The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
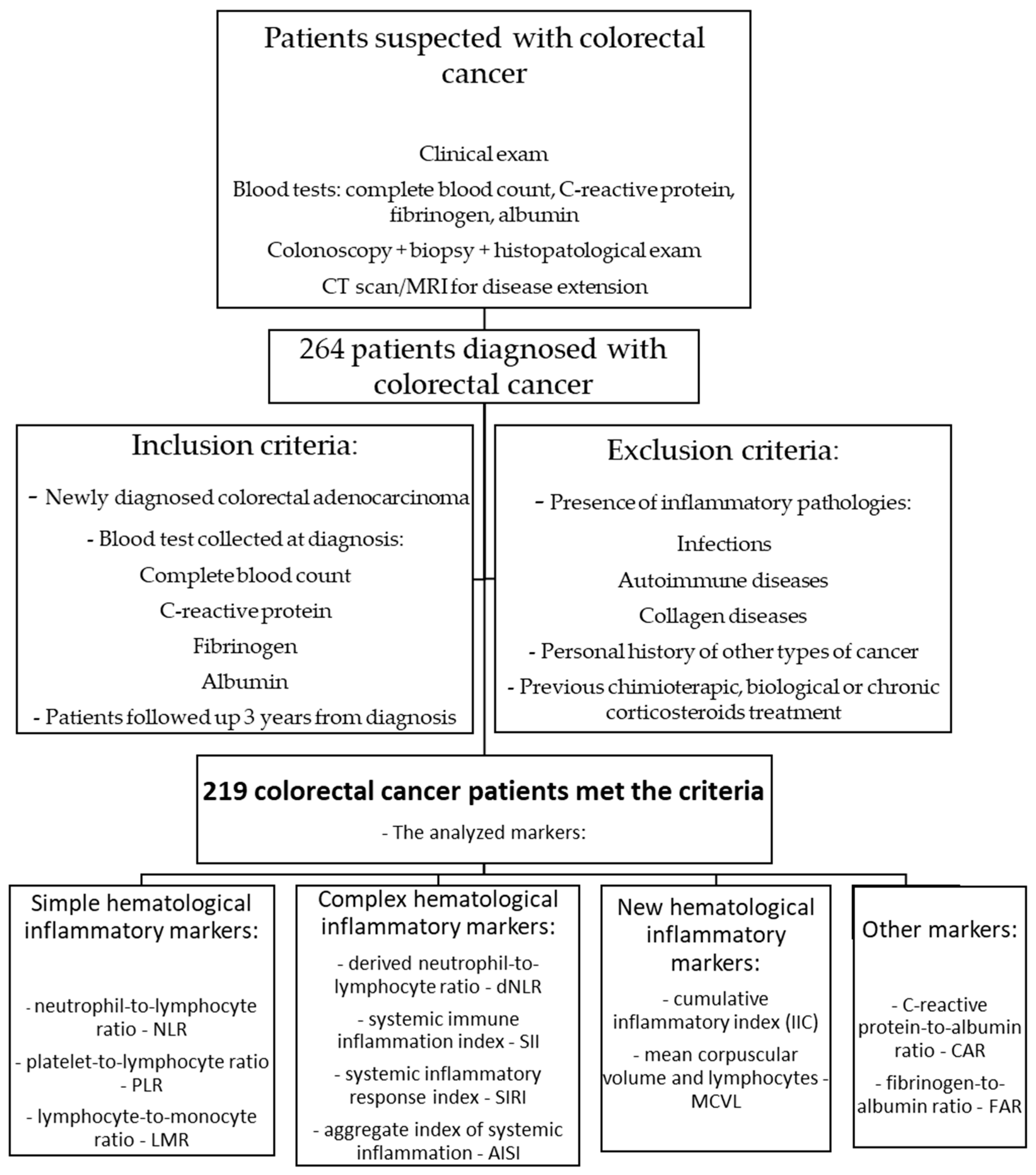
2.1. Inclusion Criteria for Patients and Study Design
2.2. Statistical Analysis
3. Results
3.1. Correlation of Inflammatory Markers with Clinicopathological and Tumor Characteristics in Colorectal Cancer Patients
3.1.1. Correlation of Inflammatory Markers with TNM Stages
3.1.2. Correlation of Inflammatory Markers with Pathological Tumor Differentiation Grade (G)
3.1.3. Correlation of Inflammatory Markers with Clinicopathological Characteristics of Colorectal Patients
3.2. Diagnostic and Prognostic Value of Analyzed Inflammatory Markers in Colorectal Cancer Patients
3.3. Comparison Between Inflammatory Markers Regarding Mean Survival Time in Colorectal Cancer Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| LMR | Lymphocyte-to-monocyte ratio |
| SII | Systemic immune inflammation index |
| SIRI | Systemic inflammatory response index |
| AISI | Aggregate index of systemic inflammation |
| dNLR | Derived neutrophil-to-lymphocyte ratio |
| IIC | Cumulative inflammatory index |
| MCVL | Mean corpuscular volume and lymphocytes |
| TNM | Tumor, node, metastasis |
References
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Hislop, G. Trends and risk factors for colorectal cancer. Br. Columbia Med. J. 2000, 42, 131–135. [Google Scholar]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Șerban, R.E.; Boldeanu, M.V.; Florescu, D.N.; Ionescu, M.; Șerbănescu, M.S.; Boldeanu, L.; Florescu, M.M.; Stepan, M.D.; Obleagă, V.C.; Constantin, C.; et al. Comparison between Substance P and Calcitonin Gene-Related Peptide and Their Receptors in Colorectal Adenocarcinoma. J. Clin. Med. 2024, 13, 5616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavel, C.; Diculescu, M.M.; Ilie, M.; Plotogea, O.M.; Sandru, V.; Enache, V.; Gheonea, D.I.; Jichitu, A.; Constantinescu, A.; Serban, R.E.; et al. Immunohistochemistry Analysis in Inflammatory Bowel Disease-Should We Bring to Light Interleukin-10? Biomedicines 2025, 13, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef]
- Vigano, A.; Bruera, E.; Jhangri, G.S.; Newman, S.C.; Fields, A.L.; Suarez-Almazor, M.E. Clinical survival predictors in patients with advanced cancer. Arch. Intern. Med. 2000, 160, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Florescu, D.N.; Boldeanu, M.V.; Șerban, R.E.; Florescu, L.M.; Serbanescu, M.S.; Ionescu, M.; Streba, L.; Constantin, C.; Vere, C.C. Correlation of the Pro-Inflammatory Cytokines IL-1β, IL-6, and TNF-α, Inflammatory Markers, and Tumor Markers with the Diagnosis and Prognosis of Colorectal Cancer. Life 2023, 13, 2261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Mishalian, I.; Bayuh, R.; Eruslanov, E.; Michaeli, J.; Levy, L.; Zolotarov, L.; Singhal, S.; Albelda, S.M.; Granot, Z.; Fridlender, Z.G. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—A new mechanism of impaired antitumor immunity. Int. J. Cancer 2014, 135, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 2020, 119, 199–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Davis, R.P.; Jenne, C.N. Platelets as Modulators of Inflammation. Semin. Thromb. Hemost. 2018, 44, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019, 39, 345–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Y.; Yang, J.; Han, X. Fibrinogen-to-Albumin Ratio is Associated with All-Cause Mortality in Cancer Patients. Int. J. Gen. Med. 2021, 14, 4867–4875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsilidis, K.K.; Branchini, C.; Guallar, E.; Helzlsouer, K.J.; Erlinger, T.P.; Platz, E.A. C-reactive protein and colorectal cancer risk: A systematic review of prospective studies. Int. J. Cancer 2008, 123, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454, Erratum in: N. Engl. J. Med. 1999, 340, 1376. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, Z.; Qiu, Y.; Zhang, J.; Wu, H.; Liang, R.; Chen, G.; Qin, G.; Li, Y.; Zou, D. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2018, 44, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barone, B.; Napolitano, L.; Reccia, P.; De Luca, L.; Morra, S.; Turco, C.; Melchionna, A.; Caputo, V.F.; Cirillo, L.; Fusco, G.M.; et al. Preoperative Fibrinogen-to-Albumin Ratio as Potential Predictor of Bladder Cancer: A Monocentric Retrospective Study. Medicina 2022, 58, 1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfensig, C.; Dominik, A.; Borufka, L.; Hinz, M.; Stange, J.; Eggert, M. A New Application for Albumin Dialysis in Extracorporeal Organ Support: Characterization of a Putative Interaction Between Human Albumin and Proinflammatory Cytokines IL-6 and TNFα. Artif. Organs 2016, 40, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Song, W.; Tian, X.; Sun, Y. Prognostic significance of platelet-to-lymphocyte ratio in patients with ovarian cancer: A meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12917. [Google Scholar] [CrossRef] [PubMed]
- Tham, T.; Olson, C.; Khaymovich, J.; Herman, S.W.; Costantino, P.D. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2018, 275, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Q.; Fan, J.; Cheng, S.; Ding, W.; Hua, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin. Chim. Acta. 2018, 479, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.; Chai, K. Systemic immune-inflammation index is associated with disease activity in patients with ankylosing spondylitis. J. Clin. Lab. Anal. 2021, 35, e23964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Tian, W.; Zhao, F.; Li, M.; Ye, Q.; Wei, Y.; Li, T.; Xie, K. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget 2018, 9, 35293–35299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, L.; Xie, H.; Yan, P. Prognostic value of the systemic inflammation response index in human malignancy: A meta-analysis. Medicine 2020, 99, e23486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.K.; Wei, Q.; Yang, Y.L.; Lu, T.Y.; Yan, Y.; Wang, F. Clinical usefulness of the lymphocyte-to-monocyte ratio and aggregate index of systemic inflammation in patients with esophageal cancer: A retrospective cohort study. Cancer Cell Int. 2023, 23, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, T.; Hao, L.; Yang, X.; Luo, C.; Wang, G.; Cai, C.L.; Qi, S.; Li, Z. Prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with non-small cell lung cancer receiving immune checkpoint inhibitors: A meta-analysis. BMJ Open 2021, 11, e049123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poenariu, I.S.; Boldeanu, L.; Ungureanu, B.S.; Caragea, D.C.; Cristea, O.M.; Pădureanu, V.; Siloși, I.; Ungureanu, A.M.; Statie, R.C.; Ciobanu, A.E.; et al. Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis. Biomedicines 2023, 11, 3137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radulescu, P.M.; Davitoiu, D.V.; Baleanu, V.D.; Padureanu, V.; Ramboiu, D.S.; Surlin, M.V.; Bratiloveanu, T.C.; Georgescu, E.F.; Streba, C.T.; Mercut, R.; et al. Has COVID-19 Modified the Weight of Known Systemic Inflammation Indexes and the New Ones (MCVL and IIC) in the Assessment as Predictive Factors of Complications and Mortality in Acute Pancreatitis? Diagnostics 2022, 12, 3118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranzani, O.T.; Zampieri, F.G.; Forte, D.N.; Azevedo, L.C.; Park, M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS ONE 2013, 8, e59321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaplan, M.; Ates, I.; Akpinar, M.Y.; Yuksel, M.; Kuzu, U.B.; Kacar, S.; Coskun, O.; Kayacetin, E. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Glapa-Nowak, A.; Szczepanik, M.; Banaszkiewicz, A.; Kwiecień, J.; Szaflarska-Popławska, A.; Grzybowska-Chlebowczyk, U.; Osiecki, M.; Kierkuś, J.; Dziekiewicz, M.; Walkowiak, J. C-Reactive Protein/Albumin Ratio at Diagnosis of Pediatric Inflammatory Bowel Disease: A Retrospective Multi-Center Study. Med. Sci. Monit. 2022, 28, e937842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tominaga, T.; Nonaka, T.; Sumida, Y.; Hidaka, S.; Sawai, T.; Nagayasu, T. The C-Reactive Protein to Albumin Ratio as a Predictor of Severe Side Effects of Adjuvant Chemotherapy in Stage III Colorectal Cancer Patients. PLoS ONE 2016, 11, e0167967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, L.; Yan, F.H.; Liu, C.; Chen, J.; Wang, D.; Zhang, C.H.; Lou, C.J.; Lian, J.; Yao, Y.; Wang, B.J.; et al. Systemic Inflammatory Biomarkers, Especially Fibrinogen to Albumin Ratio, Predict Prognosis in Patients with Pancreatic Cancer. Cancer Res. Treat. 2021, 53, 131–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, Z.; Zhang, M.; Han, Q.; Wen, J.; Luo, K.; Lin, P.; Zhang, L.; Yang, H.; Fu, J. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: The Fibrinogen/Albumin Ratio. J. Cancer 2017, 8, 1025–1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 2019, 9, 19673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, S.; Shahab, S.; Rauf, M.; Riaz, S.K.; Sheikh, A.K.; Tariq, J.; Gilani, A.; Gilani, F. Neutrophil To Lymphocyte Ratio As A Predictor Of Severity In Colorectal Adenocarcinoma. J. Ayub Med. Coll. Abbottabad 2022, 34, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Okumura, H.; Matsumoto, M.; Miyazono, F.; Hokita, S.; Aikou, T. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003, 23, 4079–4083. [Google Scholar] [PubMed]
- Hu, G.; Liu, G.; Ma, J.Y.; Hu, R.J. Lymphocyte-to-monocyte ratio in esophageal squamous cell carcinoma prognosis. Clin. Chim. Acta. 2018, 486, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.J.; Ma, J.Y.; Hu, G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin. Chim. Acta. 2018, 481, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. Progress in human tumour immunology and immunotherapy. Nature 2001, 411, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.K.; Dworacki, G.; Tsukihiro, T.; Meidenbauer, N.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin. Cancer Res. 2002, 8, 2553–2562. [Google Scholar] [PubMed]
- Vayrynen, J.P.; Tuomisto, A.; Klintrup, K.; Makela, J.; Karttunen, T.J.; Makinen, M.J. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br. J. Cancer 2013, 109, 1839–1847. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banks, R.E.; Forbes, M.A.; Kinsey, S.E.; Stanley, A.; Ingham, E.; Walters, C.; Selby, P.J. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br. J. Cancer 1998, 77, 956–964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.Y.; Ke, L.C.; Liu, Q. The pretreatment platelet-to-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer: A meta-analysis. Medicine 2018, 97, e12897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mijic, S.; Dabrosin, C. Platelet Activation In Situ in Breasts at High Risk of Cancer: Relationship with Mammographic Density and Estradiol. J. Clin. Endocrinol. Metab. 