Immune Checkpoint Inhibitor-Induced Pancreatic Injury (ICI-PI) in Adult Cancer Patients: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Protocol
2.2. Study Selection
2.3. Data Extraction
2.4. Methodological Quality
2.5. Data Analyses
3. Results
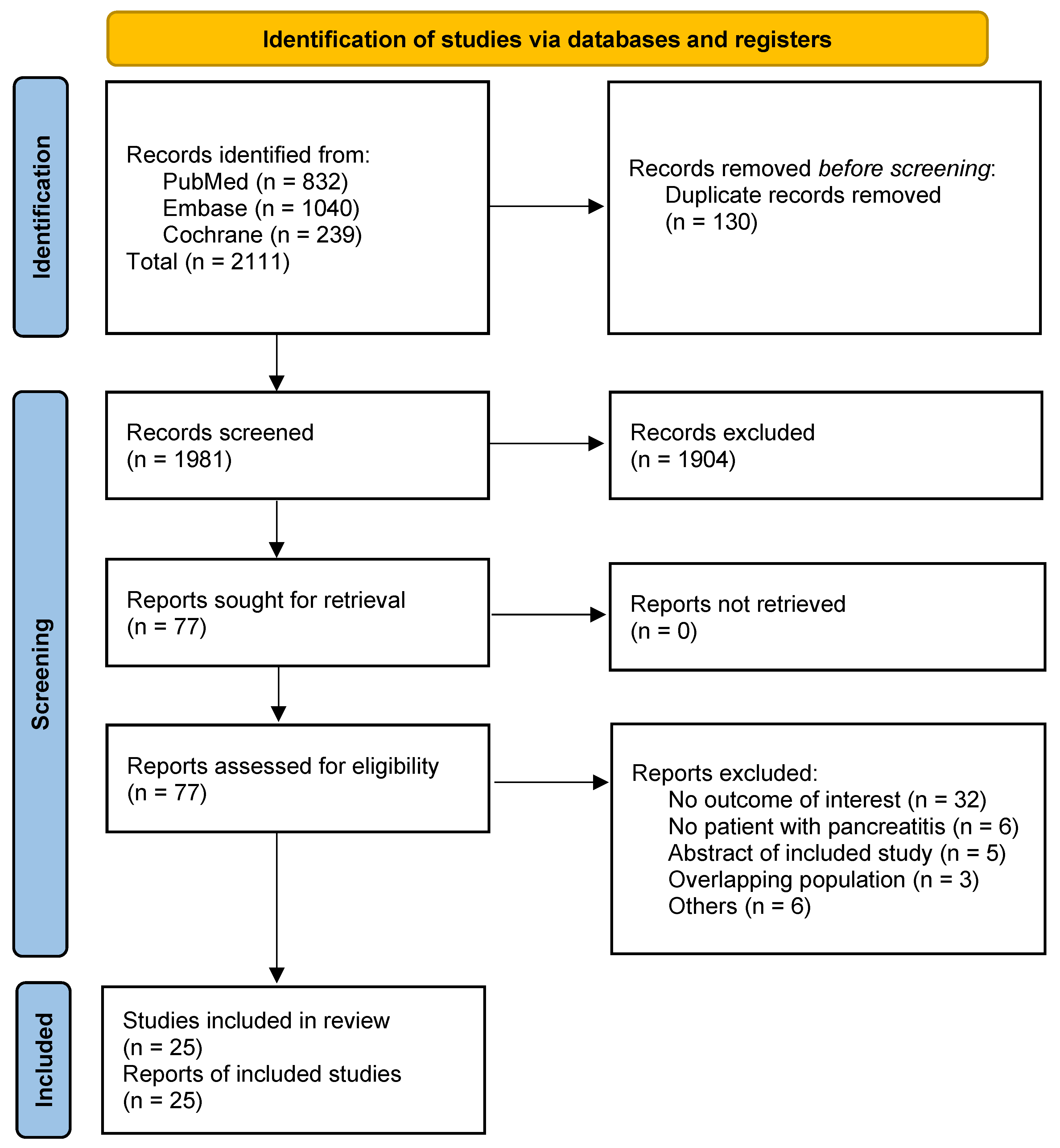
3.1. Patient Selection
3.2. Study Characteristics
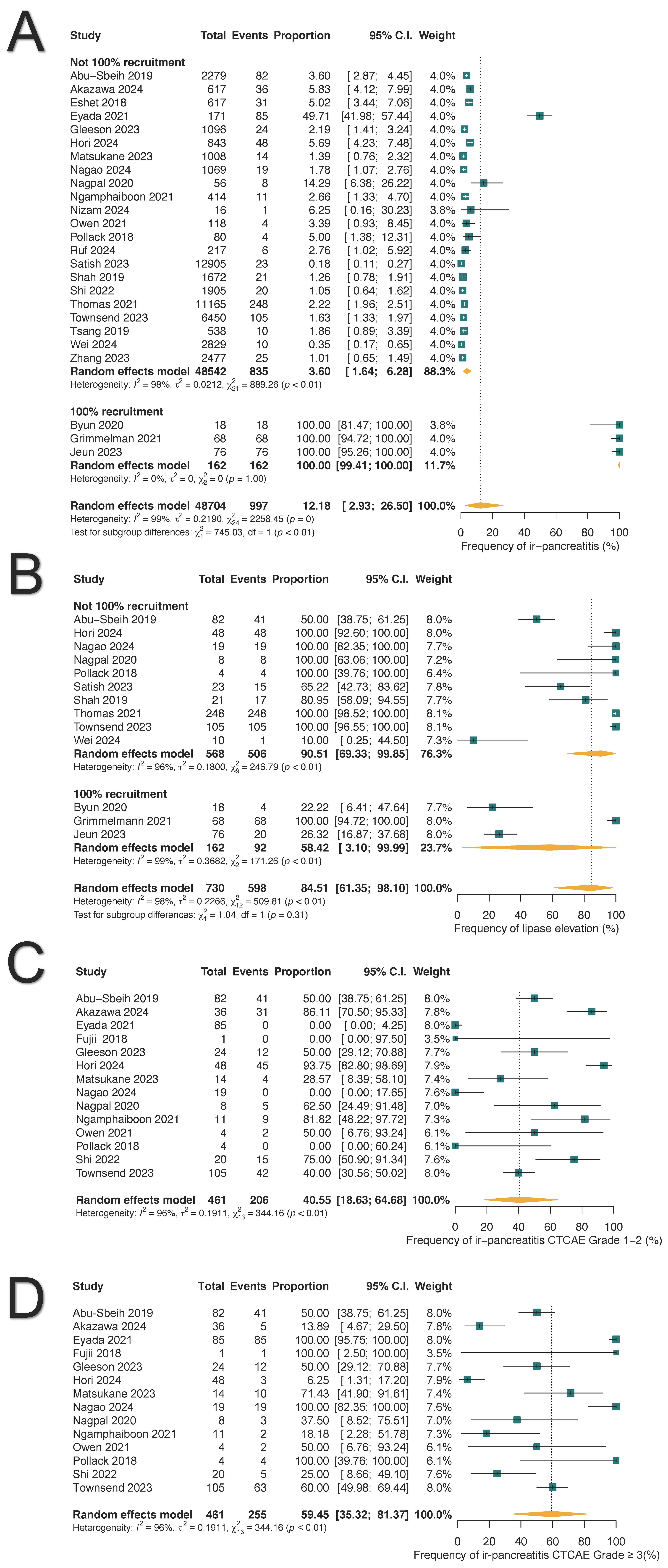
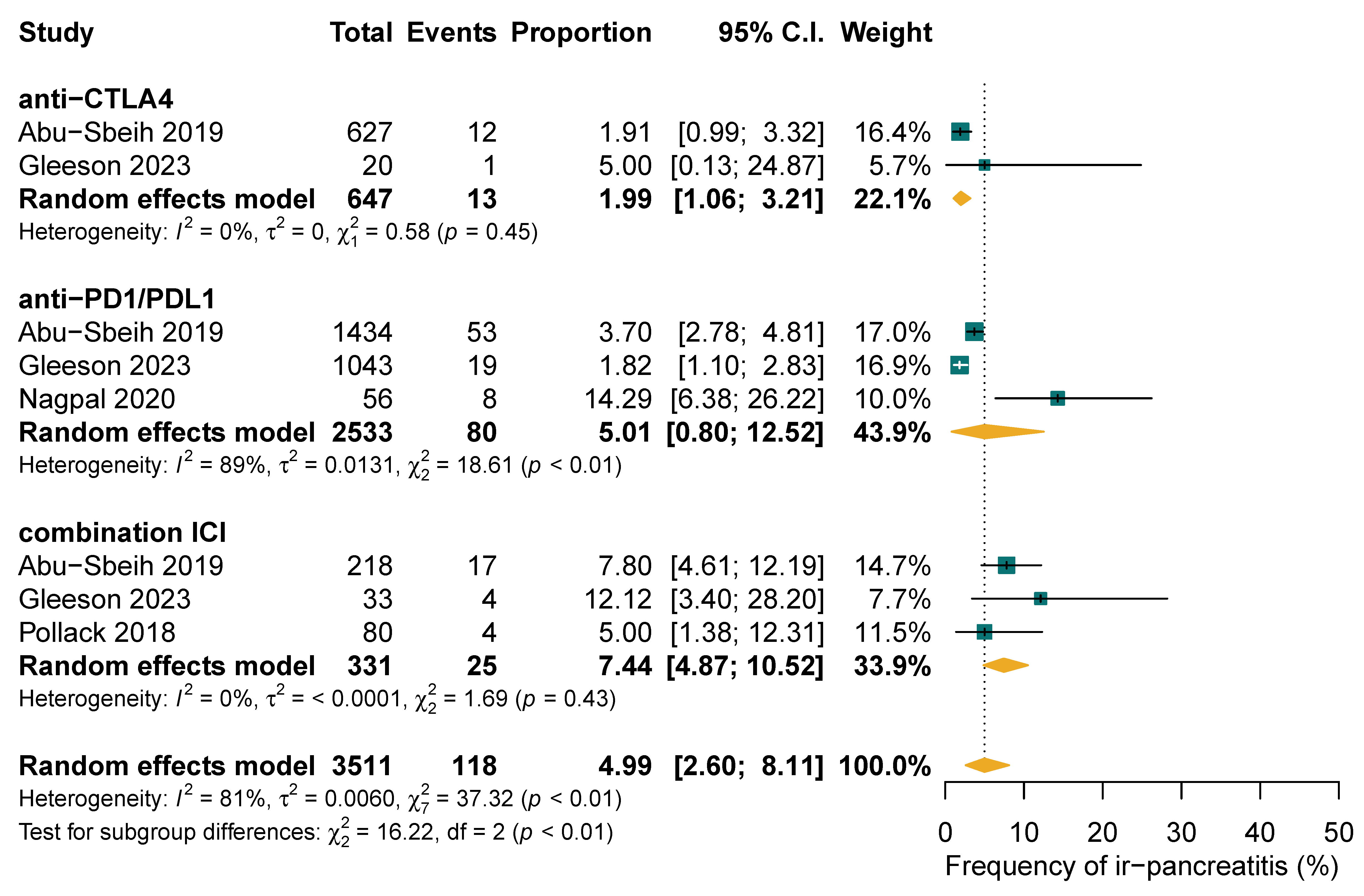
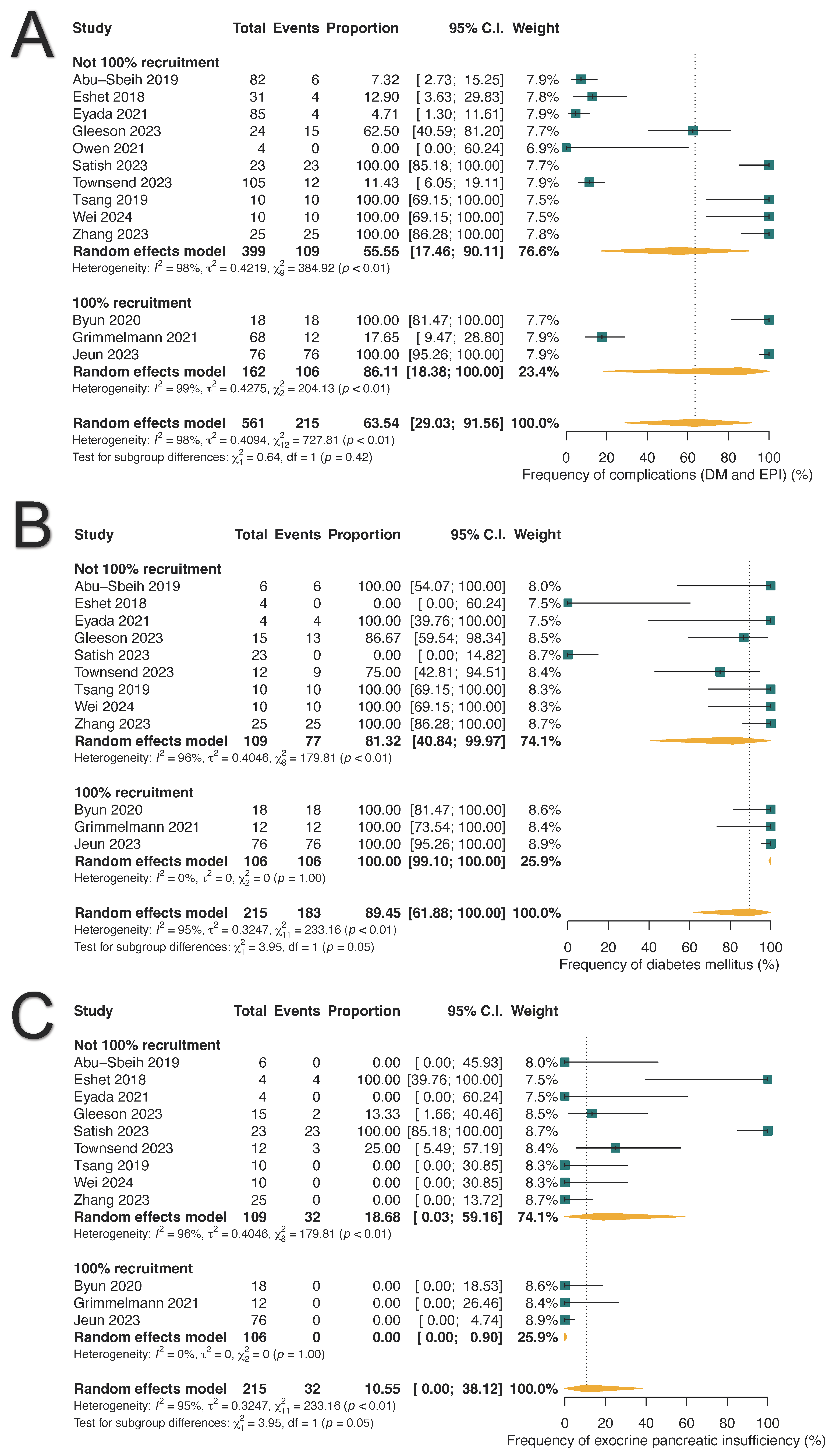
3.3. Chronic Adverse Outcomes of ICI-PI
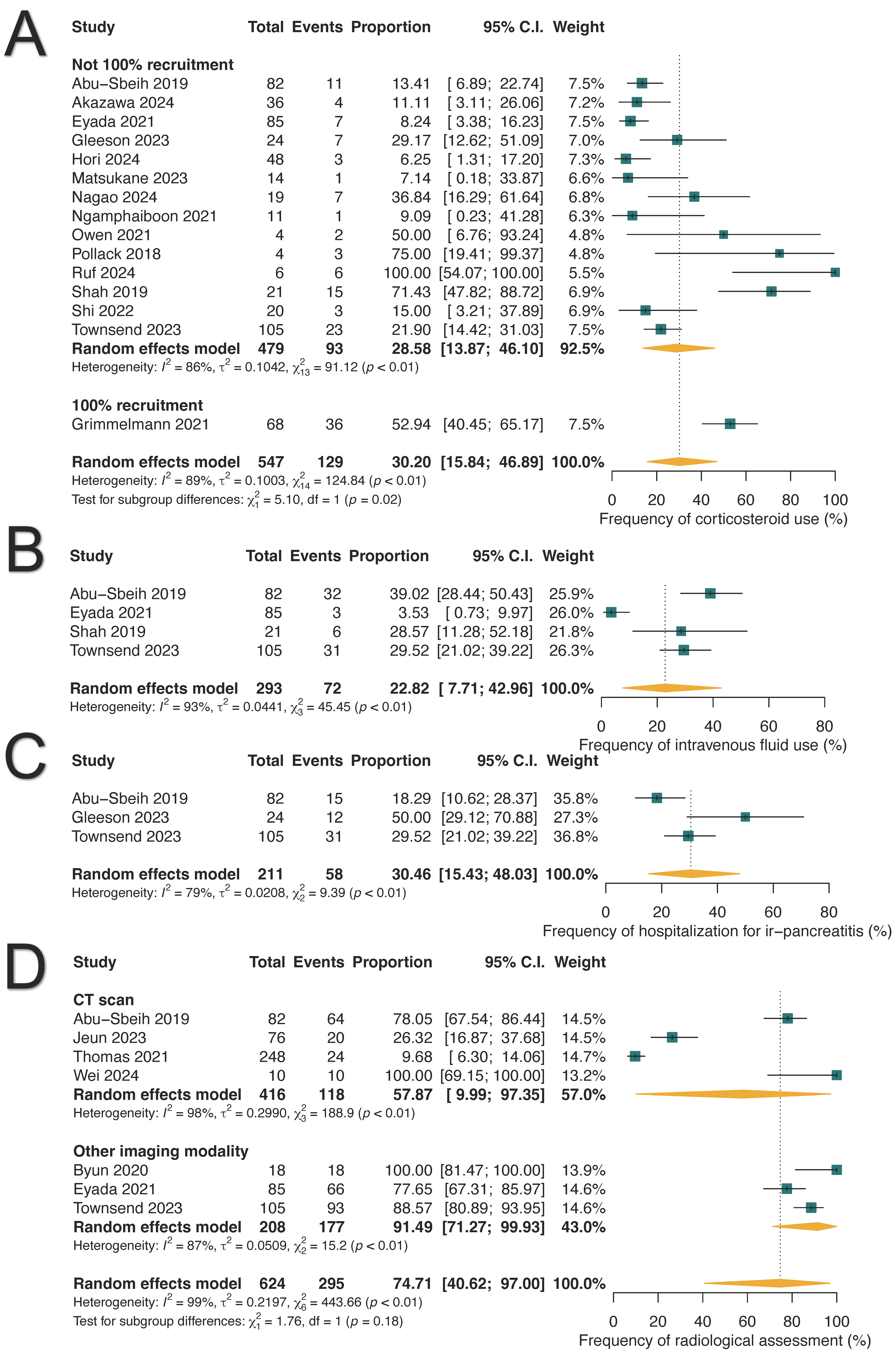
3.4. Clinical Management of ICI-PI
3.5. Survival Endpoints
3.6. Sensitivity Analysis and Bias Assessment
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.S.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the Tumor Microenvironment for Effective Immunotherapy. Med. Res. Rev. 2020, 41, 1474. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, C.H. Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice. Diabetes Metab. J. 2023, 47, 757–766. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, X.-L.; Liu, L.; Han, X.-Y.; Ji, L.-N. Type 1 Diabetes Induced by Immune Checkpoint Inhibitors. Chin. Med. J. 2020, 133, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Murakami, K.; Koiwai, A.; Kawamura, K.; Yoshino, Y.; Takasu, A.; Kin, R.; Katayama, T.; Endo, K.; Kogure, T.; et al. Neutrophil Infiltration and Acinar-Ductal Metaplasia Are the Main Pathological Findings in Pembrolizumab-Induced Pancreatitis. Intern. Med. 2022, 61, 3675–3682. [Google Scholar] [CrossRef]
- Yoneda, S.; Imagawa, A.; Hosokawa, Y.; Baden, M.Y.; Kimura, T.; Uno, S.; Fukui, K.; Goto, K.; Uemura, M.; Eguchi, H.; et al. T-Lymphocyte Infiltration to Islets in the Pancreas of a Patient Who Developed Type 1 Diabetes After Administration of Immune Checkpoint Inhibitors. Diabetes Care 2019, 42, e116–e118. [Google Scholar] [CrossRef]
- Nwankwo, O.C.; Lara-Salazar, F.M.; Lara-Salazar, S.; Abdulrahim, A.O.; Chijioke, I.; Singh, J.; Koradia, I.; Gomez, N.M.; Prakash, R.; Gopagoni, R.; et al. Immune Checkpoint Inhibitors in Cancer Treatment and Incidence of Pancreatitis. Cureus 2024, 16, e68043. [Google Scholar] [CrossRef]
- Wei, H.-H.; Lai, Y.-C.; Lin, G.; Lin, C.-W.; Chang, Y.-C.; Chang, J.W.-C.; Liou, M.-J.; Chen, I.-W. Distinct Changes to Pancreatic Volume Rather than Pancreatic Autoantibody Positivity: Insights into Immune Checkpoint Inhibitors Induced Diabetes Mellitus. Diabetol. Metab. Syndr. 2024, 16, 26. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Tang, T.; Lu, Y.; Thirumurthi, S.; Altan, M.; Jazaeri, A.A.; Dadu, R.; Coronel, E.; Wang, Y. Clinical Characteristics and Outcomes of Immune Checkpoint Inhibitor-Induced Pancreatic Injury. J. Immunotherapy Cancer 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.S.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of Pancreatitis with the Use of Immune Checkpoint Inhibitors (ICI) in Advanced Cancers: A Systematic Review and Meta-Analysis. Pancreatology 2019, 19, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.Z.; Bhayana, V. Lipase or Amylase for the Diagnosis of Acute Pancreatitis? Clin. Biochem. 2017, 50, 1275–1280. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published on June 14th, 2010. Grading for Pancreatitis on Page 48. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 17 January 2025).
