cGAS and STING in Host Myeloid Cells Are Essential for Effective Cyclophosphamide Treatment of Advanced Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Cell Lines
2.2. Construction of BM Chimeras
2.3. Tumor Model and CTX Therapy
2.4. Flow Cytometry Analysis of Cells from Dissociated Tumors
2.5. Statistical Analysis
3. Results
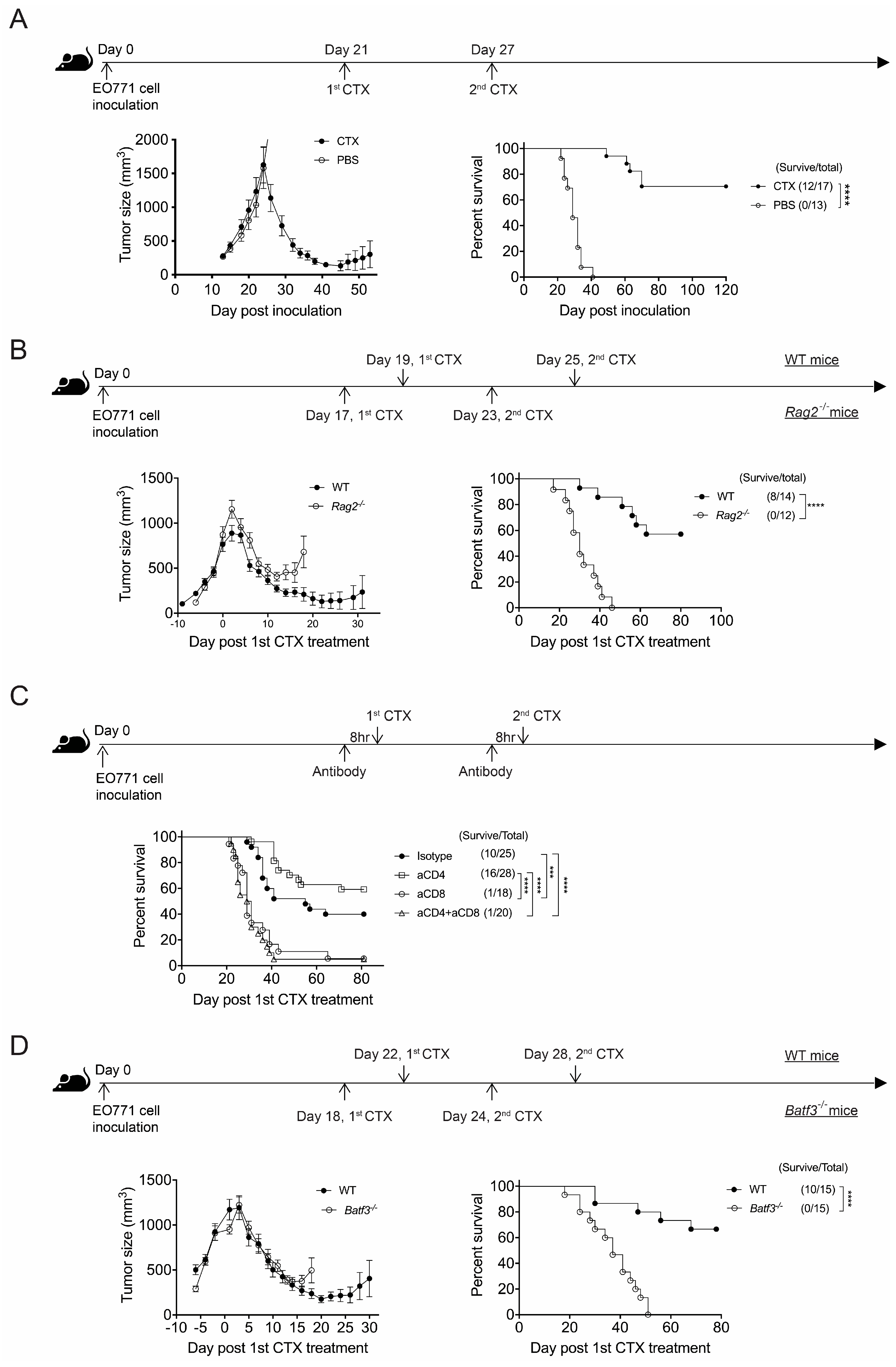
3.1. CTX Therapy Promotes Long-Term Survival of Mice with Advanced EO771 Breast Cancer but Requires CD8+ T Cell Immunity
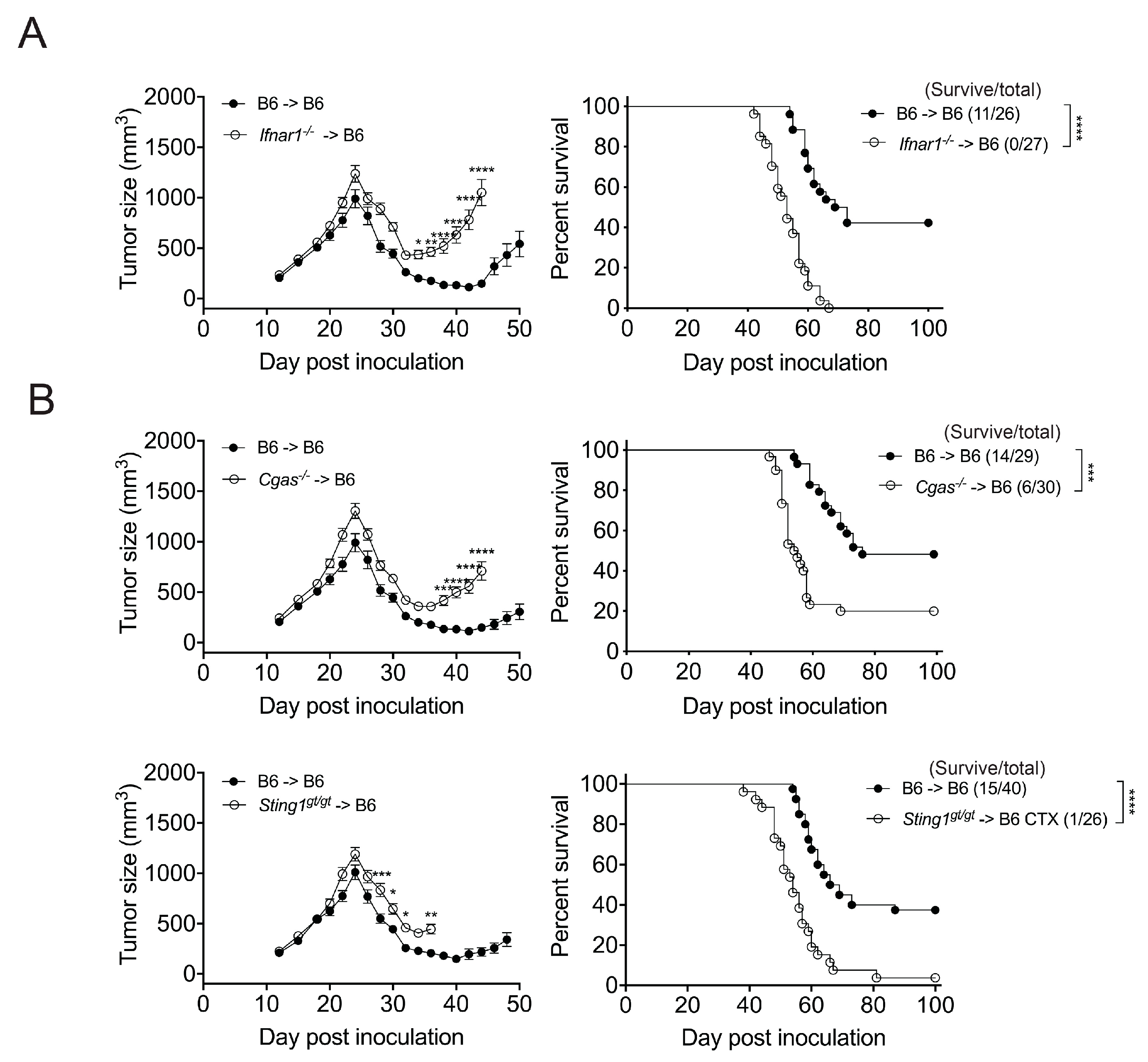
3.2. The Effect of CTX Therapy Requires the IFNar1 and cGAS/STING of Bone Marrow-Derived Cells
3.3. STING and IFNar1 of Distinct Myeloid Cells Are Essential for CTX Efficacy
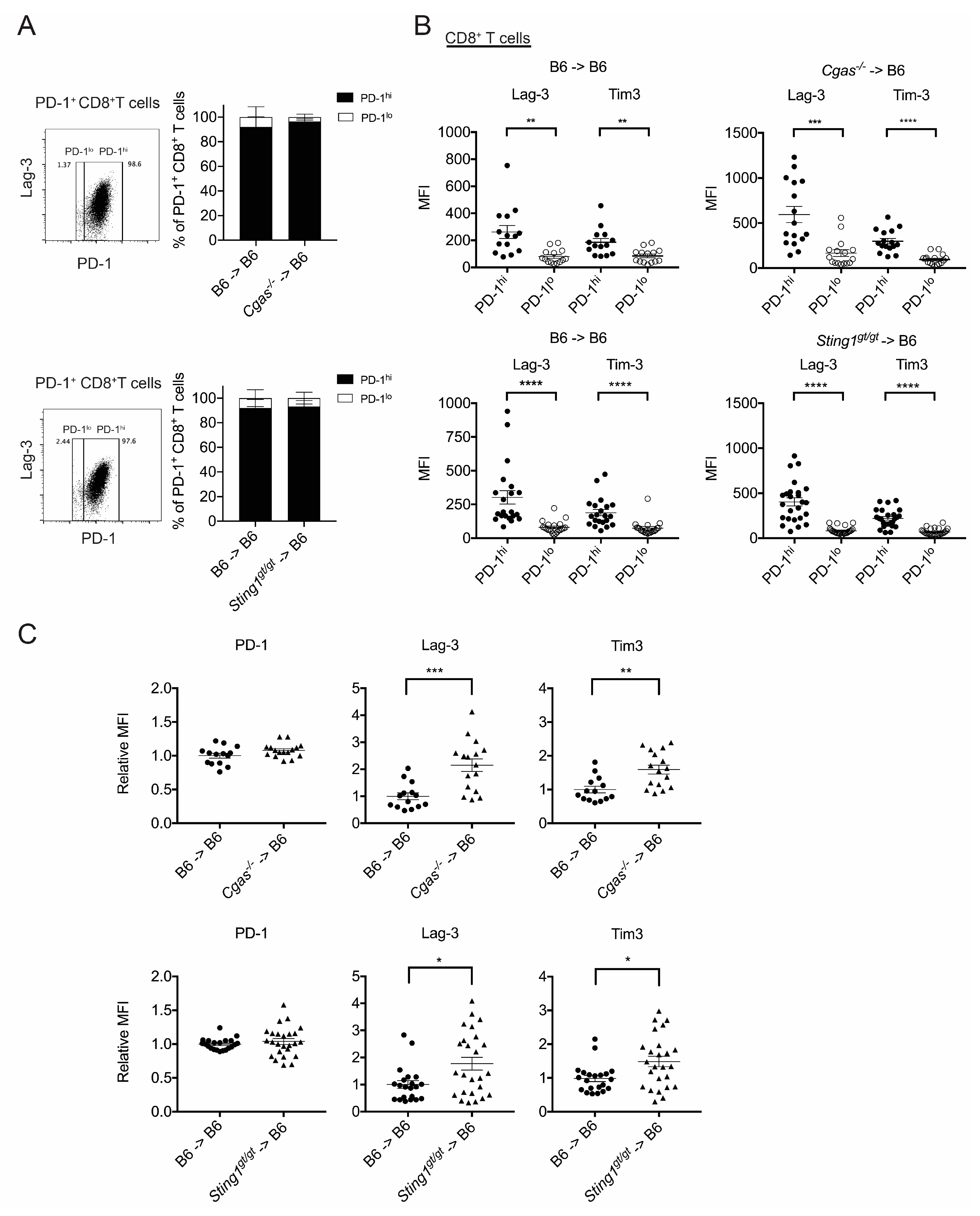
3.4. cGAS and STING of BM-Derived Cells Positively Modulate the CD8+ T Cell Response
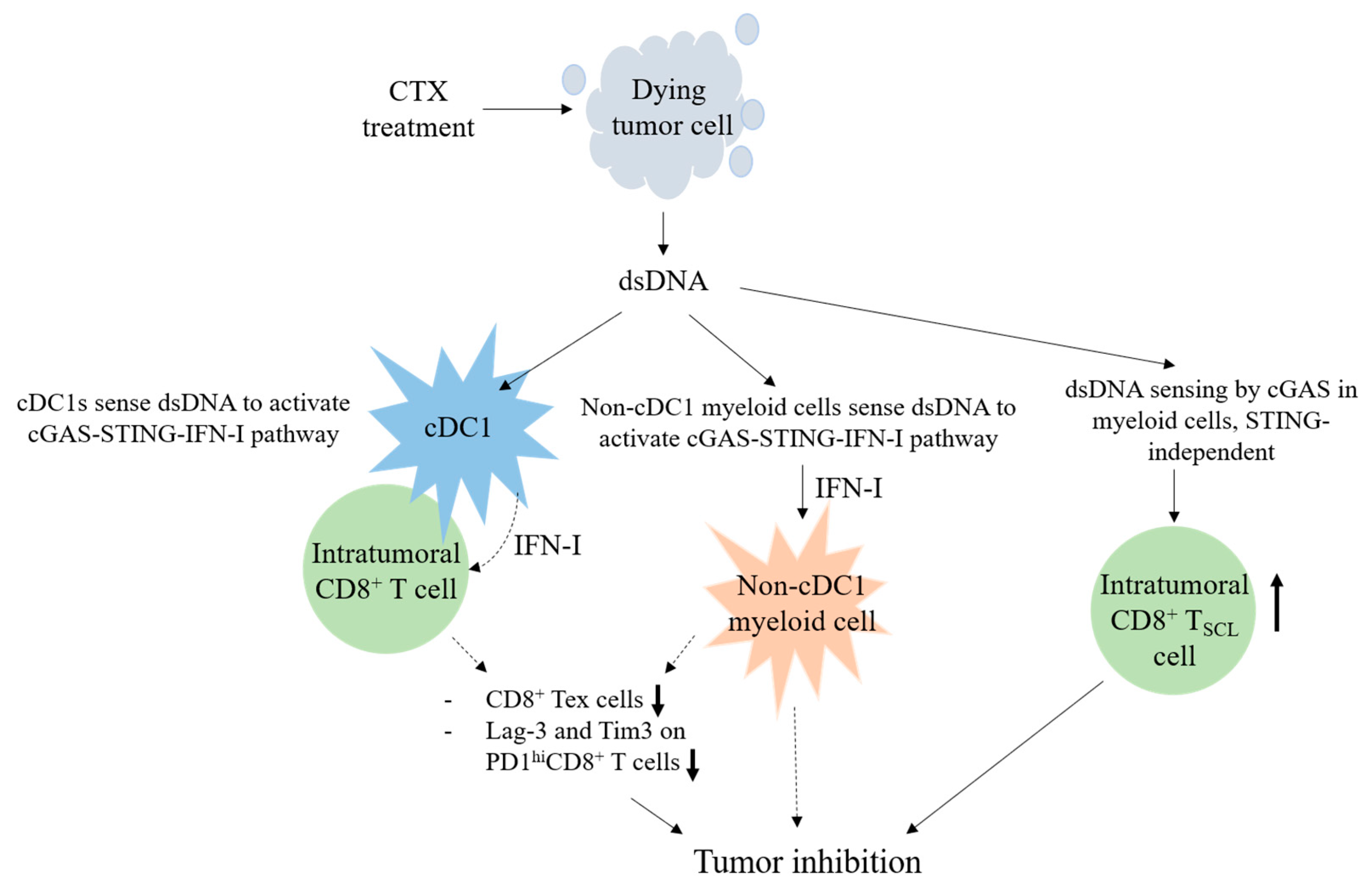
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Osawa, T.; Davies, D.; Hartley, J.A. Mechanism of cell death resulting from DNA interstrand cross-linking in mammalian cells. Cell Death Dis. 2011, 2, e187. [Google Scholar] [CrossRef][Green Version]
- van der Most, R.G.; Currie, A.J.; Cleaver, A.L.; Salmons, J.; Nowak, A.K.; Mahendran, S.; Larma, I.; Prosser, A.; Robinson, B.W.; Smyth, M.J.; et al. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS ONE 2009, 4, e6982. [Google Scholar] [CrossRef]
- Vergato, C.; Doshi, K.A.; Roblyer, D.; Waxman, D.J. Type-I interferon signaling is essential for robust metronomic chemo-immunogenic tumor regression in murine breast cancer. Cancer Res. Commun. 2022, 2, 246–257. [Google Scholar] [CrossRef]
