Ex-Vivo Drug-Sensitivity Testing to Predict Clinical Response in Non-Small Cell Lung Cancer and Pleural Mesothelioma: A Systematic Review and Narrative Synthesis
Simple Summary
Abstract
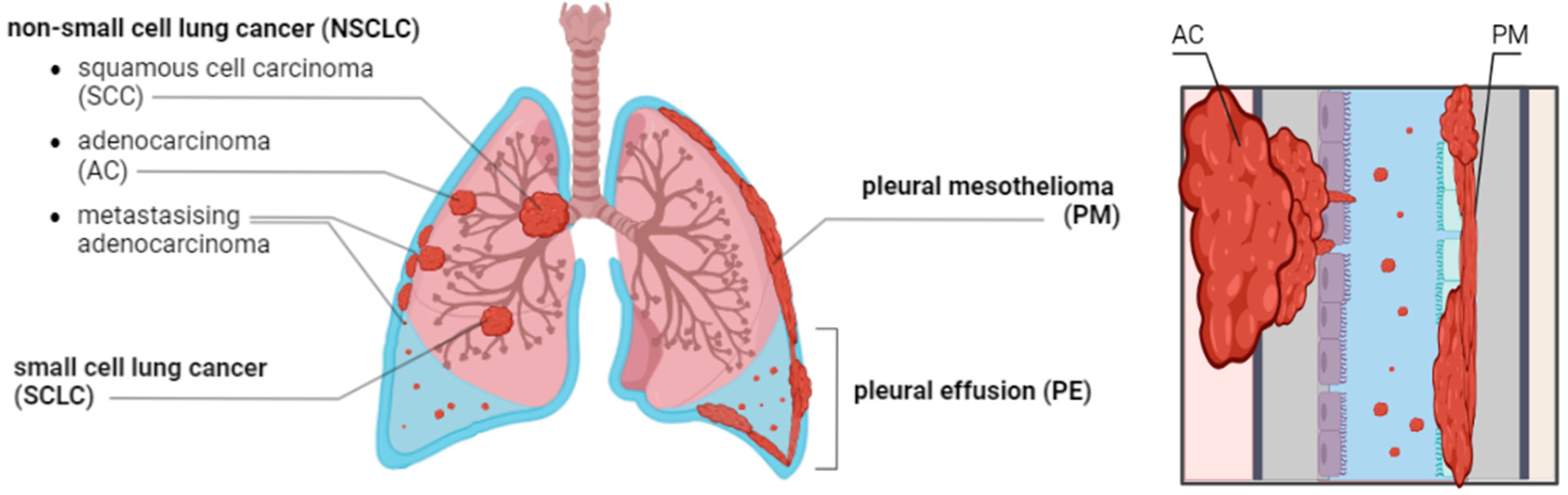
1. Introduction
2. Materials and Methods
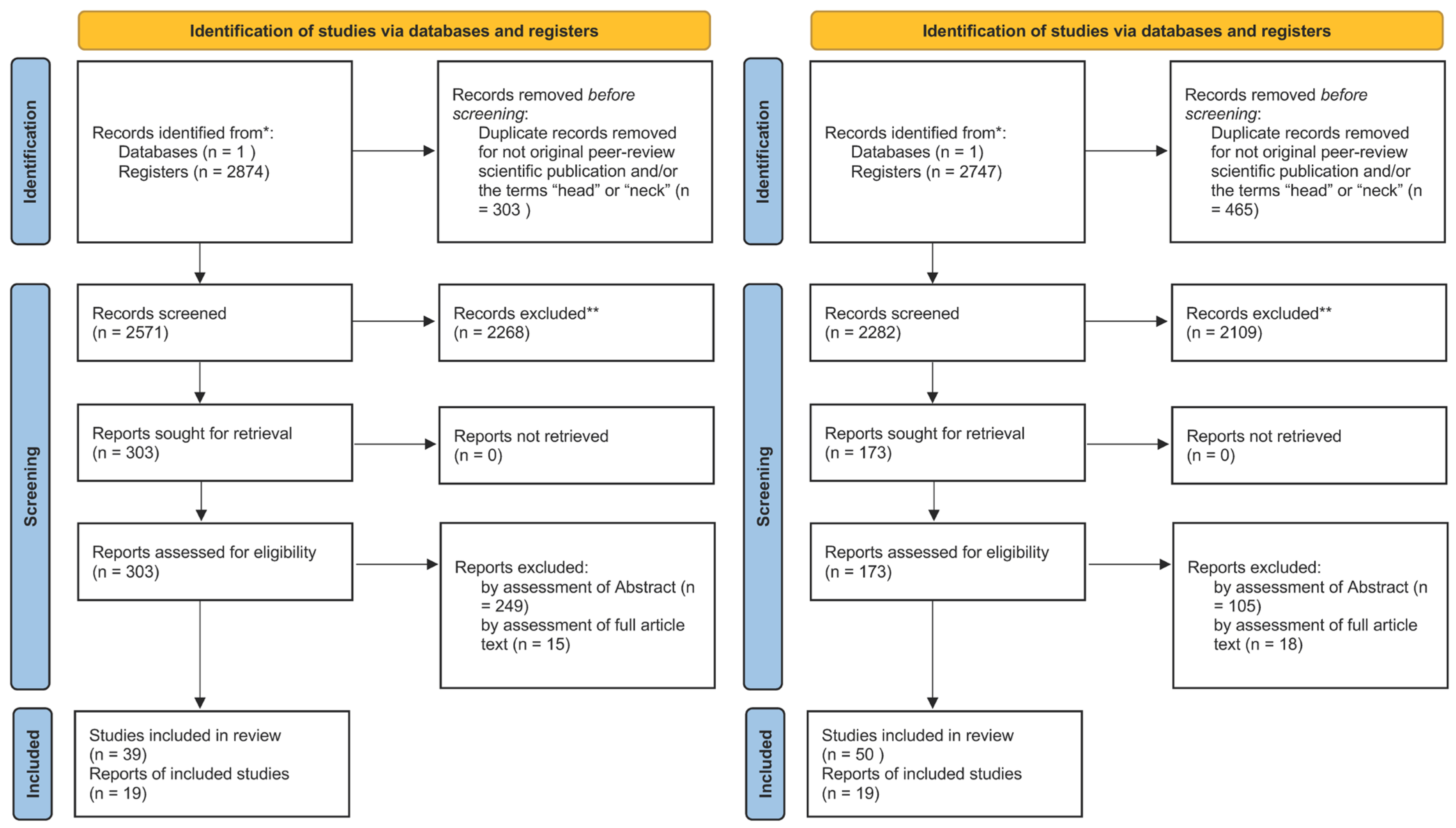
2.1. Search Strategy and Inclusion Criteria
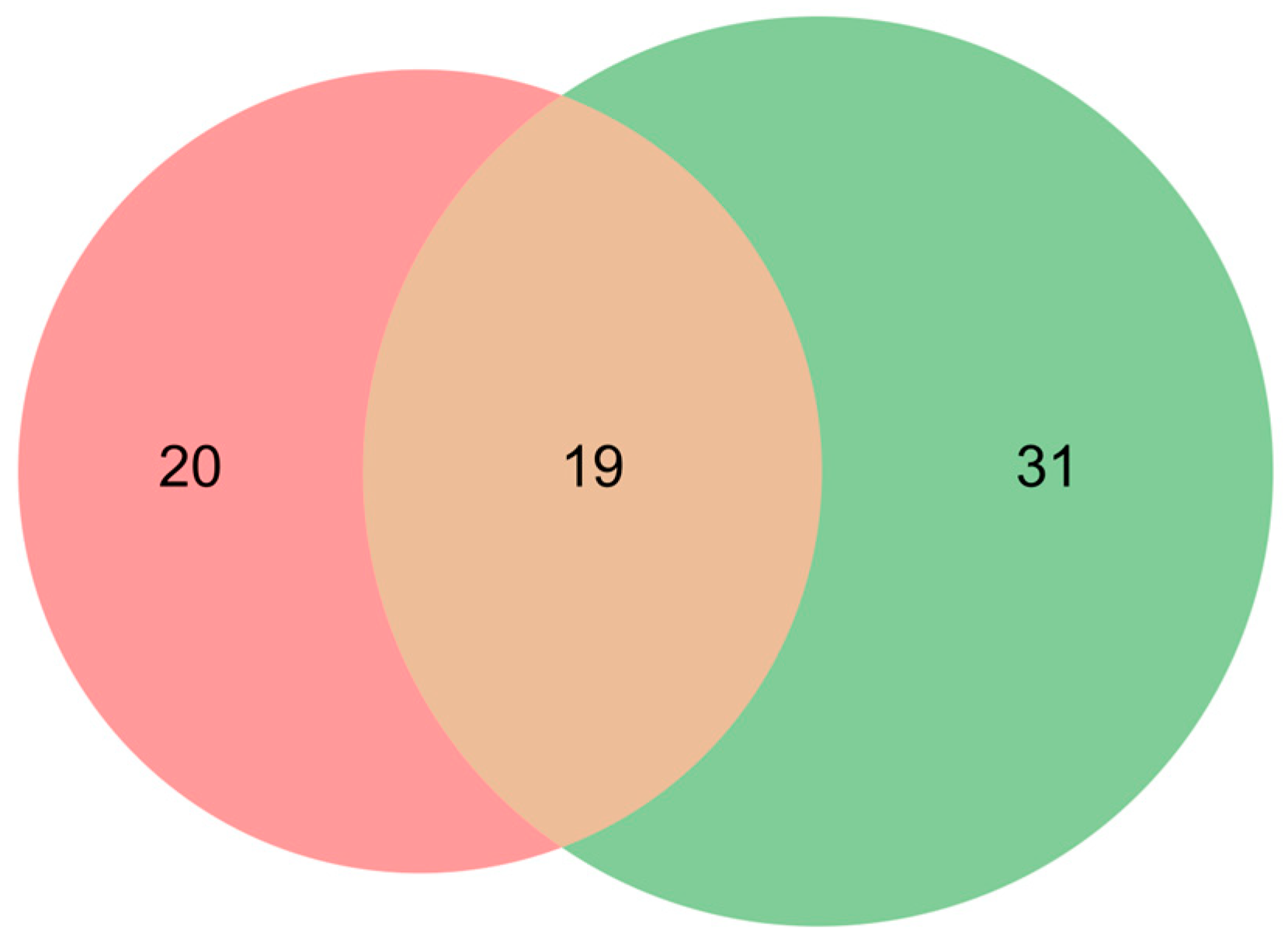
2.2. Study Selection
2.3. Data Extraction
2.4. Thematic Analysis
3. Results
3.1. Historical Progress Towards the Use of 3D Multicellular Model Systems with the Aim to Mimic the Papillary Growth of Cancer Cells In Vivo
3.2. Ex Vivo Drug-Sensitivity Identifies Optimal Therapy, Suggests More Effective Treatments, and Prevents the Use of Drugs to Which the Tumor Is Resistent
3.3. Ex Vivo Drug Sensitivity Results Can Predict Patient Response and Survival
3.4. Development of 3D Ex Vivo Cell Culture Models with Extracellular Matrix Components to Better Mimic the Tumor Microenvironment
3.5. Cancer Cells from Pleural Effusions Show Growth Advantages Ex Vivo, as Compared to Cells from Biopsies
| Reference | Number of Patients | Cell Origin | Cell Culture Model | Ex Vivo and Clinical Parameters Correlated | Observed Correlation Between Ex Vivo and Patient Response (Correlation Value) | Statistical Method | Patient Treatment in Clinic |
|---|---|---|---|---|---|---|---|
| Shie et al., 2023 [27] | 20 | AC, SCLC (biopsy) | 2D, 3DLdECM | DST vs. TR | Yes (85% accuracy 1) | N/A | ERL, GEF, AFA, PEM, GEM |
| Wu et al., 2020 [18] | 1 | NSCLC (TKI-resistant) (PE) | 2D PO | DST vs. TR | Yes, qualitative (N/A) | N/A | ICO, Cis, GEM, PEM, DTX |
| Papp et al., 2020 [32] | 14 | AC (biopsy, PE) | 3D | DST vs. TR | Yes (93% accuracy 1) | N/A | Cis, CAR, VNR, GEM, PTX, PEM, ERL, GEF |
| Kim et al., 2019 [33] | 10 | AC (biopsy, PE) | 2D collagen IV | DST vs. TR, PFS | Yes (100% accuracy 1) | N/A | OS, GEF, ENT, CRZ |
| Vinayanuwattikun et al., 2019 [23] | 11 | NSCLC (PE) | 2D | DST vs. TR | Yes, qualitative (N/A) | N/A | TXT, GEM, ERL, PEM, VNR, CAR, PTX |