- U.S. Department of Health and Human Services. Grading for Pancreatitis. In Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017; p. 35. [Google Scholar]
- U.S. Department of Health and Human Services. Grading for Hyperlipasemia. In Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017; p. 88. [Google Scholar]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. JCO 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, R.J.; De Groot, J.S.; Fabriek, B.O.; Hew, M.N.; May, A.M.; Suijkerbuijk, K.P.M. Corticosteroids for Immune-Related Adverse Events and Checkpoint Inhibitor Efficacy: Analysis of Six Clinical Trials. JCO 2024, 42, 3713–3724. [Google Scholar] [CrossRef]
- Cook, S.; Samuel, V.; Meyers, D.E.; Stukalin, I.; Litt, I.; Sangha, R.; Morris, D.G.; Heng, D.Y.C.; Pabani, A.; Dean, M.; et al. Immune-Related Adverse Events and Survival Among Patients with Metastatic NSCLC Treated with Immune Checkpoint Inhibitors. JAMA Netw. Open 2024, 7, e2352302. [Google Scholar] [CrossRef]
- Van Buren, I.; Madison, C.; Kohn, A.; Berry, E.; Kulkarni, R.P.; Thompson, R.F. Survival Among Veterans Receiving Steroids for Immune-Related Adverse Events After Immune Checkpoint Inhibitor Therapy. JAMA Netw. Open 2023, 6, e2340695. [Google Scholar] [CrossRef]
- Bai, X.; Hu, J.; Betof Warner, A.; Quach, H.T.; Cann, C.G.; Zhang, M.Z.; Si, L.; Tang, B.; Cui, C.; Yang, X.; et al. Early Use of High-Dose Glucocorticoid for the Management of irAE Is Associated with Poorer Survival in Patients with Advanced Melanoma Treated with Anti–PD-1 Monotherapy. Clin. Cancer Res. 2021, 27, 5993–6000. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; Van Den Berkmortel, F.W.P.J.; Van Den Eertwegh, A.J.M.; De Groot, J.W.B.; Boers-Sonderen, M.J.; Van Der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1–Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]
- Verheijden, R.J.; Burgers, F.H.; Janssen, J.C.; Putker, A.E.; Veenstra, S.P.G.R.; Hospers, G.A.P.; Aarts, M.J.B.; Hehenkamp, K.W.; Doornebosch, V.L.E.; Verhaert, M.; et al. Corticosteroids and Other Immunosuppressants for Immune-Related Adverse Events and Checkpoint Inhibitor Effectiveness in Melanoma. Eur. J. Cancer 2024, 207, 114172. [Google Scholar] [CrossRef] [PubMed]
- Koseki, M.; Nishimura, Y.; Elias, E.; Estaris, J.; Chesta, F.; Takaoka, K.; Shao, T.; Horita, N.; Fujiwara, Y. Pancreatitis in Patients with Cancer Receiving Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Targ. Oncol. 2024, 19, 867–877. [Google Scholar] [CrossRef]
- Oliveira, C.; Mainoli, B.; Duarte, G.S.; Machado, T.; Tinoco, R.G.; Esperança-Martins, M.; Ferreira, J.J.; Costa, J. Immune-Related Serious Adverse Events with Immune Checkpoint Inhibitors: Systematic Review and Network Meta-Analysis. Eur. J. Clin. Pharmacol. 2024, 80, 677–684. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, W.; Pang, L.; Zeng, L.; Liu, S.; Liu, J. Pancreatic Adverse Events of Immune Checkpoint Inhibitors Therapy for Solid Cancer Patients: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1166299. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Shi, C.; Liu, X.; Lv, S.; Wang, X.; Li, W. Pancreatic Injury Following Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 955701. [Google Scholar] [CrossRef]
- Kramer, S.; van Hee, K.; Blokzijl, H.; van der Heide, F.; Visschedijk, M.C. Immune Checkpoint Inhibitor-Related Pancreatitis: A Case Series, Review of the Literature and an Expert Opinion. J. Immunother. 2023, 46, 271. [Google Scholar] [CrossRef]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 1st ed.; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-53662-8. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- JBI Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 29 December 2024).
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting Proportional Meta-Analysis in Different Types of Systematic Reviews: A Guide for Synthesisers of Evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Schwarzer, G.; Rücker, G. Meta-Analysis of Proportions. In Meta-Research; Evangelou, E., Veroniki, A.A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2345, pp. 159–172. ISBN 978-1-07-161565-2. [Google Scholar]
- Townsend, M.J.; Liu, M.; Giobbie-Hurder, A.; Sack, J.S.; LeBoeuf, N.R.; Hodi, F.S.; McNabb-Baltar, J.; Grover, S. Pancreatitis and Hyperlipasemia in the Setting of Immune Checkpoint Inhibitor Therapy. J. Natl. Compr. Cancer Network JNCCN 2023, 21, 831–840.e3. [Google Scholar] [CrossRef]
- Akazawa, Y.; Nosaka, T.; Takahashi, K.; Naito, T.; Matsuda, H.; Ohtani, M.; Nakamoto, Y. 967 Analysis of Risk Factors and Long-Term Prognosis in Patients with Immune Checkpoint Inhibitor-Induced Pancreatic Injury. Gastroenterology 2024, 166, S-231. [Google Scholar] [CrossRef]