- Wu, J.; Waxman, D.J. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8+ T-cell responses and immune memory. Oncoimmunology 2015, 4, e1005521. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Waxman, D.J. Medium dose intermittent cyclophosphamide induces immunogenic cell death and cancer cell autonomous type I interferon production in glioma models. Cancer Lett. 2020, 470, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, G.; Sistigu, A.; Valentini, M.; Mattei, F.; Sestili, P.; Spadaro, F.; Sanchez, M.; Lorenzi, S.; D’Urso, M.T.; Belardelli, F.; et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011, 71, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Larmonier, N.; Schmitt, E.; Parcellier, A.; Cathelin, D.; Garrido, C.; Chauffert, B.; Solary, E.; Bonnotte, B.; Martin, F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004, 34, 336–344. [Google Scholar] [CrossRef]
- Motoyoshi, Y.; Kaminoda, K.; Saitoh, O.; Hamasaki, K.; Nakao, K.; Ishii, N.; Nagayama, Y.; Eguchi, K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol. Rep. 2006, 16, 141–146. [Google Scholar] [CrossRef]
- Radojcic, V.; Bezak, K.B.; Skarica, M.; Pletneva, M.A.; Yoshimura, K.; Schulick, R.D.; Luznik, L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol. Immunother. 2010, 59, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.C.; Blazar, B.R.; Mellor, A.L.; Munn, D.H.; Zhou, G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood 2010, 115, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; Kadima, A.N.; EL-Naggar, S.A.; Rubinstein, M.P.; Chen, Y.; Gillanders, W.E.; Cole, D.J. Defining the Ability of Cyclophosphamide Preconditioning to Enhance the Antigen-specific CD8+ T-cell Response to Peptide Vaccination: Creation of a Beneficial Host Microenvironment Involving Type I IFNs and Myeloid Cells. J. Immunother. 2007, 30, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, G.; Mattei, F.; Di Pucchio, T.; Santini, S.M.; Bracci, L.; Belardelli, F.; Proietti, E. Cyclophosphamide induces type I interferon and augments the number of CD44hi T lymphocytes in mice: Implications for strategies of chemoimmunotherapy of cancer. Blood 2000, 95, 2024–2030. [Google Scholar] [CrossRef]