2021, 106, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Richard, F.; Hamy, A.S.; Jongen, L.; Wildiers, H.; Ardui, J.; Punie, K.; Smeets, A.; Berteloot, P.; Vergote, I.; et al. Body Mass Index and Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2021, 113, 146–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, Z.; Xin, R.; Li, L.; Lv, L.; Wu, X. Platelet-to-lymphocyte ratio associated with the clinicopathological features and prognostic value of breast cancer: A meta-analysis. Int. J. Biol. Markers 2022, 37, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Acedo, A.L.; Langiu, M.; Crescence, L.; Mège, D.; Dubois, C.; Panicot-Dubois, L. Platelet and Cancer-Cell Interactions Modulate Cancer-Associated Thrombosis Risk in Different Cancer Types. Cancers 2022, 14, 730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.; Liang, C.; Xiao, F.; Yu, Q.; Wen, H.; Song, Z.; Tian, Y.; Shi, B.; Guo, Y.; Liu, D. Prognostic significance of platelet-to-lymphocyte ratio in non-small-cell lung cancer: A meta-analysis. Onco Targets Ther. 2016, 9, 869–876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, D.; Fu, Y.; Su, Q.; Wang, H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine 2016, 95, e3837, Erratum in: Medicine 2016, 95, e5074. https://doi.org/10.1097/01.md.0000490009.39850.74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yodying, H.; Matsuda, A.; Miyashita, M.; Matsumoto, S.; Sakurazawa, N.; Yamada, M.; Uchida, E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2016, 23, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta. 2018, 483, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, X.; He, Y.; Chen, X.; Liu, N.; Ding, Z.; Li, J. Prognostic role of platelet to lymphocyte ratio in prostate cancer: A meta-analysis. Medicine 2018, 97, e12504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zheng, L.; Quan, L.; Du, L. Prognostic role of platelet-to-lymphocyte ratio in oral cancer: A meta-analysis. J. Oral. Pathol. Med. 2021, 50, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, Z.; Zhou, K.; Jiang, S. The Predictive Role of Systemic Inflammation Response Index (SIRI) in the Prognosis of Stroke Patients. Clin. Interv. Aging. 2021, 16, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zinellu, A.; Collu, C.; Nasser, M.; Paliogiannis, P.; Mellino, S.; Zinellu, E.; Traclet, J.; Ahmad, K.; Mangoni, A.A.; Carru, C.; et al. The Aggregate Index of Systemic Inflammation (AISI): A Novel Prognostic Biomarker in Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2021, 10, 4134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, K.; Shi, D.; Yang, L.; Wang, Z.; Li, Y.; Gao, F.; Liu, Y.; Ma, X.; Zhou, Y. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann. Med. 2022, 54, 1667–1677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, C.; Jiang, H.; Wang, L.; Jiang, Z.; Jin, C. Baseline (derived) neutrophil-lymphocyte ratio associated with survival in gastroesophageal junction or gastric cancer treated with ICIs. Front. Oncol. 2025, 15, 1404695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.H.; Zhai, E.T.; Yuan, Y.J.; Wu, K.M.; Xu, J.B.; Peng, J.J.; Chen, C.Q.; He, Y.L.; Cai, S.R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Q.K.; Chen, P.; Hu, W.M.; Sun, P.; He, W.Z.; Jiang, C.; Kong, P.F.; Liu, S.S.; Chen, H.T.; Yang, Y.Z.; et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J. Transl. Med. 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Cao, D.; Huang, Y.; Xiong, Q.; Tan, D.; Liu, L.; Lin, T.; Wei, Q. The Prognostic and Clinicopathological Significance of Systemic Immune-Inflammation Index in Bladder Cancer. Front. Immunol. 2022, 13, 865643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, M.; Chen, L.; Kong, X.; Wang, X.; Fang, Y.; Li, X.; Wang, J. The Systemic Inflammation Response Index as an Independent Predictor of Survival in Breast Cancer Patients: A Retrospective Study. Front. Mol. Biosci. 2022, 9, 856064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Kong, X.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Pretreatment Systemic Inflammation Response Index in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy as a Useful Prognostic Indicator. Cancer Manag. Res. 2020, 12, 1543–1567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, K.; Inai, K.; Takeuchi, D.; Shinohara, T.; Nakanishi, T. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ. J. 2015, 79, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, H.; Tang, Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis. Markers 2017, 2017, 7089493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, R.; English, L.; Liu, W.K.; Tree, A.C.; Ayres, B.; Watkin, N.; Pickering, L.M.; Afshar, M. Red cell differential width (RDW) as a predictor of survival outcomes with palliative and adjuvant chemotherapy for metastatic penile cancer. Int. Urol. Nephrol. 2020, 52, 2301–2306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Xin, S.; Xu, B. Value Research of NLR, PLR, and RDW in Prognostic Assessment of Patients with Colorectal Cancer. J. Healthc. Eng. 2022, 2022, 7971415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, K.C.; Lin, Y.M.; Liu, C.C.; Wu, K.L.; Lee, K.C. High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis. Cancers 2022, 14, 945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallerstein, R.O., Jr. Laboratory evaluation of anemia. West. J. Med. 1987, 146, 443–451. [Google Scholar] [PubMed] [PubMed Central]
- Yildirim, A.; Duygulu, M.E.; Fidan, E. CDK4/6 Inhibitor-Associated Mean Corpuscular Volume Change: A Potential Parameter for Predicting Survival in Metastatic Breast Cancer? J. Coll. Physicians Surg. Pak. 2024, 34, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.L.; Zhou, X.; Xiao, D.C. Is red blood cell distribution width a prognostic factor for colorectal cancer? A meta-analysis. Front. Surg. 2022, 9, 945126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Fu, W.; Wang, H.; Bhatia, S.K.; Hammer, D.A.; Kowalska, M.A.; Muschel, R.J. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004, 64, 8613–8619. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Nordestgaard, B.G. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit. Rev. Clin. Lab. Sci. 2011, 48, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Zanoni, D.K.; Pillai, A.; Ganly, I.; Morris, L.G.; Shah, J.P.; Wong, R.J.; Patel, S.G. Host Factors Independently Associated With Prognosis in Patients With Oral Cavity Cancer. JAMA Otolaryngol. 2020, 146, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Carrero, J.J.; Zagai, U.; Evans, M.; Ingre, C.; Pawitan, Y.; Fang, F. Blood biomarkers and prognosis of amyotrophic lateral sclerosis. Eur. J. Neurol. 2020, 27, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Watson, W.S.; O’Gorman, P.; Preston, T.; Scott, H.R.; McArdle, C.S. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr. Cancer. 2001, 39, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Iseki, Y.; Ikeya, T.; Hirakawa, K. Prognostic Significance of the Preoperative Ratio of C-Reactive Protein to Albumin in Patients with Colorectal Cancer. Anticancer Res. 2016, 36, 995–1001. [Google Scholar] [PubMed]
- Nakazaki, H. Preoperative and postoperative cytokines in patients with cancer. Cancer 1992, 70, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Cromi, A.; Siesto, G.; Giudici, S.; Serati, M.; Formenti, G.; Franchi, M. Prognostic significance of preoperative plasma fibrinogen in endometrial cancer. Gynecol. Oncol. 