| Hillerdal et al., 2017 [11] | 8 | AC, PM (PE) | 3D | DST vs. TR | Yes (78% accuracy 1) | N/A | CAR, Cis, GEM, DOX, PEM, VNR |
| Chen et al., 2018 [19] | 24 | Lung cancer tissue | 2D | PFS and OS of DST sensitive vs. resistant | Yes (overall), p = 0.046 (PFS), p = 0.036 (OS) (TXT only), p = 0.041 (PFS), p = 0.040 (OS) | Wilcoxon | Cis, PEM, OP, EP, CAR, VNR, VNC, TXT, GEM, PTX |
| Karekla et al., 2017 [20] | 25 | NSCLC (biopsy) | 2D | DST vs. MST | Yes, (p = 0.019) | Kaplan–Meier, Cox regression | Cis |
| Inoue et al., 2018 [24] | 75 | NSCLC (biopsy) | 3D collagen I | DST sensitive vs. 5-year OS, DFS | Yes, 5-year OS 82% (p = 0.039); DFS 68% (p = 0.089) | Kaplan–Meier, Wilcoxon | CAR, PTX |
| Roscilli et al., 2016 [34] | 6 | AC (PE) | 2D | DST vs. TR | Yes (100% accuracy 1) | N/A | Cis, CAR, TXT, VNR, GEM, GEF, ERL |
| Szulkin et al., 2014 [22] | 16 | PM, healthy patient (PE) | 2D | DST sensitive vs. OS | Yes (p = 0.005) | Unpaired t-test | 31 different, e.g., Cis, CAR, GEM, PEM, PTX, DOC, VNC, VNR, EP |
| Higashiyama et al., 2010 [35] | 81 | NSCLC (biopsy) | 3D collagen I | DST vs. TR | Yes (70% accuracy 1) | N/A | Cis, CAR, PTX, DOC, VNR, GEM |
| Kawamura et al., 2007 [36] | 49 | Metastatic biopsies, PE | 3D collagen I | DST sensitive vs. MST | Yes (p = 0.027) | Kaplan–Meier, Cox regression | DOC, PTX, CPT-11, VNR, GEM, Cis, CAR, VDS, EP |
| Moon et al., 2007 [21] | 34 | NSCLC (biopsy) | 2D | CRR, PFS 2, OS of DFS sensitive vs. resistant | CRR p = 0.036 PFS p = 0.060 OS p = 0.025 | Kaplan–Meier, Cox regression | Cis, CAR, PTX, DOC, GEM, VNR |
| Higashiyama et al., 2001 [37] | 25 | NSCLC | 3D collagen I | DST vs. TR | p = 0.001 | Chi-squared test | Cis, CAR, ETO, 5-FU, MMC, VDS |
| Shaw et al., 1996 [26] | 21 | NSCLC (lung, metastatic biopsy, PE) | 2D | DST sensitive vs. CRR | CRR p = 0.86 OS p = 0.34 | Fisher’s exact | 12 different drugs, e.g., Cis, EP, CTX, VNC, … |
| Shaw et al., 1993 [25] | 90 3 | NSCLC (lung and metastatic biopsy) | 2D | DST sensitive vs. CRR, OS of DST sensitive vs. resistant | CRR p = 0.076 OS p = 0.34 | Fisher’s exact test, Kaplan–Meier, Mantel-Haenszel | 12 different, e.g., Cis, EP, VNC, DOX, VBL, MM-C, … |