- Byun, D.J.; Braunstein, R.; Flynn, J.; Zheng, J.; Lefkowitz, R.A.; Kanbour, S.; Girotra, M. Immune Checkpoint Inhibitor-Associated Diabetes: A Single-Institution Experience. Diabetes Care 2020, 43, 3106–3109. [Google Scholar] [CrossRef] [PubMed]
- Eshet, Y.; Baruch, E.N.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Kuznetsov, T.; Ben-Betzalel, G.; Daher, S.; Gluck, I.; Asher, N.; Apter, S.; et al. Clinical Significance of Pancreatic Atrophy Induced by Immune-Checkpoint Inhibitors: A Case–Control Study. Cancer Immunol. Res. 2018, 6, 1453–1458. [Google Scholar] [CrossRef]
- Eyada, M.; Issac, A.; Abraham, F.; Jacob, J.S.; Chari, S.; Wang, Y.; Thomas, A.S. Clinical Profiles of Asymptomatic Immune Check Point Inhibitor Induced Pancreatitis (Ici-Pi), A Type 3 Autoimmune Pancreatitis. Gastroenterology 2021, 160, S-664. [Google Scholar] [CrossRef]
- Gleeson, F.C.; Kottschade, L.; Dunleavy, K.A.; Carr, R.M.; Hartgers, M.; Levy, M.J.; Ma, W.W.; McWilliams, R.; Egan, A. Incidence and effect duration of immune checkpoint inhibitor related pancreas adverse events. Gastroenterology 2023, 164, S-1049. [Google Scholar] [CrossRef]
- Grimmelmann, I.; Momma, M.; Zimmer, L.; Hassel, J.C.; Heinzerling, L.; Pföhler, C.; Loquai, C.; Ruini, C.; Utikal, J.; Thoms, K.-M.; et al. Lipase Elevation and Type 1 Diabetes Mellitus Related to Immune Checkpoint Inhibitor Therapy—A Multicentre Study of 90 Patients from the German Dermatooncology Group. Eur. J. Cancer 2021, 149, 1–10. [Google Scholar] [CrossRef]
- Hori, Y.; Naitoh, I.; Naiki-Ito, A.; Kawai, T.; Yoshida, M.; Kato, A.; Kachi, K.; Sahashi, H.; Adachi, A.; Toyohara, T.; et al. Incidence of Pancreatic Injury and Pancreatitis in Patients Treated with Immune Checkpoint Inhibitors. Clin. Transl. Gastroenterol. 2024, 15, e00667. [Google Scholar] [CrossRef] [PubMed]
- Jeun, R.; Iyer, P.C.; Best, C.; Lavis, V.; Varghese, J.M.; Yedururi, S.; Brady, V.; Oliva, I.C.G.; Dadu, R.; Milton, D.R.; et al. Clinical Outcomes of Immune Checkpoint Inhibitor Diabetes Mellitus at a Comprehensive Cancer Center. Immunotherapy 2023, 15, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Matsukane, R.; Suetsugu, K.; Hata, K.; Matsuda, K.; Nakao, S.; Minami, H.; Watanabe, H.; Hirota, T.; Egashira, N.; Ieiri, I. Systematic Surveillance of Immune-Related Adverse Events in Clinical Practice and Impact of Subsequent Steroid Medication on Survival Outcomes. Int. J. Clin. Oncol. 2023, 28, 860–871. [Google Scholar] [CrossRef]
- Nagao, K.; Sakai, A.; Tsumura, H.; Iemoto, T.; Hirata, Y.; Hori, H.; Ogisu, K.; Kakuyama, S.; Ikegawa, T.; Hirata, T.; et al. Pancreatic Injury in Patients Treated with Immune Checkpoint Inhibitors: A Retrospective Multicenterstudy. J. Gastroenterol. 2024, 59, 424–433. [Google Scholar] [CrossRef]
- Nagpal, S.; Peeraphatdit, T.; Reid, P. Clinical Characteristics of Patients with Atezolizumab Induced Pancreatic Injury. Gastroenterology 2020, 158, S-872. [Google Scholar] [CrossRef]
- Ngamphaiboon, N.; Ithimakin, S.; Siripoon, T.; Sintawichai, N.; Sriuranpong, V. Patterns and Outcomes of Immune-Related Adverse Events in Solid Tumor Patients Treated with Immune Checkpoint Inhibitors in Thailand: A Multicenter Analysis. BMC Cancer 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Nizam, A.; Rader, R.K.; Tzeng, A.; Wei, W.; Sheng, I.Y.-F.; Martin, A.; Wee, C.E.; Gilligan, T.D.; Gupta, S.; Ornstein, M.C. Safety and Efficacy Outcomes in Immune Checkpoint Inhibitor-Treated Patients with Metastatic Urothelial Carcinoma Requiring Treatment Interruption or Discontinuation Due to Immune-Related Adverse Events. Clin. Genitourin. Cancer 2024, 22, 368–379. [Google Scholar] [CrossRef]
- Owen, C.N.; Bai, X.; Quah, T.; Lo, S.N.; Allayous, C.; Callaghan, S.; Martínez-Vila, C.; Wallace, R.; Bhave, P.; Reijers, I.L.M.; et al. Delayed Immune-Related Adverse Events with Anti-PD-1-Based Immunotherapy in Melanoma. Ann. Oncol. 2021, 32, 917–925. [Google Scholar] [CrossRef]
- Pollack, M.H.; Betof, A.; Dearden, H.; Rapazzo, K.; Valentine, I.; Brohl, A.S.; Ancell, K.K.; Long, G.V.; Menzies, A.M.; Eroglu, Z.; et al. Safety of Resuming Anti-PD-1 in Patients with Immune-Related Adverse Events (irAEs) during Combined Anti-CTLA-4 and Anti-PD1 in Metastatic Melanoma. Ann. Oncol. 2018, 29, 250–255. [Google Scholar] [CrossRef]
- Ruf, T.; Kramer, R.; Forschner, A.; Leiter, U.; Meier, F.; Reinhardt, L.; Dücker, P.; Ertl, C.; Tomsitz, D.; Tietze, J.K.; et al. Second-Line Therapies for Steroid-Refractory Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitors. Eur. J. Cancer 2024, 203, 114028. [Google Scholar] [CrossRef]