2010, 119, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Sohda, M.; Sakai, M.; Yamaguchi, A.; Watanabe, T.; Nakazawa, N.; Ubukata, Y.; Kuriyam, K.; Sano, A.; Yokobori, T.; Ogawa, H.; et al. Pre-treatment CRP and Albumin Determines Prognosis for Unresectable Advanced Oesophageal Cancer. In Vivo 2022, 36, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, Q.; Liu, W.; Yang, Y.; Yang, B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer 2020, 20, 691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Artigas, A.; Wernerman, J.; Arroyo, V.; Vincent, J.L.; Levy, M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J. Crit. Care. 2016, 33, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology 1988, 8, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Tan, Y.A.; Gao, Q.F.; Li, S.Q.; Zhang, J.; Chen, Q.G.; Jiang, Y.H.; Zhang, L.; Ying, H.Q.; Wang, X.Z. Circulating fibrinogen to pre-albumin ratio is a promising biomarker for diagnosis of colorectal cancer. J. Clin. Lab. Anal. 2019, 33, e22635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Zhang, D.; Zeng, S.; Wu, T.; Wang, Y.; Zhang, H.; Wang, B.; Hu, X. Prognostic Value of Preoperative Albumin-to-Fibrinogen Ratio in Patients with Bladder Cancer. J. Cancer 2021, 12, 5864–5873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otowa, Y.; Nakamura, T.; Yamamoto, M.; Kanaji, S.; Matsuda, Y.; Matsuda, T.; Oshikiri, T.; Sumi, Y.; Suzuki, S.; Kakeji, Y. C-reactive protein to albumin ratio is a prognostic factor for patients with Stage II/III esophageal squamous cell cancer. Dis. Esophagus 2017, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, H.; Aoyama, T.; Tamagawa, A.; Komori, K.; Maezawa, Y.; Kano, K.; Murakawa, M.; Atsumi, Y.; Hara, K.; Kazama, K.; et al. Influence of the Preoperative C-Reactive Protein-to-Albumin Ratio on Survival and Recurrence in Patients With Esophageal Cancer. Anticancer Res. 2020, 40, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zheng, C.; Ding, M.; Zhang, L.; Wang, L.; Xie, M.; Zhou, J. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer 2017, 17, 285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Patient Characteristics | No. of Cases | Total | |
|---|---|---|---|
| Age | ≥70 Years old | 128 (58.4%) | 219 (100%) |
| <70 years old | 91 (41.5%) | ||
| Gender | Male | 127 (57.9%) | 219 (100%) |
| Female | 92 (42%) | ||
| Tumor Localization | Rectum | 50 (22.8%) | 219 (100%) |
| Sigmoid | 71 (32.4%) | ||
| Descending | 18 (8.21%) | ||
| Transverse | 21 (9.5%) | ||
| Ascending | 49 (22.3%) | ||
| Cecum | 10 (4.5%) | ||
| TNM Classification | I | 32 (14.6%) | 219 (100%) |
| II | 80 (36.5%) | ||
| III | 59 (26.9%) | ||
| IV | 48 (21.9%) | ||
| T Stage | T1 | 12 (5.4%) | 219 (100%) |
| T2 | 21 (9.5%) | ||
| T3 | 143 (65.2%) | ||
| T4 | 43 (19.6%) | ||
| N Stage | N0 | 122 (55.7%) | 219 (100%) |
| N1 | 64 (29.2%) | ||
| N2 | 33 (15%) | ||
| M Stage | M0 | 171 (78%) | 219 (100%) |
| M1 | 48 (21.9%) | ||
| Tumor Pathological Grade | G1 | 42 (19.1%) | 219 (100%) |
| G2 | 125 (57%) | ||
| G3 | 52 (23.7%) | ||
| TNM Stage | I | II | III | IV | p-Value |
|---|---|---|---|---|---|
| No. of Patients | 32 | 80 | 59 | 48 | |
| Marker | |||||
| NLR | 3.73 (±1.79) | 3.88 (±2.86) | 4.3 (±2.5) | 4.73 (±2.12) | 0.023 * |
| PLR | 182.74 (±82.09) | 184.49 (±94.51) | 205.34 (±131.8) | 237.6 (±113.22) | 0.017 * |
| LMR | 3.76 (±2.71) | 3.38 (±1.33) | 3.25 (±1.57) | 2.88 (±1.45) | 0.048 * |
| SII | 1041.1 (±901.67) | 1204.74 (±773.89) | 1364.86 (±1249.61) | 1913.19 (±1241.55) | 0.041 * |
| SIRI | 2.3 (±2.09) | 2.36 (±1.58) | 2.52 (±3.37) | 3.72 (±2.81) | 0.036 * |
| AISI | 571.49 (±490.74) | 802.47 (±707.6) | 972.18 (±923.89) | 1503.51 (±1217.65) | 0.060 * |
| dNLR | 2.5 (±1.38) | 2.53 (±1.13) | 2.74 (±1.68) | 2.92 (±1.03) | 0.077 * |
| IIC | 4.73 (±2.29) | 5.03 (±4.54) | 5.61 (±3.39) | 6.32 (±3.32) | 0.046 * |
| MCVL | 70.72 (±33.03) | 51.2 (±20.13) | 49.66 (±15.12) | 48.99 (±22.65) | 0.036 * |
| CAR | 5.0 (±2.68) | 5.75 (±4.82) | 7.07 (±6.5) | 21.89 (±18.85) | <0.001 * |
| FAR | 97.1 (±36.63) | 103.1 (±24.79) | 121.15 (±31.22) | 145.4 (±38.28) | 0.003 * |
| Tumor Invasion | T1 | T2 | T3 | T4 | p-Value |
|---|---|---|---|---|---|
| No. of Patients | 12 | 21 | 143 | 43 | |