- Viaud, S.; Flament, C.; Zoubir, M.; Pautier, P.; LeCesne, A.; Ribrag, V.; Soria, J.C.; Marty, V.; Vielh, P.; Robert, C.; et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011, 71, 661–665. [Google Scholar] [CrossRef]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Gaston, J.; Cheradame, L.; Yvonnet, V.; Deas, O.; Poupon, M.F.; Judde, J.G.; Cairo, S.; Goffin, V. Intracellular STING inactivation sensitizes breast cancer cells to genotoxic agents. Oncotarget 2016, 7, 77205–77224. [Google Scholar] [CrossRef]
- Ewens, A.; Mihich, E.; Ehrke, M.J. Distant Metastasis from Subcutaneously Grown E0771 Medullary Breast Adenocarcinoma. Anticancer Res. 2005, 25, 3905–3915. [Google Scholar]
- Huang, S.W.; Lai, Y.G.; Liao, H.T.; Chang, C.L.; Ma, R.Y.; Chen, Y.H.; Liou, Y.H.; Wu, Z.Q.; Wu, Y.C.; Liu, K.J.; et al. Syngeneic natural killer cell therapy activates dendritic and T cells in metastatic lungs and effectively treats low-burden metastases. eLife 2025, 13, RP99010. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.H.; Hu, Y.; Watson, P.H.; Liu, H.; Geiger, T.R.; Anver, M.R.; Haines, D.C.; Martin, P.; Green, J.E.; et al. Immunocompetent mouse allograft models for development of therapies to target breast cancer metastasis. Oncotarget 2017, 8, 30621–30643. [Google Scholar] [CrossRef]
- Stranges, P.B.; Watson, J.; Cooper, C.J.; Choisy-Rossi, C.M.; Stonebraker, A.C.; Beighton, R.A.; Hartig, H.; Sundberg, J.P.; Servick, S.; Kaufmann, G.; et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 2007, 26, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.H.; Wang, S.W.; Chang, C.L.; Huang, P.L.; Hou, M.S.; Lai, Y.G.; Lee, G.A.; Jiang, S.T.; Tsai, C.Y.; Liao, N.S. Adipocyte IL-15 regulates local and systemic NK cell development. J. Immunol. 