- Satish, D.; Lin, I.-H.; Flory, J.; Gerdes, H.; Postow, M.A.; Faleck, D.M. Exocrine Pancreatic Insufficiency Induced by Immune Checkpoint Inhibitors. Oncologist 2023, 28, 1085–1093. [Google Scholar] [CrossRef]
- Shah, R.; Sleiman, J.; Simons-Linares, R.; Faisal, M.; Song, J.-M.; Philpott, J.; Funchain, P. Variations of Diagnosis and Management of Immune Checkpoint Inhibitor Pancreatic Injury (ICIPI) and Immune Checkpoint Inhibitor Pancreatitis: A Single Institution Experience. Am. J. Gastroenterol. 2019, 114, S58. [Google Scholar] [CrossRef]
- Shi, Y.; Fang, J.; Zhou, C.; Liu, A.; Wang, Y.; Meng, Q.; Ding, C.; Ai, B.; Gu, Y.; Yao, Y.; et al. Immune Checkpoint Inhibitor-Related Adverse Events in Lung Cancer: Real-World Incidence and Management Practices of 1905 Patients in China. Thorac. Cancer 2022, 13, 412–422. [Google Scholar] [CrossRef]
- Shirwaikar Thomas, A.; Yedururi, S.; Eyada, M.; Wang, Y.; Chari, S.T. Symptomatic Type 3 Autoimmune Pancreatitis/Immune Checkpoint Inhibitor Pancreas Injury (ICI-PI) Leads to Chronic Pancreatic Injury. Pancreas 2021, 50, 1098. [Google Scholar] [CrossRef]
- Tsang, V.H.M.; McGrath, R.T.; Clifton-Bligh, R.J.; Scolyer, R.A.; Jakrot, V.; Guminski, A.D.; Long, G.V.; Menzies, A.M. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct from Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 5499–5506. [Google Scholar] [CrossRef]
- Zhang, Z.; Sharma, R.; Hamad, L.; Riebandt, G.; Attwood, K. Incidence of Diabetes Mellitus in Patients Treated with Immune Checkpoint Inhibitors (ICI) Therapy—A Comprehensive Cancer Center Experience. Diabetes Res. Clin. Pract. 2023, 202, 110776. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Esteves, K.N.; Shank, K.R.; Deutsch, A.J.; Gunturi, A.; Chamorro-Pareja, N.; Colling, C.A.; Zubiri, L.; Perlman, K.; Ouyang, T.; Villani, A.-C.; et al. Identification of Immune Checkpoint Inhibitor–Induced Diabetes. JAMA Oncol. 2024, 10, 1409. [Google Scholar] [CrossRef] [PubMed]
- De Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van Der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune Checkpoint Inhibitors and Type 1 Diabetes Mellitus: A Case Report and Systematic Review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Chen, X.; Affinati, A.H.; Lee, Y.; Turcu, A.F.; Henry, N.L.; Schiopu, E.; Qin, A.; Othus, M.; Clauw, D.; Ramnath, N.; et al. Immune Checkpoint Inhibitors and Risk of Type 1 Diabetes. Diabetes Care 2022, 45, 1170–1176. [Google Scholar] [CrossRef]
- Hilder, R.; Tsai, K.; Quandt, Z.; Isaacs, D.; Drakaki, A.; Xing, Y.; In, G.K.; Angell, T.E.; Lechner, M.G. Safety and Efficacy of Immune Checkpoint Inhibitor Cancer Therapy in Patients with Preexisting Type 1 Diabetes Mellitus. Front. Endocrinol. 2023, 14, 1242830. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Zhang, Y.; Fang, W.; Yang, Y.; Huang, Y.; Zhang, L. Reporting of Immune Checkpoint Inhibitor Therapy–Associated Diabetes, 2015–2019. Diabetes Care 2020, 43, e79–e80. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Varnier, R.; Fontaine-Delaruelle, C.; Freymond, N.; Essongue, A.; Bouali, A.; Boschetti, G.; Lebosse, F.; Tartas, S.; Milley, S.; Cugnet-Anceau, C.; et al. Evolving Practices in Immune-Related Adverse Event Management: Insights from the IMMUCARE Multidisciplinary Board. JCO Oncol. Pr. 2024, 21, 342–350. [Google Scholar] [CrossRef]
| Study $ | Total Patients | Patients with ICI-PI (n/%) | Male (n/%) | Median Age; Years (IQR or Range) | Median Follow-Up; Months (Range) |
|---|---|---|---|---|---|
| Abu-Sbeih, 2019 [11] | 2279 | 82/3.6 | 54/65.9 | 57 (14) † | NA |
| Akazawa, 2014 [37] | 617 | 36/5.8 | 468/75.9 * | 70 * | NA |
| Byun, 2020 [38] | 18 | 18/100 | 10/55.6 | 64 (27–78) | 22.6 (0.9–33.4) |
| Eshet, 2018 [39] | 617 | 31/5 | 20/64.5 | 63 (35–80) | 14.5 (6.5–20) |
| Eyada, 2021 [40] | 171 | 85/49.7 | 62/72.9 | 61 (4) † | 2.5 |
| Gleeson, 2023 [41] | 1096 | 24/2.2 | 629/57.4 * | 68 (59–75) * | NA |
| Grimmelmann, 2021 [42] | 68 | 68/100 | 33/48.5 | 58 (20–90) | 13 (8.1–22.8) |
| Hori, 2024 [43] | 843 | 48/5.7 | 596/70.7 * | 71 (26–91) * | NA |
| Jeun, 2023 [44] | 76 | 76/100 | 43/56.6 | 60 (32–83) | 45.9 (4.3–102.9) |
| Matsukane, 2023 [45] | 1008 | 14/1.4 | 732/72.6 * | 68 (14–89) * | NA |
| Nagao, 2024 [46] | 1069 | 19/1.8 | 15/78.9 | 67 (57–78) | 12.3 * |
| Nagpal, 2020 [47] | 56 | 8/14.3 | 4/50 | 76 (61–88) | NA |
| Ngamphaiboon, 2021 [48] | 414 | 11/2.7 | 287/69.3 * | 63 (17–97) * | NA |
| Nizam, 2024 [49] | 16 | 1/6.3 | 11/68.7 * | 73 (47–79) * | NA |
| Owen, 2021 [50] | 118 | 4/3.4 | 80/67.8 * | 64 (30–89) * | 21 * |
| Pollack, 2018 [51] | 80 | 4/5 | 44/55 * | 56 (25–89) * | 14.3 * |
| Ruf, 2024 [52] | 217 | 6/2.8 | 124/57.1 * | 66 (23–89) * | NA |