| Marker | |||||
| NLR | 4.22 (±2.55) | 4.23 (±1.73) | 4.74 (±2.34) | 5.12 (±2.33) | 0.067 * |
| PLR | 165.48 (±73.18) | 187.21 (±102.52) | 192.87 (±103.66) | 272.31 (±129.86) | 0.033 * |
| LMR | 3.99 (±1.57) | 3.59 (±2.25) | 2.94 (±1.52) | 2.8 (±1.22) | 0.250 * |
| SII | 982.46 (±691.25) | 1113.24 (±1092.64) | 1250.75 (±1172.48) | 2152.78 (±1452.35) | 0.029 * |
| SIRI | 2.07 (±1.53) | 2.37 (±1.82) | 2.56 (±1.8) | 3.56 (±2.74) | 0.810 * |
| AISI | 566.85 (±474.38) | 556.0 (±367.61) | 913.01 (±459.32) | 1544.59 (±1055.29) | 0.022 * |
| dNLR | 2.43 (±1.15) | 2.65 (±0.995) | 2.81 (±2.51) | 3.26 (±1.28) | 0.063 * |
| IIC | 4.82 (±3.4) | 5.16 (±4.77) | 5.7 (±2.4) | 6.61 (±4.23) | 0.056 * |
| MCVL | 73.16 (±34.43) | 63.33 (±31.21) | 53.53 (±20.58) | 49.2 (±19.5) | 0.041 * |
| CAR | 2.99 (±1.89) | 7.62 (±4.73) | 10.28 (±9.06) | 18.1 (±10.29) | <0.001 * |
| FAR | 93.1 (±45.91) | 100.35 (±30.34) | 114.83 (±34.97) | 136.03 (±30.71) | 0.015 * |
| Lymph Node Metastasis | N0 | N1 | N2 | p-Value |
|---|---|---|---|---|
| No. of Patients | 122 | 64 | 33 | |
| Marker | ||||
| NLR | 3.99 (±2.67) | 4.12 (±2.0) | 4.4 (±2.17) | 0.182 * |
| PLR | 198.19 (±118.61) | 200.52 (±91.68) | 227.41 (±122.6) | 0.226 * |
| LMR | 3.56 (±2.38) | 3.29 (±1.47) | 2.97 (±1.29) | 0.483 * |
| SII | 1275.8 (±1058.29) | 1485.43 (±963.45) | 1657.65 (±1310.36) | 0.004 * |
| SIRI | 2.49 (±1.98) | 2.95 (±2.31) | 3.04 (±2.43) | 0.080 * |
| AISI | 872.33 (±623.72) | 1099.39 (±999.32) | 1175.45 (±1128.72) | 0.004 * |
| dNLR | 2.57 (±1.42) | 2.64 (±0.999) | 2.87 (±1.34) | 0.157 * |
| IIC | 4.96 (±2.48) | 5.19 (±4.12) | 6.2 (±3.5) | 0.043 * |
| MCVL | 56.17 (±25.06) | 51.53 (±25.21) | 48.12 (±17.34) | 0.058 * |
| CAR | 6.62 (±6.27) | 12.14 (±7.03) | 14.24 (±13.82) | <0.001 * |
| FAR | 105.52 (±33.45) | 127.3 (±35.17) | 135.28 (±33.83) | <0.001 * |
| Distant Metastasis | M0 | M1 | p-Value |
|---|---|---|---|
| No. of Patients | 171 | 48 | |
| Marker | |||
| NLR | 3.91 (±2.47) | 4.73 (±2.12) | 0.840 * |
| PLR | 193.64 (±110.1) | 237.6 (±113.22) | 0.097 * |
| LMR | 3.53 (±2.12) | 2.88 (±1.45) | 0.062 * |
| SII | 1249.03 (±1218.04) | 1913.19 (±1241.55) | 0.039 * |
| SIRI | 2.42 (±1.63) | 3.72 (±2.81) | 0.050 * |
| AISI | 838.64 (±414.27) | 1503.51 (±1217.65) | 0.018 * |
| dNLR | 2.55 (±1.36) | 2.92 (±1.03) | 0.719 * |
| IIC | 5.04 (±3.68) | 6.12 (±3.32) | 0.089 * |
| MCVL | 54.09 (±25.04) | 49.66 (±15.12) | 0.053 * |
| CAR | 5.86 (±4.17) | 21.89 (±18.85) | 0.002 * |
| FAR | 108.24 (±30.96) | 145.4 (±38.28) | 0.027 * |
| Pathological Differentiation Grade | G1 | G2 | G3 | p-Value |
|---|---|---|---|---|
| No. of Patients | 42 | 125 | 52 | |
| Marker | ||||
| NLR | 4.23 (±3.07) | 4.13 (±2.41) | 3.86 (±1.76) | 0.469 * |
| PLR | 183.15 (±89.11) | 186.18 (±100.18) | 217.15 (±121.88) | 0.15 * |
| LMR | 3.63 (±1.77) | 3.41 (±2.31) | 3.14 (±1.3) | 0.363 * |
| CAR | 6.87 (±5.13) | 8.83 (±4.34) | 12.72 (±9.5) | 0.007 * |
| FAR | 104.01 (±39.6) | 115.89 (±34.66) | 127.48 (±33.75) | <0.001 * |
| SII | 1302.63 (±1096.02) | 1389.87 (±1193.15) | 1434.45 (±1334.79) | 0.907 * |
| SIRI | 2.66 (±1.95) | 2.69 (±2.28) | 2.85 (±2.5) | 0.617 * |
| AISI | 912.31 (±853.6) | 972.95 (±864.01) | 1013.32 (±951.82) | 0.664 * |
| dNLR | 2.77 (±1.95) | 2.65 (±1.17) | 2.49 (±0.887) | 0.372 * |
| IIC | 4.99 (±2.92) | 5.24 (±3.73) | 5.4 (±3.87) | 0.467 * |
| MCVL | 55.93 (±24.18) | 50.95 (±25.27) | 48.13 (±18.25) | 0.054 * |
| Age | Gender | Primary Tumor Location | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | ≥70 Years Old 128 Patients | <70 Years Old 91 Patients | p-Value | Men 127 Patients | Woman 92 Patients | p-Value | Left Colon 138 Patients | Right Colon 80 Patients | p-Value |