2014, 193, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Browder, T.; Butterfield, C.E.; Kraling, B.M.; Shi, B.; Marshall, B.; O’Reilly, M.E.; Folkman, J. Antiangiogenic Scheduling of Chemotherapy Improves Efficacy against Experimental Drug-resistant Cancer. Cancer Res. 2000, 60, 1878–1886. [Google Scholar]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 Deficiency Reveals a Critical Role for CD8α+ Dendritic Cells in Cytotoxic T Cell Immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef]
- Abram, C.L.; Roberge, G.L.; Hu, Y.; Lowell, C.A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 2014, 408, 89–100. [Google Scholar] [CrossRef]
- Dalod, M.; Scheu, S. Dendritic cell functions in vivo: A user’s guide to current and next- generation mutant mouse models. Eur. J. Immunol. 2022, 52, 1712–1749. [Google Scholar] [CrossRef]
- Colpitts, S.L.; Stoklasek, T.A.; Plumlee, C.R.; Obar, J.J.; Guo, C.; Lefrancois, L. Cutting edge: The role of IFN-alpha receptor and MyD88 signaling in induction of IL-15 expression in vivo. J. Immunol. 2012, 188, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, C.J.; Wolf, N.; Chang, I.-C.; Kirn, G.; Marcus, A.; Ndubaku, C.O.; McWhirter, S.M.; Raulet, D.H. NK cells mediate clearance of CD8+ T cell–resistant tumors in response to STING agonists. Sci. Immunol. 2020, 5, eaaz2738. [Google Scholar] [CrossRef]
- Santana Carrero, R.M.; Beceren-Braun, F.; Rivas, S.C.; Hegde, S.M.; Gangadharan, A.; Plote, D.; Pham, G.; Anthony, S.M.; Schluns, K.S. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc. Natl. Acad. Sci. USA 2019, 116, 599–608. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Castiglioni, A.; Yang, Y.; Williams, K.; Gogineni, A.; Lane, R.S.; Wang, A.W.; Shyer, J.A.; Zhang, Z.; Mittman, S.; Gutierrez, A.; et al. Combined PD-L1/TGFbeta blockade allows expansion and differentiation of stem cell-like CD8 T cells in immune excluded tumors. Nat. Commun. 2023, 14, 4703. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, X.; Feng, K.; Gao, R.; Xue, Z.; Zhang, S.; Zhang, Y.; Corse, E.; Hu, Y.; Han, W.; et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat. Cancer 2022, 3, 108–121. [Google Scholar] [CrossRef]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef]
- Gebhardt, T.; Park, S.L.; Parish, I.A. Stem-like exhausted and memory CD8+ T cells in cancer. Nat. Rev. Cancer 2023, 23, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Schaeuble, K.; Chennupati, V.; Fuertes Marraco, S.A.; Calderon-Copete, S.; Pais Ferreira, D.; Carmona, S.J.; Scarpellino, L.; Gfeller, D.; Pradervand, S.; et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 2019, 50, 195–211.e10. [Google Scholar] [CrossRef]
- Jneid, B.; Bochnakian, A.; Hoffmann, C.; Delisle, F.; Djacoto, E.; Sirven, P.; Denizeau, J.; Sedlik, C.; Gerber-Ferder, Y.; Fiore, F.; et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci. Immunol. 2023, 8, eabn6612. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Wang, M.; Wang, X.; Chen, S.; Sun, Z.; Ren, X.; Huang, G.; Sumer, B.D.; Yan, N.; et al. STING licensing of type I dendritic cells potentiates antitumor immunity. Sci. Immunol. 2024, 8, eabn6612. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Lorenzi, S.; Mattei, F.; Sistigu, A.; Bracci, L.; Spadaro, F.; Sanchez, M.; Spada, M.; Belardelli, F.; Gabriele, L.; Schiavoni, G. Type I IFNs control antigen retention and survival of CD8alpha+ dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J. Immunol. 