| Satish, 2023 [53] | 12,905 | 23/0.2 | 16/69.6 | 62 (8) † | NA |
| Shah, 2019 [54] | 1672 | 21/1.3 | NA | NA | NA |
| Shi, 2022 [55] | 1905 | 20/1 | 1442/75.7 * | 63 (56–68) * | NA |
| Shirwaikar, 2021 [56] | 11,165 | 248/2.2 | NA | NA | NA |
| Townsend, 2023 [36] | 6450 | 105/1.6 | 59/56.2 | 64 (24–88) | 22.1 (1.2–100.6) |
| Tsang, 2019 [57] | 538 | 10/1.9 | 9/90 | 62 (43–79) | NA |
| Wei, 2024 [10] | 2829 | 10/0.4 | 8/80 | 63 (40–88) | NA |
| Zhang, 2023 [58] | 2477 | 25/1 | 14/56 | 65 | NA |
| Study $ | Tumor Sites (n/%) | Disease Stage (n/%) | Median Time of Onset, Days (Range) | Median Time to Resolution, Days (Range) | Patients with Elevated Lipase Levels (n/%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | GI | Thoracic/ H&N | GU/Gynae | Others | Metastatic | Localized | ||||
| Abu-Sbeih, 2019 [11] | 30/36.6 | 5/6.1 | 11/13.4 | 24/29.3 | 12/14.6 | 72/87.8 | 10/12.2 | NA | Steroid-55(11) † IVF-55(51) † | 41/50 |
| Akazawa, 2014 [37] | NA | NA | NA | NA | NA | 36/100 | 0/0 | 105.5 | NA | NA |
| Byun, 2020 [38] | 5/27.8 | 2/11.1 | 1/5.6 | 8/44.4 | 2/11.1 | 16/88.9 | 2/11.1 | 109.5 | NA | 4/22 |
| Eshet, 2018 [39] | 21/67.7 | 0 | 10/32.3 | 0 | 0 | NA | NA | 270 # | NA | NA |
| Eyada, 2021 [40] | 10/12.8 | 0 | 10/11.8 | 40/47 | 25/29.4 | 63/74.1 | 22/26 | NA | NA | NA |
| Gleeson, 2023 [41] | 288/26.3 * | 27/2.5 * | 223/20.3 * | 185/16.9 * | 74/6.7 * | NA | NA | NA | NA | NA |
| Grimmelmann, 2021 [42] | 68/100 | 0 | 0 | 0 | 0 | 68/100 | 0 | 135 | NA | 68/100 |
| Hori, 2024 [43] | 89/10.6 * | 164/19.5 * | 429/50.9 * | 136/16 * | 25/3 * | 48/100 | 0 | NA | NA | 48/100 |
| Jeun, 2023 [44] | 23/30.2 | 0/0 | 12/15.8 | 11/14.5 | 30/39.5 | NA | NA | 86.1 | NA | 20/26 |
| Matsukane, 2023 [45] | 147/14.6 * | 178/17.7 * | 494/49 * | 166/16.5 * | 23/2 * | NA | NA | 66 | NA | NA |
| Nagao, 2024 [46] | 4/21.1 | 1/5.3 | 4/21.1 | 10/52.5 | 0 | 335/31.4 * | 734/68.6 * | 92 (19–706) | NA | 19/100 |
| Nagpal, 2020 [47] | 0 | 0 | 2/25 | 5/62.5 | 1/12.5 | NA | NA | 130.5 (1–434) | NA | 8/100 |
| Ngamphaiboon, 2021 [48] | 33/8 * | 66/15.9 * | 235/56.8 * | 60/14.5 * | 20/5 * | 408/98.6 * | 6/1.4 * | 30 (14–254) | 84 (1–707) | NA |
| Nizam, 2024 [49] | 0 | 0 | 0 | 1/100 | 0 | 1/100 | 0 | NA | NA | NA |
| Owen, 2021 [50] | 4/100 | 0 | 0 | 0 | 0 | 107/90.7 * | 11/9.3 * | NA | NA | NA |
| Pollack, 2018 [51] | 4/100 | 0 | 0 | 0 | 0 | 4/100 | 0 | NA | NA | 4/100 |
| Ruf, 2024 [52] | 206/94.9 * | 3/1.4 * | 2/0.9 * | 6/2.8 * | 0 * | NA | NA | 63 | NA | NA |
| Satish, 2023 [53] | 8/34.8 | 2/8.7 | 4/17.4 | 6/26.1 | 3/13 | NA | NA | 390 (252–578) # | NA | 15/65 |
| Shah, 2019 [54] | NA | NA | NA | NA | NA | NA | NA | NA | NA | 17/81 |
| Shi, 2022 [55] | 0 | 0 | 20/100 | 0 | 0 | 1488/78.1 * | 417/21.9 * | 92.5 | NA | NA |
| Shirwaikar, 2021 [56] | NA | NA | NA | NA | NA | NA | NA | NA | NA | 248/100 |
| Townsend, 2023 [36] | 33/31.4 | 5/4.8 | 18/17.2 | 12/11.4 | 37/35.2 | NA | NA | 84 | NA | 105/100 |
| Tsang, 2019 [57] | 10/100 | 0 | 0 | 0 | 0 | 10/100 | 0 | 175 | NA | NA |
| Wei, 2024 [10] | 3/30 | 1/10 | 3/30 | 3/30 | 0 | NA | NA | 295.5 | NA | 1/10 |
| Zhang, 2023 [58] | 8/32 | 1/4 | 8/32 | 8/32 | 0 | NA | NA | 83.3 | NA | NA |
| Number of Events (%) | 95% CI | |
|---|---|---|
| Chronic pancreatic complications | 215 (63.45%) | 29.03–91.56% |
| Diabetes mellitus | 183 (89.45%) | 61.88–100.0% |
| Insulin use | 18 (80.07%) | 24.49–100.0% |
| Oral hypoglycemic use | 4 (19.93%) | 0.0–75.51% |
| Exocrine pancreatic insufficiency | 32 (10.55%) | 0.0–38.12% |
| Pancrelipase use | 25 (50.42%) | 0.0–100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.L.; Riya, I.J.; Piya, I.J.; Muniz, T.P.; Butler, M.O.; Saibil, S.D. Immune Checkpoint Inhibitor-Induced Pancreatic Injury (ICI-PI) in Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1080. https://doi.org/10.3390/cancers17071080
Lee CL, Riya IJ, Piya IJ, Muniz TP, Butler MO, Saibil SD. Immune Checkpoint Inhibitor-Induced Pancreatic Injury (ICI-PI) in Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(7):1080. https://doi.org/10.3390/cancers17071080
Chicago/Turabian StyleLee, Cha Len, Israt Jahan Riya, Ifrat Jahan Piya, Thiago Pimentel Muniz, Marcus Otho Butler, and Samuel David Saibil. 2025. "Immune Checkpoint Inhibitor-Induced Pancreatic Injury (ICI-PI) in Adult Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 17, no. 7: 1080. https://doi.org/10.3390/cancers17071080
APA StyleLee, C. L., Riya, I. J., Piya, I. J., Muniz, T. P., Butler, M. O., & Saibil, S. D. (2025). Immune Checkpoint Inhibitor-Induced Pancreatic Injury (ICI-PI) in Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 17(7), 1080. https://doi.org/10.3390/cancers17071080







