Spain-The BELIEVE Study. Cancers, 17(7), 1137. https://doi.org/10.3390/cancers17071137