| Wilbur et al., 1992 [38] | 25 | NSCLC (tissue, PE) | 3D | DST sensitive vs. CRR | Yes (p = 0.04) | Wilcoxon | Cis, CPP, DOX, EP, 5-FL, IM, MM-C, VBL, VNC |
| Ajani et al., 1987 [39] | 14 | Lung (tissue, PE) | 2D CAM | DST vs. CRR | Yes (93% accuracy 1) | N/A | DOX, 4-HC, 5-FU Cis, EP, MM-C, VNC, BCNU, BLM |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Jeon, D.S.; Kim, H.C.; Kim, S.H.; Kim, T.J.; Kim, H.K.; Moon, M.H.; Beck, K.S.; Suh, Y.G.; Song, C.; Ahn, J.S.; et al. Five-Year Overall Survival and Prognostic Factors in Patients with Lung Cancer: Results from the Korean Association of Lung Cancer Registry (KALC-R) 2015. Cancer Res. Treat. 2023, 55, 103–111. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Yang, H.; Testa, J.R.; Carbone, M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr. Treat. Options Oncol. 2008, 9, 147–157. [Google Scholar] [CrossRef]
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [CrossRef]
- Hillerdal, C.-O.; Ötvös, R.; Szatmári, T.; Own, S.A.; Hillerdal, G.; Dackland, Å.-L.; Dobra, K.; Hjerpe, A. Ex Vivo Evaluation of Tumor Cell Specific Drug Responses in Malignant Pleural Effusions. Oncotarget 2017, 8, 82885–82896. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, R.; Szulkin, A.; Hiilerdal, C.-O.; Celep, A.; Yousef-Fadhel, E.; Skribek, H.; Hjerpe, A.; Szekely, L.; Dobra, K. Drug Sensitivity Profiling and Molecular Characteristics of Cells from Pleural Effusions of Patients with Lung Adenocarcinoma. Genes Cancer 2015, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Devarasetty, M.; Herberg, S.; Petty, W.J.; Marini, F.; Miller, L.; Kucera, G.; Dukes, D.K.; Ruiz, J.; Skardal, A.; et al. Pleural Effusion Aspirate for use in 3D Lung Cancer Modeling and Chemotherapy Screening. ACS Biomater. Sci. Eng. 2019, 5, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Akerlund, E.; Gudoityte, G.; Moussaud-Lamodiere, E.; Lind, O.; Bwanika, H.C.; Lehti, K.; Salehi, S.; Carlson, J.; Wallin, E.; Fernebro, J.; et al. The drug efficacy testing in 3D cultures platform identifies effective drugs for ovarian cancer patients. NPJ Precis. Oncol. 2023, 7, 111. [Google Scholar] [CrossRef]