| NLR | 4.29 (±2.77) | 3.8 (±1.77) | 0.172 * | 4.12 (±2.32) | 4.04 (±2.55) | 0.843 * | 3.99 (±2.26) | 4.28 (±2.66) | 0.172 * |
| PLR | 205.89 (±107.67) | 199.59 (±118.38) | 0.128 * | 192.74 (±105.15) | 217.81 (±119.94) | 0.048 * | 193.62 (±102.2) | 220.94 (±126.32) | 0.128 * |
| LMR | 3.3 (±2.2) | 3.52 (±1.71) | 0.222 * | 3.63 (±2.51) | 3.32 (±1.54) | 0.365 * | 3.51 (±2.21) | 3.17 (±1.61) | 0.222 * |
| SII | 1457.9 (±1440.07) | 1305.56 (±922.49) | 0.08 * | 1365.26 (±1185.67) | 1435.1 (±1341.65) | 0.529 * | 1316.88 (±1138.06) | 1540.77 (±1424.36) | 0.058 * |
| SIRI | 2.87 (±1.17) | 2.48 (±1.89) | 0.254 * | 2.72 (±2.08) | 2.69 (±2.42) | 0.177 * | 2.58 (±2.28) | 2.96 (±2.35) | 0.254 * |
| AISI | 1031.32 (±616.57) | 918.32 (±820.4) | 0.092 * | 947.6 (±825.02) | 1035.12 (±795.86) | 0.698 * | 908.38 (±779.14) | 1125.13 (±827.19) | 0.062 * |
| dNLR | 2.73 (±1.51) | 2.49 (±0.925) | 0.06 * | 2.66 (±1.35) | 2.6 (±1.24) | 0.845 * | 2.57 (±1.31) | 2.76 (±1.29) | 0.06 * |
| IIC | 5.61 (±4.06) | 4.8 (±2.86) | 0.196 * | 5.38 (±4.03) | 5.2 (±3.32) | 0.884 * | 5.18 (±3.47) | 5.49 (±3.89) | 0.498 * |
| MCVL | 51.35 (±22.98) | 54.38 (±23.49) | 0.498 * | 53.21 (±23.39) | 53.06 (±23.25) | 0.669 * | 55.09 (±25.9) | 49.77 (±17.7) | 0.196 * |
| GLS | 9.59 (±6.57) | 9.12 (±6.88) | 0.345 * | 9.55 (±6.62) | 9.18 (±6.81) | 0.616 * | 9.53 (±6.68) | 9.27 (±6.81) | 0.345 * |
| FAR | 117.61 (±37.61) | 114.75 (±33.97) | 0.119 * | 110.67 (±36.08) | 124.29 (±34.76) | 0.018 * | 115.53 (±38.85) | 118.64 (±30.48) | 0.119 * |
| Marker | Survival Time | χ2 | p-Value | |
|---|---|---|---|---|
| High Levels | Low Levels | |||
| NLR | 30 months (76 patients) | 34 months (143 patients) | 13.68 | <0.001 * |
| PLR | 31.3 months (87 patients) | 33.6 months (132 patients) | 3.8 | 0.051 * |
| LMR | 34.7 months (99 patients) | 30.7 months (120 patients) | 4.41 | 0.036 * |
| SII | 31 months (83 patients) | 33.7 months (136 patients) | 5.31 | 0.021 * |
| SIRI | 30 months (79 patients) | 33.7 months (140 patients) | 5.59 | 0.017 * |
| AISI | 29.1 months (69 patients) | 34.03 months (150 patients) | 16.1 | <0.001 * |
| dNLR | 29.6 months (78 patients) | 34.2 months (141 patients) | 15 | <0.001 * |
| IIC | 29.5 months (77 patients) | 34.1 months (142 patients) | 19.2 | <0.001 * |
| MCVL | 34.3 months (104 patients) | 30.8 months (115 patients) | 4.37 | <0.001 * |
| CAR | 26.4 months (76 patients) | 35.5 months (143 patients) | 78.5 | <0.001 * |
| FAR | 27.6 months (79 patients) | 35.1 months (140 patients) | 42.64 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, R.-E.; Popescu, D.-M.; Boldeanu, M.-V.; Florescu, D.N.; Șerbănescu, M.-S.; Șandru, V.; Panaitescu-Damian, A.; Forțofoiu, D.; Șerban, R.-C.; Gherghina, F.-L.; et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers 2025, 17, 990. https://doi.org/10.3390/cancers17060990
Șerban R-E, Popescu D-M, Boldeanu M-V, Florescu DN, Șerbănescu M-S, Șandru V, Panaitescu-Damian A, Forțofoiu D, Șerban R-C, Gherghina F-L, et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers. 2025; 17(6):990. https://doi.org/10.3390/cancers17060990
Chicago/Turabian StyleȘerban, Robert-Emmanuel, Dragoș-Marian Popescu, Mihail-Virgil Boldeanu, Dan Nicolae Florescu, Mircea-Sebastian Șerbănescu, Vasile Șandru, Afrodita Panaitescu-Damian, Dragoș Forțofoiu, Rebecca-Cristiana Șerban, Florin-Liviu Gherghina, and et al. 2025. "The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma" Cancers 17, no. 6: 990. https://doi.org/10.3390/cancers17060990
APA StyleȘerban, R.-E., Popescu, D.-M., Boldeanu, M.-V., Florescu, D. N., Șerbănescu, M.-S., Șandru, V., Panaitescu-Damian, A., Forțofoiu, D., Șerban, R.-C., Gherghina, F.-L., & Vere, C.-C. (2025). The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers, 17(6), 990. https://doi.org/10.3390/cancers17060990