2011, 186, 5142–5150. [Google Scholar] [CrossRef]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8alpha+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Mattiuz, R.; Brousse, C.; Ambrosini, M.; Cancel, J.C.; Bessou, G.; Mussard, J.; Sanlaville, A.; Caux, C.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; et al. Type 1 conventional dendritic cells and interferons are required for spontaneous CD4+ and CD8+ T-cell protective responses to breast cancer. Clin. Transl. Immunol. 2021, 10, e1305. [Google Scholar] [CrossRef]
- Ataide, M.A.; Komander, K.; Knopper, K.; Peters, A.E.; Wu, H.; Eickhoff, S.; Gogishvili, T.; Weber, J.; Grafen, A.; Kallies, A.; et al. BATF3 programs CD8+ T cell memory. Nat. Immunol. 2020, 21, 1397–1407. [Google Scholar] [CrossRef]
- Qiu, Z.; Khairallah, C.; Romanov, G.; Sheridan, B.S. Cutting Edge: Batf3 Expression by CD8 T Cells Critically Regulates the Development of Memory Populations. J. Immunol. 2020, 205, 901–906. [Google Scholar] [CrossRef]
- Carozza, J.A.; Bohnert, V.; Nguyen, K.C.; Skariah, G.; Shaw, K.E.; Brown, J.A.; Rafat, M.; von Eyben, R.; Graves, E.E.; Glenn, J.S.; et al. Extracellular cGAMP is a cancer cell-produced immunotransmitter involved in radiation-induced anti-cancer immunity. Nat. Cancer 2020, 1, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duran, M.A.; Dhanota, N.; Chatila, W.K.; Bettigole, S.E.; Kwon, J.; Sriram, R.K.; Humphries, M.P.; Salto-Tellez, M.; James, J.A.; et al. Metastasis and Immune Evasion from Extracellular cGAMP Hydrolysis. Cancer Discov. 2021, 11, 1212–1227. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef]
- Li, J.; Hubisz, M.J.; Earlie, E.M.; Duran, M.A.; Hong, C.; Varela, A.A.; Lettera, E.; Deyell, M.; Tavora, B.; Havel, J.J.; et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature 2023, 620, 1080–1088. [Google Scholar] [CrossRef]