- Kaur, G.; Doroshow, J.H.; Teicher, B.A. Format (2D vs 3D) and media effect target expression and response of patient-derived and standard NSCLC lines to EGFR inhibitors. Cancer Treat. Res. Commun. 2021, 29, 100463. [Google Scholar] [CrossRef]
- Murakami, S.; Tanaka, H.; Nakayama, T.; Taniura, N.; Miyake, T.; Tani, M.; Kushima, R.; Yamamoto, G.; Sugihara, H.; Mukaisho, K.I. Similarities and differences in metabolites of tongue cancer cells among two- and three-dimensional cultures and xenografts. Cancer Sci. 2021, 112, 918–931. [Google Scholar] [CrossRef]
- Tidwell, T.R.; Rosland, G.V.; Tronstad, K.J.; Soreide, K.; Hagland, H.R. Metabolic flux analysis of 3D spheroids reveals significant differences in glucose metabolism from matched 2D cultures of colorectal cancer and pancreatic ductal adenocarcinoma cell lines. Cancer Metab. 2022, 10, 9. [Google Scholar] [CrossRef]
- Wu, M.; Hong, G.; Chen, Y.; Ye, L.; Zhang, K.; Cai, K.; Yang, H.; Long, X.; Gao, W.; Li, H. Personalized drug testing in a patient with non-small-cell lung cancer using cultured cancer cells from pleural effusion. J. Int. Med. Res. 2020, 48, 300060520955058. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Ma, S.; Li, C.; Xu, C.; Shen, Y.; Zhao, J.; Miao, L. Evaluation of the in vitro Chemosensitivity and Correlation with Clinical Outcomes in Lung Cancer using the ATP-TCA. Anticancer. Agents Med. Chem. 2018, 18, 139–145. [Google Scholar] [CrossRef]
- Karekla, E.; Liao, W.J.; Sharp, B.; Pugh, J.; Reid, H.; Quesne, J.L.; Moore, D.; Pritchard, C.; MacFarlane, M.; Pringle, J.H. Ex Vivo Explant Cultures of Non-Small Cell Lung Carcinoma Enable Evaluation of Primary Tumor Responses to Anticancer Therapy. Cancer Res. 2017, 77, 2029–2039. [Google Scholar] [CrossRef]