- Hosea, R.; Hillary, S.; Naqvi, S.; Wu, S.; Kasim, V. The two sides of chromosomal instability: Drivers and brakes in cancer. Signal Transduct. Target. Ther. 2024, 9, 75. [Google Scholar] [CrossRef]
- Lu, C.; Guan, J.; Lu, S.; Jin, Q.; Rousseau, B.; Lu, T.; Stephens, D.; Zhang, H.; Zhu, J.; Yang, M.; et al. DNA Sensing in Mismatch Repair-Deficient Tumor Cells Is Essential for Anti-tumor Immunity. Cancer Cell 2021, 39, 96–108 e106. [Google Scholar] [CrossRef] [PubMed]
- Pantelidou, C.; Sonzogni, O.; De Oliveria Taveira, M.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Efficacy Depends on CD8+ T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.; Mao, A.J.; Lensink-Vasan, M.; Wang, L.; Vance, R.E.; Raulet, D.H. Tumor-Derived cGAMP Triggers a STING-Mediated Interferon Response in Non-tumor Cells to Activate the NK Cell Response. Immunity 2018, 49, 754–763 e754. [Google Scholar] [CrossRef] [PubMed]
- Schadt, L.; Sparano, C.; Schweiger, N.A.; Silina, K.; Cecconi, V.; Lucchiari, G.; Yagita, H.; Guggisberg, E.; Saba, S.; Nascakova, Z.; et al. Cancer-Cell-Intrinsic cGAS Expression Mediates Tumor Immunogenicity. Cell Rep. 2019, 29, 1236–1248 e1237. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Zhang, J.; Wang, Y.; Simoneau, A.; Yang, H.; Levine, A.S.; Zou, L.; Chen, Z.J.; Lan, L. cGAS suppresses genomic instability as a decelerator of replication forks. Sci. Adv. 2020, 6, eabb8961. [Google Scholar] [CrossRef]
- Cui, S.; Yu, Q.; Chu, L.; Cui, Y.; Ding, M.; Wang, Q.; Wang, H.; Chen, Y.; Liu, X.; Wang, C. Nuclear cGAS Functions Non-canonically to Enhance Antiviral Immunity via Recruiting Methyltransferase Prmt5. Cell Rep. 2020, 33, 108490. [Google Scholar] [CrossRef]
- Ma, D.; Yang, M.; Sun, C.; Cui, X.; Xiong, G.; Wang, Q.; Jing, W.; Chen, H.; Lv, X.; Liu, S.; et al. cGAS suppresses hepatocellular carcinoma independent of its cGAMP synthase activity. Cell Death Differ. 2024, 31, 722–737. [Google Scholar] [CrossRef]