- Moon, Y.W.; Choi, S.H.; Kim, Y.T.; Sohn, J.H.; Chang, J.; Kim, S.K.; Park, M.S.; Chung, K.Y.; Lee, H.J.; Kim, J.H. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer 2007, 109, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Szulkin, A.; Otvös, R.; Hillerdal, C.-O.; Celep, A.; Yousef-Fadhel, E.; Skribek, H.; Hjerpe, A.; Székely, L.; Dobra, K. Characterization and Drug Sensitivity Profiling of Primary Malignant Mesothelioma Cells from Pleural Effusions. BMC Cancer 2014, 14, 709. [Google Scholar] [CrossRef]
- Vinayanuwattikun, C.; Prakhongcheep, O.; Tungsukruthai, S.; Petsri, K.; Thirasastr, P.; Leelayuwatanakul, N.; Chanvorachote, P. Feasibility Technique of Low-passage In Vitro Drug Sensitivity Testing of Malignant Pleural Effusion from Advanced-stage Non-small Cell Lung Cancer for Prediction of Clinical Outcome. Anticancer. Res. 2019, 39, 6981–6988. [Google Scholar] [CrossRef]
- Inoue, M.; Maeda, H.; Takeuchi, Y.; Fukuhara, K.; Shintani, Y.; Funakoshi, Y.; Funaki, S.; Nojiri, T.; Kusu, T.; Kusumoto, H.; et al. Collagen gel droplet-embedded culture drug sensitivity test for adjuvant chemotherapy after complete resection of non-small-cell lung cancer. Surg. Today 2018, 48, 380–387. [Google Scholar] [CrossRef]
- Shaw, G.L.; Gazdar, A.F.; Phelps, R.; Ilona Linnoila, R.; Ihde, D.C.; Johnson, B.E.; Oie, H.K.; Pass, H.I.; Steinberg, S.M.; Ghosh, B.C.; et al. Individualized Chemotherapy for Patients with Non-Small Cell Lung Cancer by Prospective Identification of Neuroendocrine Markers and in Vitro Drug Sensitivity Testing. Cancer Res. 1993, 53, 5181–5187. [Google Scholar] [PubMed]
- Shaw, G.L.; Gazdar, A.F.; Phelps, R.; Steinberg, S.M.; Linnoila, R.I.; Johnson, B.E.; Oie, H.K.; Russell, E.K.; Ghosh, B.C.; Pass, H.I.; et al. Correlation of in vitro drug sensitivity testing results with response to chemotherapy and survival: Comparison of non-small cell lung cancer and small cell lung cancer. J. Cell Biochem. Suppl. 1996, 24, 173–185. [Google Scholar] [CrossRef]
- Shie, M.Y.; Fang, H.Y.; Kan, K.W.; Ho, C.C.; Tu, C.Y.; Lee, P.C.; Hsueh, P.R.; Chen, C.H.; Lee, A.K.; Tien, N.; et al. Highly Mimetic Ex Vivo Lung-Cancer Spheroid-Based Physiological Model for Clinical Precision Therapeutics. Adv. Sci. 2023, 10, e2206603. [Google Scholar] [CrossRef] [PubMed]
- Clohessy, J.G.; Pandolfi, P.P. The Mouse Hospital and Its Integration in Ultra-Precision Approaches to Cancer Care. Front. Oncol. 2018, 8, 340. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinska, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Maelandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Meijer, T.G.; Naipal, K.A.; Jager, A.; van Gent, D.C. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci. OA 2017, 3, FSO190. [Google Scholar] [CrossRef]
- Kodack, D.P.; Farago, A.F.; Dastur, A.; Held, M.A.; Dardaei, L.; Friboulet, L.; von Flotow, F.; Damon, L.J.; Lee, D.; Parks, M.; et al. Primary Patient-Derived Cancer Cells and Their Potential for Personalized Cancer Patient Care. Cell Rep. 2017, 21, 3298–3309. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Steib, A.; Abdelwahab, E.M.; Meggyes-Rapp, J.; Jakab, L.; Smuk, G.; Schlegl, E.; Moldvay, J.; Sarosi, V.; Pongracz, J.E. Feasibility study of in vitro drug sensitivity assay of advanced non-small cell lung adenocarcinomas. BMJ Open Respir. Res. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.Y.; Kim, D.H.; Joo, H.; Yun, M.R.; Jung, D.; Yun, J.; Heo, S.G.; Ahn, B.; Park, C.W.; et al. Patient-Derived Cells to Guide Targeted Therapy for Advanced Lung Adenocarcinoma. Sci. Rep. 2019, 9, 19909. [Google Scholar] [CrossRef] [PubMed]
- Roscilli, G.; De Vitis, C.; Ferrara, F.F.; Noto, A.; Cherubini, E.; Ricci, A.; Mariotta, S.; Giarnieri, E.; Giovagnoli, M.R.; Torrisi, M.R.; et al. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity. J. Transl. Med. 2016, 14, 61. [Google Scholar] [CrossRef]
- Higashiyama, M.; Oda, K.; Okami, J.; Maeda, J.; Kodama, K.; Imamura, F.; Minamikawa, K.; Takano, T.; Kobayashi, H. Prediction of Chemotherapeutic Effect on Postoperative Recurrence by in Vitro Anticancer Drug Sensitivity Testing in Non-Small Cell Lung Cancer Patients. Lung Cancer 2010, 68, 472–477. [Google Scholar] [CrossRef]