- Banerjee, D.; Langberg, K.; Abbas, S.; Odermatt, E.; Yerramothu, P.; Volaric, M.; Reidenbach, M.A.; Krentz, K.J.; Rubinstein, C.D.; Brautigan, D.L.; et al. A non-canonical, interferon-independent signaling activity of cGAMP triggers DNA damage response signaling. Nat. Commun. 2021, 12, 6207. [Google Scholar] [CrossRef]
- Su, J.; Coleman, P.; Ntorla, A.; Anderson, R.; Shattock, M.J.; Burgoyne, J.R. Sensing Cytosolic DNA Lowers Blood Pressure by Direct cGAMP-Dependent PKGI Activation. Circulation 2023, 148, 1023–1034. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-G.; Liao, H.-T.; Chen, Y.-H.; Huang, S.-W.; Liou, Y.-H.; Wu, Z.-Q.; Liao, N.-S. cGAS and STING in Host Myeloid Cells Are Essential for Effective Cyclophosphamide Treatment of Advanced Breast Cancer. Cancers 2025, 17, 1130. https://doi.org/10.3390/cancers17071130
Lai Y-G, Liao H-T, Chen Y-H, Huang S-W, Liou Y-H, Wu Z-Q, Liao N-S. cGAS and STING in Host Myeloid Cells Are Essential for Effective Cyclophosphamide Treatment of Advanced Breast Cancer. Cancers. 2025; 17(7):1130. https://doi.org/10.3390/cancers17071130
Chicago/Turabian StyleLai, Yein-Gei, Hao-Ting Liao, Yung-Hsiang Chen, Shih-Wen Huang, Yae-Huei Liou, Zhen-Qi Wu, and Nan-Shih Liao. 2025. "cGAS and STING in Host Myeloid Cells Are Essential for Effective Cyclophosphamide Treatment of Advanced Breast Cancer" Cancers 17, no. 7: 1130. https://doi.org/10.3390/cancers17071130
APA StyleLai, Y.-G., Liao, H.-T., Chen, Y.-H., Huang, S.-W., Liou, Y.-H., Wu, Z.-Q., & Liao, N.-S. (2025). cGAS and STING in Host Myeloid Cells Are Essential for Effective Cyclophosphamide Treatment of Advanced Breast Cancer. Cancers, 17(7), 1130. https://doi.org/10.3390/cancers17071130