- Kawamura, M.; Gika, M.; Abiko, T.; Inoue, Y.; Oyama, T.; Izumi, Y.; Kobayashi, H.; Kobayashi, K. Clinical evaluation of chemosensitivity testing for patients with unresectable non-small cell lung cancer (NSCLC) using collagen gel droplet embedded culture drug sensitivity test (CD-DST). Cancer Chemother. Pharmacol. 2007, 59, 507–513. [Google Scholar] [CrossRef]
- Higashiyama, M.; Kodama, K.; Yokouchi, H.; Takami, K.; Nakagawa, H.; Imamura, F.; Minamigawa, K.; Kobayashi, H. Cisplatin-Based Chemotherapy for Postoperative Recurrence in Non-Small Cell Lung Cancer Patients: Relation of the in Vitro Chemosensitive Test to Clinical Response. Oncol. Rep. 2001, 8, 279–283. [Google Scholar] [CrossRef]
- Wilbur, D.W.; Camacho, E.S.; Hilliard, D.A.; Dill, P.L.; Weisenthal, L.M. Chemotherapy of Non-Small Cell Lung Carcinoma Guided by an in Vitro Drug Resistance Assay Measuring Total Tumour Cell Kill. Br. J. Cancer 1992, 65, 27–32. [Google Scholar] [CrossRef]
- Ajani, J.A.; Baker, F.L.; Spitzer, G.; Kelly, A.; Brock, W.; Tomasovic, B.; Singletary, S.E.; McMurtrey, M.; Plager, C. Comparison between Clinical Response and in Vitro Drug Sensitivity of Primary Human Tumors in the Adhesive Tumor Cell Culture System. J. Clin. Oncol. 1987, 5, 1912–1921. [Google Scholar] [CrossRef]
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 2020, 12, 2727. [Google Scholar] [CrossRef]
- Rezk, R.; Marín-García, R.; Gad, A.K.B. The Fibrillar Matrix: Novel Avenues for Breast Cancer Detection and Treatment. Engineering 2021, 7, 1375–1380. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125 Pt 13, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.; Rainero, E. Cross-Talk Between the Tumor Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol. 2020, 10, 239. [Google Scholar] [CrossRef]
- ang, M.; McKay, D.; Pollard, J.W.; Lewis, C.E. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res. 2018, 78, 5492–5503. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- van Ooijen, H.; Verron, Q.; Zhang, H.; Sandoz, P.A.; Frisk, T.W.; Carannante, V.; Olofsson, K.; Wagner, A.K.; Sandström, N.; Önfelt, B. A thermoplastic chip for 2D and 3D correlative assays combining screening and high-resolution imaging of immune cell responses. Cell Rep. Methods 2025, 5, 100965. [Google Scholar] [CrossRef]
- Alsaed, B.; Smolander, J.; Laitinen, H.; Lin, L.; Bobik, N.; Lahtinen, L.; Räsänen, M.; Jansouz, S.; Peltonen, K.; Jokinen, E.; et al. Ex Vivo Modeling of Precision Immuno-Oncology Responses in Lung Cancer. Sci. Adv. 2024, 10, eadq6830. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zipprick, J.; Demir, E.; Krynska, H.; Köprülüoğlu, S.; Strauß, K.; Skribek, M.; Hutyra-Gram Ötvös, R.; Gad, A.K.B.; Dobra, K. Ex-Vivo Drug-Sensitivity Testing to Predict Clinical Response in Non-Small Cell Lung Cancer and Pleural Mesothelioma: A Systematic Review and Narrative Synthesis. Cancers 2025, 17, 986. https://doi.org/10.3390/cancers17060986
Zipprick J, Demir E, Krynska H, Köprülüoğlu S, Strauß K, Skribek M, Hutyra-Gram Ötvös R, Gad AKB, Dobra K. Ex-Vivo Drug-Sensitivity Testing to Predict Clinical Response in Non-Small Cell Lung Cancer and Pleural Mesothelioma: A Systematic Review and Narrative Synthesis. Cancers. 2025; 17(6):986. https://doi.org/10.3390/cancers17060986
Chicago/Turabian StyleZipprick, Jenny, Enes Demir, Hanna Krynska, Sıla Köprülüoğlu, Katharina Strauß, Marcus Skribek, Rita Hutyra-Gram Ötvös, Annica K. B. Gad, and Katalin Dobra. 2025. "Ex-Vivo Drug-Sensitivity Testing to Predict Clinical Response in Non-Small Cell Lung Cancer and Pleural Mesothelioma: A Systematic Review and Narrative Synthesis" Cancers 17, no. 6: 986. https://doi.org/10.3390/cancers17060986
APA StyleZipprick, J., Demir, E., Krynska, H., Köprülüoğlu, S., Strauß, K., Skribek, M., Hutyra-Gram Ötvös, R., Gad, A. K. B., & Dobra, K. (2025). Ex-Vivo Drug-Sensitivity Testing to Predict Clinical Response in Non-Small Cell Lung Cancer and Pleural Mesothelioma: A Systematic Review and Narrative Synthesis. Cancers, 17(6), 986. https://doi.org/10.3390/cancers17060986








